Abstract
Background
Molecular epidemiological definitions that are based on staphylococcal cassette chromosome mec (SCCmec) typing and phylogenetic analysis of methicillin-resistant Staphylococcus aureus (MRSA) isolates are considered a reliable way to distinguish between healthcare-associated MRSA (HA-MRSA) and community-associated MRSA (CA-MRSA). However, there is little information regarding the clinical features and outcomes of bacteremia patients with MRSA carrying different SCCmec types.
Methods
From January 1 through December 31, 2006, we recorded the demographic data and outcomes of 159 consecutive adult MRSA bacteremia patients from whom isolates for SCCmec analysis were collected. All participants were patients at a tertiary care center in Taiwan.
Principal Findings
The following SCCmec types were identified in MRSA isolates: 30 SCCmec II (18.9%), 87 SCCmec III (54.7%), 22 SCCmec IV (13.8%), and 20 SCCmec V (12.6%). The time from admission to the first MRSA-positive blood culture for patients infected with isolates with the SCCmec III element (mean/median, 50.7/26 days) was significantly longer than for patients infected with isolates carrying SCCmec IV or V (mean/median, 6.7/3 days for SCCmec IV; 11.1/10.5 days for SCCmec V) (P<0.05). In univariate analysis, community onset, soft tissue infection, and deep-seated infection were predictors for SCCmec IV/V. In multivariate analysis, length of stay before index culture, diabetes mellitus, and being bedridden were independent risk factors associated with SCCmec II/III.
Conclusions
These findings are in agreement with previous studies of the genetic characteristics of CA-MRSA. MRSA bacteremia with SCCmec II/III isolates occurred more among patients with serious comorbidities and prolonged hospitalization. Community onset, skin and soft tissue infection, and deep-seated infection best predicted SCCmec IV/V MRSA bacteremia.
Introduction
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has been isolated mainly from skin or soft tissue infections [1], [2], although severe invasive infections caused by CA-MRSA strains, such as pyomyositis, osteomyelitis, necrotizing fasciitis, severe pneumonia, and sepsis, have also been reported [3]–[7]. CA-MRSA strains share a common pulsed field gel electrophoresis (PFGE) pattern, show greater susceptibility to most non-β-lactam antibiotics, and carry staphylococcal cassette chromosome mec (SCCmec) type IV or V elements; these characteristics are not typical of healthcare-associated MRSA (HA-MRSA) strains [1].
In recent years, CA-MRSA has emerged as an important cause of healthcare-associated and nosocomial bacteremia in many countries [8]–[10]. In contrast to the US and Europe, the major CA-MRSA strains in Taiwan are ST59, SCCmec IV or ST59, SCCmec VT; the major clone in nosocomial MRSA infections in Taiwan is SCCmec III: ST239, followed by SCCmec II: ST 5 [11]–[16]. Molecular epidemiological definitions, based on staphylococcal cassette chromosome mec (SCCmec) typing and phylogenetic analysis of MRSA isolates, are considered the most reliable way to distinguish between HA-MRSA and CA-MRSA [17]. In a study of MRSA bacteremia by Seybold et al., CA-MRSA strains (as defined by PFGE) were associated with injection drug use and with skin and soft tissue infection [8]. In their study of CA-MRSA strains defined by antibiogram phenotype, Popovich et al. concluded that demographic data and risk factors could not reliably distinguish patients infected with CA-MRSA strains from those infected with HA-MRSA strains [9].
SCCmec II and III, which are larger elements, may not be suited to CA-MRSA strains; these elements show a different distribution of antibiotic resistance genes and toxin genes compared to SCCmec IV and V [17]. In this study, we compared the demographic characteristics, clinical features, and outcomes of patients with MRSA isolates with different SCCmec types.
Materials and Methods
Patient Selection
This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Institutional Review Board (No. 200705068R) of National Taiwan University Hospital (NTUH), Taipei, Taiwan. The Institutional Review Board waived the need for informed consent from participants because the study involved very minimal risk to the subjects, did not include intentional deception, and did not involve sensitive populations or topics; this waiver does not adversely affect the rights and welfare of the subjects.
NTUH is a university hospital with 2500 beds that provides primary and tertiary care in northern Taiwan. From January 1 through December 31, 2006, all patients age >16 years with MRSA bacteremia admitted to NTUH were identified from information in a laboratory database.
