Cells, Pathways and Cytokines
Cells and Pathways
Of the multiple cell lineages undergoing reconstitution in the setting of hematopoietic stem cell transplantation (HSCT), this overview will focus on reconstitution of the T cell arm of the immune system. In clinical HSCT, the autologous setting is free of constraints imposed by reactions to allogeneic antigens, but markers are lacking to distinguish reconstitution from transferred cells versus stem or progenitor cells left residual in the host. In allogeneic transplants, the converse is true – the stem cell origins of the reconstituted populations can be determined, but the biology of such reconstitution is confounded by concomitant allogeneic responses. Because of these constraints, the basic biology of T cell immune reconstitution was first delineated in mouse models in which the availability of congenic strains allowed stem cell origins of reconstituted cell populations to be investigated without the presence of confounding allogenic reactions. Two pathways of T cell regeneration were identified: the thymic-dependent maturation of new T cells from marrow progenitors, and thymic-independent expansion of mature peripheral T cells.1 These initial studies also determined that no other pathways existed by which substantial peripheral populations of T cells were generated, and also established a means for investigating T cell reconstitution in humans by analyzing the expression of isoforms of the leukocyte common antigen CD45 as markers of naïve and memory populations which correlated with the pathway of regeneration. They also demonstrated that homeostatic expansion of mature T cells occurred in lymphopenic hosts. Studies of such expansion have formed central concepts of homeostasis – that T cell levels are maintained by a balance between cytokine-consuming cells and homeostatic cytokines that support the maturation, proliferative expansion and survival of T cells.2
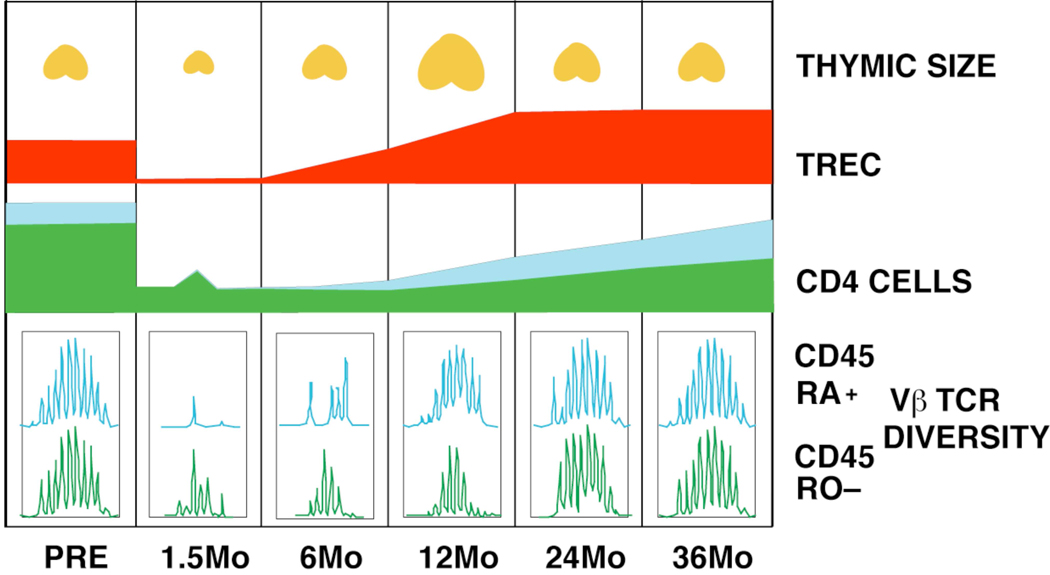
In translating these murine findings to humans, the recovery of CD4+ T cells in pediatric patients who were severely lymphopenic following chemotherapy treatment were initially tracked (Figure 1). There was a strong correlation between the recovery of total CD4+ T cell numbers and the recovery of naive, CD45RA+CD45RO- CD4+ T cells after as few as six months.3 Furthermore, recovery of naive CD4+ cells was associated with a marked expansion of thymus volume, consistent with a dynamic regulation of the thymus.
Figure 1.
Model representing the post transplant time course of CD4+ T cell recovery in a patient with a robust renewal of thymopoiesis. Thymic enlargement peaks at 12 months post transplant and is accompanied by an increasing number of CD45RA+CD62L+ naive and TREC-bearing cells in the second year. Recovery of TCR repertoire diversity in the memory subpopulations follows and is dependent upon recovery of adequate numbers of naive cells.
