In a rapidly developing field, one can always anticipate that different interpretations of similar data will co-exist. Stem cell transplanters can be a contentious lot, especially in the absence of controlled randomized trials. Thus, while improvements in the basic understanding of acute GVHD has led to many testable hypotheses in the management of GVHD, there remains little consensus regarding the most effective and least toxic approach to GVHD prevention. In the 1980’s the comparison would have been between cyclosporine based regimens and ex vivo T cell depletion. While ex vivo T cell depletion is still used in some settings, pharmacologic based therapy and in vivo T cell depletion with serotherapy now predominate. This review is meant to highlight the advantages and disadvantages of the “standard of care” and assess the prospects for future regimens that may be more effective.
The case for calcineurin inhibitors plus methotrexate
Rainer Storb, MD
Graft-versus-host disease (GVHD) has been the Achilles heel of allogeneic hematopoietic cell transplantation (HCT) since its first description in the 1950s (reviewed in 1). It is caused by immune reactions of donor T cells against disparate host histocompatibility antigens. Most GVH reactions are undesirable, and cause disease in skin, gut, and liver as principal target organs. One reaction, directed at hematopoietic tissue targets, is desirable and indispensable for the cure of hematologic malignancies but difficult to separate from GVHD. Donor T-cell immunity can be triggered by both major and minor histocompatibility differences. The latter observation made in the late 1960s in dogs given marrow grafts from DLA-identical littermates2,3 was unpredicted and prompted the search for immunosuppressive drugs capable of averting or at least mitigating the often violent GVH reactions.
Most then known immunosuppressive agents, alkylators and antimetabolites, were screened for efficacy in animal models during the late 1960s and early 1970s, either given alone or in combinations. Many of them showed toxicities to the gastrointestinal tract, the liver, or the marrow graft, which led to their elimination from further investigation. One of the drugs, the folate antagonist methotrexate (MTX), emerged the winner. Studies, first by Uphoff in mice 4 and then by the Seattle group in dogs5, showed significant reduction in GVHD severity, prolongation of survival, and occasional long-term survivors, even among major histocompatibility complex (MHC)-mismatched graft recipients. Presumably, this resulted from a dramatic effect of MTX on grafted donor lymphocytes attempting to replicate in response to encountering antigens on host target tissues. For best effect, MTX dosing was begun not before 24 hours after HCT had elapsed. Owing to its gastrointestinal side-effects, further MTX doses were spaced, eventually resulting in the day 1, 3, 6, 11, and then once weekly schedule that entered clinical trials in the late 1960s and remained “standard” through the 1970s6. Even under the best of circumstances, HLA-identical sibling marrow grafts for aplastic anemia patients conditioned with cyclophosphamide, a 15% incidence of grade III and a 10% incidence of grade IV acute GVHD were seen 7,8.
A clinical pilot study with the first calcineurin inhibitor, cyclosporine (CSP), was reported by Powles et al. in 19789. Disappointingly, canine studies showed equivalency between MTX and CSP in regards to acute GVHD prevention 10. This was confirmed in three prospective clinical trials in patients given HLA-identical sibling grafts for hematologic malignancies11. Mortality from GVHD-related complications continued to remain high.
Consequently, combinations and different schedules of several antimetabolites and CSP were explored in dogs in hopes of identifying superior regimens12. Of all combinations and schedules tested, a short course of MTX with an extended course of CSP showed clear evidence of synergism between the two drugs and resulted in impressive improvement of survival in recipient dogs13. Two small randomized, prospective clinical trials, one in patients with aplastic anemia and the other in patients with acute or chronic myelogenous leukemia given HLA-identical sibling grafts, showed MTX/CSP to be significantly better both in preventing acute GVHD and improving survival than either drug alone14,15. Both trials were published in 1986 with follow-up reported in 200516,17. Grade IV acute GVHD was not seen in patients given MTX/CSP in either study, and grade III disease was the exception, while patients given single agent prophylaxis experienced 38% (MTX) and 26% (CSP) grades III-IV acute GVHD, respectively. Disappointingly, no improvements were seen with regard to chronic GVHD. Moreover, some concern was raised that patients with acute myelocytic leukemia given MTX/CSP might have a slightly higher rate of leukemic relapse than their CSP-treated counterparts, but differences were not statistically significant, possibly due to small patient numbers in each arm.
When MTX/CSP was used in early unrelated HCT (“archaic” HLA typing: donors chosen by serologic testing for HLA-A and –B and mutual non-reactivity of their cells in mixed leukocyte culture) and HLA-mismatched related grafts, results were somewhat disappointing, and comparatively high rates of severe acute GVHD were seen. Attempts to improve outcomes of HCT by adding prednisone to the MTX/CSP combination did not yield convincingly positive results18–20.
In the early 1990s, another calcineurin inhibitor, tacrolimus (FK-506), promised wonders in the liver transplantation field 21. However, studies in the canine HCT model showed tacrolimus alone to be no better than either MTX or CSP alone, even when it was combined with prednisone22,23. Not surprisingly and reminiscent of the CSP experience more than a decade earlier, combining tacrolimus with a short course of MTX led to impressive improvement in survival of canine recipients22. These preclinical findings led to several Phase I/II clinical trials, which were followed by two multicenter, randomized, prospective trials comparing MTX/CSP to MTX/tacrolimus, one in HLA-identical sibling recipients24 and the other in recipients of HLA-matched unrelated grafts25; all patients in the two trials had hematologic malignancies. Both trials showed reductions in the overall incidence of acute (but not chronic) GVHD among patients in the MTX/tacrolimus arms compared to MTX/CSP. However, surprisingly, survival of MTX/tacrolimus-treated patients was not better than that of patients given MTX/CSP; in fact, it was slightly worse in the trial involving HLA-identical sibling grafts. The outcomes of the two trials prompted some transplant centers to become proponents of MTX/tacrolimus, while others continued using MTX/CSP. As for use of the latter, Figures 1 and 2 give examples of survivals in patients with aplastic anemia and chronic myelogenous leukemia given MTX/CSP prophylaxis after HLA-matched related or unrelated HCT.
