Abstract
Objective
Our study examined the relationship between variant stereociliary bundles of cochlear outer hair cells (OHCs) and auditory function to analyze assessment criteria for rotated stereociliary bundles in the guinea pig cochlea.
Methods
Auditory brainstem response and distortion product otoacoustic emission (DPOAE) were recorded on 100 guinea pigs. Variant hair cells were identified and counted by scanning electron and light microscopy.
Results
The most common variation observed was rotation of stereociliary bundles in the first-row OHCs (OHC1), with most 13.3% variant OHC1 rotated 90 degrees and a few 2.5% rotated 180 degrees. Occasionally, the length and angle of the 2 arms of an OHC deviated from the norm. The auditory brainstem response threshold of affected animals increased only slightly, 20- to 30-dB sound pressure level. More importantly, amplitude of DPOAE increased significantly (40.5 dB sound pressure level).
Conclusion
Our study suggests that rotation of stereociliary bundles in the cochlear OHC was found to be prevalent in 28% of the animals. We established the assessment criteria for rotated stereociliary bundles that were more than 10% OHC1 rotated. This hair bundle seemed to be rotated by 90 degrees from the normal orientation and was accompanied with changes of auditory function. Increased amplitude of DPOAE is associated with the variation of rotated OHC that might result in hearing loss.
Keywords: Auditory brainstem response, Cochlear outer hair cell, Distortion product of otoacoustic emission, Guinea pigs, Stereociliary bundle, Variation
Variant stereociliary bundles of cochlear outer hair cells (OHCs) in guinea pigs were discovered fortuitously in approximately 50% of the animals examined by several investigators (1). We also found this phenomenon frequently in our previous observations of guinea pigs, even in human newborns (2). Accordingly, the anomaly has been described in a subset of animals obtained from animal suppliers, including golden hamsters from Japan (3) and guinea pigs from the U.K. and China (1,3-5).
Planar cell polarity (PCP) refers to the polarization of cells within the plane of a cell sheet. A distinctive epithelial PCP in vertebrates is the uniform orientation of stereociliary bundles of the sensory hair cells in the mammalian cochlea (6,7). Planar cell polarity is a fundamental aspect in the development of the inner ear. Not only is the uniform orientation of stereociliary bundles on hair cells one of the clearest examples of planar polarity in a mammalian system, the cochlea is also an ideal model system to study this (8,9). Yoshida and Liberman (10) histologically examined the cochleae from 100 albino guinea pigs and observed 24 of these to have rotated stereociliary bundles, with males and females almost equally represented in the affected population. This congenital anomaly was strictly limited to first-row OHCs, of which 15 to 50% were found to be rotated up to a possible 180 degrees from the normal orientation. Compound action potentials (CAPs) were measured on mice that displayed abnormal hair bundles to analyze whether this structural alteration had a functional consequence. Indeed, this structural change resulted in CAP thresholds increased by 5 to 10 dB on average when compared with thresholds of control animals. The level of CAP threshold increase was correlated with a quantitation of the degree of stereociliary rotation to demonstrate that first-row OHCs play a greater role in amplifying signals within the cochlea than do the second- and third-row OHCs (10).
Another study found that OHCs from av6J mutant (6J/6J) mice appear rotated from normal position in the basal turn. Additionally, in av2J mice, OHCs are severely deformed and show abnormal organization of the stereocilia (11), whereas some of the cells have rotated stereocilia bundles, with the tip of the V pointing at an angle of up to 90 degrees away from the normal orientation.
Normal cochleae were apparent in the first row; however, 50% OHCs were rotated bundles, but they were considered normal by this investigator. It was concluded by this author that the irregular arrangement and natural loss of stereociliary bundles of the OHCs are not considered to be pathologic (12).
In this current study, assessment of cochlear OHC rotation was not based on criteria from previous literature. Rather, our study involved the assessment of hearing acuity in 100 guinea pigs that were then independently examined for cochlear morphology and histology. We measured hearing acuity by both auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) testing. It is well established that DPOAE analysis can discern alterations of OHC functionality that are not detected by ABR analysis (9). The cochlea of each animal was examined by electron microscopy for stereociliary bundle misorientation that was mapped to specific cochlear areas. The histologic results of cochlear damage were compared with the DPOAE analysis to see if both methods concomitantly pinpointed similar areas of stereociliary bundle misorientation. The goal is to use DPOAE analysis to discern subtle changes in stereociliary bundle misorientation.
