Abstract
Persons coinfected with tuberculosis (TB) and HIV are at high risk of death, in part due to suboptimal utilization of HIV-specific health care. We sought to better understand HIV-associated health care utilization and mortality in a retrospective cohort of TB/HIV coinfected cases reported in North Carolina 1993–2003. In this cohort, HIV was newly diagnosed during TB presentation for 34.2% of coinfected patients. Patients had advanced HIV (median CD4 104 cells/mm3) at TB diagnosis. Of 260 patients previously known to be HIV positive, 32.3% had seen a physician for HIV care in the previous 6 months and only 18.5% were taking antiretrovirals when TB was diagnosed; 34.8% of patients started antiretrovirals during TB treatment. Twenty-seven (5%) patients died prior to starting TB treatment; of those who survived, 13.6% (70/515) died prior to completing TB treatment, and 42.7% (220/515) died during a median 1408 days of follow-up. CD4 count (relative risk [RR] 0.53 per 100 cell increase, 95% confidence interval [CI] 0.34, 1.02) and highly active antiretroviral therapy (HAART) use during TB therapy (RR 0.37, 95% CI 0.13, 1.02) were independently associated with decreased mortality, while age greater than 45 (RR 2.18, 95% CI 1.11, 4.29) was independently associated with increased mortality during TB treatment. We conclude that TB/HIV coinfected patients had low utilization rates of HIV-specific care prior to TB diagnosis. Many did not receive potentially lifesaving HIV treatment while on TB therapy, and mortality was high as a result. Interventions to enhance utilization of HIV-related health care and integration of TB and HIV services should be studied to improve outcomes.
Introduction
Tuberculosis (TB) and HIV are closely intertwined across the world. HIV coinfection makes individuals with underlying latent TB infection much more likely to develop active TB than their uninfected counterparts.1,2 In 2000, it was estimated that 29% of TB cases in the United States were attributable to HIV infection.3 That same year, TB was the second most common opportunistic infection reported in new AIDS cases in New York City.4 In addition, TB has been shown to increase HIV viral replication in vitro,5 increase the risk for developing other opportunistic infections,6 and may even accelerate the course of HIV disease.7,8
TB diagnosis is often more difficult among HIV-infected persons, often resulting in delayed diagnosis and delayed institution of antituberculous therapy. HIV-infected patients with TB and low CD4+ T-lymphocyte counts (<100 cells/mm3) more often present with atypical chest radiographs and negative acid-fast sputum smears compared to their HIV-negative counterparts.9 HIV infection has also been associated with an increased risk of death during TB treatment in the United States and other industrialized countries.10–14 Low CD4+ T-lymphocyte count (<200 cells/mm3), previous AIDS-defining illness, multi-drug–resistant TB,15,16 the presence of extrapulmonary TB (especially meningitis),17 and a history of injection drug use18 have all been associated with increased mortality in TB/HIV coinfected patients.
There are potential opportunities to positively intervene and prevent HIV-associated TB disease. Among known HIV-infected populations, testing for and treating latent TB infection can reduce the risk of TB activation, as can treatment with highly active antiretroviral therapy (HAART). Conversely, the diagnosis of TB can lead to life-saving HIV/AIDS care.13,18–20 However, many persons with TB/HIV have advanced HIV disease at the time of TB presentation, and frequently are either unaware of their HIV status, or are not receiving HIV care.19,21 TB/HIV coinfected patients in North America are also more likely to have social problems complicating care, including homelessness, illicit drug use, unemployment, or incarceration, which may further complicate their ability to access HIV-specific care.13 Patients with TB in North Carolina are almost exclusively managed through the public health system, so understanding the barriers to care and outcomes in coinfected patients is vital to improving the quality of care in our state, and relevant to other large public health programs in other areas. We therefore examined health care utilization and mortality in a retrospective cohort of TB/HIV coinfected persons reported between 1993–2003 in North Carolina.
Methods
Data collection
Surveillance data for all HIV-seropositive TB cases in North Carolina reported January 1, 1993 to December 31, 2003 were obtained from the North Carolina TB Control Program. The National Death Index was linked with these surveillance data to ascertain the date of death for any TB case that died prior to December 31, 2004. TB cases that did not die prior to December 31, 2004 were right-censored as of that date. Thirty-five patients stopped TB treatment because they were either lost to follow-up or moved; 11 of these were found to have died using the National Death Index and the remainder were right-censored as noted above.