Finally, an inverse correlation was observed between age and early recovery of CD4+ T cells in the peripheral blood. These data supported the conclusion that thymic-dependent T cell production was primarily responsible for the repopulation of peripheral blood CD4+ T cells in young patients following acute T cell depletion associated with therapy. Most importantly, this work confirmed that the approach of utilizing CD45 isoform expression on CD4+ T cells, as established from murine basic research, could be used to investigate immune reconstitution in humans.
To assess the contributions of thymus dependent and independent pathways in adults, CD45 isoforms expressed by CD4+ T cells following chemotherapy were characterized.4 In contrast to observations in pediatric patient populations, lymphopenic adults (ages over 30) generated few new CD45RA+ CD4+ cells during the first year post therapy. Instead, CD45RO+ CD4+ cells increased rapidly in number, recovering the majority of CD4+ T cells to pretreatment levels within the first 3 months after chemotherapy.5 Thus, these studies established the existence of an early second primary pathway of CD4+ T cell regeneration in humans, namely a thymus independent, peripheral expansion pathway marked by production of T cells of memory phenotype. These studies left unresolved the issue of T cell reconstitution in adults over prolonged periods of time.
In characterizing T cell reconstitution in adults over prolonged periods of time, it was confirmed that early recovery of CD4+ T cells following autologous HSCT consisted primarily of cells with an activated (HLA-DR+) effector memory (CD62L– CCR7– CD45RA–) phenotype, but that this peripheral expansion only transiently increased the peripheral T cell population.6 In contrast, a renewal of thymopoiesis – as assessed by naïve cells, TREC frequency, thymus size and repertoire rediversification – resulted in recovery of normal numbers of total CD4+ T cell levels by the end of the second year. When thymopoiesis was not reactivated, levels of total CD4 cells remained below normal even 4 – 5 years after transplant. Furthermore, thymus-dependent CD4 production reestablished central memory (CM) populations, identified as CD62L+ CCR7+ CD45RA–.6 Thus the recovery of substantial thymus activity was necessary for CD4+ T cell reconstitution over time.
In studying the recovery of CD8+ T cells over a prolonged time period after autologous HSCT, it was observed that the kinetics of repopulation differed markedly from that of CD4+ T cells. Four patterns of CD8+ T cell recovery were identified. In the first, found in more than half of the patients, the CD28- CD8+ cells not only expanded as a massive early spike in total CD8 numbers, but were the dominant elements in persistently elevated CD8 populations observed throughout the first 2 years post transplant. Furthermore, these CD28- cells had a limited oligoclonal TCR repertoire that also was consistent over time, and due to the high proportion of these cells, dominated the overall spectratype of the CD8 cells. It was found that a recovery pattern dominated by a disproportionate expansion of CD28- (effector memory) CD8 cells correlated with CMV IgG seropositive status pretreatment. A second pattern, observed in approximately one third of the patients, bore more resemblance to CD4+ T cell recovery. In this pattern, phenotypically naïve (CD28+CD45RA+ CD11adull) CD8+ T cells gradually predominated in the second year of recovery, providing the CD8 cells as a whole with a broadly diverse T cell receptor repertoire and high TREC frequencies. A third subset of patients displaying features of both Patterns I and II was identified, demonstrating that these were independent pathways of recovery. Finally, a subset of patients displayed a fourth pattern. These lacked major increases in either early effector populations or late recovery of naïve CD8+ T cells; the total numbers of CD8 cells remained low and total repertoire diversity was limited throughout the follow-up period. The observation of severe long term deficits in total numbers and in repertoire diversity in both CD4 and CD8 numbers demonstrated the limits of homeostatic expansion in adults.
Cytokines
It has been found that blood levels of IL-7 and IL-15 follow the pattern of a determining homeostatic cytokine. For example, in patients undergoing T-replete allogeneic transplant for treatment of leukemia or lymphoma, plasma levels of IL-15 increase during each successive round of cyto-reductive adjuvant therapy, reaching a 50 – 100 fold increase on the day of transplant, the days of greatest CD8 and NK depletion. Concurrent with the rapid recovery of NK and CD8+ memory T cells, IL-15 levels in the peripheral blood decreased. This inverse correlation of cells and cytokine is consistent with the consumption kinetics of a homeostatic cytokine.
IL7 is also a primary homeostatic cytokine for general T cell populations.7, 8 It is the first of the primary homeostatic cytokines to be identified as such, and is the first to be introduced into clinical trials. In these trials, rhIL-7 produced a marked dose-dependent increase in the numbers of circulating CD4+ and CD8+ T cells that persisted in follow-up assays at 6 to 12 weeks post treatment4, 9 Furthermore, rhIL-7 therapy disproportionately increased CCR7+CD27+CD45RA+ naive and CCR7+CD27+CD45RA- central memory (CM) cells, which represent the most diverse components of the mature TCR pool. Because of the extent of this population shift, IL-7 administration led to an overall increase in TCR diversity in CD4+ and CD8+ T-cells. Thus rhIL-7 can induce thymus-independent T cell growth in naïve and CM populations and enhance repertoire diversity in peripheral T cell populations.