Figure 1.
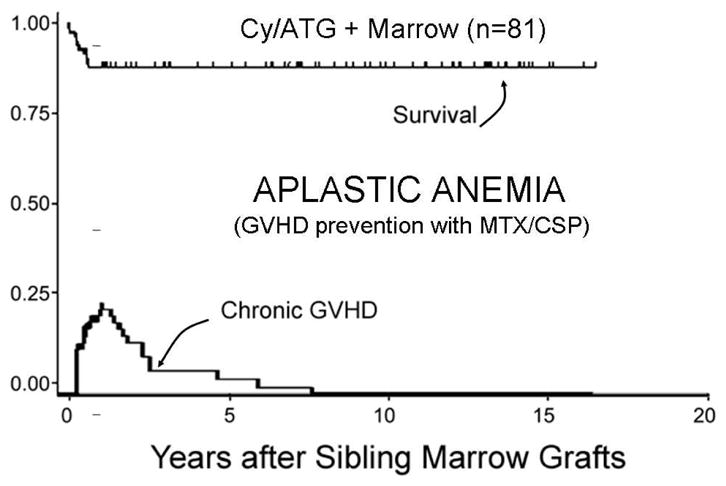
Survival and chronic GVHD in patients with aplastic anemia (2–63 years old) given HLA-matched related grafts and receiving MTX/CSP after conditioning with cyclophosphamide and anti-thymocyte globulin (Cy/ATG)87. Similar results have been reported in children by Locatelli et al.88.
Figure 2.
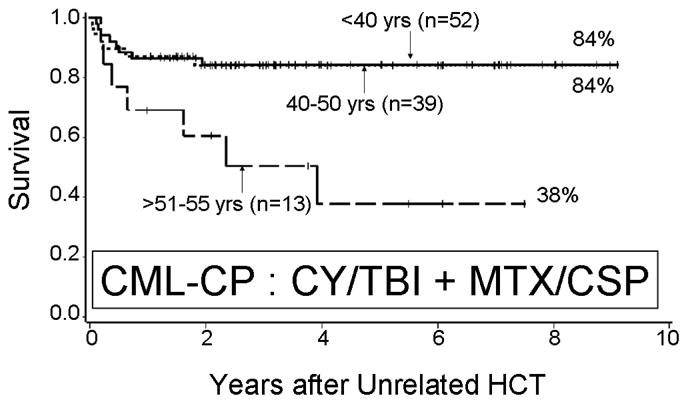
Survival of patients with chronic myelogenous leukemia in chronic phase (CML-CP) given HLA-matched unrelated HCT and receiving MTX/CSP after conditioning with cyclophosphamide and total body irradiation (Cy/TBI)89. This represents a subgroup of patients who received prophylaxis with fluconazole and ganciclovir.
Another agent, mycophenolate mofetil (MMF), whose metabolite mycophenolic acid inhibits proliferation of lymphocytes, was also highly synergistic in dogs when combined with CSP, both in preventing acute GVHD26 and enhancing hematopoietic engraftment after nonmyeloablative conditioning27. The combination is now widely used for patients receiving reduced-intensity conditioning regimens (reviewed in 28). Serious (grade 3–4) acute GVHD was seen in 4–5% of HLA-matched related29 and 10 % of HLA-matched unrelated recipients30 given nonmyeloablative conditioning. However, prevention of acute GVHD was not improved with MMF/CSP in human myeloablative recipients when compared to MTX/CSP31,32. Yet another study showed improved prevention of acute GVHD when the agent rapamycin (sirolimus) was combined with MTX/CSP33, while a more recent study did not confirm this result34. Of note, combining the non-toxic drug ursodiol with MTX/CSP (tacrolimus) or MMF/CSP GVHD prevention, while not reducing the overall incidence of acute GVHD, has led to a remarkable reduction in liver GVHD and perhaps also non-relapse mortality35.
Conclusions
The combination of MTX and a calcineurin inhibitor originated in preclinical canine studies from the early 1980s and 1990s, and has been in clinical use for more a quarter century. It has led to outstanding survivals after HLA-identical sibling grafts, for example in patients with aplastic anemia and chronic myelogenous leukemia. It has been slightly less effective for unrelated transplantations and noticeably less effective for HLA-mismatched grafts. For patients with hematologic malignancies, MTX/CSP (tacrolimus) seems to have struck a reasonable balance between preventing undesirable GVH reactions and retaining desirable graft-versus-tumor effects. Yet, the drug combination is far from ideal. The immunosuppressive agents have numerous side effects, have to be given for extended periods of time, delay immunologic recovery, and, moreover, are not uniformly effective, with many patients still dying from acute GVHD and associated complications. So far, attempts at improving on MTX/CSP (tacrolimus) have met with only equivocal success. The ideal GVHD prevention would consist of causing apoptotic death of all host-reactive donor lymphocytes within days of HCT while leaving donor T cells with memory for pathogens unscathed, thereby inducing graft-host tolerance while allowing for rapid recovery of other immune functions. An example of such an approach has been reported for HLA-haploidentical related grafts with administration of high-doses of cyclophosphamide 3 and 4 days after HCT36. The concern with this and similar approaches in patients with hematologic cancer, however, is an increased risk of relapse of malignancies given the current lack of understanding of how to discriminate between desirable graft-versus-tumor effects and undesirable GVHD. Until that understanding has been attained, MTX/CSP (tacrolimus) may continue to be standard of care.
The case against calcineurin inhibitors and methotrexate
Corey Cutler, MD
Despite advances in all other aspects of transplant-related technology over the past 20 years, the relatively toxic regimen of a calcineurin inhibitor and methotrexate remains as the standard of care. The recent impetus to find newer agents and regimens for GVHD prophylaxis has been driven largely by the adverse effects of methotrexate, as well as insufficient control of GVHD afforded by this drug.