MATERIALS AND METHODS
Animals
One hundred pigmented guinea pigs, each having a normal Preyer reflex, normal tympanic membrane, and weighing 400 to 500 g, were obtained from the Animal Center of the Fourth Military Medical University. The data on prevalence of the stereocilia anomaly in a sample of 100 guinea pigs from 4 separate experiments were conducted between 2000 and 2006 (Table 1). Animals were provided by the Experimental Animal Center of the Medical College of Xian Jiaotong University. All procedures were performed in accordance with the US Public Health Services Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Xian Jiaotong University.
TABLE 1.
Data on prevalence of the stereocilia anomaly in a sample of 100 guinea pigs from 4 separate experiments that were conducted between 2000 and 2006
| ID | Group history (dates) |
Group size, N (ears) |
Stereocilia anomaly (n [%]) |
Stereocilia normal (n [%]) |
|---|---|---|---|---|
| 1 | OHCs damage and DPOAE (2001.4) |
20 (both ears) | 8 (40) | 12 (60) |
| 2 | GM ototoxicity (2002.6) |
20 (both ears) | 5 (25) | 15 (75) |
| 3 | Inner ears proteomics (2005.12) |
50 (left ear) | 12 (24) | 38 (76) |
| 4 | CAPs assayed surgery (2006.3) |
10 (both ears) | 3 (30) | 7 (70) |
| Total | 100 (75 ears) | 30 (28) | 76 (72) |
CAP indicates compound action potential; GM, gentamicin; OHC, outer hair cell; DPOAE, distortion product otoacoustic emission.
ABR Assay
All 100 animals were assessed by ABR analysis in both ears. For this analysis, each animal was anesthetized with barbital sodium (40 mg kg−1, i.p.) and then placed in a soundproof room. A recording electrode was inserted into the calvarium, a reference electrode into the mastoid being tested, and a ground electrode into the opposite mastoid. The animals were stimulated with a click. Auditory brainstem response thresholds were measured with a SmartEP system (Intelligent Hearing Systems, Miami, FL, USA) and Wave III was used as a marker, essentially as described (13). Stimuli were presented at a rate of 25 milliseconds and averaged across 512 sweeps. Responses were band-pass filtered at 100 and 3,000 Hz and amplified 100,000-fold. A threshold was defined as the minimal stimulus level that gave a recognizable waveform on a normalized scale.
DPOAE Assay
We also used DPOAE testing to specifically assess OHC functionality. All 100 animals were tested in both ears. Animals were anesthetized and placed in a soundproof room, and their external auditory meatus was cleaned, and animals were microscopically examined for external ear canal and middle ear obstruction. Only guinea pigs with clearly visible healthy tympanic membranes were included. Therefore, 21 of the 121 were excluded for unhealthy tympanic membranes. Before recording, an operating room microscope (Olympus x10; Tokyo, Japan) was used to place the stimulus probe and microphone in the test ear. The coupler was placed close to the tympanic membrane. Distortion product-grams were acquired, and 2 or more assessments were obtained for each animal in both ears.
We used the HIS SmartEP3.30 USBez Software (Intelligent Hearing Systems), and for DPOAE measurement, DPOAE measurements were conducted for pure tones from 2 to 36 KHz. An Etymotic 10B+ (Etymotic Research, Elk Grove Village, IL, USA) probe was inserted into the external ear canal and used with 2 different types of transducers depending on the range of the stimulation frequency. For frequencies ranging from 2 to 16 kHz, Etymotic ER2 stimulus response signals were sampled at a rate of 128 kHz using a 16-bit D/A converter; L1 and L2 amplitudes were set to the same level. Frequencies were acquired with an F2-F1 ratio of 1:22. Stimuli were then presented starting from the lowest frequencies tested and increasing to the highest frequencies tested. Five stimulation levels ranging from 65 to 25 dB sound pressure level in 10-dB steps were used. A total of 4 blocks were acquired with each block consisting of 32 sweeps.
