Abstract
Intrinsic Signal Optical Imaging (ISOI) can be used to map cortical function and organization. Because its detected signal lasts 10+ sec consisting of three phases, trials are typically collected using a long (tens of seconds) stimulus delivery interval (SDI) at the expense of efficiency, even when interested in mapping only the first signal phase (e.g., ISOI initial dip). It is unclear how the activity profile can change when stimuli are delivered at shorter intervals, and whether a short SDI can be implemented to improve efficiency. The goals of the present study are two-fold: characterize the ISOI activity profile when multiple stimuli are delivered at 4 sec intervals, and determine whether successful mapping can be attained from trials collected using an SDI of 4 sec (offering >10× increase in efficiency). Our results indicate that four stimuli delivered 4 sec apart evoke an activity profile different from the triphasic signal, consisting of signal dips in a series at the same frequency as the stimuli despite a strong rise in signal prior to the 2nd–4th stimuli. Visualization of such signal dips is dependent on using a baseline immediately prior to every stimulus. Use of the 4-sec SDI is confirmed to successfully map activity with a similar location in peak activity and increased areal extent and peak magnitude compared to using a long SDI. Additional experiments were performed to begin addressing issues such as SDI temporal jittering, response magnitude as a function of SDI duration, and application for successful mapping of cortical function topography.
Keywords: intrinsic signal optical imaging, stimulus delivery interval, interstimulus interval, rat, whisker, barrel, primary somatosensory cortex
Introduction
The functional imaging technique Intrinsic Signal Optical Imaging (ISOI; (Grinvald et al., 1986); (Frostig et al., 1990); (Ts’o et al., 1990); for a recent review see (Frostig and Chen-Bee, 2009)) using red illumination is commonly employed for mapping cortical activity and plays an important role in furthering our understanding of brain function and organization. The detected ISOI signal evoked by a brief stimulus is slow in onset (within half a second of stimulus onset), long in duration (10+ seconds), and, at least in the rat (Chen-Bee et al., 2007), is comprised of three signal phases consisting of a dip in signal below baseline followed by an overshoot in signal above baseline and another dip below baseline (referred to hereafter as the initial dip, overshoot, and undershoot). Capture of the entire signal is not necessary; rather, the ISOI initial dip is better suited and preferentially exploited for successful ISOI mapping of brain function. While typically not serving any mapping purpose, the latter signal phases are still taken into consideration; long intervals (tens of seconds) between stimulus deliveries are typically employed in order to allow not just the ISOI initial dip but also any remaining ISOI signal phases to return to baseline. Such practice is at the expense of data collection efficiency, substantially slowing the rate at which trials can be accumulated and hence undesirably prolonging an experiment; also, the occurrence of stimulus deliveries is considerably sparse compared to the stimulation experienced by awake and behaving subjects. We wondered whether the stimulus delivery interval (SDI) could be shortened as a means to improve data collection efficiency (as well as offer progress towards a regimen of stimulus deliveries more analogous to behaviorally relevant stimulation). For example, rather than wait tens of seconds until completion of the entire preceding signal, a given stimulus could be delivered after completion of only the preceding signal’s initial dip, which would require just a few seconds but would be at the risk of coinciding stimulus delivery with lingering signal. At present, the profile of ISOI activity is unknown when a series of multiple stimuli are delivered at short intervals; furthermore, it is also unclear whether trial collection using a short SDI can be successfully used for ISOI mapping of brain function.
Thus, the goals of the current study are two-fold. We used ISOI to image brain activity in the rat barrel cortex evoked by brief (1 sec) stimulation of a single whisker and addressed two questions. First, to investigate the consequences of delivering a stimulus without waiting for the entire completion of previously evoked signal, we characterized the activity profile of four stimuli delivered 4 sec apart as compared to a single stimulus delivery. Second, to determine whether a short SDI can be employed for successful ISOI mapping of brain activity, within the same subject we compared the activity map obtained from collecting 128 trials using a short (4 sec) versus long (tens of seconds) SDI. Additional experiments were performed to begin addressing the temporal jittering of the short SDI, the response magnitude as a function of SDI duration, and the actual application of the short SDI for successfully fulfilling the mapping needs of an imaging study. The collective results of the present study indicate that multiple stimuli delivered at 4 sec intervals can evoke an activity profile with stereotypical properties different than the triphasic activity profile, and trial collection using a short SDI of 4 sec can indeed be used for successful ISOI mapping of brain function with substantial improvement in efficiency.
The subset of the present data acquired using a long SDI is part of a large data pool presented elsewhere in a previous publication (Chen-Bee et al., 2007).
Materials and Methods
Most of the surgical preparation, imaging, and data analysis used in the present study are described in detail elsewhere (Chen-Bee et al., 2007). Summary and additional details are provided below.