The use of post-transplant methotrexate may increase the risk of significant complications of transplantation, including oropharyngeal mucositis and diffuse alveolar hemorrhage/idiopathic pneumonia syndrome.37,38 As an antiproliferative agent, methotrexate use causes a delay in the time to neutrophil and platelet engraftment after transplantation.39 Finally, as an agent that can independently induce tissue injury, there is a theoretical concern that methotrexate may paradoxically be implicated in the steps of GVHD initiation by exacerbating tissue injury and augmenting the cytokine cascade associated with GVHD.40
On the Use of Tacrolimus
Cyclosporine and tacrolimus share a final common pathway of inhibition of IL-2 mediated T cell expansion and cytotoxicity. The two compounds have non-overlapping toxicity profiles, with tacrolimus generally being considered less toxic within its suggested therapeutic serum concentration range. Several lines of evidence suggest that this agent is more effective than cyclosporine in GVHD prophylaxis. In a retrospective review of 777 patients who underwent unrelated donor transplantation, the rate of grade II-IV acute GVHD was 36.4% in comparison with 57.8% for cyclosporine patients, and this led to an improvement in overall survival.41 There is also anecdotal evidence of the utility of drug switching from cyclosporine to tacrolimus in cases of resistant GVHD.42–44
Two large North American phase III studies have been performed that compared the combination of tacrolimus and methotrexate with the combination of cyclosporine and methotrexate in matched, related,24 and unrelated donor marrow transplantation,25 as well as a third smaller trial in Japan.45 In the matched, related donor setting, 329 patients were randomized to receive either tacrolimus with methotrexate or cyclosporine and methotrexate and the incidence of grade II–IV acute GVHD was 31.9% in the tacrolimus arm and 44.4% in the cyclosporine arm (adjusted HR 1.61 for cyclosporine, p=0.01). Differences in toxicity and adverse renal outcomes were related to the dosing strategy of tacrolimus, where trough levels up to 40 ng/ml were considered acceptable, and resulted in an excess of treatment-related deaths. In contrast, 27% of deaths in the cyclosporine arm were related to GVHD, whereas only 10% of deaths in the tacrolimus arm were related to GVHD. Randomization in this trial was imbalanced, with more patients with advanced malignancies assigned to the tacrolimus arm (and who also had a trend to receive more intense conditioning regimens) and as such, no difference in overall survival was demonstrable among standard risk patients. A matched pair analysis demonstrated that differences in the combination of diagnosis, disease status, and age between the two treatment arms within the advanced disease group of patients confounded the analysis of survival in advanced stage patients.46
In the unrelated donor randomized trial, the rate of grade II-IV acute GVHD was 56% among patients randomized to tacrolimus and 74% among patients randomized to receive cyclosporine (p=0.0002). In addition, there was a decrease in the severity of acute GVHD in the tacrolimus group (p=0.005), leading to a decrease in the cumulative dose of corticosteroids by day 105 in the tacrolimus arm (p=0.016), and a four-fold decrease in the rate of GVHD related-death (13.3 vs. 3.3%). The incidence of adverse renal outcomes in the tacrolimus treated patients in this trial was more modest compared to the related donor trial, likely related to a slightly narrower serum target trough concentration. However, analysis of drug concentration and outcomes suggested that 10–20 ng/ml may be the optimal level.47,48
The Japanese study, reported by Hiraoka et al,45 randomly assigned 131 patients to receive either tacrolimus or cyclosporine. 112 of these patients (56 in each group) received concomitant methotrexate, and the remaining patients received either corticosteroids or no additional immunosuppression, in a balanced fashion. The incidence of acute GVHD in the tacrolimus arm was significantly lower than the cyclosporine arm (17.5 vs. 48%, p<0.0001), and these findings were consistent in both related and alternative donor subgroups. While treatment discontinuation due to medication adverse events was higher in the tacrolimus group, no patient required hemodialysis. This is likely attributable to the lower serum trough concentration goals (20–25 ng/ml for 2–3 weeks, 10–15 ng/ml thereafter) in this trial.
How Effective Can Therapy Be, If It Cannot Be Delivered?
In order for any therapy to be effective, it must be delivered in a consistent manner, according to plan. Unfortunately, methotrexate doses are commonly omitted after transplantation due to ongoing toxicity or the concern over increased toxicity related to its ongoing usage. In two large randomized North American trials,24,25 the rate of successful delivery of methotrexate (4 doses totaling 45 mg/m2) was only 67.4% (66.2% in combination with tacrolimus, 68.5% in combination with cyclosporine). This inability to deliver effective prophylaxis is highly relevant, since the omission of the 4th dose of methotrexate may be associated with an increased risk of acute GVHD.39,49,50
Advances beyond calcineurin-methotrexate combinations
Given the inadequacies of methotrexate as a GVHD prophylaxis agent, several groups have embarked on research efforts to replace this compound. Several programs are highlighted here.
Etanercept
While most strategies to prevent and treat acute GVHD focus on blocking T cell proliferation, targeting the cytokine pathways that lead to the upregulation of alloreactive T cells is seldom considered. The first cytokine blockade protocol added interleukin-1 receptor antagonist to conventional tacrolimus/methotrexate in a randomized double-blind trial.51. No benefit was observed in this study despite promising results in phase II trials.52 Another cytokine worth targeting is tumor necrosis factor (TNFα). TNFα is thought to be one of the critical cytokine regulators involved in the GVHD cytokine cascade, and as such represents a potential target in GVHD prophylaxis. The TNFα inhibitor etanercept has been demonstrated to be efficacious in the treatment of acute GVHD.53 Since it has been demonstrated that levels of the TNFα receptor (used as a surrogate for circulating TNFα) rise in advance of acute GVHD, the Michigan group have now tested etanercept as part of a GVHD prophylaxis regimen.