Regulation of thymopoiesis
As noted, prolonged lymphopenia is associated with return of enhanced thymus function in some adults. Insulin-like Growth Factor (IGF-1) is a known regulator of thymic function; exogenous IGF-1 has been shown to affect thymic and peripheral T-cell populations in murine bone marrow transplant models.10, 11 Current studies indicate that IGF-1 controls thymic function through its effect on thymic epithelial cells (TEC). Similarly, KGF enhances thymopoiesis through receptors expressed by TEC.12–15 Recently, it has been shown that interruption of androgen signaling16 exerts its effect to enhance thymus activity in the transplant setting by increased expression of CCL25, a molecule important for the immigration of thymocytes precursors from marrow into the thymus.17 Taken together, these three molecules which increase thymus production of T cells indicate that thymus epithelial cells constitute a primary site of control with respect to modulation of thymus function. Naturally occurring pathways of such regulation remain largely uncharacterized.
What’s Broken and Why It Matters
Impaired CD4+ T cell reconstitution after allogeneic stem cell transplantation
The reconstitution of effective T cell immunity is one of the most important elements of successful allogeneic stem cell transplantation. The most problematic element in T cell reconstitution is the restoration of functionally competent donor-derived CD4+ T cells which is critical for the prevention of opportunistic infections and is most directly affected by the development of graft versus host disease (GVHD). Defects in thymic production and peripheral homeostatic expansion have both been shown to contribute to the impairment in CD4+ T cell regeneration.18, 19 With respect to the former, the generation of new CD4+ T cells has been shown to be constrained by direct thymic damage resulting from the conditioning regimen and/or age-related involution.20, 21 Additionally, in patients with GVHD, thymic production of naive T cells is further compromised due to direct T cell-mediated epithelial damage along with the reduced production of cytokines necessary for thymopoiesis.15, 22 This places a larger burden on mature CD4+ T cells that are transferred with the marrow graft for the maintenance of T cell immunity. GVHD, however, also deleteriously affects expansion of these cells in the periphery of transplant recipients.23 This has been attributed to the propensity of these cells to have shortened survival24, 25 and to be more prone to undergo apoptotic cell death26, 27 due to the over expression of death receptors and the under expression of prosurvival protein (i.e. bcl-2, bcl-XL). Why expansion and survival of CD4+ T cells is impaired, particularly in the setting of GVHD, is still not well understood. Aside from the effects of GVHD itself, the administration of immune suppressive agents, such as calcineurin inhibitors, steroids and other drugs for either prevention or treatment of GVHD may also affect immune reconstitution after allogeneic HSCT.
CD4+ T cell reconstitution in the periphery is constrained by the host microenvironment
A central question in understanding the failure of effective CD4+ T cell reconstitution in the periphery is whether the quantitative reduction in CD4+ T cell numbers is due to an intrinsic defect in these cells or whether the environmental milieu that arises from GVHD deleteriously affects CD4+ T cell expansion. To address this question, several studies have employed adoptive transfer models to assess the capability of T cells from GVHD recipients to survive when removed from their original environment. Dulude and colleagues [7] demonstrated that host-tolerant T cells have a limited capability to expand when transferred into GVHD mice. In contrast, T cells obtained from animals undergoing GVHD were competent to expand in thymectomized secondary hosts, supporting the premise that these T cells were capable of significant expansion once removed from the GVHD milieu. Additional work by Gorski et al.28 has shown that the adoptive transfer of GVHD splenic T cells into irradiated hosts of the same MHC type as the original recipient results in a marked attenuation of GVHD coupled with the expansion of donor-derived T cells and restoration of normal CD4/CD8 ratios. Moreover, using CDR3 spectratyping as an approach to examine the T cell repertoire, these studies demonstrated that the repertoire skewing and contraction that is characteristic of GVHD is not a fixed defect. Rather, T cells from GVHD animals were capable of significant molecular diversity after their transfer into secondary hosts. These data indicate that skewing and holes in the T cell repertoire are not necessarily fixed in GVHD animals, but rather subdominant T cell populations persist in these mice and have the capacity for expansion and the ability to contribute to normalization of the T cell repertoire once they are removed from the GVHD milieu. Collectively, these studies strongly suggest that the defects in the GVHD microenvironment are responsible for quantitative and qualitative failure of effective CD4+ T cells reconstitution during GVHD.