Data Collection
All patients were evaluated using a structured form. The clinical course of the infection and the infection foci were evaluated and recorded based on information supplied by primary care physicians and medical records. Diagnosis of the infection focus was based on clinical, bacteriological, and radiological results. The infection was considered “deep-seated” if any of the following were present: infective endocarditis, mycotic aneurysm, osteomyelitis, septic arthritis, pyomyositis, necrotizing pneumonia/empyema, or abscess formation in any deep organ, such as the liver or kidney. Modified Duke criteria were used to evaluate infective endocarditis [18]. Catheter-related bacteremia was defined by a semi-quantitative culture of the vascular catheter tip that yielded more than 15 MRSA colonies in the absence of other sources of bacteremia [19]. The other sites of infection at the onset of bacteremia were defined according to the US Centers for Disease Control and Prevention criteria [20]. If no infection focus could be identified, the bacteremia was classified as primary bacteremia.
The following data were recorded for each patient: age, sex, underlying illness, hospitalization history or outpatient department involvement within the previous year, existence of a percutaneous device catheter, time from admission until a MRSA-positive culture, initial laboratory findings, and outcome.
“Health care–associated” was defined using previously published definitions [8], [9], which include hospital-onset infection or the presence of any of the following HA-MRSA risk factors within the year prior to the index culture: (1) residence in a longterm care facility, (2) prior admission to an acute care facility (3) use of central intravenous catheters or longterm venous access devices, (4) use of urinary catheters, (5) use of other longterm percutaneous devices (6) prior surgical procedures, and/or (7) need for any form of dialysis [8], [9].
Microbiological Laboratory Procedures
Identification of S.aureus was based on colony morphology, Gram staining, a positive catalase reaction, slide agglutination test results (bioMérieux, Marcy l'Etoile, France), and/or results obtained with the Phoenix system (Becton Dickinson, Sparks, MD, USA). Antibiotic susceptibility testing for S. aureus in this study included oxacillin, vancomycin, minocycline, levofloxacin, ciprofloxacin, tetracycline, trimethoprim/sulfamethoxazole, gentamicin, clindamycin, and rifampin and was performed according to standard microbiological methods [21]. Resistance to oxacillin was confirmed by PCR for the mecA gene. The presence of the PVL gene lukF-lukS was determined by PCR using a primer that was described previously [22], and the presence of 10 staphylococcal enterotoxin virulence genes (sea, seb, sec, sed, see, seg, seh, sei, sej, and tst) was also determined by PCR using the protocol of Jarraud et al. [23]. The presence of the SCCmec elements (I–V) and the mecA gene was determined by methods described previously [14], [24]–[26]. Analysis of the polymorphic X-region of the protein A gene (spa) and multilocus sequence typing (MLST) for the major pulso type was performed as described previously [27]–[28]. Analysis of PFGE patterns was performed using GelCompar software (Applied Maths, Austin, TX, USA). We performed spa typing and PFGE typing of all isolates, but only performed MLST on major pulsotypes in different SCCmectypes.
Statistical Analysis
Mean values and standard deviations were calculated for continuous variables. Percentages were used for categorical variables. For univariate analysis, SCCmec types were compared using the chi-square test or Fisher's exact test as indicated for categorical variables and the analysis of variance (ANOVA) test with least-significant-difference post-hoc tests for continuous variables. Due to their similar biological features, we considered SCCmec II and III to be one group and SCCmec IV and SCCmec V to be another group. The associations between potential risk factors and SCCmec II/III or SCCmec IV/V in patients with MRSA bacteremia were also investigated using univariate and multivariate logistical regression. Crude and adjusted odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated. Cumulative survival after the day of the first MRSA-positive blood culture was calculated using the Kaplan-Meier method. Differences in cumulative survival for patients with different SCCmec types were tested with the log-rank test. The effect of infection with CA-MRSA on outcome was evaluated using a multivariate Cox proportional hazards regression model adjusted for age, sex, and underlying comorbidities. The data were analyzed using SPSS software for Windows (Release 15.0; SPSS, Chicago, IL).
