Abstract
Background
Selective memory deficits occur in individuals with human immunodeficiency virus (HIV) infection and those with chronic alcoholism, but the potential compounded effect of these conditions is seldom considered, despite the high prevalence of alcohol use disorders in HIV infection.
Methods:
Here, we examined component processes of working and episodic memory in HIV infection and chronic alcoholism (ALC) in 4 subject groups (HIV, ALC, HIV + ALC, and normal controls) at baseline and 1-year follow-up. Accuracy scores, response times, and rate of information processing were assessed with subtests of the computerized neuropsychological test battery, the MicroCog.
Results:
Although individuals with either HIV infection or alcoholism generally performed at normal levels, individuals comorbid with HIV infection and alcoholism were impaired relative to controls and to the single diagnosis groups on selective memory processes. Immediate episodic memory was impaired, whereas working memory remained intact. Ability to retain information over time was not impaired in the clinical groups. Little performance change between groups was detected over 1 year. Results could not be explained by amount of alcohol consumed over a lifetime, CD4 cell count, AIDS diagnosis, or HAART medication.
Conclusions:
This study provides behavioral support for adverse synergism of HIV infection and chronic alcoholism on brain function and is consistent with neuroimaging reports of compromised hippocampal and associated memory structures related to episodic memory processes in these 2 conditions.
Keywords: HIV Infection, Alcoholism, Comorbidity, Memory, MicroCog
Heavy alcohol use is reported in more than 50% of HIV-infected clinic patients (Conigliaro et al., 2006; Cook et al., 2001; Miguez et al., 2003; Samet et al., 2004) and is associated with increased immune suppression (Wang and Watson, 1995; Wang et al., 2002). With the widespread use of highly active antiretroviral therapy (HAART), the incidence of cognitive and motor deficits related to HIV infection has declined (Sacktor et al., 2002); however, specific neuropsychological deficits persist (Cysique et al., 2004; Sacktor et al., 2002) and may be exacerbated by other conditions, notably alcohol use disorders, that affect neural systems.
Cognitive impairments ranging from attentional deficits to motor abnormalities have been associated with both HIV infection (Bornstein et al., 1993; Heaton et al., 1995; Rothlind et al., 2005) and chronic alcoholism (Fama et al., 2007; Fein et al., 1990; Oscar-Berman, 2000; Oscar-Berman and Marinkovic, 2003; Parsons, 1993; Parsons and Nixon, 1998; Sullivan et al., 2000, 2002b). Although the underlying mechanisms of HIV infection and chronic alcoholism differ, each condition results in untoward effects on the nervous system and has demonstrable effects on cortical, limbic, striatal, and cerebellar brain systems (Chang et al., 2001; Meyerhoff, 2001; Oscar-Berman and Hutner, 1993; Oscar-Berman and Marinkovic, 2003; Sullivan, 2000; Sullivan et al., 1995b).
Despite the number of reports documenting cognitive deficits associated with HIV infection and chronic alcoholism separately, few studies have addressed the combined effects of these diseases. Farinpour and colleagues (Farinpour et al., 2000) reported impaired verbal working memory, and Martin and colleagues (Martin et al., 2001, 2004) reported greater cognitive impulsivity in studies of individuals with HIV infection and drug use disorders, including alcoholism, compared with seronegative drug users. A study of comorbidity for HIV infection and current heavy alcohol consumption reported synergistic effects for these conditions on motor and visuospatial tasks (Rothlind et al., 2005). Examination of HIV-infected individuals with past alcohol use disorders (Green et al., 2004) provided evidence for additive and interactive effects of previous alcohol abuse and HIV infection on verbal reasoning, auditory processing, and reaction time tasks. Using a novel Match-to-Sample Stroop Task, Schulte and colleagues (2005) demonstrated that HIV-infected individuals with alcoholism had normally reduced reaction times when a valid match cue introduced a Stroop stimulus but were disproportionately slow when the cue was invalid, indicating impairment in attentional disengagement.
Common to both HIV infection and chronic alcoholism are reports of impaired memory and executive functions (Fama et al., 2004; Fein et al., 2006; Oscar-Berman, 1990; Oscar-Berman et al., 2004; Parsons and Nixon, 1993; Parsons and Prigatano, 1977; Pitel et al., 2007a,b; Sullivan et al., 2000, 2002b). Pitel and colleagues (2007a) examined both memory and executive function abilities in abstinent alcoholics and demonstrated that executive function impairments did not account for the majority of variance in memory scores, concluding the memory impairment to be “genuine” rather than solely the result of executive function deficits.
Over the past 3 decades, rigorous neuropsychological studies have revealed that memory, once thought to be a single process, actually comprises multiple processes, each contributing to different aspects of memory. These component processes include working memory, which refers to the ability to hold information for a short time as is needed for remembering a number while dialing a phone (Baddeley, 1992), and episodic memory, which refers to the ability to encode, store, and retrieve information for periods longer than 30 seconds (Tulving, 2002).