Critical molecules necessary for CD4+ T cell survival
The major survival signals that have been identified for both naive and memory CD4+ T cells are cytokines and MHC molecules29. With respect to cytokines, as noted, IL-7 has been shown to play the pivotal role in T cell homeostasis and is necessary for survival of CD4+ T cells.29, 30 Several lines of evidence, however, argue against a deficiency of IL-7 being responsible for the impaired CD4+ T cell reconstitution observed during GVHD. First of all, IL-7 levels have been shown to be markedly elevated in recipients undergoing GVHD.31 Furthermore, administration of IL-7 in experimental GVHD models has not been shown to enhance CD4+ T cell reconstitution.32, 33 Finally, recent data indicate that high levels of stromal cell-derived IL-7 actually function in a negative feedback loop to curtail homeostatic expansion of CD4+ T cells.34 Thus, these data lead one to conclude that deprivation of IL-7 per se does not appear to be an adequate explanation for the impaired reconstitution of these cells.
While naïve T cells require self-peptide/MHC signals for survival,35 memory T cells do not share this requirement although the lack of MHC ligands may affect memory T cell function.36 Destruction of lymphoid niches by GVHD or reductions in the numbers of class II expressing cells in the periphery may therefore inhibit T:MHC contacts necessary for survival, and is a potential explanation for the observed reduction in CD4+ T cells. Recent studies indicate that dendritic cells appear to be a likely candidate based on data demonstrating that an increase in CD11c+ cells is associated with increased homeostatic expansion of CD4+ T cells. In fact, work by Guimond et al.34 has demonstrated that IL-7 production by BM-derived DCs binds to IL-7R+ expressed on CD4+ T cells and leads to the homeostatic expansion of these cells under lymphopenic conditions. Plasmacytoid DCs, in particular, may be primarily responsible for regulating the CD4 T cell niche which is of potential clinical significance given data showing that a deficiency of these cells is associated with impaired T cell reconstitution and an increase in transplant-related mortality.37
Blockade of IL-6 signaling results in a recalibration of effector and regulatory CD4+ T cells
While a major goal in allogeneic stem cell transplantation is the restoration of normal numbers of CD4+ T cells, the specific subset composition of these cells may also be critical in the re-establishment of functional T cell immunity. GVHD is characterized by the preponderance of effector CD4+ T cells (e.g. TH1 and TH17) that are capable of secreting inflammatory cytokines or mediating direct tissue damage.38 In contrast, a majority of studies have shown that CD4+ foxp3+ regulatory T cells (Tregs) that serve to mitigate inflammatory responses are significantly reduced in GVHD recipients.39, 40 This resulting imbalance in the ratio of effector to Tregs contributes to the proinflammatory milieu. Cytokines that are produced during GVHD have been shown to be critical in directing the differentiation of naïve T cells to either effector T cells or Tregs. IL-6 is of particular interest with respect to GVHD biology since it occupies a unique position at the crossroads where the fate of naïve T cells to become either regulatory cells or proinflammatory T cells is determined. In the presence of IL-6 and transforming growth factor-β (TGF-β), naïve T cells differentiate into TH17 cells, whereas in its absence these same cells are induced to become Tregs.41 The question of whether blockade of IL-6 signaling would alter the subset composition of CD4+ T cells in GVHD recipients was therefore examined. These studies revealed that treatment of recipient mice with an anti-IL-6R antibody significantly prolonged survival and inhibited the severity of GVHD.42 This was attributable to marked increase in regulatory T cells which occurred in both thymic-dependent and thymic-independent manners. Moreover, it was observed that a commensurate reduction in the absolute number of proinflammatory TH1 and TH17 cells in GVHD target organs occurred. Thus, overproduction of IL-6 during GVHD results in a qualitative imbalance in CD4+ T cells towards an effector phenotype, whereas blockade of IL-6 signaling serves to recalibrate the effector and regulatory arms of the immune system and thereby mitigate the severity of GVHD. These studies therefore indicate that in addition to impairment of immune reconstitution in the setting of GVHD, a second link of the two exists in that the successful reconstitution of certain subpopulations and functions can decrease the severity of GVHD.