Cochlear Surface Preparation
After each guinea pig was killed, its auditory vesicles were excised through the temporal bone. The left cochlea was slowly perfused with 0.5% AgNO3 (staining fluid, 10–20 ml), washed with distilled water 3 times, and then fixed with 10% (phosphate-buffered saline) formaldehyde solution. After exposure to daylight for 2 to 4 hours, cochleas were extracted and stretched under a dissecting microscope and sealed with glycerin (Sigma-Aldrich, Inc., St Louis, MO, USA). Inner hair cells and OHCs were observed and counted under a light microscope.
Scanning Electron Microscopy
The right cochlea was used for this procedure (because treatment of the left is described in the previous section) (Table 1). Each guinea pig was decapitated, and the cochleae were quickly removed from the skull and immersed in fixative (2.5% glutaldehyde in 0.1 mol/L phosphate buffer; Sigma-Aldrich). The round and oval windows were immediately opened, and a small hole was drilled in the apex and basal turn of the cochlea. The fixative was perfused through the cochlea via the oval window or round window and small hole. Specimens were postfixed in the same fixative for 3 hours to several days at 4°C. After rinsing in buffer, the cochleae were microdissected and removed from the bone, vestibular membrane, and tectorial membrane using a surgical dissecting microscope (Olympus x10). The cochleae were then treated with 1% OsO4 for 1 hour (Sigma), rinsed again, and dehydrated in increasing concentrations (30–100%) of ethanol. The basilar membrane was exposed at a critical point by drying and then coated with gold. All specimens were viewed with an H-600 SEM (Hitachi, Tokyo, Japan) scanning electron microscope.
RESULTS
Histologic Examination of Cochleae Reveals Frequency and Location of Variant Stereociliary Bundles
Observations via light microscope were performed in 4 separate experiments to identify variant stereociliary bundles in bilateral cochlear OHCs in 28% of the animals tested. Specifically, among the 28 guinea pigs (two ears from each guinea pig to obtain 75 ears), 14 cochlear surface preparations were intact. To examine the cochleae thoroughly and systematically, we divided the cochleae into designated quadrants: apical turn, second turn, third turn, and basal turn. Detailed analysis of each quadrant revealed that variant stereociliary bundles were found mainly in the first row of cochlear OHCs and existed in every turn of the cochlea with greater (60% in the basal turn) and lesser frequency (5% in the apex; Table 2). All of these variant forms of stereociliary bundles were either irregular or rotated (62.28% were frequently rotated by 90 degrees, 11.45% were less frequently rotated by 180 degrees; Table 2) (Figs. 1 and 2). The rotated OHCs found in Table 1 in Group 11 ear samples were counted and summarized in Figures 3 and 4. It seems that 90-degree rotation is the most common of the rotational conformations and becomes less frequent toward the apex (Figs. 3 and 4).
TABLE 2.
Number of OHC variants at each turn of the cochlea (Table 1; 1 group; n = 8)
| Animals | Basal | Third | Second | First | Total | |
|---|---|---|---|---|---|---|
| 1 | 245 | 104 | 130 | 55 | 534 | Severe |
| 38 | 139 | 83 | 8 | 3 | 233 | Severe |
| 7 | 105 | 40 | 36 | 23 | 204 | Moderate |
| 60 | 174 | 5 | 5 | 2 | 186 | Moderate |
| 23 | 63 | 18 | 9 | 4 | 94 | Moderate |
| 34 | 40 | 19 | 7 | 4 | 70 | Mild |
| 17 | 32 | 12 | 6 | 2 | 56 | Mild |
| 21 | 42 | 1 | 6 | 3 | 41 | Mild |
| Total | 840 | 282 | 207 | 96 | 1418 | Mild |
| Average | 60% | 20% | 15% | 5% | — | — |
— indicates no data presented.
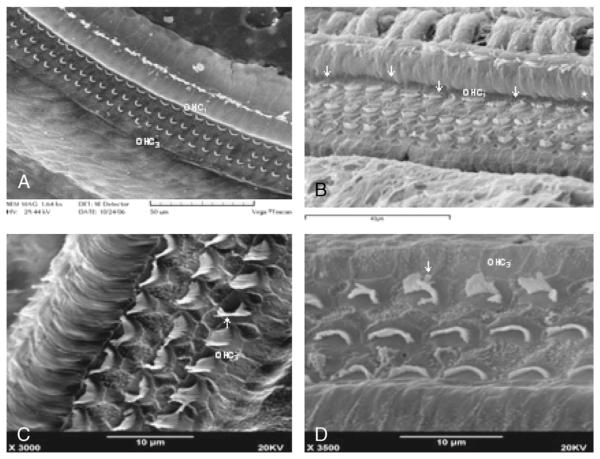
FIG. 1.