Subjects and surgical preparation
Principles of laboratory animal care were followed, and all performed procedures were in compliance with the National Institutes of Health guidelines and approved by the University of California Irvine Animal Care and Use Committee. At the start of an experiment, each adult male Sprague Dawley rat received a bolus intraperitoneal injection of sodium pentobarbital (Nembutal, 55 mg/kg b.w.) followed by an intramuscular injection of atropine (0.05 mg/kg b.w.) into the hind leg. Supplemental Nembutal injections were administered as necessary to maintain mild corneal reflexes and additional atropine injections were administered every 6 hours. Body temperature was monitored and maintained at 37°C with an electric homeothermic blanket. The exposed left skull overlying the subregion of primary somatosensory cortex responsive to the large facial whiskers was thinned to ~150 μm thickness with a dental drill, and a saline-filled well of petroleum jelly sealed with a coverslip was built over the thinned skull region (Masino et al., 1993).
ISOI data acquisition
Each rat was placed under a 12-bit CCD camera combined with an inverted 50 mm lens such that a cortical region of 6.5 × 4.9 mm beneath the thinned skull region mapped onto a final array of 191 × 144 pixels (i.e., 1 pixel = 34 × 34 μm cortical region). An image of the surface vasculature was taken for later reference (Fig. 7B) using green illumination before the CCD camera was focused 600 μm below the cortical surface. For data collection, the imaged region was continuously illuminated with a red light-emitting diode (635 nm max, 15 nm full width at half-height) powered by a 6V battery. During each imaging trial, frames were captured at 10 Hz rate (i.e., 100-msec frames). A single stimulus delivery consisted of 5 rostral-caudal deflections at 5 Hz delivered to a single whisker (C2). Additional details about stimulus delivery and trial collection are provided below according to experimental condition. With noted exceptions, a total of 128 stimulation trials were collected per experimental condition. At the end of each experiment, trials were separated according to condition and then summed, and the summed data collapsed into 500-msec frames (referred to hereafter as a data file) to increase the signal-to-noise ratio.
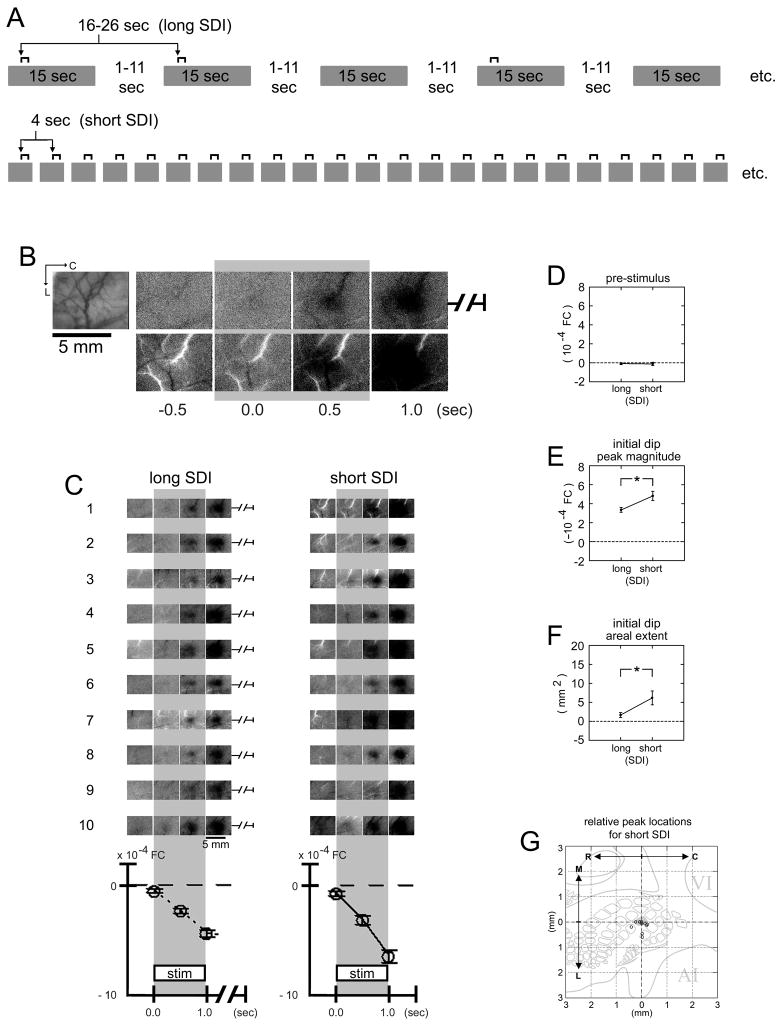
Fig. 7.