Examination of components of memory and identification of the specific mnemonic and nonmnemonic processes that may underlie these memory deficits would provide information concerning neural mechanisms involved in these processes. Although dissociable, the different processes of memory can interact and be mutually helpful or disruptive. In addition to differences in function, components of memory are supported by different brain systems (Gabrieli, 1998; Squire and Zola-Morgan, 1991), with frontal based neural systems particularly implicated in working memory and medial temporal neural systems particularly implicated in episodic memory. Neuroimaging studies have indicated hippocampal system compromise in HIV infection (Castelo et al., 2006) and in chronic alcoholism (Agartz et al., 1999; Cardenas et al., 2005; Laakso et al., 2000; Sullivan et al., 1995a), suggesting that episodic memory functions would be especially vulnerable in these conditions. Despite reports of impaired memory processes and anatomical substrates subserving these processes in HIV infection and alcoholism, little attention has been given to the possible confounding effects of these conditions on components of working and episodic memory.
Although cognitive performance is generally measured with accuracy scores, time taken to process information is an important component to consider when assessing cognitive processes. Altered time-accuracy trade-offs have been consistently demonstrated in alcoholism (Nixon and Bowlby, 1996; Sullivan et al., 2002a). Alcoholics performed normally but needed more time to do these tasks than normal controls. This phenomenon has also been observed in HIV-infected individuals. Chang and colleagues (Chang et al., 2001) reported that although accuracy scores were at the level of control subjects on working memory tasks, reaction times were slower.
Here, we used the MicroCog: Assessment of Cognitive Functioning (Powell et al., 1993), a computerized test battery that assesses component processes of working and episodic memory and records both accuracy scores and response times, in 4 groups of subjects (HIV, ALC, HIV + ALC, NC) at baseline and 1-year follow-up. We tested the hypotheses that (i) the groups would show a step-wise performance profile, with the comorbid (HIV + ALC) group performing worse than the single diagnosis groups (HIV-only and ALC-only) and the single diagnosis group performing worse than the controls (NC), (ii) episodic memory processes would be more compromised than working memory processes in the comorbid group, and (iii) the comorbid group would demonstrate the least improvement over time (i.e., dampened practice effects from baseline to follow-up) compared with the other groups.
METHODS
Participants
We examined 164 participants at baseline: 40 with HIV infection (HIV; 28 men, 12 women), 38 with alcoholism (ALC; 24 men, 14 women), 47 comorbid for both conditions (HIV + ALC; 38 men, 9 women), and 39 normal controls (NC; 22 men, 17 women) on sub-tests of the MicroCog. Subjects were recruited from HIV clinics and local substance abuse treatment programs. Controls were recruited from the local community. One-year follow-up testing was completed on 124 participants: 34 with HIV infection (HIV; 23 men, 11 women), 23 with alcoholism (ALC; 14 men, 9 women), 37 comorbid for both conditions (HIV + ALC; 31 men, 6 women), and 30 normal controls (NC; 17 men, 13 women).
All participants were screened using the structured psychiatric diagnostic interview for the DSM-IV (SCID) (First et al., 1998) and structured questionnaires on health status. Subjects were excluded if they had a significant history of psychiatric or neurological disorder not related to their primary diagnosis, a history of past or present alcohol or drug abuse or dependence in the NC group, recent (within the last 3 months) substance dependence other than alcohol in the patient groups, or a serious medical condition or HIV-related opportunistic infection. The Beck Depression Inventory-II (Beck et al., 1996) assessed severity of depression symptoms in all groups. HIV subjects were also rated on the Karnofsky Scale (Karnofsky, 1949). All subjects underwent a semi-structured interview (Skinner, 1982; Skinner and Sheu, 1982) to quantify lifetime alcohol consumption and periods of heavy drinking (defined as >4 drinks for women and >6 drinks for men per day). Blood sample for confirmation of HIV status via HIV Ab, plasma viral load, and CD4 cell count was conducted.
Subjects were assigned to one of the following 4 groups based on clinical assessment: (i) HIV-infected individuals who had never met criteria for alcohol abuse or dependence and had never consumed more than 6 drinks per day for men or 4 drinks per day for women on average over any 30 day period (HIV), (ii) individuals who met lifetime criteria for alcohol dependence within the past 3 years and were seronegative for HIV (ALC), (iii) HIV-infected individuals who also met criteria for alcohol abuse or dependence within the past 3 years (HIV + ALC), and (iv) control individuals who were neither HIV positive nor met alcohol abuse or dependence criteria or other Axis-I diagnosis in their lifetime (NC). Duration of abstinence at baseline was not different in the alcohol groups (ALC group 153 ± 219 days; HIV + ALC group 168 ± 258 days) and included a subset of active drinkers in each group. The HIV-infected groups did not differ on duration of illness or viral load (HIV group: duration 9.0 ± 6.6 years, log viral load 3.1 ± 1.0; HIV + ALC group: duration 10.7 ± 6.4 years; log viral load 3.3 ± 1.1).
