Dear Editor:
Highly active antiretroviral therapy (HAART) is effective in lowering viral load in adults and children when taken as prescribed. It is less clear how often or for how long patients can miss doses, or how patterns of inadequate adherence to different treatment regimens with different potency or dosing frequency influence virologic control and immune status.1,2 There is also lack of clarity in how best to measure patient adherence to treatment. Adolescents, who account for the majority of new HIV infections,3 often have difficulty adhering to new medication regimens.4,5 It has previously been demonstrated that adolescents starting HAART with “perfect” self-reported adherence at weeks 4–16 had better short (24 week) and long-term (144 week) virologic control.6 Perfect adherence was defined as the adolescent reporting no antiretroviral doses missed in the 3 days prior to each of four study visits. This measure was restrictive as it could not distinguish between adolescents who might only have missed one dose at one study visit, all doses at one visit, or all doses at all study visits. The goal in this report was to assess other measures of early self-reported adherence in their ability to predict long-term virologic, immunologic, and adherence outcomes.
Pediatric AIDS Clinical Trials Group (PACTG) Protocol 381 was an observational study of 120 adolescents 11–22 years of age infected with HIV through risk behaviors, initiating HAART and followed for up to 3 years.6 The study opened to enrollment in March 1999 and closed to follow-up in November 2004. The study was approved by the Institutional Review Board at each site and informed consent obtained from all participants.
Adherence to each study drug at all study visits was assessed by self-report using the PACTG standardized Adherence Questionnaire. Each participant was asked to identify which antiretrovirals they were taking and how often, and how many doses of each treatment they had missed on each of the 3 days prior to the study visit. The missed doses information was converted to percent doses taken of expected (pi) across the 3 days, calculated as:
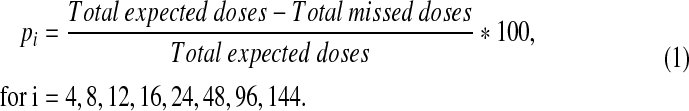 |
(1) |
Five adherence summary measures were calculated for each participant using data from the first five study visits up to week 24: p4 to p24: (1) perfect (100% adherence at all time points versus <100%); (2) mean of all time points, reflecting average adherence levels; (3) standard deviation (SD) of all time points reflecting variability; (4) skewness of all time points, reflecting asymmetry (e.g., a few visits with low (or high) versus many with high (or low) adherence); and (5) area under the curve (AUC) created by plotting the adherence at each time point and calculating the area under this curve.
Three outcomes at 48, 96, and 144 weeks of follow-up were defined: (1) controlled viral load: HIV-1 RNA ≤400 copies per milliliter by week 24 and sustained to each time point versus a confirmed HIV-1 RNA >400 copies per milliliter or off study, (2) immunologic reconstitution: CD4 cell count ≥100 cells/mm3 above CD4 count at entry versus CD4 <100 cells/mm3 above entry or off study, and (3) good adherence: on HAART with self-reported percent adherence greater than 95% versus adherence 95% or less or off HAART. Analyses included participants who stayed on HAART at least 24 weeks. If self-reported adherence at a visit before week 24 was unavailable, the value from the previous (or week 8 if missing week 4) visit was imputed. Ability of each summary measure to predict each outcome was assessed using logistic regression, modeling mean, AUC, SD, and skewness as continuous predictors and also modeling the mean categorized into three levels (<75%, 75%–95% and >95%).
Of the 120 adolescents (49% male, 71% black non-Hispanic, 67% >18 years, 89% CDC Disease Category A) who started HAART (58% on efavirenz and 38% on unboosted nelfinavir-based regimens), only 41 (34%) completed 144 weeks of follow-up on HAART. Twenty-four remained on their initial regimen. Thirty-two (33%) of the 96 who switched from their initial regimen onto a different combination changed because of poor adherence. Fifty-seven of the 79 participants (72%) who came off HAART completely either had poor adherence or were lost-to-follow-up and none came off because of improved health status. Among participants on HAART who completed the self-report adherence form, the percent reporting no missed doses in the 3 days prior to a study visit ranged from 57% to 74% and remained relatively constant throughout follow-up (visits were every 12 weeks after week 24).