Results
Patients, Risk Factors, and Clinical Features
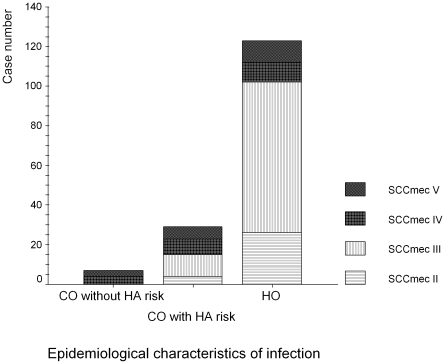
During the study period, there were 159 consecutive adult patients with MRSA bacteremia from whom isolates were collected for microbiological analysis (101 men, 63.5%; 58 women, 36.5%). Twelve patients from whom isolates were not collected were excluded from the study. There were no differences between the study group and the excluded group in terms of sex, age, and ward distribution. The mean age of the 159 adult patients was 67.3±16.5 years. The MRSA SCCmec types were as follows: 30 SCCmec II (18.9%), 87 SCCmec III (54.7%), 22 SCCmec IV (13.8%), and 20 SCCmec V (12.6%). All SCCmec V isolates belonged to SCCmec type VT [13]. Seven patients matched the criteria for CA-MRSA bacteremia (4.4%), including 4 (57.1%) MRSA isolates with SCCmec IV and 3 (42.9%) with SCCmec V. In the remaining 152 (95.6%) patients with HA-MRSA bacteremia, 29 (18.2%) patients had community-onset infections and 123 (77.4%) had hospital-onset infections. For 29 patients with community-onset HA-MRSA bacteremia, the SCCmec type distribution was as follows: 4 (13.8%) SCCmec II, 11 (37.9%) SCCmec III, 8 (27.6%) SCCmec IV, and 6 (20.7%) SCCmec V. In 123 patients with hospital-onset HA-MRSA bacteremia, the distribution of SCCmec types was 26 (21.1%) SCCmec II, 76 (61.3%) SCCmec III, 10 (8.1%) SCCmec IV, and 11 (8.9%) SCCmec V. The distribution of each SCCmec type in a heathcare setting (either community-onset or hospital-onset, with or without healthcare associated factor) is shown in Figure 1.
Figure 1. The distribution of SCCmec: community-onset (CO) or hospital-onset (HO) and healthcare-associated (HA) factor.
MRSA Bacteremia Patient Characteristics Associated with Specific MRSA SCCmec Types
Table 1 summarizes the demographic data and comorbidities of all patients. The mean age of patients with MRSA SCCmec V (56.9 years) was significantly lower than that of patients with MRSA SCCmec II (69.9) and MRSA SCCmec III (69.0) (P<0.05). MRSA carrying SCCmec II/III was more likely to occur in the ICU, and MRSA carrying SCCmec IV/V was more likely to occur in the community (P<0.05). The time from admission to the first MRSA-positive blood culture for patients infected with isolates with the SCCmec III element (mean/median, 50.7/26 days) was significantly longer than for patients infected with isolates carrying SCCmec IV or V (mean/median, 6.7/3 days for SCCmec IV; 11.1/10.5 days for SCCmec V) (P<0.05). Compared to patients with SCCmec V isolates, MRSA bacteremia patients with SCCmec III isolates were more likely to be bedridden, but their infections were less likely to be associated with underlying disease (P<0.05). Patients with SCCmec III isolates were also more likely to have diabetes compared to those with MRSA SCCmec IV (P<0.05). Compared to patients with SCCmec IV isolates, those with MRSA carrying SCCmec II were more likely to have had surgery within the previous three months (P<0.05)
Table 1. Comparison of demographic data and comorbidities of MRSA bacteremia patients with different SCCmec types.