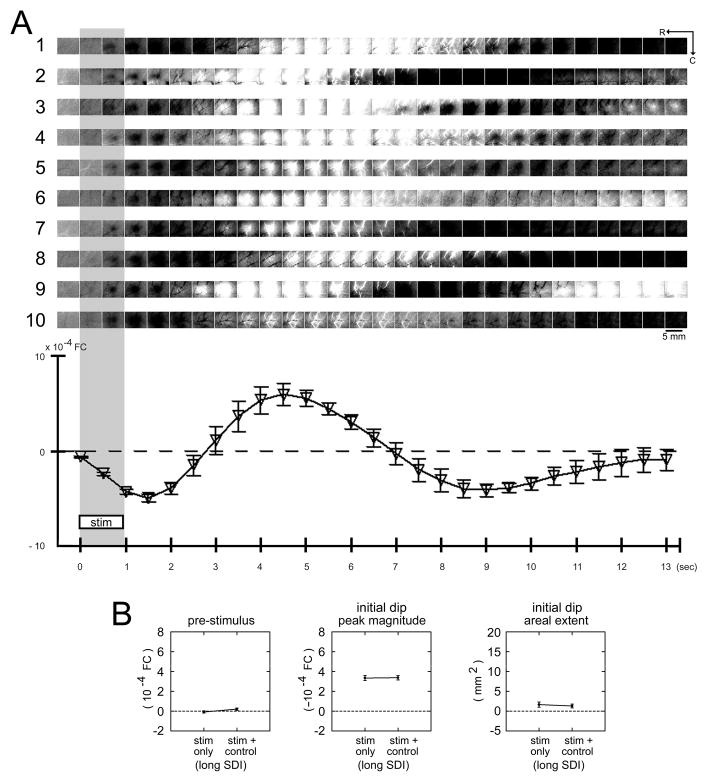
Activity maps from trial collection using a long versus short SDI within the same rat. (A) Schematic of trial collection. For each rat, trials using a long versus short SDI were collected as described in Table 1, Columns 1 and 3, respectively. (B) Results from a representative case (same as in Fig. 1C). Picture of the surface vasculature as seen through the thinned skull is provided for the imaged region. Images of pre- and post-stimulus activity are shown for the long (top row) versus short (bottom row) SDI. Stimulus delivery is indicated with a gray background bar. For the short SDI, the pre-stimulus image (−0.5 sec) appeared quite similar to that for the long SDI except for some ‘white’ vessel presence. Also, areal extent and signal strength of the evoked initial dip appeared stronger, along with some ‘white’ vessel presence. (C) Summary of results for 10 rats. First case provided is the same as in (B). Plots of average intrinsic signal (means and standard errors) for the first three post-stimulus time points are provided. Note the greater initial dip signal for the short SDI (right column) compared to the long SDI (left column). (D–F) Means and standard errors of pre-stimulus FC value and initial dip peak magnitude and areal extent. No significant difference (two-tailed paired t(9) = −0.20, p = 0.847) in pre-stimulus FC value was found between the two types of SDIs (long versus short; D). In contrast, a significant increase in the initial dip peak magnitude (two-tailed paired t(9) = 3.50, p = 0.007; E) and areal extent (two-tailed paired t(9) = 3.23, p = 0.010; F) was found for the short SDI. Asterisks denote p < 0.05. (G) Initial dip peak location. Initial dip peak locations for the short SDI are plotted relative to those for the long SDI (relative coordinate of 0,0 = perfect co-localization) and superimposed on an appropriately scaled schematic of cortical layer IV anatomical topography that includes portions of primary somatosensory (barrel of stimulated whisker is shaded in gray), visual (VI), and auditory (AI) cortices, with the relative origin centered above the barrel of the stimulated whisker. The peak locations were observed to co-register (within 0.2 mm away; i.e., within the stimulated whisker barrel) for half of the cases, with the remaining cases observed to be 0.2–0.5 mm away (i.e., within surrounding septa or adjacent whisker barrels).
Experimental Design 1 - activity profile for multiple stimuli delivered at 4 sec intervals
To characterize the activity profile for multiple stimulus deliveries separated by a short interval, imaging was performed in two groups of rats. For the first group (n = 10 rats), activity evoked by a single stimulus was imaged (Fig. 1A) using trial collection parameters as detailed in Table 1, Column 1 (because of the trial collection parameters, the average interval between consecutive stimulus deliveries was in the order of tens of seconds). For the second group (n = 8 rats), activity evoked by a series of four stimuli delivered 4 sec apart (referred to hereafter as 4-stimuli series) was imaged (Fig. 3A) using extended imaging trials as detailed in Table 1, Column 4. The experiment day began with a quick confirmation that a single stimulus evoked a typical ISOI triphasic signal (all details same as in Table 1, Column 3, except only 64 stimulation trials were collected). Then, for the experiment proper, extended trials were collected in which the 4-stimuli series was delivered in each trial. The extended trial duration enabled the continuous capture, and therefore characterization, of activity throughout the delivery of the 4-stimuli series and for 15.5 sec thereafter (Fig. 3A). The interval between the stimuli within the 4-stimuli series was set to 4 sec because trial collection using a short SDI of 4 sec would permit data collection at a substantially accelerated rate while still ensuring that a given stimulus delivery occurred after termination of the preceding ISOI initial dip. Also, a 4 sec interval was a good candidate for revealing potential consequences of coinciding a given stimulus delivery with the latter segment of the preceding evoked signal because the ISOI overshoot phase maximizes approximately 4 sec after stimulus onset (see Fig. 2).