Ten of the 40 HIV and 15 of the 47 HIV + ALC subjects had met CDC criteria for AIDS (CD4 < 200, AIDS defining symptom) sometime during the course of their illness. Of the 40 HIV-infected individuals 25 were on HAART and 6 were on other HIV treatment; and in the HIV + ALC group, 22 of 47 subjects were on HAART and 5 individuals were on other HIV treatment. All HIV-infected individuals had CD4 > 100 cells per mm3 at initial visit and a Karnofsky score >70. All data were obtained in compliance with the regulations of both SRI International and Stanford University through Institutional Review Board review and approval. Written informed consent was obtained from all participants. Demographics for the 4 subject groups at baseline and follow-up are in Table 1.
Table 1.
Demographic Characteristics of Subjects (mean, standard deviation, range) at Baseline and Follow-Up Testing
| Group | Age (yrs) | Education (yrs) | NART IQ† | Lifetime consumption (kg) | Beck Depression Inventory–II | CD4 cell count |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| HIV infection | 41.8 | 14.0 | 58.4 | 10.6 | 527.9 | |
| (HIV n = 40) | (9.7) | (2.8) | – | (58.2) | (8.6) | (264.1) |
| 19 to 59 | 6 to 19 | 0 to 247 | 0 to 36 | 46 to 1227 | ||
| Alcoholic | 42.8 | 13.4 | 847.1 | 11.7 | ||
| (ALC n = 38) | (9.4) | (1.9) | – | (703.2) | (9.8) | NA |
| 22 to 57 | 8 to 18 | 33 to 3656 | Oto 37 | |||
| HIV + Alcoholism | 44.9 | 13.0 | 914.7 | 12.1 | 437.0 | |
| (HIV + ALC n = 47) | (7.0) | (2.2) | – | (660.0) | (8.2) | (216.1) |
| 25 to 58 | 8 to 18 | 25 to 2861 | 0 to 30 | 100 to 1072 | ||
| Control | 40.4 | 15.0 | 45.4 | 2.3 | ||
| (NC n = 39) | (10.1) | (2.1) | – | (75.0) | (2.9) | NA |
| 20 to 72 | 12 to 19 | 0 to 301 | 0 to 12 | |||
| Baseline ANOVAs Post-hoc t-tests |
ns |
p = 0.0008 ALC = ALC+HIV < NC |
p = 0.0001 ALC = HIV + ALC > HIV = NC |
p = 0.0001 HIV = ALC = HIV + ALC > NC |
ns | |
| Follow-up | ||||||
| HIV infection | 44.0 | 14.0 | 109.7 | 60.9 | 10.3 | 517.6 |
| (HIV n = 34) | (9.8) | (2.9) | (8.8) | (59.5) | (7.7) | (245.2) |
| 21 to 60 | 6 to 19 | 89 to 121 | 0 to 248 | 0 to 34 | 151 to 1177 | |
| Alcoholic | 44.4 | 13.5 | 108.5 | 770.0 | 9.3 | |
| (ALC n = 23) | (9.2) | (2.1) | (7.1) | (769.1) | (11.2) | NA |
| 23 to 58 | 8 to 18 | 96 to 121 | 40 to 3685 | 0 to 42 | ||
| HIV + alcoholism | 45.7 | 13.1 | 106.6 | 853.9 | 9.4 | 469.1 |
| (HIV + ALC n = 37) | (7.4) | (2.3) | (8.3) | (644.9) | (8.0) | (306.8) |
| 26 to 59 | 8 to 18 | 91 to 122 | 26 to 2609 | 0 to 26 | 108 to 1323 | |
| Control | 42.0 | 15.1 | 112.6 | 51.3 | 2.5 | |
| (NC n = 30) | (10.5) | (2.0) | (1.3) | (87.2) | (4.4) | NA |
| 21 to 73 | 12 to 18 | 92 to 124 | 0 to 318 | 0 to 20 | ||
| Follow-up ANOVAs Post-hoc t-tests |
p = 0.0074 ALC = ALC + HIV < NC |
p = 0.032 ALC = ALC + HIV < NC |
p = 0.0001 ALC = HIV + ALC > HIV = NC |
p = 0.0016 HIV = ALC = HIV + ALC > NC |
ns | |
NA = Not applicable.
NART, National Adult Reading Test was given at follow-up testing and not at baseline.