As part of a public health quality improvement project, North Carolina TB Control staff retrospectively abstracted HIV-specific clinical (e.g., opportunistic infections, CD4+ T-lymphocyte count) and health care utilization data from public health charts. These charts are maintained for all TB patients by the county public health clinic responsible for management of that patient. Such charts were available for 433 individuals; 110 charts were unavailable. If a person had a second case of TB, relapse, or reinfection, only data from the first episode were included in the analysis. HIV risk factor data (excepting illicit drug use) are not routinely collected by the TB control program and were not available in the charts or the surveillance database. Data obtained from chart review were entered independently twice (to reduce errors) into a Microsoft Access database (Microsoft, Redmond, WA). The database was then linked to the previously described surveillance/mortality data, deidentified by North Carolina public health staff, and the deidentified database was provided to the principal investigator for analysis. Exemption for use of this deidentified database was granted by the Institutional Review Boards of Duke University Medical Center and the University of North Carolina-Chapel Hill School of Public Health.
We defined the date of TB presentation as the initial date of hospitalization or clinic visit during which the diagnosis of TB was made (e.g., a specimen collected from this visit grew Mycobacterium tuberculosis, TB medications were begun during this visit, or the patient was referred directly from this setting to a health department for TB treatment). The date of TB diagnosis was defined as the date that TB medications were started. HIV status known prior to TB presentation was determined if any of the following were present: (1) records from previous visits to the health department indicated a prior diagnosis of HIV; (2) record of a positive HIV-antibody test that indicated a positive test on a date prior to the date of TB presentation; (3) hospital discharge summary indicated a diagnosis of HIV prior to the date of TB presentation. Individuals were considered to have seen a physician for HIV-specific care in the 6 months prior to TB diagnosis if a physician had: (1) provided antiretroviral medications; (2) provided opportunistic infection prophylaxis; (3) checked CD4 count or viral load; or (4) provided health maintenance care in patients who did not qualify for antiretroviral or opportunistic infection prophylactic therapy. HAART was defined as an antiretroviral regimen that included two nucleoside reverse transcriptase inhibitors (NRTI) and either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI). Boosted PI monotherapy, such as ritonavir/lopinavir, was included as HAART. Non-HAART therapy was defined as any of the following: dual NRTIs; any other two-drug combination (non-boosted PI + NRTI or NNRTI + NRTI); or monotherapy.
Data analysis
Statistical analysis was performed using SAS 9.1.3. (SAS Institute, Inc., Cary, NC). Categorical variables were compared using the χ2 or Fisher's exact test as appropriate, with a p value of <0.05 indicating statistical significance. Continuous variables were compared using the Wilcoxon or Kruskal-Wallis test, as appropriate. For univariate and multivariate analysis of risk factors for death during TB treatment and survival analysis, analysis was restricted to patients with a known CD4 T-lymphocyte count; patients of Asian and Native American race/ethnicity were also excluded due to small numbers (n = 3). Relative risk (RR) and 95% confidence intervals (CI) were calculated for risk factors for death during TB treatment, which was analyzed as a binary variable.22 If univariate analysis showed an association between the risk factor and death during TB treatment (p < 0.05), or was supported by previously published evidence, it was included in multivariate binomial regression analysis. Long-term survival was estimated using the Kaplan-Meier method, and group comparisons in survival were made with the log-rank test, in which p value <0.05 indicated statistical significance. Hazard ratios (HR) were determined for all-cause mortality using the Cox Proportional Hazards method.23 All p values were two-sided.
Results
Subject characteristics
Between January 1, 1993 and December 31, 2003, there were 5332 cases of TB reported in North Carolina, of which 553 (10.4%) had known HIV coinfection. Of these, 10 reports represented reinfection or relapse; only data from the first report were included in this analysis (n = 543). Among those with TB/HIV, 91.7% (498/543) had culture-confirmed TB compared to 79.9% (3807/4762) culture-confirmed among those who were HIV negative/unknown. The proportion of reported TB cases with known HIV status increased over the study period, from 42% in 1993 to 78% in 2003.