Etanercept consists of two p75 portions of the TNFα receptor covalently linked to the Fc portion of human IgG and has a half-life of approximately 3 days after subcutaneous dosing. In this phase II trial, etanercept (0.4 m/kg, 25 mg maximum) was given twice weekly commencing 1 week prior to transplantation, as induction-type immunotherapy, and continued for 18 doses. In addition, tacrolimus and mini-dose methotrexate (20 mg/m2 total) was administered, and three different myeloablative regimens were used. Overall, the rate of grade II–IV acute GVHD was 23%, and was as low as 11% when fludarabine and busulfan were used for conditioning (unpublished data kindly provided by S. Choi). 100 day treatment-related mortality was 16%, with no patient in the fludarabine – busulfan arm succumbing by day 100. Interestingly, outcomes were inversely related to the levels of TNFα, with patients who had the lowest levels having the best outcomes, with the lowest rates of acute GVHD. While this regimen does not completely eliminate methotrexate, the inclusion of the cytokine blocking drugs allows for a meaningful reduction in the dose of methotrexate that ultimately leads to a reduction in treatment-related mortality, and thus is promising.
Anti-Thymocyte Globulin
There are conflicting single center data on the value of additional ATG and many centers use this agent routinely in unrelated donor transplantation, while others do not. The use of ATG in the peri-transplant period can deplete both host and donor immune cells, and while designed to reduce the incidence and severity of acute GVHD, ATG may also increase malignant relapse rates by delaying anti-tumor immunity development.
In a large randomized trial, patients who underwent myeloablative allogeneic stem cell transplantation from 8/8-matched unrelated donors were randomly assigned to be given ATG in addition to cyclosporine and methotrexate. In this experience, there was a significant reduction in the rate of grade II–IV and grade III–IV acute GVHD favoring ATG use (33% vs. 51%, p=0.01; 11.7% vs. 24.5%, p=0.05).54 There was a slight survival advantage in the ATG arm, which did not attain statistical significance (59.2% vs. 51.9%, p=0.47).
Substitutions In Lieu of Methotrexate
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) has been studied extensively as an agent for the prevention of acute GVHD. MMF is the 2-morpholinoethyl ester of mycophenolic acid (MPA), a potent, selective, and reversible inhibitor of inosine monophosphate dehydrogenase that inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation into DNA. Because T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, MPA has potent cytostatic effects on lymphocytes. Absorbed well after oral administration, the optimal dose for use in transplantation is unclear, with some centers administering it twice and others three times daily. The Seattle group suggested an optimal dose of 45 mg/m2/day in a phase I/II trial, where GVHD rates were similar when compared with historical controls.32 The dose limiting toxicities include reversible myelosuppression and mucosal toxicity of the gastrointestinal tract causing diarrhea, which can often clinically and histologically resemble GVHD itself.55
MMF has been used for GVHD prophylaxis in combination with cyclosporine, .32,56 cyclosporine with methotrexate,57 and tacrolimus.58 In a retrospective review involving 93 patients, Neumann et al compared their single center experience of cyclosporine with MMF in comparison with cyclosporine and methotrexate.59 There were trends towards a reduced rate of grade II-IV acute GVHD (38% vs. 61%) and improved overall survival (76% vs. 55%), although neither of these comparisons were statistically significant. However, there was a statistically significant reduction in the time to neutrophil engraftment (12 vs. 18 days, p < 0.0001).
In a small, single center randomized trial, the combination of cyclosporine and MMF was compared with cyclosporine and methotrexate after busulfan-based conditioning therapy and marrow transplantation. 40 patients were randomized prior to the early closure of the study. The trial was discontinued prematurely because the combination of MMF and cyclosporine was associated with faster hematopoietic engraftment (11 vs. 18 days, p<0.001), and decreased incidence of oropharyngeal mucositis (median OMAS score 0.25 vs. 1.0, p=0.004) as well as a reduction in the severity of the oropharyngeal mucositis (21% vs. 65%, p=0.008). There was a similar incidence of acute GVHD, and comparable survival as compared to cyclosporine and methotrexate.31 With a superior toxicity profile and comparative GVHD outcomes, this regimen can be considered superior to, or at least equivalent to cyclosporine and methotrexate.
A second small trial, evaluated the combination of tacrolimus and methotrexate in comparison with tacrolimus and MMF for patients undergoing related or unrelated donor transplantation. In an intent-to-treat analysis of 42 patients randomized to MMF and 47 patients randomized to methotrexate, no differences in the incidence of grade II-IV GVHD were noted (79% in both arms), and long term outcome was the same in both arms as well.60 As in the prior MMF trial, this study demonstrated a significant reduction in the incidence and severity of oropharyngeal mucositis, MMF patients used less narcotics and parenteral nutrition and had shorter hospital stays (supplemental unpublished data kindly provided by J. Perkins).
Sirolimus
Used extensively at DFCI since 2000, sirolimus is the first commercially available mTOR inhibitor. It has immunomodulatory properties that extend far beyond T cell inhibition to include effects on antigen presentation cells, the thymus and preservation of regulatory T cell subsets after transplantation.61
The initial experience demonstrated the safety of this compound when added to tacrolimus and methotrexate in mismatched related and unrelated donor transplantation. In this trial involving 39 high-risk patients, the rate of grade II–IV acute GVHD was only 26%.33 In subsequent phase II studies, the substitution of sirolimus for methotrexate, in combination with tacrolimus, was shown to lead to a rate of grade II–IV acute GVHD of 20.5%, with grade III–IV acute GVHD occurring in under 5% of patients.62 No difference in outcome between recipients of related or matched unrelated donors was noted. These findings have been confirmed by several groups,63–65 however the Seattle group, using sirolimus in combination with methotrexate and cyclosporine or tacrolimus, were unable to replicate these findings.34 This smaller experience is flawed for several reasons, including the use of concomitant methotrexate and busulfan, both of which increase treatment-related toxicity in this setting,66 the timing of administration of the immunosuppressive regimen,63 and the use of cyclosporine when there is known synergy between sirolimus and tacrolimus.67 The value of sirolimus in GVHD prophylaxis has also been extended to the reduced intensity setting,68,69 HLA-haploidentical transplantation,70 umbilical cord blood transplantation,71 and pediatric transplantation.72 Regimens that have tested sirolimus on combination with mycophenolate rather than tacrolimus also appear to be promising,73 whereas a combination evaluating everolimus appeared less so.74
Despite an increase in an incidence of endothelial-related injuries, the combination of sirolimus and tacrolimus is associated with improved overall survival and a reduction in treatment-related mortality.66 One of the more striking differences associated with this combination is the reduction in oropharyngeal mucositis, which is one of the most serious patient-reported adverse effects related to transplantation.75 In a case-control study, the incidence and severity of mucositis was reduced, the use of parenteral nutrition was reduced, and patients required less narcotic analgesia when compared with patients who received methotrexate for GVHD prophylaxis.37 Thus there appears to be a benefit in GVHD and morbidity after transplantation with this regimen.