Groups did not differ significantly in age [F(3,160) = 1.89, p = 0.133] but did in years of formal education [F(3,160) = 5.90, p = 0.0008] and IQ estimate scores [F(3,111) = 3.04, p = 0.032] [National Adult Reading Test (NART); Nelson, 1982]. Follow-up analyses indicated that the ALC and HIV + ALC groups had fewer years of education and lower NART-IQ estimates than the control group (years of education: ALC vs. NC, t(75) = 3.48, p = 0.008; HIV + ALC vs. NC, t(84) = 4.29, p = 0.0001; NART: ALC vs. NC, t(49) = 2.03, p = 0.048; HIV + ALC vs. NC, t(61) = 3.05, p = 0.004).
Examination of baseline subjects who did and did not return for follow-up yielded few demographic differences. The 10 HIV + ALC subjects who did not return for follow-up had a shorter period of alcohol sobriety prior to initial testing [t(45) = 2.72, p = 0.01; 118 days vs. 352 days] and modestly more days of lifetime heavy drinking [t(45) = 1.96, p = 0.06, 6,205 days vs. 4,154 days] than the HIV + ALC subjects who remained in the study. The subgroup of 6 HIV subjects who did not return was modestly younger [t(38) = 1.97, p = 0.06; 34.8 years vs. 43.0 years] than the retained HIV group.
Neuropsychological Measures
The MicroCog
Assessment of Cognitive Functioning (Powell et al., 1993) is a computerized battery designed as a screening measure for cognitive impairment in adults. Accuracy scores and response times are calculated for each subtest. Instructions at the beginning of test administration state that both accuracy and speed are important but that accuracy is more important than speed (Elwood, 2001). We selected subtests from this battery to specifically examine the constructs of working and episodic memory. All subjects reported normal or corrected to normal vision at testing.
Working Memory
Digits Forward
Sequences of digits were presented 1 at a time on the computer screen, each digit remained on the screen for 1 second. After the sequence was completed, the subject typed the sequence just seen on the monitor using the number pad. The first trial consisted of 5 digits and, depending on whether the subject responded correctly or incorrectly, the next trial contained one more or one fewer number than the previous trial. The test continued to a maximum span of 9 digits or was discontinued after 3 consecutive incorrect trials. A subject's score was the sum of the number of digits within correctly sequenced trials (e.g., a correct sequence of 5 numbers received a score of 5); scores could range from 0 to 71.
Digits Backward
Sequences of digits were presented in the same manner as for Digits Forward, but instead subjects typed the numbers in reverse order of presentation. The beginning sequence span was 4 digits, maximum span was 9 digits, and total scores could range from 0 to 66.
Alphabet Tracking
Letters were presented at a rate of 1/s. Subjects pressed the enter key when they saw the letter “A” and then when they saw the letter “B” and so on in sequential order from “A” to “O.” If a target letter was missed, subjects were cued on the screen that they missed the target and the letter sequence containing the missed target was repeated. Each letter could be missed up to 3 times, resulting in scores per letter ranging from 0 to 3 points. The subtest was discontinued after 3 misses on any target letter trial. Total scores could range from 0 to 45. This subtest has been reported to be highly correlated (r = 0.85) with the Mental Control subtest of the Wechsler Memory Scale–III (Elwood, 2001; Wechsler, 1997).
Math Computation
Eight, single operand (addition, subtraction, multiplication, or division) equations were presented and subjects were to type the correct answer from left to right on the number pad. Each correct answer received one point, resulting in total scores from 0 to 8. This task is moderately correlated (r = 0.62) with the Arithmetic subtest of the Wechsler Adult Intelligence Scale–Revised (Green et al., 1994; Wechsler, 1981).
Verbal Episodic Memory
Address
Subjects were asked to read and remember a name and address, and were allowed as much time as needed before proceeding to the next task. Questions pertaining to this information (4 items) are included as part of the delayed memory subtest.
Stories
Two short narratives were presented, 1 at a time on the computer. Subjects were allowed as much time as needed to read and learn each story. Following each story, subjects were presented multiple choice questions. The immediate memory condition consisted of 11 questions (5 questions about the first story and 6 questions about the second story). The delayed memory subtest consisted of 27 questions: 23 questions based on the stories (11 questions about the first story and 12 questions about the second story) and 4 questions based on the address. The time interval between immediate and delayed conditions was between 10 and 15 minutes. Percent memory retention scores were based on whether correct responses on immediate memory were also correct on delayed memory, thus retention scores could not exceed 100%. Performance on this subtest has been reported to be moderately correlated (r = 0.63) with the Logical Memory subtests of the Wechsler Memory Scale–Revised (Green et al., 1994).
Statistical Analyses
Baseline accuracy scores are presented in Table 2. Statistical analyses, however, used age- and education-adjusted scores unless otherwise noted. Accordingly, baseline and follow-up scores for each subject were standardized on the baseline scores of the control group, corrected for age and years of education and expressed as Z-scores. Information processing rates were calculated by dividing accuracy raw scores by response times, and these ratio scores were then standardized on the ratio scores of the control group at baseline. Where necessary, Z-scores were inverted (e.g., response times) so that for all measures higher scores indicated better performance.