TB cases with HIV coinfection differed from other cases in several significant ways (Table 1). While TB disproportionately afflicts black persons in North Carolina, this disparity was even more pronounced in the TB/HIV coinfected group, of whom 80.5% were black, compared to 54.6% of the TB cases with negative or unknown HIV status. Individuals with HIV were more likely to be male, U.S.-born, and have both pulmonary and extrapulmonary TB compared to their HIV-negative/HIV-unknown counterparts. Over time, an increasing proportion of TB/HIV coinfected patients were of Hispanic ethnicity (2.7% in 1993–1995, 10.1% in 1996–1999, and 18.8% in 2000–2003), but other characteristics remained relatively stable over time. Susceptibility testing was performed for 488 patients' initial isolates: 21 (4.3%) were resistant to isoniazid, 4 (0.8%) were resistant to rifampin, 2 (0.4%) were resistant to both isoniazid and rifampin, 6 (1.2%) were resistant to pyrazinamide, and 8 (1.6%) were resistant to ethambutol. No patient had extensively drug-resistant TB during this period.
Table 1.
Demographic and Clinical Features of HIV-Positive and HIV-Negative/HIV-Unknown Patients Diagnosed with Tuberculosis in North Carolina, 1993–2003
| Variable | HIV-positive n (%) n = 543 | HIV negative/unknown n (%) n = 4762 | p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 425 (78.3) | 3048 (64.0) | <0.0001 | |
| Female | 118 (21.7) | 1714 (36.0) | ||
| Age | ||||
| ≤24 | 11 (2.0) | 540 (11.3) | <0.0001 | |
| 25–44 | 377 (69.4) | 1231 (25.9) | <0.0001 | |
| 45–64 | 150 (27.6) | 1372 (28.8) | 0.58 | |
| ≥65 | 5 (0.9) | 1619 (34.0) | <0.0001 | |
| Race/ethnicity | ||||
| White, non-Hispanic | 47 (8.7) | 1310 (27.5) | <0.0001 | |
| Hispanic | 56 (10.3) | 500 (10.5) | 0.94 | |
| Black, non-Hispanic | 437 (80.5) | 2600 (54.6) | <0.0001 | |
| Asian | 1 (0.2) | 304 (6.4) | <0.0001 | |
| Native American | 2 (0.4) | 42 (0.9) | 0.31 | |
| Unknown | 0 | 6 (0.1) | 1.0 | |
| U.S.-born | ||||
| Yes | 467 (86.0) | 3876 (81.4) | 0.01 | |
| No | 76 (14.0) | 882 (18.5) | 0.01 | |
| Unknown | 0 | 4 (0.1) | 1.0 | |
| Site of disease | ||||
| Pulmonary | 378 (69.6) | 3724 (78.2) | <0.0001 | |
| Extrapulmonary | 92 (16.9) | 862 (18.1) | 0.56 | |
| Pulmonary/extrapulmonary | 73 (13.4) | 176 (3.7) | <0.0001 | |
| Injection drug use | 54 (9.9) | 41 (0.9) | <0.0001 | |
| Noninjection illicit drug use | 166 (30.6) | 312 (6.6) | <0.0001 | |
| Excess alcohol use | 214 (39.4) | 930 (19.5) | <0.0001 |
Median CD4 count for patients with known CD4 count (n = 316) was 104 cells/mm3 (interquartile range, 39–216 cells/mm3). Over time, an increasing proportion of patients had available CD4 counts (40.8% in 1993–1995, 61.4% in 1996–1999, and 73.5% in 2000–2003), but the median CD4 count did not change significantly over time (p = 0.37). Fifty-four patients (9.9%) had documentation of one or more opportunistic infections prior to TB presentation (23 Pneumocystis pneumonia, 33 thrush, 8 esophageal candidiasis, 2 cytomegalovirus, 1 cryptococcal meningitis, 1 cerebral toxoplasmosis, 6 disseminated Mycobacterium avium complex—numbers do not sum to 54 because several patients had more than one infection).