The issue of whether sirolimus-based GVHD prophylaxis is better than tacrolimus and methotrexate is currently being addressed in a randomized, controlled trial conducted by the BMT CTN. This trial will enroll 312 patients and attempt to demonstrate a 15% improvement in GVHD-free survival at 114 days from the time of transplantation. This endpoint was chosen to highlight both the improved rate of GVHD prophylaxis as well as the reduction in treatment-related mortality seen in prior clinical trials.
Elimination of Calcineurin Inhibitors and Methotrexate
Cyclophosphamide
Post-transplant cyclophosphamide was used initially in the 1980’s to prevent GVHD via inhibition of rapidly dividing T cells in a manner similar to methotrexate.76 Stem cells contain high levels of aldehyde dehydrogenase which converts 4-hydroxycyclophosphamide into a non-alkylating metabolite, thus sparing the stem cell from the anti-proliferative activity of this agent. In addition, the gastrointestinal epithelium also contains high levels of aldehyde dehydrogenase, thus affording a protective effect against gastrointestinal mucositis when this cytotoxic agent is given shortly after intensive conditioning regimens. The Hopkins group is testing the use of high dose, post-transplantation cyclophosphamide as sole prophylaxis of GVHD after HLA-matched BMT.77 Cyclophosphamide is given at a dose of 50 mg/kg on days 3 and 4 after transplantation, after oral busulfan and cyclophosphamide (100 mg/kg total) conditioning in patient with advanced hematologic malignancies. Over 100 patients have been treated in this manner, with bone marrow as the preferred stem cell source (unpublished data kindly provided by L. Luznik). The median time to neutrophil engraftment in this experience is 23 days (MRD donors, n=78) and 25 days (unrelated donors, n= 39), without the use of exogenous colony stimulating factors. Grade II–IV acute GVHD occurred in 41% (MRD) and 46% (unrelated) of patients, however grade III–IV GVHD was uncommon, occurring in 11 and 8% of patients respectively. This regimen was very safe as the treatment-related mortality rates at 100 days were 6% and 13% for MRD and unrelated donor recipients, respectively. With a median follow-up of 26 months among surviving patients, the cumulative incidence of chronic GVHD is only 10% Overall survival exceeds 50% at 2 years in a group of patients with advanced malignancies. As predicted, oropharyngeal mucositis was not severe and was reduced in comparison with a cyclosporine and methotrexate cohort (personal communication, E. Fuchs). While GVHD rates here appear similar to historical controls, this regimen with surprisingly low toxicity may also be considered an alternative to methotrexate after transplantation, and deserves to be tested in a randomized trial. In haploidentical transplantation, supplemental tacrolimus is being administered.
With the recent elevation of chronic GVHD as a major concern in allogeneic transplantation, it will become important to develop GVHD prophylaxis regimens that address this concern as well. There is mounting evidence demonstrating the important role of CD4+CD25+FoxP3+ T cells (regulatory T cells) in the pathophysiology of chronic GVHD78–80. Regulatory T cell function is dependent on IL-2,81–83 and since calcineurin inhibitors act by inhibiting the T cell response to IL-2, calcineurin inhibitors are far more damaging to regulatory T cell populations than drugs such as sirolimus or mycophenolate.84,85 The autoimmune manifestations of chronic GVHD have been linked to the failure of clonal deletion induced by calcineurin inhibition,86 suggesting that early exposure to these agents may in fact be deleterious for prevention of chronic GVHD. Thus, strategies that increase regulatory T cell numbers or function may be associated with a reduction in chronic GVHD. In the German experience using sirolimus, MMF and ATG, in addition to low rates of acute GVHD, the rate of chronic GVHD was similarly low (30%) although it is difficult to attribute this to the lack of the calcineurin inhibitor or the use of ATG in the preparative regimen. 73
Summary and Future Directions
There are numerous strategies that have been demonstrated to be effective in prevention against acute GVHD. In smaller randomized trials, many of these approaches have been shown to be equivalent or superior, in some respects, when compared with the standard of cyclosporine and methotrexate. While larger randomized trials are required for establishment of a new standard of care. The need is for an effective regimen that eliminates BOTH acute and chronic GVHD, allows effective immunological recovery, and maintains graft-versus-leukemia effects.
Table 1.
Summary of Randomized Trials of Novel GVHD Regimens
| Sample Size | Gr. II–IV Acute GVHD | Gr. III–IV Acute GVHD | Chronic GVHD | |
|---|---|---|---|---|
| Ratanatharathorn, 199824 | ||||
| CsA/Mtx | 164 | 44.4% | 17.1% | 55.9% |
| Tac/Mtx | 165 | 31.9%* | 13.3% | 49.4% |
| Nash, 200025 | ||||
| CsA/Mtx | 90 | 74% | 25.5% | 70% |
| Tac/Mtx | 90 | 56%* | 17.7%* | 76% |
| Hiraoka, 200145 | ||||
| CsA/Mtx | 65 | 48.0% | 21.1% | 47.8% |
| Tac/Mtx | 66 | 17.5%* | 9.5% | 47.3% |
| Bolwell, 200431 | ||||
| CsA/Mtx | 19 | 37% | -- | 64% |
| CsA/MMF | 21 | 48% | -- | 63% |
| Finke, 200854 | ||||
| CsA/Mtx | 98 | 51.0% | 24.5% | 58.8% |
| CsA/Mtx/ATG | 103 | 33.0%* | 11.7% | 30.8%* |
| Perkins, 200860 | ||||
| Tac/Mtx | 47 | 79% | 4% | 45% |
| Tac/MMF | 42 | 78% | 19%* | 38% |
Acknowledgments
Supported in part by NCI grant CA142106 (J.H.A.) and CA78902, CA18029, CA15704 and HL36444 (RS)
Footnotes
Conflict of Interest Statement: Drs Antin and Cutler have received research funding support from Wyeth and Astellas.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rainer Storb, Email: rstorb@fhcrc.org, Member and Head, Program in Transplantation Biology, Fred Hutchinson Cancer Research Center, Professor of Medicine, University of Washington, 1100 Fairview Ave North, D1-100, Seatttle, WA 98109, (206) 667-4407, (206) 667-6124 fax.