One hundred two adolescents stayed on HAART at least 24 weeks. Twenty were missing one of the five adherence assessments and an additional 8 were missing at least two. The adherence percentage was imputed from the previous visit (or the week 8 value if missing week 4) for these missing values. Shown in Table 1 are week 24 adherence measures by outcome. For all long-term outcomes at all time points, adolescents with improved outcomes were more likely to have perfect adherence (higher % in fourth column of Table 1), higher mean adherence (higher median week 4–24 adherence) and lower variability (lower median SD) over the first 24 weeks. AUC correlated strongly with mean adherence (r = 0.98) and gave similar results and skewness was not predictive of any outcomes (not shown). For controlled viral load, higher week 24 mean adherence predicted virologic control out to week 144 and lower SD to week 96. Higher week 24 mean adherence was marginally predictive of improved CD4 counts at week 96 and week 144. Higher week 24 mean adherence predicted good self-reported adherence at all subsequent weeks, while the perfect measure and lower SD were significant predictors at weeks 48 and 96. Modeling week 24 mean adherence using three levels (<75%, 75%–95% and >95%) gave results similar to those modeling it as a continuous measure.
Table 1.
Relationship of Week 24 Adherence Summary Measures and Long-Term Outcomes
| |
|
|
Week 24 adherence summary measure |
|||||
|---|---|---|---|---|---|---|---|---|
| |
|
|
Perfect |
Mean |
SD |
|||
| Week | Long-term outcome | na | %b | pc | Medd | pe | Medf | pe |
| 48 | Controlled viral load | |||||||
| Yes | 59 | 42 | 0.21 | 96 | 0.003 | 8 | 0.011 | |
| No | 43 | 30 | 80 | 25 | ||||
| 96 | Yes | 44 | 48 | 0.058 | 98 | 0.001 | 5 | 0.007 |
| No | 58 | 29 | 83 | 17 | ||||
| 144 | Yes | 29 | 45 | 0.32 | 96 | 0.038 | 5 | 0.15 |
| No | 73 | 34 | 93 | 12 | ||||
| 48 | Improved reconstitution | |||||||
| Yes | 35 | 40 | 0.61 | 97 | 0.118 | 8 | 0.12 | |
| No | 66 | 35 | 92 | 10 | ||||
| 96 | Yes | 35 | 46 | 0.17 | 98 | 0.045 | 5 | 0.20 |
| No | 66 | 32 | 90 | 14 | ||||
| 144 | Yes | 24 | 42 | 0.56 | 96 | 0.082 | 9 | 0.34 |
| No | 77 | 35 | 93 | 10 | ||||
| 48 | Good adherence | |||||||
| Yes | 55 | 53 | <0.001 | 100 | <0.001 | 0 | 0.001 | |
| No | 47 | 19 | 80 | 28 | ||||
| 96 | Yes | 45 | 53 | 0.003 | 100 | 0.011 | 0 | 0.025 |
| No | 57 | 25 | 89 | 15 | ||||
| 144 | Yes | 31 | 48 | 0.13 | 98 | 0.037 | 5 | 0.17 |
| No | 71 | 32 | 93 | 12 | ||||
Number of patients with (Yes) and without (No) each long-term outcome at weeks 48, 96, and 144.
Percent with perfect adherence (no missed doses at study visits 4, 8, 12, 16, and 24) among those with (Yes) and without (No) each long-term outcome.
p value from logistic regression on long-term outcome with adherence modeled as “perfect” (100%) or “not perfect” (<100%).
Median across 102 patients of week 4–24 mean summary measure of adherence.
p value from logistic regression on long-term outcome with summary measure modeled as a continuous covariate.
Median across 102 patients of SD summary measure of adherence.
SD, standard deviation.
Using the subset of participants staying on their initial efavirenz (n = 47) or unboosted nelfinavir (n = 34) regimen at least 24 weeks, the percent of participants with suppressed viral load was higher in the efavirenz arm within each adherence level (<75%, 75%–95% and >95%). At week 24 the percent with suppressed viral load in the efavirenz arm was 15% higher than the nelfinavir arm (86% versus 71%) among those with mean adherence greater than 95% and by 46% (63% versus 17%) among those with mean adherence less than 75% (p = 0.034). Similarly at week 48, the rate of viral suppression in the efavirenz arm was higher by 20% (82% versus 62%) in those with mean adherence greater than 95% and by 33% (50% versus 17%) in those with mean adherence less than 75% (p = 0.053).