Health care utilization
Among the 433 HIV-seropositive patients with charts available for review, we were able to determine that 60% (260/433) were known to be HIV-seropositive prior to TB presentation. A visit to a health department prior to TB presentation was documented for 212 (49.0%) of patients, 100 (47.2%) of whom visited for screening/treatment for sexually transmitted diseases, 65 (30.7%) visited for TB screening, and 47 (22.2%) visited for other reasons. HIV testing was offered during the previous health department visit to 65/122 (53.3%) of patients whose HIV status was unknown at the time of the visit, including only 50/78 (64.1%) of persons visiting for screening/treatment for sexually transmitted diseases. The proportion of patients previously known to be HIV-positive did not vary significantly over the three time periods (1993–1995, 1996–1999, 2000–2003; p = 0.56).
Of those known to be positive, only 32.3% (84/260) had a documented health care provider visit for HIV care in the 6 months prior to their TB presentation. At TB presentation, 18.5% (48/260) of those with previously known HIV-positive status were taking antiretrovirals, of whom 24 were taking HAART. For patients with known HIV-positive status and CD4 count less than 200, only 22.2% (45/203) were documented to be taking Pneumocystis prophylaxis at TB presentation, with no temporal trend. For individuals with known HIV-positive status and CD4 count less than 50, 8.4% (7/83) were documented to be taking Mycobacterium avium prophylaxis at the time of TB diagnosis; this proportion increased from 0% in 1993–1995 to 20.7% in 2000–2003 (p = 0.0056).
Documentation of tuberculin skin testing performed prior to TB presentation was noted in 63.5 % (165/260) of known HIV-positive patients, and 99 (60%) patients with skin testing documentation had a prior positive result. However, only 28 (28.3%) of these had documentation of prior treatment for latent tuberculosis infection (and adherence data were not routinely available for these 28). Most patients (63.9%, 347/543) were diagnosed with TB in the hospital; 30 (5.5%) were diagnosed at a health department, 28 (5.2%) at an outpatient clinic, 6 (1.1%) in a correctional facility, and for 132 (24.3%) the place where TB was diagnosed could not be determined from available data.
During TB treatment, 34.8% (166/477) of patients who survived to TB diagnosis and were not already on antiretrovirals were started on some kind of antiretroviral therapy (105 HAART, 61 non-HAART), 79.4% (177/223) of those with CD4 200 or less were placed on Pneumocystis prophylaxis and 35.4% (35/99) of those with CD4 50 or less were placed on Mycobacterium avium complex prophylaxis (Note: denominators differ from the previous section because subjects newly diagnosed with HIV and who had CD4 counts subsequently assessed are included here). Among those who started antiretroviral therapy during TB treatment, the median time between initiation of TB treatment and antiretroviral initiation was 1 month (range, 0–11 months). Appropriate use of Pneumocystis prophylaxis was stable over time, while prescription of appropriate Mycobacterium avium complex prophylaxis increased over time: 12.0% in 1993–1995, 34.2% in 1996–1999, and 52.8% in 2000–2003. Patients whose HIV status was known prior to TB presentation were actually less likely to be started on HAART during TB treatment than patients who were diagnosed with HIV concurrently with TB diagnosis (22.8% with known status versus 33.6% with HIV status unknown prior to TB presentation started on HAART, p = 0.02). The rate of starting HAART during TB treatment increased over time (0% in 1993–1995, 22.2% 1996–1999, and 37.1% 2000–2003, p < 0.0001), but even in 2000–2003, less than half (48.9%) of patients with CD4 less than 200 were started on HAART prior to completion of TB treatment.
Morbidity and mortality
Twenty-seven (5.0%) patients died prior to TB diagnosis, and 1 never received treatment and was lost to follow-up. Seventy-six (14.8%) of the remaining 515 patients had one or more opportunistic infections diagnosed concurrently with TB presentation (8 Pneumocystis, 65 thrush, 11 esophageal candidiasis, 4 cytomegalovirus, 1 cryptococcal infection, 3 toxoplasmosis, 4 disseminated Mycobacterium avium complex). An additional 55 patients (10.7%) had one or more documented opportunistic infections during TB treatment (9 Pneumocystis, 37 thrush, 10 esophageal candidiasis, 4 cryptococcal infections, 5 toxoplasmosis, 5 disseminated Mycobacterium avium complex). Clinical manifestations possibly attributable to immune reconstitution syndrome were noted in 66 (12.8%) patients; these included new fevers (n = 46), increased chest pain while on treatment (n = 17), paradoxical worsening of the chest radiograph (n = 17), and enlarging lymph nodes with pain or drainage (n = 12). Seventy (13.6%) of the 515 patients who survived long enough to receive at least 1 dose of TB treatment died prior to completion of TB treatment; of these, 22 died during the first 2 months after starting TB treatment and 48 died later.