Joseph H. Antin, Email: jantin@partners.org, Professor of Medicine, Harvard Medical School, Dana-Farber Cancer Institute, 44 Binney St., D1B12, Boston, MA 02115, (617) 632-2525, (617) 632-5168 fax.
Corey Cutler, Email: corey_cutler@dfci.harvard.edu, Assistant Professor of Medicine, Harvard Medical School, Dana-Farber Cancer Institute, 44 Binney St., D1B13, Boston, MA 02115, (617) 632-4719, (617) 632-5168 fax.
References
- 1.Van Bekkum D, De Vries M. Radiation Chimeras. New York: Academic Press; 1967. [Google Scholar]
- 2.Epstein RB, Storb R, Ragde H, Thomas ED. Cytotoxic typing antisera for marrow grafting in littermate dogs. Transplantation. 1968;6:45–58. doi: 10.1097/00007890-196801000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Storb R, Rudolph RH, Thomas ED. Marrow grafts between canine siblings matched by serotyping and mixed leukocyte culture. J Clin Invest. 1971;50:1272–1275. doi: 10.1172/JCI106605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uphoff DE. Alteration of homograft reaction by A-methopterin in lethally irradiated mice treated with homologous marrow. Proc Soc Exp Biol Med. 1958;99:651–653. doi: 10.3181/00379727-99-24450. [DOI] [PubMed] [Google Scholar]
- 5.Storb R, Epstein RB, Graham TC, Thomas ED. Methotrexate regimens for control of graft-versus-host disease in dogs with allogeneic marrow grafts. Transplantation. 1970;9:240–246. doi: 10.1097/00007890-197003000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Thomas ED, Storb R, Clift RA, et al. Bone marrow transplantation. N Engl J Med. 1975;292:832. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Prentice RL, Thomas ED. Treatment of aplastic anemia by marrow transplantation from HLA identical siblings. Prognostic factors associated with graft versus host disease and survival. J Clin Invest. 1977;59:625–632. doi: 10.1172/JCI108680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308:302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 9.Powles RL, Barrett AJ, Clink H, Kay HE, Sloane J, McElwain TJ. Cyclosporin A for the treatment of graft-versus-host disease in man. Lancet. 1978;2:1327–1331. doi: 10.1016/s0140-6736(78)91971-2. [DOI] [PubMed] [Google Scholar]
- 10.Deeg HJ, Storb R, Weiden PL, Graham T, Atkinson K, Thomas ED. Cyclosporin-A: effect on marrow engraftment and graft-versus-host disease in dogs. Transplant Proc. 1981;13:402–409. [PubMed] [Google Scholar]
- 11.Storb R, Deeg HJ, Fisher L, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71:293–298. [PubMed] [Google Scholar]
- 12.Storb R, Kolb HJ, Deeg HJ, et al. Prevention of graft-versus-host disease by immunosuppressive agents after transplantation of DLA-nonidentical canine marrow. Bone Marrow Transplant. 1986;1:167–177. [PubMed] [Google Scholar]
- 13.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of intolerance. Transplantation. 1982;34:30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Storb R, Deeg HJ, Farewell V, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–125. [PubMed] [Google Scholar]
- 15.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 16.Sorror ML, Leisenring W, Deeg HJ, Martin PJ, Storb R. Re: Twenty-year follow-up in patients with aplastic anemia given marrow grafts from HLA-identical siblings and randomized to receive methotrexate/cyclosporine or methotrexate alone for prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:567–568. doi: 10.1016/j.bbmt.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Sorror ML, Leisenring W, Deeg HJ, Martin PJ, Storb R. Twenty-year follow-up of a controlled trial comparing a combination of methotrexate plus cyclosporine with cyclosporine alone for prophylaxis of graft-versus-host disease in patients administered HLA-identical marrow grafts for leukemia. Biol Blood Marrow Transplant. 2005;11:814–815. doi: 10.1016/j.bbmt.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 19.Chao NJ, Snyder DS, Jain M, et al. Equivalence of 2 effective graft-versus-host disease prophylaxis regimens: results of a prospective double-blind randomized trial. Biol Blood Marrow Transplant. 2000;6:254–261. doi: 10.1016/s1083-8791(00)70007-3. [DOI] [PubMed] [Google Scholar]
- 20.Storb R, Pepe M, Anasetti C, et al. What role for prednisone in prevention of acute graft-versus-host disease in patients undergoing marrow transplants? Blood. 1990;76:1037–1045. [PubMed] [Google Scholar]
- 21.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 22.Storb R, Raff RF, Appelbaum FR, et al. FK-506 and methotrexate prevent graft-versus-host disease in dogs given 9.2 Gy total body irradiation and marrow grafts from unrelated dog leukocyte antigen-nonidentical donors. Transplantation. 1993;56:800–807. doi: 10.1097/00007890-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, Seidel K, Fitzsimmons WE, Sale G, Storb R. Glucocorticoids fail to enhance the effect of FK506 and methotrexate in prevention of graft-versus-host disease after DLA-nonidentical, unrelated marrow transplantation. Bone Marrow Transplant. 1997;20:137–141. doi: 10.1038/sj.bmt.1700863. [DOI] [PubMed] [Google Scholar]
- 24.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 25.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 26.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 27.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 28.Sandmaier B, Storb R. Reduced-intensity conditioning followed by hematopoietic cell transplantation for hematologic malignancies. In: Appelbaum F, Forman S, Negrin R, Blume K, editors. Thomas’ Hematopoietic Cell Transplantation. Oxford, UK: Wiley-Blackwell; 2009. pp. 1043–1058. [Google Scholar]
- 29.Burroughs L, Mielcarek M, Leisenring W, et al. Extending postgrafting cyclosporine decreases the risk of severe graft-versus-host disease after nonmyeloablative hematopoietic cell transplantation. Transplantation. 2006;81:818–825. doi: 10.1097/01.tp.0000203556.06145.5b. [DOI] [PubMed] [Google Scholar]