Table 2.
Mean, SD, Range for Baseline Accuracy Scores of MicroCog Subtests
| HIV | ALC | HIV + ALC | NC | |
|---|---|---|---|---|
| Working memory | ||||
| Alphabet tracking (max = 45) | 44.1 | 43.4 | 42.8 | 44.8 |
| (2.1) | (5.0) | (6.9) | (0.5) | |
| 33 to 45 | 15 to 45 | 1 to 45 | 43 to 45 | |
| Digits forward (max = 71) | 28.3 | 26.6 | 24.4 | 28.1 |
| (9.1) | (1.4) | (7.7) | (9.7) | |
| 10 to 49 | 11 to 48 | 11 to 51 | 10 to 52 | |
| Digits backward (max = 66) | 22.1 | 22.7 | 18.7 | 25.5 |
| (9.8) | (8.7) | (8.4) | (11.5) | |
| 4 to 46 | 4 to 45 | 0 to 43 | 9 to 47 | |
| Math calculation (max = 8) | 5.3 | 4.6 | 4.7 | 5.5 |
| (2.3) | (2.5) | (2.3) | (1.9) | |
| 0 to 8 | 0 to 8 | 0 to 8 | 0 to 8 | |
| Episodic memory | ||||
| Immediate memory (max = 11) | 9.0 | 8.3 | 7.7 | 8.8 |
| (1.6) | (1.9) | (2.0) | (1.5) | |
| 5 to 11 | 5 to 11 | 4 to 11 | 5 to 11 | |
| Delayed memory (max = 27) | 18.8 | 18.6 | 17.3 | 19.2 |
| (3.5) | (3.4) | (4.2) | (2.9) | |
| 9 to 27 | 11 to 26 | 9 to 26 | 9 to 23 | |
| Percent retention memory (max = 100) | 85.7 | 87.8 | 84.7 | 89.2 |
| (13.4) | (10.8) | (19.7) | (13.8) | |
| 50 to 100 | 60 to 100 | 14 to 100 | 50 to 100 |
Baseline scores were assessed with ANOVAs and 2 group t-tests. Repeated measures ANOVA examined differences in baseline and follow-up scores, and significant differences were examined with follow-up t-tests. Within-group paired t-tests compared baseline and follow-up testing where appropriate. Finally, within-group analyses examined relationships between test scores and demographic variables, including depressive symptoms, lifetime alcohol consumption, and CD4 cell count with Pearson product-moment correlations or Spearman Rho correlations, depending on the nature and distribution of the scores. In light of the multiple comparisons made, group differences on test scores were reported as significant only if they met criteria for Benjamini and Hochberg's false discovery rate (FDR) procedure that protects against Type I errors while also limiting Type II errors (Benjamini and Hochberg, 1995; Benjamini et al., 2001). The FDR for between group analyses was based on 18 test scores in this study and was set to an alpha level of 5% to denote significant differences and a rate of 10% to denote statistical trends. Follow-up tests were evaluated based on an FDR calculated for the 6 group comparisons made in each case (HIV vs. ALC; HIV vs. HIV + ALC; HIV vs. NC; ALC vs. HIV + ALC; and HIV + ALC vs. NC).
RESULTS
Accuracy Scores
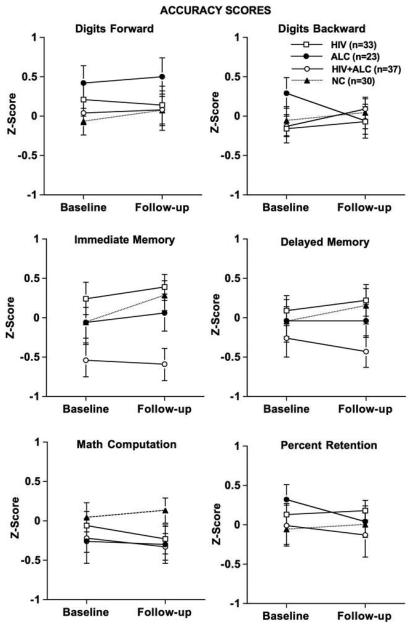
Figure 1 depicts age- and education-corrected accuracy Z-scores for each group at baseline and follow-up for each test measure.
Fig. 1.
Accuracy scores: Age- and education-corrected Z-scores for baseline and follow-up for all groups.
Group x test session interactions derived from repeated measures ANOVA were not forthcoming for accuracy scores. A group difference was observed on the immediate episodic memory score [F(3,120) = 5.39, p = 0.002] (Fig. 1). The HIV + ALC group had significantly lower scores at follow-up compared with the HIV and NC groups [HIV + ALC vs. HIV t(69) = 3.68, p = 0.0005; HIV + ALC vs. NC t(65) = 3.08, p = 0.003]. The NC, ALC, and HIV groups did not differ among each other. Group differences were not observed on delayed memory or amount of information retained from immediate memory. Additionally, working memory scores were not significantly different between the groups.