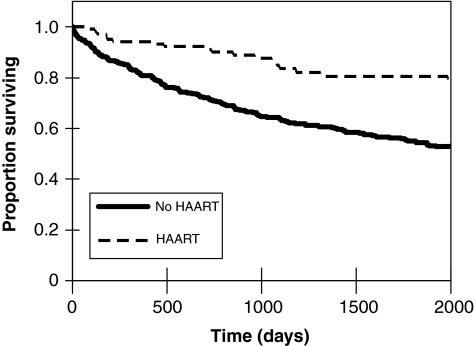
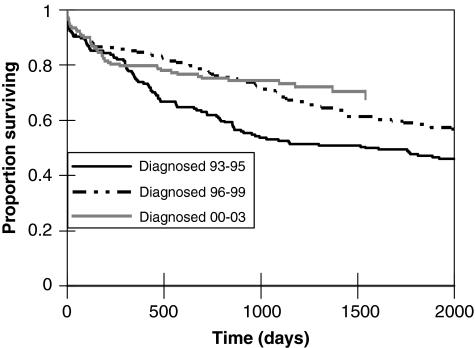
Predictors of mortality during TB treatment among the 307 patients for whom CD4 counts were available are outlined in Table 2. These predictors were also associated with poor long-term survival after TB presentation. After a median 1408 days of follow-up after presentation, 42.7% (220/515) of patients with TB/HIV had died. Receipt of HAART during TB treatment was a powerful predictor of long-term survival after TB presentation (Fig. 1). The relationship between year of TB presentation and long-term survival was more complex (Fig. 2). Despite the presumed greater availability of HAART over time, survival during the first year after TB presentation did not differ significantly among persons diagnosed in 1993–1995, 1996–1999, and 2000–2003 (p = 0.10). However, long-term survival improved over time among persons who survived at least a year after diagnosis; TB/HIV patients who survived at least a year after being diagnosed with TB in 2000–2003 had significantly better survival than similar patients diagnosed in 1996–1999 (p = 0.016, log-rank test), who in turn had nonsignificantly better survival than similar patients diagnosed in 1993–1995 (p = 0.18, log-rank test). In multivariable survival analysis, age greater than 45 years (HR 1.44, 95% CI 0.96–2.15), baseline CD4 count (HR 0.76 per 100 cell increase, 95% CI 0.65–0.88), and having HAART started during TB treatment (HR 0.43, 95% CI 0.26–0.72) were independently associated with long-term survival after TB diagnosis (a hazard ratio >1 indicating greater risk of death).
Table 2.
Univariate and Multivariate Analyses of Predictors of Death During Tuberculosis Treatment in TB/HIV Coinfected Patients in North Carolina, 1993–2003 (n = 307)
| Covariate | Univariate RR (95%CI) | Multivariate RR (95% CI) |
|---|---|---|
| Age | ||
| <45 | 1.00 (reference) | 1.00 (reference) |
| ≥45 | 1.85 (0.91, 3.76) | 2.18 (1.11, 4.29) |
| Race/ethnicity | ||
| White | 1.00 (reference) | |
| Hispanic | 0.56 (0.14, 2.28) | |
| Black | 0.55 (0.20, 1.48) | |
| Gender | ||
| Male | 1.00 (reference) | |
| Female | 1.35 (0.60–3.03) | |
| Site of disease | ||
| Pulmonary | 1.00 (reference) | |
| Extrapulmonary | 0.75 (0.23, 2.44) | |
| Pulmonary and extrapulmonary | 1.86 (0.79, 4.36) | |
| CD4 count (per 100 cells/mm3) | 0.55 (0.36, 0.86) | 0.53 (0.34, 0.81) |
| HAART use during TB treatment | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.37 (0.13, 1.04) | 0.37 (0.13, 1.02) |
Only patients who survived long enough to receive at least one dose of TB treatment with available CD4 counts were included in this analysis. Patients of Asian and Native American races were also excluded due to low numbers (n = 3).