- 30.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 31.Bolwell B, Sobecks R, Pohlman B, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34:621–625. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 32.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 34.Furlong T, Kiem HP, Appelbaum FR, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14:531–537. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruutu T, Volin L, Parkkali T, Juvonen E, Elonen E. Cyclosporine, methotrexate, and methylprednisolone compared with cyclosporine and methotrexate for the prevention of graft-versus-host disease in bone marrow transplantation from HLA-identical sibling donor: a prospective randomized study. Blood. 2000;96:2391–2398. [PubMed] [Google Scholar]
- 36.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler C, Li S, Kim HT, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant. 2005;11:383–388. doi: 10.1016/j.bbmt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:223–229. doi: 10.1053/bbmt.2001.v7.pm11349809. [DOI] [PubMed] [Google Scholar]
- 39.Cutler C, Antin J. Omission of day+11 methotrexate after matched-related allogeneic peripheral blood stem cell transplantation does not increase the risk of graft-vs.-host disease. Biol Blood Marrow Transplant. 2002;8:85. [Google Scholar]
- 40.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 41.Yanada M, Emi N, Naoe T, et al. Tacrolimus instead of cyclosporine used for prophylaxis against graft-versus-host disease improves outcome after hematopoietic stem cell transplantation from unrelated donors, but not from HLA-identical sibling donors: a nationwide survey conducted in Japan. Bone Marrow Transplant. 2004;34:331–337. doi: 10.1038/sj.bmt.1704596. [DOI] [PubMed] [Google Scholar]
- 42.Furlong T, Storb R, Anasetti C, et al. Clinical outcome after conversion to FK 506 (tacrolimus) therapy for acute graft-versus-host disease resistant to cyclosporine or for cyclosporine-associated toxicities. Bone Marrow Transplant. 2000;26:985–991. doi: 10.1038/sj.bmt.1702639. [DOI] [PubMed] [Google Scholar]
- 43.Carnevale-Schianca F, Martin P, Sullivan K, et al. Changing from cyclosporine to tacrolimus as salvage therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:613–620. doi: 10.1016/s1083-8791(00)70026-7. [DOI] [PubMed] [Google Scholar]
- 44.Kanamaru A, Takemoto Y, Kakishita E, et al. FK506 treatment of graft-versus-host disease developing or exacerbating during prophylaxis and therapy with cyclosporin and/or other immunosuppressants. Japanese FK506 BMT Study Group. Bone Marrow Transplant. 1995;15:885–889. [PubMed] [Google Scholar]
- 45.Hiraoka A, Ohashi Y, Okamoto S, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28:181–185. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- 46.Horowitz MM, Przepiorka D, Bartels P, et al. Tacrolimus vs. cyclosporine immunosuppression: results in advanced-stage disease compared with historical controls treated exclusively with cyclosporine. Biol Blood Marrow Transplant. 1999;5:180–186. doi: 10.1053/bbmt.1999.v5.pm10392964. [DOI] [PubMed] [Google Scholar]
- 47.Przepiorka D, Nash RA, Wingard JR, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant. 1999;5:94–97. doi: 10.1053/bbmt.1999.v5.pm10371361. [DOI] [PubMed] [Google Scholar]
- 48.Wingard JR, Nash RA, Przepiorka D, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant. 1998;4:157–163. doi: 10.1053/bbmt.1998.v4.pm9923414. [DOI] [PubMed] [Google Scholar]
- 49.Kumar S, Wolf RC, Chen MG, et al. Omission of day +11 methotrexate after allogeneic bone marrow transplantation is associated with increased risk of severe acute graft-versus-host disease. Bone Marrow Transplant. 2002;30:161–165. doi: 10.1038/sj.bmt.1703616. [DOI] [PubMed] [Google Scholar]
- 50.Nash RA, Pepe MS, Storb R, et al. Acute graft-versus-host disease: analysis of risk factors after allogeneic marrow transplantation and prophylaxis with cyclosporine and methotrexate. Blood. 1992;80:1838–1845. [PubMed] [Google Scholar]
- 51.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100:3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 52.Antin JH, Weinstein HJ, Guinan EC, et al. Recombinant interleukin-1 receptor antagonist in the treatment of steroid-resistant graft-versus-host disease. Blood. 1994;84:1342–1348. [PubMed] [Google Scholar]
- 53.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 55.Papadimitriou JC, Cangro CB, Lustberg A, et al. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11:295–302. doi: 10.1177/106689690301100406. [DOI] [PubMed] [Google Scholar]
- 56.Bornhauser M, Schuler U, Porksen G, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67:499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 57.Kasper C, Sayer HG, Mugge LO, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant. 2004;33:65–69. doi: 10.1038/sj.bmt.1704299. [DOI] [PubMed] [Google Scholar]
- 58.Osunkwo I, Bessmertny O, Harrison L, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10:246–258. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Neumann F, Graef T, Tapprich C, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005;35:1089–1093. doi: 10.1038/sj.bmt.1704956. [DOI] [PubMed] [Google Scholar]
- 60.Perkins J, Alsina M, Anasetti C, et al. A Randomized, Controlled Trial of Graft-Versus-Host Disease (GVHD) Prophylaxis Comparing Tacrolimus and Mycophenolate Mofetil to Tacrolimus and Methotrexate: Analysis of GVHD, Relapse and Survival. ASH Annual Meeting Abstracts. 2008;112:2235. [Google Scholar]
- 61.Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:471–476. doi: 10.1038/sj.bmt.1704604. [DOI] [PubMed] [Google Scholar]