There are regular OHCs in the normal groups (A). Variant forms of stereociliary bundles were irregular or rotated often frequently by 90 degrees and less frequently by 180 degrees in the first-row OHC1 (B; arrows). There is less rotation in the OHC3 (C, D).
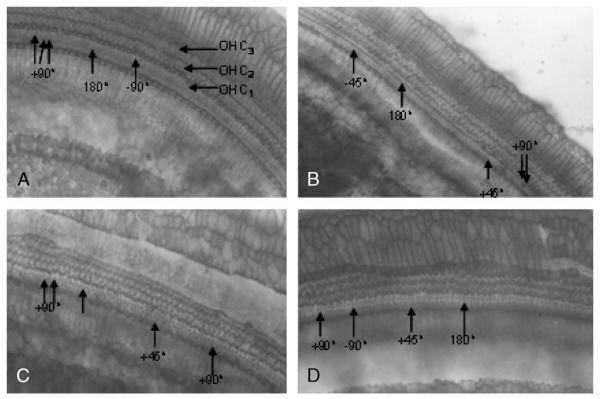
FIG. 2.
Light micrographs showing the orientation of hair cells in the cochlear anomaly in guinea pigs. First-row OHCs (OHC1), second-row OHCs (OHC2), and third-row OHCs (OHC3). Surface view of organ of Corti of a control guinea pig showing abnormal OHCs (arrow-heads), particularly in the OHC1. The rotation of OHC bundles can be in the 180-, +90-, −90-, +45-, and −45-degree direction.
FIG. 3.
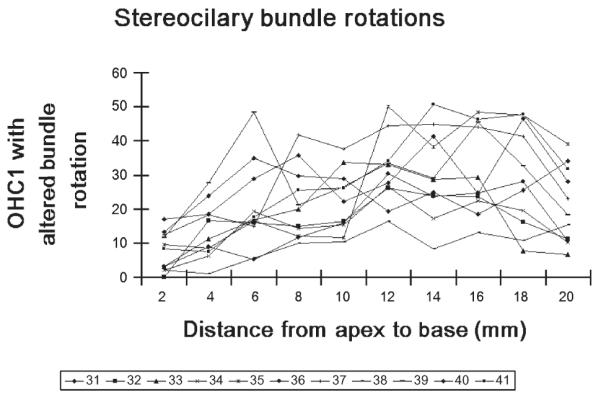
The percentage of rotated OHCs’ stereociliary bundle in 31 to 41 guinea pigs.
FIG. 4.
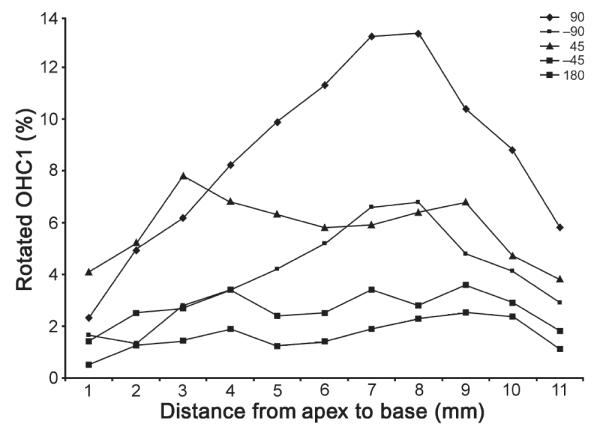
The percentage of rotated first-row OHCs in all the affected ears analyzed in the present study (series 1, 180 degrees; series 2, 90 degrees; series 3, −90 degrees; series 4, 45 degrees; series 5, −45 degrees).