Response Times
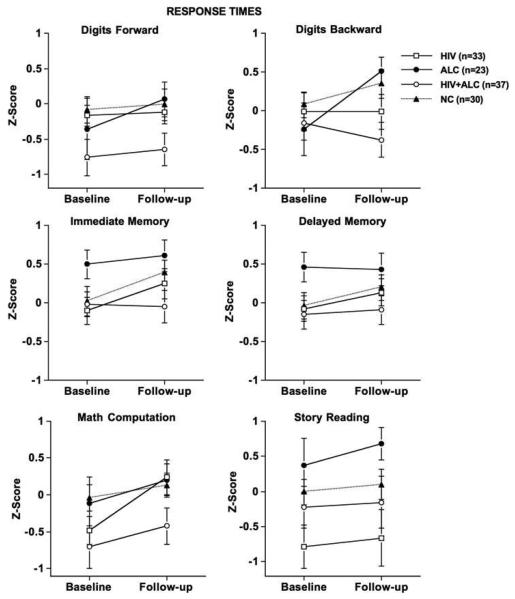
Figure 2 depicts age- and education-corrected Z-scores for response times at baseline and follow-up testing. A modest test session difference was observed [F(3, 120) = 6.98, p = 0.009], with the HIV group taking significantly less time to complete the immediate episodic memory task at follow-up than at baseline [t(33) = 3.20, p = 0.003]. Group differences were not observed on any response times associated with the working memory tasks.
Fig. 2.
Response times: Age- and education-corrected Z-scores for baseline and follow-up for all groups.
Information Processing Rate
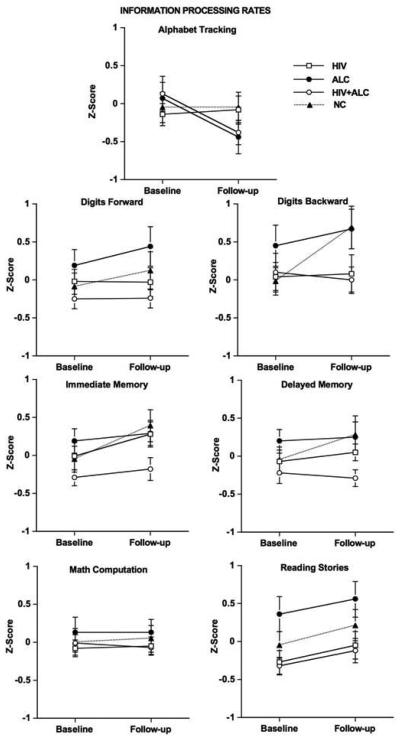
Group x test session interactions were not significant for baseline or follow-up information processing rates. Figure 3 depicts information processing rates for each test measure for each group at baseline and follow-up. A test session main effect was observed in information processing rate for immediate memory [F(1,120) = 10.81, p = 0.001], with modestly higher processing rate at follow-up compared with baseline for the NC [t(29) = 2.57, p = 0.016] and HIV [t(33) = 2.25, p = 0.031] groups. Modest differences were noted for group and test session variables on rate of information processing for story reading [group: F(3,120) = 3.57, p = 0.016; test session F(1,120) = 6.13, p = 0.015], with the HIV + ALC group having modestly lower rates of processing than the ALC group at baseline and follow-up (baseline: HIV + ALC vs. ALC t(58) = 2.91, p = 0.006; follow-up: HIV + ALC vs. ALC t(58) = 2.67, p = 0.01). Information processing rates for the working memory tasks were not statistically different among the groups.
Fig. 3.
Information processing rates: Age- and education-corrected Z-scores for baseline and follow-up for all groups.
Possible Moderating Demographic and Disease-Related Variables
Neither depressive symptoms, as assessed with the BDI-II, nor lifetime alcohol consumption, estimated with the timeline interview, correlated significantly in any patient group. Group differences were not significant for accuracy scores, response times, or information processing rates in individuals with AIDS versus those without AIDS. Higher CD4 cell counts in the HIV + ALC group at baseline correlated with greater improvement in percent memory retention (r = 0.49, p = 0.003) over time. Change in CD4 cell count between sessions, however, was not related to cognitive scores examined.
DISCUSSION
In support of our primary hypothesis, the comorbid group (HIV + ALC) performed worse than the other 3 groups (HIV, ALC, NC) on specific memory tasks, and the deficits were selective rather than global and nonspecific. In contrast to episodic memory, working memory processes did not differ between groups. Examination of accuracy scores revealed selective impairments in immediate episodic memory processes in the HIV + ALC group compared with the HIV-only and control groups. By contrast, percent retention, the ability to retain information once learned, was at normal levels for the HIV + ALC group. This is consistent with Nixon and colleagues (1987) who reported episodic memory impairment with normal retention levels over time in middle-aged detoxified male alcoholics. This pattern of spared and impaired memory processes is suggestive of an encoding rather than a retrieval deficit in these individuals.