- 62.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolff D, Andree H, Hilgendorf I, Casper J, Freund M, Junghanss C. Sirolimus in Combination with Tacrolimus in Allogeneic Stem Cell Transplantation-Timing and Conditioning Regimen May Be Crucial. Biol Blood Marrow Transplant. 2008;14:942–943. doi: 10.1016/j.bbmt.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Claxton DF, Ehmann C, Rybka W. Control of advanced and refractory acute myelogenous leukaemia with sirolimus-based non-myeloablative allogeneic stem cell transplantation. Br J Haematol. 2005;130:256–264. doi: 10.1111/j.1365-2141.2005.05600.x. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura R, Rodriguez R, Palmer J, et al. Tacrolimus and Sirolimus as GVHD Prophylaxis for Sibling Donor Hematopoietic Stem Cell Transplant (HCT) Using Three Conditioning Regimens; Fludarabine-Melphalan, FTBI-VP16, and Busulfan-Cyclophosphamide. Biol Blood Marrow Transplant. 2009;15:89a. [Google Scholar]
- 66.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112:4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koenen H, Michielsen E, Verstappen J, Fasse E, Joosten I. Superior T-cell suppression by rapamycin and FK506 over rapamycin and cyclosporine A because of abrogated cytotoxic T-lymphocyte induction, impaired memory responses, and persistent apoptosis. Transplantation. 2003;75:1581–1590. doi: 10.1097/01.TP.0000053752.87383.67. [DOI] [PubMed] [Google Scholar]
- 68.Ho VT, Aldridge J, Kim HT, et al. Comparison of Tacrolimus and Sirolimus (Tac/Sir) versus Tacrolimus, Sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:844–850. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alyea EP, Li S, Kim HT, et al. Sirolimus, Tacrolimus and Reduced-Dose Methotrexate as Graft Versus Host Disease (GVHD) Prophylaxis after Non-Myeloablative Stem Cell Transplantation:Low Incidence of Acute GVHD Compared with Tacrolius/Methotrexate or Cyclosporine/Prednisone. Blood. 2004;104:209a. [Google Scholar]
- 70.Claxton DF, Popescu D, Carraher M, Chiafari FA, Ehmann C, Rybka W. Sirolimus and tacrolimus allow engraftment of haploidentical and other alternative donor stem cells after non-myeloablative conditioning. Biol Blood Marrow Transplant. 2004;10:38–39. [abstract] [Google Scholar]
- 71.Cutler C, Stevenson K, Kim HT, et al. Double Umbilical Cord Blood Transplantation with Reduced Intensity Conditioning and Sirolimus-Based GVHD Prophylaxis. 2009 doi: 10.1038/bmt.2010.192. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulsipher MA, Wall DA, Grimley M, et al. A Phase I/II study of the safety and efficacy of the addition of sirolimus to tacrolimus/methotrexate graft versus host disease prophylaxis after allogeneic haematopoietic cell transplantation in paediatric acute lymphoblastic leukaemia (ALL) Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07889.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schleuning M, Judith D, Jedlickova Z, et al. Calcineurin inhibitor-free GVHD prophylaxis with sirolimus, mycophenolate mofetil and ATG in Allo-SCT for leukemia patients with high relapse risk: an observational cohort study. Bone Marrow Transplant. 2009;43:717–723. doi: 10.1038/bmt.2008.377. [DOI] [PubMed] [Google Scholar]
- 74.Platzbecker U, Pabst C, Kiani A, et al. Graft Versus Host Disease Prophylaxis with Everolimus and Tacrolimus in Patients with Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukaemia (AML) Receiving Allogeneic Peripheral Blood Stem Cell Transplantation (PBSCT) ASH Annual Meeting Abstracts. 2006;108:817a. [Google Scholar]
- 75.Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–39. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 76.Santos GW, Tutschka PJ, Brookmeyer R, et al. Cyclosporine plus methylprednisolone versus cyclophosphamide plus methylprednisolone as prophylaxis for graft-versus-host disease: a randomized double-blind study in patients undergoing allogeneic marrow transplantation. Clin Transplant. 1987;1:21–28. [Google Scholar]
- 77.Luznik L, Chen AR, Kaup M, et al. Post-Transplantation High-Dose Cyclophosphamide (Cy) Is Effective Single Agent GVHD Prophylaxis That Permits Prompt Immune Reconstitution after Myeloablative HLA Matched Related and Unrelated Bone Marrow Transplantation (BMT) Blood. 2006;108:2891s. [Google Scholar]
- 78.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 79.Zorn E. CD4+CD25+ regulatory T cells in human hematopoietic cell transplantation. Seminars in Cancer Biology. 2006;16:150–159. doi: 10.1016/j.semcancer.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 81.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 82.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 83.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 84.Baan CC, van der Mast BJ, Klepper M, et al. Differential Effect of Calcineurin Inhibitors, Anti-CD25 Antibodies and Rapamycin on the Induction of FOXP3 in Human T Cells. Transplantation. 2005;80:110–117. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 85.Zeiser RS, Nguyen VH, Beilhack A, et al. Inhibition of CD4+CD25+ regulatory T cell function by calcineurin dependent interleukin-2 production. Blood. 2006:2006–2001. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hess AD. Modulation of graft-versus-host disease: role of regulatory T lymphocytes. Biol Blood Marrow Transplant. 2006;12:13–21. doi: 10.1016/j.bbmt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Kahl C, Leisenring W, Deeg HJ, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. Br J Haematol. 2005;130:747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]
- 88.Locatelli F, Bruno B, Zecca M, et al. Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood. 2000;96:1690–1697. [PubMed] [Google Scholar]
- 89.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]