Auditory Function and OHC Stereociliary Bundle Variation
Auditory brainstem response thresholds of 8 guinea pigs increased slightly (20–30 dB sound pressure level), but with no statistically significant difference between animals with various degrees of stereociliary rotation alteration (mild to severe) and animals with normal cochlear morphology. To resolve this and increase the sensitivity of functional measurement, DPOAE analysis was used to discern altered stereociliary functionality that is not readily detected by ABR analysis. Recent studies have provided evidence that DPOAE analysis can detect stereociliary bundle impairment localized to a specific cochlear region. Indeed, DPOAE amplitudes increased significantly in these 8 guinea pigs, especially in high-frequency areas that correlated with histologic analysis that mapped the greatest variation to the base of the cochlea. Thus, the severe variant group exhibited the greatest amplitude at the highest frequency tested for DPOAE analysis when compared with animals of normal cochlear morphology (Fig. 2).
In contrast to other studies, we observed that abnormal OHC stereociliary bundles were frequently (60.28%) rotated toward the round window and rarely (34.18%) in the opposite direction (toward the copula of the cochlea). Some 11.45% of bundles were rotated up to 180 degrees (Table 3). Although the extent of OHC stereociliary bundle variation was very severe in many animals, bundle rotation existed in the first-row OHCs only.
TABLE 3.
Quantitation of rotation angle of variant OHCs in the base of the cochlea (Table 1; 1 group; n = 8)
| Animals | +90 degrees (positive) |
−90 degrees (negative) |
180 degrees |
Ratio |
|---|---|---|---|---|
| 60 | 93 | 57 | 28 | 3:2:1 |
| 52 | 101 | 62 | 30 | 3:2:1 |
| 34 | 59 | 14 | 7 | 4:2:1 |
| 1 | 54 | 30 | 8 | 7:4:1 |
| 34 | 56 | 32 | 6 | 9:5:1 |
| 38 | 61 | 57 | 13 | 5:4:1 |
| 15 | 101 | 37 | 7 | 14:5:1 |
| 7 | 41 | 29 | 8 | 5:3:1 |
| 21 | 30 | 16 | 12 | 5:3:2 |
| 23 | 47 | 18 | 5 | 9:3:1 |
| 17 | 10 | 10 | 2 | 5:5:1 |
| Average value | 60.28% | 34.18% | 11.45% | 5:3:1 |
Tables 3 summarizes different rotated OHC number in the base of cochlea, and the average ratio was 5:3:1. According to degrees of stereociliary bundle variations, they could be classified as follows: severe, variant OHC ≥50%; moderate, ≥20%; light, individual OHC variations. In this study, 3 guinea pigs (6 ears) had severe variation, 2 (3 ears) were moderate, and 3 (5 ears) were light. OHC indicates outer hair cell.
DISCUSSION
The results of our electron microscopic analysis revealed that 28% of the animals had stereociliary bundle misorientation, inconsistent with other studies (Table 4) (1,14) reporting this in 50% of the animals. This discrepancy can be attributed to a significantly smaller sample size of animals examined in the previously cited studies. Another study of stereocilia bundle rotation that used light microscopy and the same sample size as our study reported 24% of the animals with stereociliary bundle misorientation (10). The histologic analysis of Yoshida and Liberman of cochleae from 100 albino guinea pigs revealed congenital anomalies in roughly equal prevalence in males and females. In these affected animals, 15 to 50% of the first-row OHCs showed distinctly abnormal orientation of the W-shaped stereociliary array. These abnormal hair bundles could be rotated by up to 180 degrees from the normal quasi-radial orientation. Second- and third-row OHCs seemed normal in all cases. Our study confirmed this previously published result by Yoshida and Liberman and extends the results by showing the phenomenon occurs in normal pigmented and albino guinea pigs and can be detected by the relatively sensitive and convenient DPOAE technique.
TABLE 4.