Information processing rate deficits (accuracy/response time) were observed on episodic memory tasks in the HIV + ALC group. Unlike other studies (Nixon and Parsons, 1991; Nixon et al., 1995), however, we found no evidence of an accuracy-time trade-off in our ALC-only group. Information processing rates for the single diagnosis groups were comparable to that of the control group. The MicroCog imposed no time limit on the working memory and episodic memory tasks. Indeed, subjects were instructed that, although speed was important, accuracy was more important. These instructions differ from those commonly given where speed and accuracy are both required in performance. Thus, differences in test administration may account for the lack of a speed /accuracy trade-off in the current study.
The immediate verbal episodic memory deficits observed in this study in the comorbid group lend behavioral support to in vivo neuroimaging reports of compromised medial temporal neural systems involving hippocampal and related structures in HIV infection (Castelo et al., 2006) and chronic alcoholism (Agartz et al., 1999; Cardenas et al., 2005; Sullivan and Marsh, 2003; Sullivan et al., 1995a) separately. In HIV individuals, Castelo and colleagues (Castelo et al., 2006), using fMRI, observed altered integrity of hippocampal-pre-frontal regions during episodic encoding, despite comparable accuracy scores and reaction times for the HIV group relative to age and education matched controls. These authors suggested that the HIV virus functionally affects the medial temporal as well as frontal-striatal systems. In alcoholic subjects, Sullivan and colleagues (1995a), using structural MRI, demonstrated hippocampal volume deficits, suggesting that chronic alcoholism affects hippocampal systems, even though these volume deficits were not related to episodic memory impairment in their subjects. Thus, while structural integrity is compromised in the hippocampus and associated memory regions in HIV infection and chronic alcoholism, it may be that, in comorbid individuals behavioral evidence of these changes are more easily detected because of their additive adverse effects on structures underlying episodic memory processes.
Investigating possible molecular mechanisms of the additive and synergistic neurotoxic effects of HIV infection and alcoholism, Flora and colleagues (2005) examined mice co-exposed to Tat, a regulatory protein of HIV and ethanol. Dually-treated mice demonstrated an increased level of oxidative stress and inflammatory related processes, conditions associated with cell dysregulation and cell death, in the hippocampus and striatum compared with mice that were not dually exposed. Venkatesan and colleagues (2007) have alternatively speculated that cognitive dysfunction in individuals comorbid for HIV infection and drugs of abuse, including alcohol, may be related to alterations in hippocampal neuro-genesis.
The pattern of performance on the MicroCog remained relatively the same over a period of 1 year. Although changes were generally not significant from baseline to follow-up, groups tended to show a positive slope in performance, indicative of a practice effect over time. Thus, our hypothesis that the comorbid group would not show practice effects was not supported. Lack of significant worsening in performance over time may have been due to the relatively short interval between testing sessions or lack of sensitivity of the MicroCog measure.
Consistent with a report by Martin and colleagues (2001), history of an AIDS-defining event did not distinguish the HIV groups on memory performance. With longitudinal testing, however, we did observe that greater improvement of memory retained from immediate to delayed testing was related to improvement in CD4 cell count, suggesting a link between performance and HIV disease state.
Although no impairments in working memory or episodic memory were detected in the alcohol-only and HIV-only groups, a conclusion based on this one test that dysfunction in these processes does not exist in either primary condition is not warranted. The MicroCog was designed as a screening devise for cognitive dysfunction and as such is likely not a sensitive enough measure for detecting subtle memory compromise. The ability of this test to detect deficits in the comorbid group together with the conservative statistical approach adopted for analyses lends confidence that the episodic memory impairments identified do exist and do not reflect a spurious finding.
Presence of memory impairment has real-world implications for daily functioning. For example, episodic memory deficits put affected individuals at risk for difficulties in maintaining medication regimes, following through on work and family responsibilities, and effectively using information available to them (Gorman et al., 2009). Even subtle deficits in episodic memory can adversely affect functional capabilities for activities of daily life. Identification of memory deficits and knowledge of selective processes affected may assist individuals in understanding how certain difficulties in their life are related to these disrupted processes and learning compensatory strategies to overcome affected functions, e.g., keeping notes on medication and appointments and using lists to direct daily activities. In summary, the episodic memory deficits identified in HIV + ALC, but not detected in the single diagnosis groups, suggest that disease comorbidity results in compounded untoward effects on episodic memory processes.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA012999, AA017347, AA005965, and AA017168. Portions of this research were presented at the 2006 and 2007 International Neuropsychological Society meetings.