Outcomes were “intent-to-treat,” assuming participants who went off study were failures, but conclusions were similar (although less statistically significant) only analyzing participants remaining on study and with observed data. Among participants with perfect adherence during the first 24 weeks, 33% were lost to follow-up, compared to 46% of those without perfect early adherence. Results were also similar using only observed adherence measurements during the first 24 weeks, i.e., not based on imputation for missing adherence evaluations.
Adherence to HAART and ability to stay on study in this cohort of adolescents initiating HAART was poor, resulting in disappointing rates of long-term virologic control. Self-reported mean adherence level (measured as a continuous outcome and also categorized into levels <75%, 75%–95% or >95%) was more predictive of adherence and virologic control out to 144 weeks than the perfect measure (<100% versus 100%). Decreased variability (SD) in early adherence was predictive of viral control for up to 96 weeks, but asymmetry, represented by skewness (for example not taking medications on weekends) was not a useful predictor. Results also confirmed earlier reports that more potent non-nucleoside reverse transcriptase inhibitor (NNRTI)-based HAART regimens are more “forgiving” with respect to virologic suppression than non-boosted protease inhibitor (PI)-based regimens,2,7 most likely due to longer half-lives of these agents. At the time the study was conducted, boosted PIs were not standard of care. However, even now, while not recommended as an initial regimen, use of unboosted PIs may arise in particular circumstances,8 so these findings confirming less forgiveness for this regimen versus an efavirenz-based regimen remain relevant.
The adherence measures in this study were based on self-report that may not be an accurate measure of true adherence, and information was not collected on socioeconomic status, access to medical care, housing instability or other quality of life measures which have been related to adherence in other studies.4,5,9 Despite these limitations, self-reported adherence over the first 24 weeks on HAART was predictive of longer term adherence, virologic control, and improvements in CD4 counts in adolescents initiating HAART. Health care providers should monitor self-reported adherence by patients as they start new antiretroviral regimens and over the first few months. We recommend they ask about numbers of doses missed and over how many days, rather than just asking if subjects have missed any doses (yes/no). If providers identify problems, they can intervene early to establish better adherence patterns, which will result in better longer term outcomes.10
References
- 1.Lima VD. Harrigan R. Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelo LJ. Samples C. Rogers AS. Peralta L. Friedman L. HIV infection and AIDS in adolescents: An update of the position of the Society for Adolescent Medicine. J Adolesc Heath. 2006;38:88–91. doi: 10.1016/j.jadohealth.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Merzel C. VanDevanter N. Irvine M. Adherence to antiretroviral therapy among older children and adolescents with HIV: A qualitative study of psychosocial contexts. AIDS Patient Care STDs. 2008;22:977–987. doi: 10.1089/apc.2008.0048. [DOI] [PubMed] [Google Scholar]
- 5.Rudy BJ. Murphy DA. Harris DR. Muenz L. Ellen J. Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: A study of prevalence and interactions. AIDS Patient Care STDs. 2009;23:185–194. doi: 10.1089/apc.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn PM. Rudy BJ. Lindsey JC, et al. Long-term observation of adolescents initiating HAART therapy: Three-year follow-up. AIDS Res Human Retroviruses. 2007;23:1208–1214. doi: 10.1089/aid.2006.0290. [DOI] [PubMed] [Google Scholar]
- 7.Weiser SD. Guzman D. Riley ED. Clark R. Bangsberg DR. Higher rates of viral suppression with nonnucleoside reverse transcriptase inhibitors compared to single protease inhibitors are not explained by better adherence. HIV Clin Trials. 2004;5:278–287. doi: 10.1310/LNHD-K1R7-HQP5-HJCQ. [DOI] [PubMed] [Google Scholar]
- 8.Hammer SM. Eron JJ. Reiss P, et al. Antiretroviral treatment of adult HIV infection 2008 Recommendations of the International AIDS Society—USA Panel. JAMA. 2006;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 9.Holmes WC. Bilker WB. Wang H. Chapman J. Gross R. HIV/AIDS-specific quality of life and adherence to antiretroviral therapy over time. J Acquir Immune Defic Syndr. 2007;46:323–327. doi: 10.1097/QAI.0b013e31815724fe. [DOI] [PubMed] [Google Scholar]
- 10.Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services. 2008. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Last accessed]. Aug 23, 2009. pp. 1–128.www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf January 29.