Summary of recent reports of stereociliary anomaly in the mammalian cochlea
| Research group | Percentage of anomaly of animals |
W-shaped rotated angle from the normal orientation (degrees) |
OHCs rotated of OHC1, OHC2, and OHC3 |
Percentage of rotated OHCs |
|---|---|---|---|---|
| Furness et al. (1) | Guinea pigs, 48% (11 of 23) | 180 | 47% OHC1, 11.2% OHC2, and 12.6% OHC3 |
26.6% rotated (10); 10–20% rotated (6); 10% rotated (3) |
| Comis et al. (3) | Albino guinea pigs, 50% (5 of 10) |
180 | OHC1 | 20–80% |
| Gu and Goodwin (14) | Albino guinea pigs, 50% (3 of 6) |
90 | OHC1 | — |
| Yoshida and Liberman (10) |
Albino guinea pigs, 24% (24 of 100) |
45 | 10–70% OHC1 | 40–70% |
| Li et al. (2) | C40% (8 of 20) | 90 | OHC1 | 50% |
| Curtin et al. (30) | Celsr1 mouse, 100% | 20 | OHC1, OHC2, OHC3 | 60% (apex turn), 68% (basal turn) |
| Li et al. (4) | Albino guinea pig, 25% (5 of 20) |
45 | OHC1 | 10% |
Earlier studies discussed the difficulty in detecting stereociliary bundle variations by electrophysiologic measurements (10). In fact, ABR analysis failed to demonstrate any appreciable differences (15- to 20-dB threshold increase over control animal thresholds) in affected animals even with substantial stereociliary bundle misorientation (5). The inability to readily detect the bundle rotation phenotype with ABR analysis prompted the use of CAP (10) and DPOAE analysis in our study. Together, increase in CAP threshold and changes in DPOAE threshold implicate stereocilia bundle rotation and OHC dysfunction. Compound action potential thresholds in affected animals were, on the average, elevated by 5 to 10 dB with respect to normal controls. It was observed that if 40 to 70% of first-row OHCs show rotational anomaly, this promotes a 5- to 10-dB shift in CAP thresholds, suggesting that OHC1 significantly contributes to cochlear amplification than either of the other OHC rows (10). It should be noted that CAP analysis is an invasive surgical procedure that may pose a morbidity risk to the animals tested. To facilitate testing, we used DPOAE analysis that is non-invasive and significantly easier than CAP analysis, which directly measures OHC function. The CAP is the summed electric response from spiral ganglion cells that form the afferent neural signal from the cochlea. Distortion product otoacoustic emissions are thought to arise from active elements within the OHC, and the endocochlear potential (EP) measure the ability of the stria vascularis to power the cochlea (15). The inconsistent relations between the DPOAE and OHC loss may be attributed, in part, to the inability to easily quantify other morphologic changes (e.g., stereocilia defects, altered tip links) over the entire extent of the basilar membrane in large numbers of animals or to changes in the EP that may affect the function of cells that are present. Brown (16), for example, reported that electron microscopic analysis showed OHC abnormalities that could explain the reduction in DPOAEs in the absence of OHC loss. Martin et al. (17) also reported a temporary reduction in the amplitude of the DPOAE resulting from the effect of furosemide on the EP generated by the stria vascularis in rabbits.
We have shown by corresponding histologic analysis that this procedure can correctly identify cochlear regions with the greatest stereociliary bundle misorientation (2,4). Therefore, significant increase in DPOAE amplitude accompanied by normal or slightly elevated ABR threshold might act as diagnostic reference criteria for OHC variations, including stereocilia bundle rotation. Outer hair cell stereociliary bundle variations were found mainly in the first row of the cochlea, where the differentiation of OHC was highest and the functions were the most complicated. Our study found that ABR threshold increased by 15 dB in animals with OHC variations, which coincided with nonlinear active micromechanic movement of OHC in Corti organ resulting in DPOAE that could reflect OHC functional sensitivity. For instance, many sensory epithelia are composed of population of hair cells in which all the stereociliary bundles are oriented in the same direction. When variant OHC stereociliary bundles were stimulated, populations of cells with opposite orientations are located on opposite sides of a clear reversal line, resulting in a situation in which the same stimulus leads to depolarization of 1 group of cells and hyperpolarization of the other population, their movements became more contradictory, and the nonlinearity of basilar membrane became more evident, which made amplitudes of DPOAE increase abnormally and induced a significantly increased amplitudes in frequency areas where OHC variant degrees were more severe. Therefore, a significant raised amplitude of DPOAE accompanying normal or slightly higher threshold of ABR might act as a diagnostic reference criteria for OHC variations in this article.
It has been suggested that stimulus-frequency emissions and low-level, transiently evoked emissions may differ fundamentally from distortion-product emissions in that the former arise as linear cochlear reflections of local irregularities in cochlear architecture (18,19), whereas the latter arise as nonlinear distortions in basilar membrane mechanics. It has been further suggested that the relatively large size of stimulus-frequency emissions in primate ear results from the greater overall irregularity in the cochlear architecture compared with rodent ear. The presence of severe rotational anomaly described in the report of Yoshida and Liberman (10) may offer an ideal way to test this hypothesis. Our research data suggest that a significant increase in DPOAE amplitude accompanied by normal or slightly elevated ABR threshold might act as a diagnostic reference criteria for OHC variations, including stereocilia bundle rotation.