REFERENCES
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to mulitple testing. J Royal Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bornstein RA, Nasrallah HA, Para MF, Whitacre CC, Rosenberger P, Fass RJ. Neuropsychological performance in symptomatic and asymptomatic HIV infection. AIDS. 1993;7:519–524. doi: 10.1097/00002030-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66:1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Justice AC, Gordon AJ, Bryant K. Role of alcohol in determining human immunodeficiency virus (HIV)-relevant outcomes: A conceptual model to guide the implementation of evidence-based interventions into practice. Med Care. 2006;44(8 Suppl 2):S1–S6. doi: 10.1097/01.mlr.0000223659.36369.cf. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of 2 cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Elwood RW. MicroCog: assessment of cognitive functioning. Neuropsychol Rev. 2001;11:89–100. doi: 10.1023/a:1016671201211. [DOI] [PubMed] [Google Scholar]
- Fama R, Eisen JC, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Pfefferbaum A, Sullivan EV. Upper and lower limb motor impairments in alcoholism, HIV-infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1038–1044. doi: 10.1111/j.1530-0277.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: Contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Novak RM, Harrow M. Verbal working memory in HIVseropositive drug users. J Int Neuropsy Soc. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Flora G, Pu H, Lee Y, Ravikumar R, Nath A, Henning B, Toborek M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191:2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19:186–203. doi: 10.1007/s11065-009-9095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- Green R, Green J, Harrison J, Kutner M. Screening for cognitive impairment in older individuals: Validation of a computer-based test. Arch Neurol. 1994;51:779–786. doi: 10.1001/archneur.1994.00540200055017. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsy Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, Tiihonen J. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, Harrow M. Auditory working memory in HIV-1 infection. J Int Neuropsy Soc. 2001;7:20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Research & Health. 2001;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Nixon SJ, Bowlby D. Evidence of alcohol-related efficiency deficits in an episodic learning task. Alcohol Clin Exp Res. 1996;20:21–24. doi: 10.1111/j.1530-0277.1996.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Kujawski A, Parsons OA, Yohman JR. Semantic (verbal) and figural memory impairment in alcoholics. J Clin Exp Neuropsych. 1987;9:311–322. doi: 10.1080/01688638708405053. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Parsons OA. Alcohol-related efficiency deficits using an ecologically valid test. Alcohol Clin Exp Res. 1991;15:601–606. doi: 10.1111/j.1530-0277.1991.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcohol Clin Exp Res. 1995;19:577–581. doi: 10.1111/j.1530-0277.1995.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr. 1990;101:136–155. [PubMed] [Google Scholar]
- Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No. 34. National Institutes of Health; Bethesda, MD: 2000. pp. 437–472. [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-Induced Brain Damage, NIAAA Research Monographs No. 22. National Institutes of Health; Rockville, MD: 1993. pp. 121–156. [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28:667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcoholism and the brain: an overview. Alcohol Res Health. 2003;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- Parsons O. Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol Induced Brain Damage, NIAAA Research Monograph No. 22. National Institutes of Health; Rockville, MD: 1993. pp. 173–194. [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurol Clin. 1993;11:205–218. [PubMed] [Google Scholar]
- Parsons O, Nixon S. Cognitive-functioning in sober social drinkers: A review of the research since 1986. J Stud Alcohol. 1998;59:180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Prigatano GP. Memory functioning in alcoholics. In: Birnbaum IM, Parker ES, editors. Alcohol and Human Memory. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 185–194. [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, Desgranges B, Eustache F. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res. 2007a;31:1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, Beaunieux H. Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcohol Clin Exp Res. 2007b;31:238–248. doi: 10.1111/j.1530-0277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Powell D, Kaplan E, Whitla D, Weintraubm S, Caitlin R, Funkenstein H. MicroCog Assessment of Cognitive Functioning Manual. Psych Corp; San Antonio, TX: 1993. [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsy Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of Neurovirology. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: Evidence from a Stroop match-to-sample task. Biol Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, NIAAA Research Monograph No. 34. National Institutes of Health; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin Exp Res. 2002a;26:705–713. [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002b;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L. Hippocampal volume deficits in alcoholic Korsakoff's syndrome. Neurology. 2003;61:1716–1719. doi: 10.1212/01.wnl.0000098940.31882.bb. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995a;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Deshmukh A, Desmond JE, Pfefferbaum A. Alcohol and the cerebellum: effects on balance, motor coordination, and cognition. Alcohol Health & Research World. 1995b;19:138–141. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming Gl, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. CMLS, Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O'Brien CP, Song L, Davis-Vogal A, Ho WZ. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin Exp Res. 2002;26:1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Watson RR. Is alcohol consumption a cofactor in the development of acquired immunodeficiency syndrome? Alcohol. 1995;12:105–109. doi: 10.1016/0741-8329(94)00090-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Revised. The Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. Third Edition The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]