| Characteristic, n (%) | SCCmec II n = 30 | SCCmec III n = 87 | SCCmec IV n = 22 | SCCmec V n = 20 | Total n = 159 | P |
| Sex, male | 20 (66.7) | 56 (64.4) | 12 (54.5) | 13 (65.0) | 101 (63.5) | 0.816 |
| Age, mean years ± SD | 69.9±14.9 | 69.0±15.9 | 66.6±15.6 | 57.0±19.4 | 67.3±16.5 | 0.02 |
| Community onset | 4 (13.3) | 11 (12.6) | 12 (54.5) | 9 (45.0) | 36 (22.6) | <0.001 |
| ICU onset | 15 (50) | 40 (46) | 2 (9.1) | 0 (0) | 57 (35.8) | <0.001 |
| Length of hospital say before index culture mean days ± SD, median (range) | 35.9±33.1 28 (0–157) | 50.7±71.3 26 (0–360) | 6.7±9.0 3 (0–33) | 11.1±12.2 10.5 (0–39) | 36.8±57.6 19.0 (0–360) | 0.001 |
| Comorbid condition | ||||||
| No underlying disease | 0 (0) | 0 (0) | 1 (4.8) | 2 (10) | 3 (1.9) | 0.017 |
| Diabetes mellitus | 7 (23.3) | 35 (40.2) | 2 (9.1) | 6 (30.0) | 50 (31.4) | 0.027 |
| Cancer | 10 (33.3) | 25 (28.7) | 5 (22.7) | 11 (55) | 51 (32.1) | 0.106 |
| Liver cirrhosis | 2 (6.7) | 12 (13.8) | 1 (4.5) | 4 (20) | 19 (11.9) | 0.375 |
| End-stage renal disease | 3 (10) | 16 (18.4) | 4 (18.2) | 1 (5) | 24 (15.1) | 0.409 |
| Cerebrovascular disease | 5 (16.7) | 24 (27.6) | 3 (13.6) | 2 (10) | 34 (21.4) | 0.242 |
| Charlson comorbidity score ≥3 | 17 (56.7) | 55 (63.2) | 10 (45.5) | 11 (55) | 93 (58.5) | 0.490 |
| Bed-ridden status | 7 (23.3) | 28 (32.2) | 3 (13.6) | 1 (0.5) | 39 (24.5) | 0.035 |
| Recent surgery | 10 (33.3) | 16 (18.4) | 0 (0) | 2 (10.0) | 28 (17.6) | 0.009 |
Table 2 shows the clinical characteristics and outcomes of bacteremia patients infected with MRSA with different SCCmec types. In terms of infection sites, MRSA with SCCmec IV was more often associated with superficial skin and soft tissue infections than MRSA with SCCmec II (P = 0.015). In addition, patients with MRSA carrying SCCmec IV were significantly more likely to have non-prosthetic septic arthritis/osteomyelitis (P<0.001) and deep-seated infections not related to surgery or prosthesis (P = 0.008) compared to MRSA bacteremia patients with SCCmec II and SCCmec III isolates.
Table 2. Comparison of clinical characteristics and outcomes of MRSA bacteremia patients with different SCCmec types.
| Clinical syndrome,n (%) | SCCmec II n = 30 | SCCmec III n = 87 | SCCmec IV n = 22 | SCCmec V n = 20 | Total n = 159 | P |
| Infection focus* 1 | ||||||
| Primary bacteremia | 6 (20.0) | 15 (17.2) | 6 (27.3) | 5.(25.0) | 32 (20.1) | 0.695 |
| Skin and soft tissue | 0 (0) | 6 (6.9) | 5 (22.7) | 3.(15.0) | 14 (8.8) | 0.015 |
| Central catheter related infection | 13 (43.3) | 35 (40.2) | 7 (31.8) | 3 (15.0) | 58 (36.5) | 0.152 |
| Surgical site or prosthetic infection | 5 (16.7) | 12 (13.8) | 1 (4.5) | 0 (0) | 18 (11.3) | 0.187 |
| Pneumonia | 11 (36.7) | 27 (31.0) | 3 (13.6) | 6 (30) | 47 (29.6) | 0.326 |
| Deep-seated infection* 2 | 2 (6.7) | 3 (3.4) | 6 (27.3) | 3 (15.0) | 14 (8.8) | 0.004 |
| Endocarditis | 1 (3.3) | 2 (2.3) | 3 (13.6) | 1 (5.0) | 7 (4.4) | 0.140 |
| Septic arthritis and osteomyelitis | 0 (0) | 0 (0) | 4 (18.2) | 1 (5.0) | 5 (3.1) | <0.001 |
| Necrotizing pneumonia and empyema | 1 (3.3) | 2 (2.3) | 0 (0) | 2 (10) | 5 (3.1) | 0.256 |
| Outcome | ||||||
| Persistent bacteremia* 3 | 4 (13.3) | 5 (5.7) | 1 (4.5) | 1 (5.0) | 11 (6.9) | 0.532 |
| 7-day mortality | 6 (20.0) | 8 (9.2) | 0 (0) | 0 (0) | 14 (8.8) | 0.002 |
| 30-day mortality | 12 (40) | 26 (29.9) | 4 (18.2) | 8 (40.0) | 50 (31.4) | 0.309 |
*1Some patients had more than one focus of infection.