RR, relative risk; CI, confidence interval; HAART, highly active antiretroviral therapy.
FIG. 1.
Survival rates for tuberculosis (TB)/HIV coinfected patients who received highly active antiretroviral therapy (HAART) during TB treatment in North Carolina, 1993–2003.
FIG. 2.
Survival rates for TB/HIV coinfected patients after TB presentation during three specific time periods: 1993–1995, 1996–1999, and 2000–2003.
Discussion
North Carolina TB/HIV cases reported between 1993–2003 had strikingly low rates of HIV-related health care utilization prior to TB diagnosis. In fact, only 32.3% of patients with known HIV status had been seen by a physician for their HIV during the previous 6 months. These patients presented with advanced HIV (median CD4 count 104 cells/mm3), frequently were diagnosed with concurrent opportunistic infections, and had a high risk of death either prior to TB diagnosis (5.0% of patients) or during TB treatment (12.9% of patients who survived long enough to begin treatment). Much of the morbidity in these patients was potentially preventable: 71 patients with previously documented latent TB infection had failed to take latent TB treatment, 77.8% of patients with previously diagnosed HIV and a CD4 count less than 200 were not on Pneumocystis prophylaxis (and Pneumocystis was a common concurrently diagnosed opportunistic infection), and a small minority of patients eligible for antiretrovirals were actually taking antiretrovirals at the time of TB presentation.
TB diagnosis represented an opportunity to enter (in the case of the approximately one third of TB/HIV patients who were previously unaware of their HIV infection) or reenter HIV care. We demonstrated, as have others, that HAART is associated with reduced mortality during and after TB treatment.21,24–27 Despite the demonstrated benefits of HAART, the majority of TB/HIV coinfected patients in our population did not take HAART. This unfortunate fact was illustrated by the finding that survival during the first year after TB presentation did not improve over time, despite the increasing availability of HAART during the study period. Data to explain the underutilization of HAART were not available in the sources analyzed for this study, but the data suggest several possible reasons for HAART underutilization. HIV disease severity is the primary consideration when deciding whether to begin HAART, but other issues such as a prior history of poor adherence, homelessness, and alcohol or injection drug use may also influence this decision.28 Patients who knew their HIV-positive status or had received HIV-specific care prior to their TB diagnosis were actually less likely to receive HAART during TB treatment, suggesting that previous nonadherence may have been a factor in not starting HAART in these patients. High pill burden may have reduced overall patient adherence to HAART.21,29 However, the pill burden and complexity of HIV regimens progressively decreased from 1996 to 2003, so one would expect that a greater proportion of patients diagnosed in the 2000–2003 time period would have received HAART if regimen complexity were the key issue. TB diagnosis and provision of directly observed therapy clearly provide an excellent opportunity to reengage patients in HIV-specific health care, and many patients were started on prophylaxis for opportunistic infections and antiretroviral therapy during TB treatment. HAART clearly will not prevent all opportunistic diseases, as immunologic reconstitution in response to HAART is often incomplete.30,31 Given the very high observed mortality rate in TB/HIV coinfected patients (42.7% of patients who survived to diagnosis died after a median 1408 days of follow-up), HAART should be initiated during TB treatment for patients who meet current treatment guidelines.