In a related study, we found OHC rotation in newborn humans, suggesting that OHC morphology variation normally occurs in humans and in animals. Little is known of the incidence of stereociliary bundle misorientation in the adult human population. We had found that variant OHC stereociliary bundles occurred in the apex turn of the newborn cochlea. It is possible that variant-rotated OHC might result in deafness and tinnitus (2). These disorders may be named as “deafness and tinnitus related to OHC stereociliary bundles variations.”
In conclusion, OHC stereociliary bundle variations may be common in humans and animals, thus requiring future studies to confirm these results. If the DPOAE test is correct, it might also provide a way to screen for the anomaly in a more deterministic or predictive fashion.
Therefore, rotation of stereociliary bundles and extra stereocilia on the OHC as well as the extra inner hair cell is induced by genetic variation. For example, a second noncanonical pathway uses F2 and Dv1, along with a number of relatively novel molecules, to regulate the generation of PCP (20,21). Our interests are centered in these cited studies of mutated genes that have generated the stereociliary bundle rotations that have included the rangI2 mutant, which shows the single inner and 3 outer rows of sensory hair cells with the characteristic chevrons formed by their stereocilia (22-24) (Fig. 5). With the exception of orientation, stereociliary bundles seemed normal, which suggests that the effects of Vangl2 are restricted to orientation and do not include bundle formation. Shortly after this study was published, the inner ear was used to identify several additional genes that are also involved in mammalian PCP (25,26). Whereas some of these genes are orthologs of Drosophila PCP genes, such as Celsr1, a mammalian ortholog of flamingo, others seem to be “new” PCP genes, such as protein tyrosine kinase 7, a novel single transmembrane tyrosine (27). In vitro studies demonstrated a role for Wnts and, most likely, Wnt7a in some aspects of stereociliary bundle orientation, suggesting that Wnts can play a role in the PCP signaling pathway in vertebrates (7,8,28) These researchers measured the angel of the rotated OHCs from normal orientation. Analysis revealed that most were rotated by 80 degrees, and a few were rotated to achieve 180 degrees, which was distributed predominantly at the whole cochlear turn, with frequent occurrence in the bases region of cochlea. Therefore, the stereociliary bundle can deviate from the final orientation by as much as 180 degrees. Their results indicated that, whereas most bundles arise with an orientation that is within 80 degrees of their final orientation, some bundles develop with initial orientations that deviate by as much as 160 degrees (29).
FIG. 5.
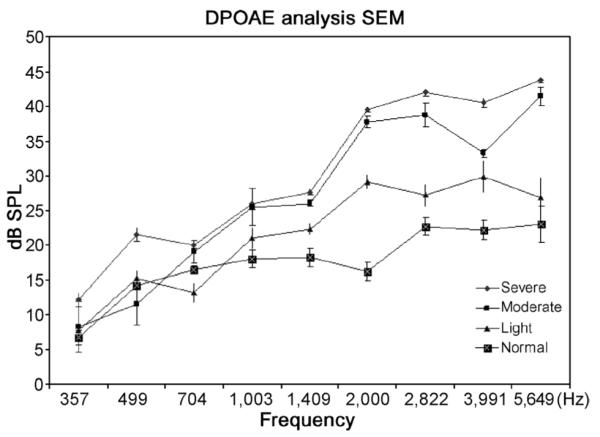
Distortion product otoacoustic emission analysis of animals with various degrees of stereociliary bundle rotation. Values are the means and standard deviations for each group (severe, 3; moderate, 2; mild, 4; normal, 6).
Cochlear generation of this polarity is important for hair cell mechanotransduction and auditory perception because stereociliary bundles are only sensitive to vibrations in their single plane of polarization. We analyze assessment criteria for rotated stereociliary bundles, which can be applied to PCP mice, guinea pigs, and others mammals.
Acknowledgments
Supported by National Natural Science Foundation of China (grant no. 39970785), International Collaborate Research Foundation of National Natural Science of China (grant no. 322200462), and National Institutes of Health (grant no. RO1DC007392).