*2Deep-seated infection not related to surgery or prosthesis.
*3Persistent bacteremia (>7 days).
SCCmec II/III vs. SCCmec IV/V
In subsequent analyses, we grouped patients with SCCmec II and SCCmec III isolates together for comparison with patients with SCCmec IV and SCCmec V isolates. In univariate analysis, age, ICU onset, length of stay before index culture, diabetes mellitus, bedridden status, recent surgery, and catheter-related infection were associated with recovery of SCCmec II/III isolates (Table 3). Community onset, skin and soft tissue infection, and deep-seated infection (not related to surgery/prosthesis) were risk factors associated with isolates carrying SCCmec IV/V (Table 3). Multivariate analysis revealed four independent factors associated with patients infected by MRSA carrying SCCmec II/III: ICU onset (OR, 16.82; 95% CI, 3.52–80.15), length of stay before index culture (OR 1.07; 95% CI, 1.03–1.10), diabetes mellitus (OR, 4.51; 95% CI, 1.51–13.42), and bedridden status (OR, 5.90; 95% CI, 1.61–21.61) (Table 3).
Table 3. Results of univariate and multivariate logistic regression analyses (SCC mec II/III isolates vs. SCCmec IV/V isolates).
| Univariate OR (95% CI) | Multivariate OR (95% CI) | |
| ICU onset | 17.74 (4.10–76.84) | 16.82 (3.52–80.15) |
| Recent surgery | 5.71 (1.29–25.24) | |
| Bedridden | 4.06 (1.35–12.23) | 5.90 (1.61–21.61) |
| Diabetes | 2.38 (1.01–5.61) | 4.51 (1.51–13.42) |
| Catheter-related infection | 2.23 (1.00–4.95) | |
| Length of hospital say before index culture | 1.07 (1.04–1.11) | 1.07 (1.03–1.10) |
| Age | 1.03 (1.01–1.04) | |
| Skin and soft tissue infection | 0.23 (0.08–0.71) | |
| Deep-seated infection | 0.16 (0.05–0.52) | |
| Community-onset | 0.15 (0.07–0.33) |
In univariate analysis, MRSA bacteremia patients with SCCmec II/III isolates had more catheter-related infections and had more often had recent surgery, and patients with SCCmec IV/V isolates had more skin and soft tissue infections and more deep-seated infections. However, these associations failed to reach significance in multivariate analysis.
Mortality Analysis
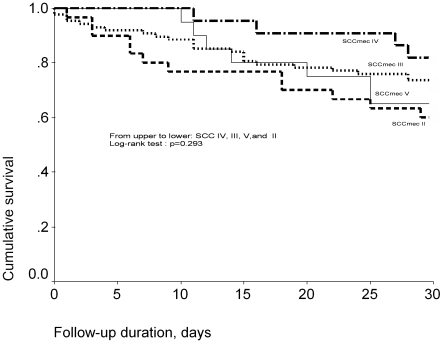
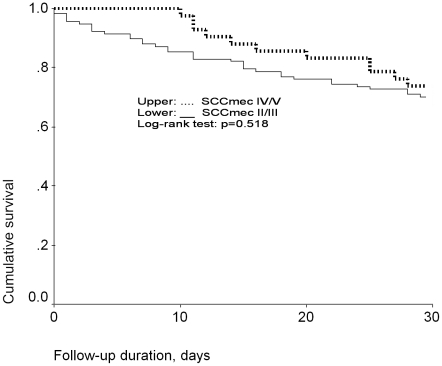
Figure 2 shows the Kaplan-Meier survival curves for MRSA bacteremia patients with isolates carrying different SCCmec types. The 30-day cumulative survival was 60%, 70.1%, 81.8%, and 60% for patients with SCCmec II, III, IV, and V isolates, respectively. There was no significant difference in survival (P = 0.293, log-rank test). The 30-day cumulative survival was 67.5% for patients with SCCmec II/III isolates and 71.4% for patients with SCCmec IV/V isolates; no significant differences emerged (P = 0. 403, log-rank test)(Figure 3).
Figure 2. Thirty-day Kaplan-Meier survival curves for MRSA bacteremia patients according to SCCmec type.
Figure 3. Thirty-day Kaplan-Meier survival curves for MRSA bacteremia patients: SCCmec II/III vs. SCCmec IV/V.