We had access to TB public health clinic records, but not to private or other public sources of HIV care. The TB clinic records were frequently missing basic, vital pieces of information about patients' HIV care, suggesting poor coordination between providers of TB care and providers of HIV care. These missing data were a significant limitation of our study. Since these records are maintained locally, data regarding prior use of health department services only pertain to that location; care received at other health departments was not documented. In addition, care from outside providers would only be included in the chart if documented by a public health provider. Because of substantial issues of stigma associated with HIV/AIDS and requests for anonymity, some of these records may have been intentionally left bare. Therefore, public health and HIV-specific care utilization may be underestimated in this cohort. Others have noted similar deficits in HIV-associated recordkeeping by TB providers.32,33 Distance and unfamiliarity with the public health system can make communication between HIV practitioners and TB public health practitioners challenging, and may account for the degree of undocumented HIV-specific data. Clear, timely communication between HIV and TB providers is crucial to providing optimum care to this complicated patient population. This is recognized in countries where there is a high prevalence of both diseases, such as South Africa, where efforts are being made to integrate the TB and HIV treatment programs.34 Similar efforts in the United States may positively impact outcomes in TB/HIV coinfected patients. As a result of this study the North Carolina TB Control Program is making significant efforts to facilitate such communication. The TB Control Program now routinely records HIV care data for all TB/HIV coinfected patients, such as the name of the HIV provider, CD4 count, and concurrent antiretrovirals. One of the state TB Medical Consultants (all experienced HIV providers) reviews each case of TB/HIV in real time to look for problematic drug interactions and advocate for the best HIV care as well as optimal TB care.
Acknowledgments
We wish to acknowledge the tuberculosis control staff and medical records staff in public health clinics across North Carolina who were very helpful in assisting with record review and retrieval. We would also like to thank Victoria Merolla for her assistance with data entry. Dr. Hamilton acknowledges support from National Institutes of Health (NIH) 5K24AI001833; Dr. Stout acknowledges support from NIH 5K23AI051409. Dr. Gadkowski acknowledges support from NIH 5T32AI007392 (AIDS training grant).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Pape JW. Jean SS. Ho JL. Hafner A. Johnson WD., Jr. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 2.Selwyn PA. Hartel D. Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 3.Corbett EL. Watt CJ. Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Hanna DB. Gupta LS. Jones LE, et al. AIDS-defining opportunistic illnesses in the HAART era in New York City. AIDS Care. 2007;19:264–272. doi: 10.1080/09540120600834729. [DOI] [PubMed] [Google Scholar]
- 5.Toossi Z. Johnson JL. Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: Potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28:1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Munsiff SS. Alpert PL. Gourevitch MN. Chang CJ. Klein RS. A prospective study of tuberculosis and HIV disease progression. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:361–366. doi: 10.1097/00042560-199812010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Manas E. Pulido F. Pena JM, et al. Impact of tuberculosis on the course of HIV-infected patients with a high initial CD4 lymphocyte count. Int J Tuberc Lung Dis. 2004;8:451–457. [PubMed] [Google Scholar]
- 8.Whalen C. Horsburgh CR. Hom D, et al. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 9.Palmieri F. Girardi E. Pellicelli AM, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30:68–74. doi: 10.1007/s15010-002-2062-9. [DOI] [PubMed] [Google Scholar]
- 10.Fielder JF. Chaulk CP. Dalvi M, et al. A high tuberculosis case-fatality rate in a setting of effective tuberculosis control: Implications for acceptable treatment success rates. Int J Tuberc Lung Dis. 2002;6:1114–1117. [PubMed] [Google Scholar]
- 11.Oursler KK. Moore RD. Bishai WR, et al. Survival of patients with pulmonary tuberculosis: Clinical and molecular epidemiologic factors. Clin Infect Dis. 2002;34:752–759. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 12.Cayla JA. Caminero JA. Rey R, et al. Current status of treatment completion and fatality among tuberculosis patients in Spain. Int J Tuberc Lung Dis. 2004;8:458–464. [PubMed] [Google Scholar]