Further understanding of the mechanisms that hinder the reconstitution of CD4+ T cells in allogeneic stem cell transplant recipients, in particular those with GVHD, will hopefully lead to more effective strategies that will result in improved immune competence. Likewise, increased information regarding the complex interactions of reconstituted subsets of T cells and allo-aggressive responses in the setting of allogeneic HSCT should give rise to new strategies for prevention of GVHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: Implications for bone marrow transplantation and immunotherapy. Immunological Reviews. 1997 Jun;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T- cell regeneration after bone marrow transplantation. Blood. 2001 Mar 1;97(5):1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 3.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. New England Journal of Medicine. 1995 Jan 19;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006 May–Jun;29(3):313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakim FT, Cepeda R, Kaimei S, et al. Constraints on CD4 recovery postchemotherapy in adults: Thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood. 1997 Nov 1;90(9):3789–3798. [PubMed] [Google Scholar]
- 6.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005 Apr;115(4):930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu YW, Memon SA, Sharrow SO, et al. Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood. 2004 Aug 15;104(4):1110–1109. doi: 10.1182/blood-2003-10-3635. [DOI] [PubMed] [Google Scholar]
- 8.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001 Mar 15;97(6):1525–1533. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]
- 9.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008 Jul 7;205(7):1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpdogan O, Muriglan SJ, Kappel BJ, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003 Jun 27;75(12):1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 11.Chu YW, Schmitz S, Choudhury B, et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood. 2008 Oct 1;112(7):2836–2846. doi: 10.1182/blood-2008-04-149435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008 Jun 15;111(12):5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krijanovski OI, Hill GR, Cooke KR, et al. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999 Jul 15;94(2):825–831. [PubMed] [Google Scholar]
- 14.Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007 Mar 15;109(6):2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min D, Taylor PA, Panoskaltsis-Mortari A, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002 Jun 15;99(12):4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 16.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001 Mar;142(3):1278–1283. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 17.Williams KM, Lucas PJ, Bare CV, et al. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008 Oct 15;112(8):3255–3263. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002 Apr 15;73(7):1154–1158. doi: 10.1097/00007890-200204150-00026. [DOI] [PubMed] [Google Scholar]
- 19.Poulin JF, Sylvestre M, Champagne P, et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003 Dec 15;102(13):4600–4607. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 20.Heitger A, Neu N, Kern H, et al. Essential role of the thymus to reconstitute naïve (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997 Jul 15;90(2):850–857. [PubMed] [Google Scholar]
- 21.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000 May 27;355(9218):1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001 Mar 1;97(5):1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 23.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999 Apr 19;189(8):1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebib NC, Deas O, Rouleau M, et al. Peripheral blood T cells generated after allogeneic bone marrow transplantation: lower levels of bcl-2 protein and enhanced sensitivity to spontaneous and CD95-mediated apoptosis in vitro. Abrogation of the apoptotic phenotype coincides with the recovery of normal naive/primed T-cell profiles. Blood. 1999 Sep 1;94(5):1803–1813. [PubMed] [Google Scholar]
- 25.Lin MT, Tseng LH, Frangoul H, et al. Increased apoptosis of peripheral blood T cells following allogeneic hematopoietic cell transplantation. Blood. 2000 Jun 15;95(12):3832–3839. [PubMed] [Google Scholar]
- 26.Alpdogan SO, Lu SX, Patel N, et al. Rapidly proliferating CD44hi peripheral T cells undergo apoptosis and delay post-transplantation T-cell reconstitution after allogeneic bone marrow transplantation. Blood. 2008 Dec 1;112(12):4755–4764. doi: 10.1182/blood-2008-02-142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brochu S, Rioux-Masse B, Roy J, Roy DC, Perreault C. Massive activation-induced cell death of alloreactive T cells with apoptosis of bystander postthymic T cells prevents immune reconstitution in mice with graft-versus-host disease. Blood. 1999 Jul 15;94(2):390–400. [PubMed] [Google Scholar]
- 28.Gorski J, Chen X, Gendelman M, et al. Homeostatic expansion and repertoire regeneration of donor T cells during graft versus host disease is constrained by the host environment. Blood. 2007 Jun 15;109(12):5502–5510. doi: 10.1182/blood-2006-12-061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002 Aug;2(8):547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dean RM, Fry T, Mackall C, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008 Dec 10;:26–35. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001 Oct 1;98(7):2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 33.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002 Oct 1;100(7):2642–2649. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 34.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009 Feb;10(2):149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999 Aug;11(2):173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 36.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002 Mar;3(3):244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 37.Mohty M, Blaise D, Faucher C, et al. Impact of plasmacytoid dendritic cells on outcome after reduced-intensity conditioning allogeneic stem cell transplantation. Leukemia. 2005 Jan;19(1):1–6. doi: 10.1038/sj.leu.2403558. [DOI] [PubMed] [Google Scholar]
- 38.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006 Jan;43(1):3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004 Oct 1;104(7):2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007 Nov 15;110(10):3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009 Jul 23;114(4):891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]