To determine whether the SCCmec type would independently affect 30-day mortality, we performed Cox-regression analysis by controlling for age, sex, and comorbidity (Charlson score). After adjustment for age, sex, and comorbidity (Charlson score), the 30-day mortality of patients with SCCmec IV/V isolates was not significantly higher than that of patients with SCCmec II/III isolates (adjusted hazard ratio, 0.793; 95% CI, 0.406–1.547; P = .496).
Genotype, Antimicrobial Susceptibility, and Virulence Gene Profile of the MRSA Isolates
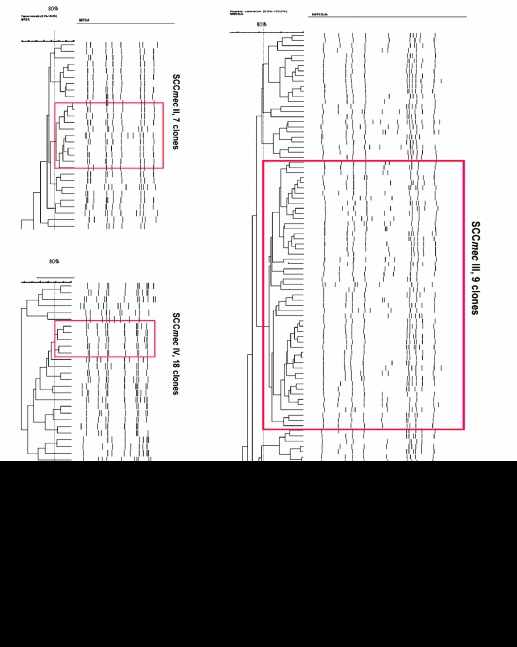
Table 4 shows the genotype, antibiotic susceptibility, and virulence gene profile of isolates with four different SCCmec types. The genotypes of the HA-MRSA strains (SCCmec II and III) were more homogeneous (the same identical spa type and pulsotypes) than those of the CA-MRSA strains (SCCmec IV and V). Figure 4 shows the PFGE of MRSA isolates containing 4 different SCCmec types. The main pulsotypes carried with SCCmec II were ST5, spa t002, and agr 2 and were positive for the sec, seg, sei, and tst genes. The main strain carrying SCCmec IV and V was ST59 and was positive for spa t437, agr 1, and seb. The major differences in virulence gene profiles and antibiotic susceptibility between SCCmec IV and SCCmec V isolates were the pvl carrier rate (9% vs. 85%) and gentamicin susceptibility (36% vs. 90%), respectively. The major strain carrying SCCmec III was spa ST239 and was positive for spa t037, agr1, and sea. Isolates with SCCmec III were often multi-drug resistant except for rifampin.
Table 4. Molecular typing, antimicrobial susceptibility, and virulence genes of MRSA isolates with different SCCmec types.
| SCCmec II n = 30 | SCCmec III n = 87 | SCCmec IV n = 22 | SCCmec V n = 20 | |
| Major spa type (%) MLST* | t002 (97%) ST5 | t037 (93%) ST239 | t437 (68%) ST59 | t437 (75%) ST59 |
| Major agr type (%) | agr 2 (93%) | agr 1 (98%) | agr 1 (77%) | agr 1 (85%) |
| Antibiotic susceptibility profile | ||||
| Oxacillin MIC≥128 µg/mL (%) | 100 | 99 | 0 | 0 |
| Vancomycin MIC = 2 µg/mL (%) | 7 | 30 | 0 | 0 |
| Susceptible rate to non-β-lactam (%) | ||||
| Erythromycin | 0 | 0 | 9 | 10 |
| Clindamycin | 0 | 18 | 23 | 15 |
| Gentamicin | 0 | 1 | 36 | 90 |
| Levofloxacin | 0 | 0 | 100 | 90 |
| Ciprofloxacin | 0 | 0 | 100 | 90 |
| Trimethoprim-sufamethoxazole | 100 | 0 | 100 | 100 |
| Minocycline | 100 | 62 | 91 | 100 |
| Tetracycline | 100 | 0 | 41 | 40 |
| Rifampin | 53 | 83 | 96 | 80 |
| Virulence gene | ||||
| pv l (%) | 0 | 0 | 9 | 85 |
| Enterotoxin gene (sea-sej,tst) | sec: 77% seg: 100% sei: 100% tst: 87% | sea: 87% seb: 2% seg: 1% sei: 1% | seb: 68% sec: 9% seg: 23% sei: 23% | seb: 80% seg: 10% sei: 10% sej: 10% |
*We only performed MLST on strains from major pulsotypes for the different SCCmec types.