- 13.Sterling TR. Zhao Z. Khan A, et al. Mortality in a large tuberculosis treatment trial: Modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006;10:542–549. [PubMed] [Google Scholar]
- 14.Borgdorff MW. Veen J. Kalisvaart NA. Nagelkerke N. Mortality among tuberculosis patients in The Netherlands in the period 1993–1995. Eur Respir J. 1998;11:816–820. doi: 10.1183/09031936.98.11040816. [DOI] [PubMed] [Google Scholar]
- 15.Girardi E. Palmieri F. Cingolani A, et al. Changing clinical presentation and survival in HIV-associated tuberculosis after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26:326–331. doi: 10.1097/00126334-200104010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri F. Pellicelli AM. Girardi E, et al. Negative predictors of survival in HIV-infected patients with culture-confirmed pulmonary tuberculosis. Infection. 1999;27:331–334. doi: 10.1007/s150100050038. [DOI] [PubMed] [Google Scholar]
- 17.Whalen C. Horsburgh CR., Jr. Hom D, et al. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS. 1997;11:455–460. doi: 10.1097/00002030-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Alpert PL. Munsiff SS. Gourevitch MN. Greenberg B. Klein RS. A prospective study of tuberculosis and human immunodeficiency virus infection: Clinical manifestations and factors associated with survival. Clin Infect Dis. 1997;24:661–668. doi: 10.1093/clind/24.4.661. [DOI] [PubMed] [Google Scholar]
- 19.Leonard MK. Larsen N. Drechsler H, et al. Increased survival of persons with tuberculosis and human immunodeficiency virus infection, 1991–2000. Clin Infect Dis. 2002;34:1002–1007. doi: 10.1086/339448. [DOI] [PubMed] [Google Scholar]
- 20.Hung CC. Chen MY. Hsiao CF, et al. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–2622. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Dean GL. Edwards SG. Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 22.McNutt LA. Wu C. Xue X. Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 23.Walker GA. Common Statistical Methods for Clinical Research with SAS Examples. Cary, NC: SAS Institute Inc.; 2002. [Google Scholar]
- 24.Nahid P. Gonzalez LC. Rudoy I, et al. Treatment outcomes of patients with HIV and tuberculosis. Am J Respir Crit Care Med. 2007;175:1199–1206. doi: 10.1164/rccm.200509-1529OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akksilp S. Karnkawinpong O. Wattanaamornkiat W, et al. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13:1001–1007. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia dO. Martinez-Gonzalez MA. Cayla JA, et al. Influence of highly active anti-retroviral therapy (HAART) on the natural history of extra-pulmonary tuberculosis in HIV patients. Int J Tuberc Lung Dis. 2002;6:1051–1057. [PubMed] [Google Scholar]
- 27.Dheda K. Lampe FC. Johnson MA. Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 28.Bogart LM. Kelly JA. Catz SL. Sosman JM. Impact of medical and nonmedical factors on physician decision making for HIV/AIDS antiretroviral treatment. J Acquir Immune Defic Syndr. 2000;23:396–404. doi: 10.1097/00126334-200004150-00006. [DOI] [PubMed] [Google Scholar]
- 29.Dworkin MS. Adams MR. Cohn DL, et al. Factors that complicate the treatment of tuberculosis in HIV-infected patients. J Acquir Immune Defic Syndr. 2005;39:464–470. doi: 10.1097/01.qai.0000152400.36723.85. [DOI] [PubMed] [Google Scholar]
- 30.Collazos J. Asensi V. Carton JA. Factors associated with poor immunologic responses despite viral suppression in markedly immunosuppressed patients. AIDS Patient Care STDS. 2007;21:378–384. doi: 10.1089/apc.2006.0136. [DOI] [PubMed] [Google Scholar]
- 31.Stout JE. Lai JC. Giner J. Hamilton CD. Reactivation of retinal toxoplasmosis despite evidence of immune response to highly active antiretroviral therapy. Clin Infect Dis. 2002;35:e37–e39. doi: 10.1086/341306. [DOI] [PubMed] [Google Scholar]
- 32.Kwara A. Roahen-Harrison S. Prystowsky E, et al. Manifestations and outcome of extra-pulmonary tuberculosis: Impact of human immunodeficiency virus co-infection. Int J Tuberc Lung Dis. 2005;9:485–493. [PubMed] [Google Scholar]
- 33.Gampper SN. George JA. Carter EJ, et al. Co-infection with Mycobacterium tuberculosis and HIV in high risk clinical care setting in Rhode Island. AIDS Care. 1998;10:221–229. doi: 10.1080/09540129850124479. [DOI] [PubMed] [Google Scholar]
- 34.Dong K. Thabethe Z. Hurtado R, et al. Challenges to the success of HIV and tuberculosis care and treatment in the public health sector in South Africa. J Infect Dis. 2007;196(Suppl 3):S491–S496. doi: 10.1086/521111. [DOI] [PubMed] [Google Scholar]