REFERENCES
- 1.Furness DN, Hackney CM, Hynd AN. Rotated stereociliary bundles and their relationship with the tectorial in the guinea pig cochlea. Acta Otolaryngol. 1990;109:66–75. doi: 10.3109/00016489009107416. [DOI] [PubMed] [Google Scholar]
- 2.Li SL, Zhu HL, Liu H, et al. Variant stereociliary bundles of cochlear out hair cells and their relationship with auditory function. J Audiol Speech Pathol. 2002;10:91–101. [Google Scholar]
- 3.Comis SD, Pickles JO, Osborne MP, et al. Tip-link organization in anomalously-oriented hair cells of the guinea pig cochlea. Hear Res. 1989;40:205–12. doi: 10.1016/0378-5955(89)90161-5. [DOI] [PubMed] [Google Scholar]
- 4.Li SL, Zheng QY, Yan LY, et al. Variant of stereociliary bundles of cochlea outer hair cells and it relationship with ototoxicity on Gentamicin. J Audiol Speech Pathol. 2005;113:256–9. [Google Scholar]
- 5.Fujita H, Orita Y. An inner ear anomaly in golden hamsters. Am J Otolaryngol. 1988;9:224–31. doi: 10.1016/s0196-0709(88)80031-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Mark S, Zhang X, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian D, Jones C, Rzadzinska A, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabdoub A, Donohue MJ, Brennan A, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–84. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 9.Seifert JRK, Mlodzik M. Frizzled/PCP signaling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–38. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida N, Liberman MC. Stereociliary anomaly in the guinea pig: effects of hair bundle rotation on cochlear. Hear Res. 1999;131:29–38. doi: 10.1016/s0378-5955(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 11.Raphael Y, Kobayashi KN, Dootz GA. Severe vestibular and auditory impairment in three alleles of Ames waltzer (av) mice. Hear Res. 2001;151:237–49. doi: 10.1016/s0378-5955(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 12.McFadden S, Dalian D, Jiang H, Woo J, Salvi R. Chinchilla models of selective cochlear hair cell loss. Hear Res. 2002;174:230–8. doi: 10.1016/s0378-5955(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 13.Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu Z, Goodwin J. Variant stereociliary bundles in the guinea pig cochlea. Chin J Otorhinolaryngol. 1993;28:82–3. [Google Scholar]
- 15.Oghalai JS. Chlorpromazine inhibits cochlear function in guinea pigs. Hear Res. 2004;198:59–68. doi: 10.1016/j.heares.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Brown AM. Acoustic distortion from rodent ears: a comparison of responses from rats, guinea pigs, and gerbils. Hear Res. 1987;31:25–38. doi: 10.1016/0378-5955(87)90211-5. [DOI] [PubMed] [Google Scholar]
- 17.Martin GK, Jassir D, Stagner BB, et al. Effects of loop diuretics on the suppression tuning of distortion product otoacoustic emissions in rabbits. J Acoust Soc Am. 1998;104:972–83. doi: 10.1121/1.423340. [DOI] [PubMed] [Google Scholar]
- 18.Zweig G, Shera CA. The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am. 1995;98:2018–47. doi: 10.1121/1.413320. [DOI] [PubMed] [Google Scholar]
- 19.Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for OAEs. J Acoust Soc Am. 1999;105:782–98. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- 20.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 21.Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–33. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- 22.Ciruna B, Jenny A, Lee D, et al. Planar cell polarity signaling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–4. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenny A, Mlodzik M. Planar cell polarity signaling. Mt Sinai J Med. 2006;73:738–50. [PubMed] [Google Scholar]
- 24.Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–78. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montcouquiol M, Rachel RA, Lanford PJ, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 26.Montcouquiol M, Bryan E, Crenshaw EB, Kelley MW. Development of planar cell polarity in the inner ear. Annu Rev Neurosci. 2006;29:363–86. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Borchers AJ, Jolicoeur C. PTK7/CCK-4 is a novel regulator of planar cell polarity in of planar cell polarity in vertebrates. Nature. 2004;430:93–8. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 28.Dabdoub A, Donohue MJ, Brennan A, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–84. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Mark S, Zhang X, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtin JA, Quint E, Tsipouri V, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]