Figure 4. Pulsed-field gel electrophoresis of different SCCmec types; red indicates the major pulsotype.
Discussion
We conducted a large one-year retrospective study in a medical center in Taiwan that included 159 consecutive adult patients with MRSA bacteremia. We recorded bacterial genotyping results, antibiotic susceptibility, and virulence gene profiling results for each patient. In this study, the percentage of MRSA bacteremia patients with community-onset, healthcare-associated MRSA with SCCmec IV/V was 48%; among patients with hospital-onset, healthcare-associated MRSA, the frequency was 17%. This finding is similar to those of previous studies in US populations that found that CA-MRSA strains (USA 300) are an important cause of healthcare-related infections [8]–[10], [29], [30]. We compared the demographic characteristics, risk factors, and outcomes of adult MRSA bacteremia patients from whom strains with different SCCmec types were isolated (SCCmec II, III, IV, and V). Microbiological study of the isolates showed that the major clones of CA-MRSA strains in Taiwan (SCCmec IV/V, spa t437, ST59 with or without the pvl gene) or HA-MRSA strains in Taiwan (SCCmec III, spa t037, ST239 and SCCmec II, spa t002, and ST5 with the tst gene) differed from those in the United States and Europe. In addition, we found the demographic and clinical characteristics of patients with MRSA bacteremia differed according to specific SCCmec subtype.
Two recent studies of MRSA bacteremia used the USA300 PFGE pattern and typical antibiotic phenotype for differentiating CA-MRSA and HA-MRSA strains [8]–[9]. The association we identified between SCCmec II/III and ICU onset and length of stay before index culture was similar to results from previous studies [8]–[10], [30]. Previous studies identified a trend between a longer hospital stay before index culture and greater risk of ICU admission in HA-MRSA bacteremia patients [8], [9]. Our cohort also showed longer hospitalization before index culture and more cases of ICU onset. In our study, the number of days the patient was bedridden and diabetes mellitus were other risk factors associated with SCCmec II/III isolates. SCCmectypes IV and V are considerably smaller than SCCmec elements I–III. Our finding support the idea that HA-MRSA strains do not survive well in the community setting and that antibiotic selective pressure or cross-transmission in the hospital were needed for the survival of HA-MRSA strains [17].
Little has been published regarding the role or association of specific MRSA genotypes with particular presentations. Fowler et al. showed that MRSA ST30 was associated with a significant trend towards higher levels of hematogenous complications [31]. Edgeworth et al. suggested that the ST239 strain was associated with an increased rate of vascular access device-related bacteremia [32]. In univariate analysis in this study, the bacteremia patients with HA-MRSA strains (t002, ST5 and t037, ST239) had more catheter-related infections, and patients with CA-MRSA strains (mainly spa t437 and ST59) showed a trend towards more skin and soft tissue infections and deep-seated infections; however, neither association reached significance in multivariate analysis. Our findings support previous reports that CA-MRSA strains are highly associated with skin and soft tissue infection, osteomyelitis, and necrotizing pneumonia, although the CA-MRSA strain in Taiwan was not USA300 [1]–[5]. Although our investigation only included patients with MRSA bacteremia, it extended the finding that certain S. aureus genotypes are more likely to be associated with some clinical syndromes or with infection severity [31]–[33]. Several studies suggest that CA-MRSA strains harboring the smaller SCCmec type IV element grow faster and achieve higher infection burdens than nosocomial MRSA strains [34]–[38]. In agreement with this observation, we found that there were more deep-seated infections involving MRSA with SCCmec IV/V than other subtypes. However, in our study MRSA isolates with SCCmec IV/V were not more lethal than SCCmec II/III isolates. This may be due to more severe comorbidities and more isolates with high vancomycin MIC (MIC = 2) in patients with MRSA carrying SCCmec II/III.
Our study had some limitations. First, the SCCmec and genotype distribution was different than that reported in other parts of the world, so our results cannot be generalized to populations in which the distribution of CA-MRSA and HA-MRSA strains differs from Taiwan. Second, differences in infection presentation and outcome may be explained by factors other than SCCmec genotype, such as virulence genes, antibiotic MIC for the bacterial isolates, adequate infection drainage, and empirical vancomycin therapy. The study size may be too small to address associations between genotype and clinical syndromes or severity.
In conclusion, in this study of 159 adult MRSA bacteremia patients, specific demographic and clinical risk factors were found to predict recovery from bacteremia caused by MRSA with different SCCmec types. MRSA isolates carrying SCCmec II/III were found more frequently in patients with significant comorbidities and prolonged hospitalization. Community onset, skin and soft tissue infection, and deep-seated infection best predicted MRSA isolates carrying SCCmec IV/V.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by a grant from the National Science Council (96-2314-B-002-031-)in Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 2.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Bocchini CE, Hulten KG, Mason EO, Jr, Gonzalez BE, Hammerman WA, et al. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–440. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 4.Pannaraj PS, Hulten KG, Gonzalez BE, Mason EO, Jr, Kaplan SL. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953–60. doi: 10.1086/507637. [DOI] [PubMed] [Google Scholar]
- 5.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 6.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 7.Adem PV, Montgomery CP, Husain AN, Koogler TK, Arangelovich V, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005;353:1245–1251. doi: 10.1056/NEJMoa044194. [DOI] [PubMed] [Google Scholar]
- 8.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 9.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787–794. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 10.Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13:236–242. doi: 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 13.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005;43:4719–4730. doi: 10.1128/JCM.43.9.4719-4730.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JL, Chen SY, Wang JT, Wu GH, Chiang WC, et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis. 2008;46:799–806. doi: 10.1086/527389. [DOI] [PubMed] [Google Scholar]
- 15.Chen FJ, Lauderdale TL, Huang IW, Lo HJ, Lai JF, et al. Methicillin-resistant Staphylococcus aureus in Taiwan. Emerg Infect Dis. 2005;11:1760–1763. doi: 10.3201/eid1111.050367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YC, Su LH, Wu TL, Lin TY. Changing molecular epidemiology of methicillin-resistant Staphylococcus aureus bloodstream isolates from a teaching hospital in Northern Taiwan. J Clin Microbiol. 2006;44:2268–2270. doi: 10.1128/JCM.00776-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluytmans-Vandenbergh MF, Kluytmans JA. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin Microbiol Infect. 2006;12(Suppl 1):9–15. doi: 10.1111/j.1469-0691.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 18.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 19.Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1998;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 21.Bannerman TL. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology, 8th ed. Washington, DC: American Society of Microbiology Press; 2003. pp. 384–404. [Google Scholar]
- 22.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 23.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–36. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, et al. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–2651. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano T, Higuchi W, Otsuka T, Baranovich T, Enany S, et al. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob Agents Chemother. 2008;52:837–45. doi: 10.1128/AAC.01001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmsen D, Claus H, Witte W, Rothganger J, Claus H, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 30.Davis SL, Rybak MJ, Amjad M, Kaatz GW, McKinnon PS. Characteristics of patients with healthcare-associated infection due to SCCmec type IV methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2006;27:1025–1031. doi: 10.1086/507918. [DOI] [PubMed] [Google Scholar]
- 31.Fowler VG, Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196:738–747. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 32.Edgeworth J, Yadegarfar G, Pathak S, Batra R, Cockfield JD, et al. An outbreak in an intensive care unit of a strain of methicillin-resistant Staphylococcus aureus sequence type 239 associated with an increased rate of vascular access device–related bacteremia. Clin Infect Dis. 2007;44:493–501. doi: 10.1086/511034. [DOI] [PubMed] [Google Scholar]
- 33.Seybold U, Blumberg HM. Reading the tea leaves or deciphering DNA microarrays: are certain methicillin-resistant Staphylococcus aureus clones adapted to cause specific infections? Clin Infect Dis. 2007;44:502–505. doi: 10.1086/511047. [DOI] [PubMed] [Google Scholar]
- 34.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 35.Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent F, Lelievre H, Cornu M, Vandenesch F, Carret G, et al. Fitness and competitive growth advantage of new gentamicin-susceptible MRSA clones spreading in French hospitals. J Antimicrob Chemother. 2001;47:277–283. doi: 10.1093/jac/47.3.277. [DOI] [PubMed] [Google Scholar]
- 37.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]