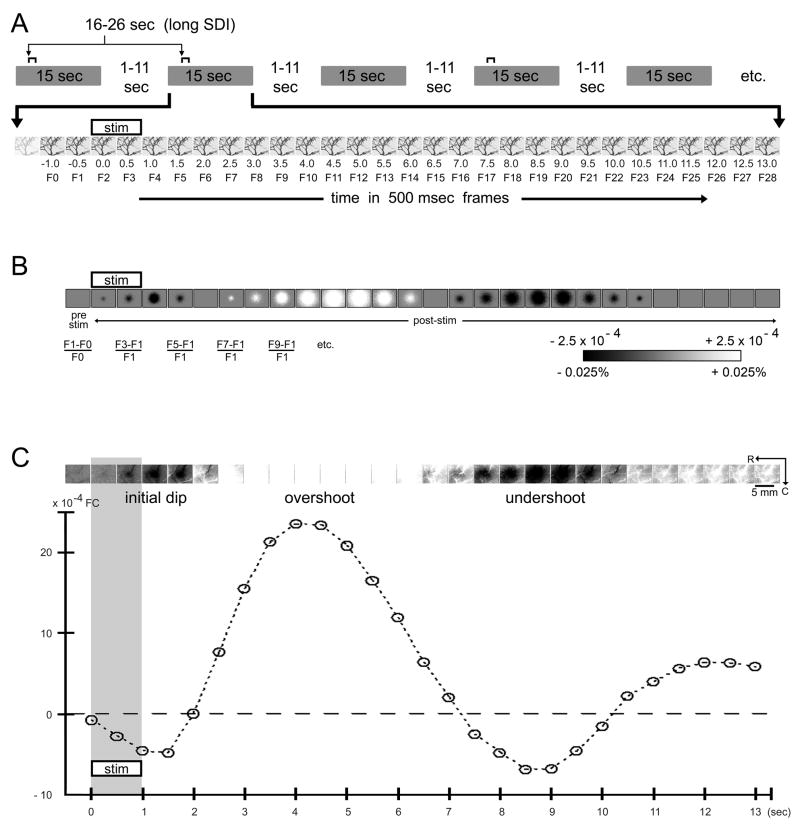
Fig. 1.
Single stimulus delivery and trial collection using a long SDI (stim+control trials). (A) Schematic of trial accumulation. Trial collection parameters are detailed in Table 1, Column 1. Because an equal number of control trials were collected, stimulus delivery intervals (SDIs) were in essence centered at 42 sec with a jitter range of 20+ sec. One stimulation trial is enlarged to illustrate the temporal relationship between stimulus delivery and the 500-msec frames (dark streaks depict large dural and cortical surface blood vessels). (B) Schematic of visualizing intrinsic signal activity. Post-stimulus frames are converted to fractional change (FC) values relative to pre-stimulus data. For details see Materials and Methods; grayscale bar applies to all applicable figures. (C) Visualization and plot of intrinsic signal activity for a representative rat. A single stimulus delivery using a long SDI evoked a triphasic signal. Activity was relatively stable prior to stimulus onset, evidenced by a homogeneous pre-stimulus image with only subtle contributions from blood vessels (light or dark streaks). The post-stimulus intrinsic signal time course from the location of peak initial dip activity was extracted and plotted.
Table 1.
Summary of experiment details.
| Column 1 | Column 2 | Column 3 | Column 4 | Column 5 | |
|---|---|---|---|---|---|
| Long SDI With Jitter (stim+control trials) | Short SDI No Jitter | Long SDI With Jitter (stim trials only) | Extended Trials | Short SDI With Jitter | |
| see Fig. 1A | see Fig. 7A | see Fig. 3A | |||
| sample ize (n) | 10 rats | 10 rats | 8 rats | 3 rats | |
| single trial parameters | 15-sec trials 150 frames/trial |
3-sec trials 30 frames/trial |
15-sec trials 150 frames/trial |
30-sec trials 300 frames/trial |
2-sec trials 20 frames/trial |
| Stimulus Delivery (whisker C2) | single (5 deflections at 5 Hz for 1 sec) | single | single | 4-stimuli series (four single stimulus deliveries 4 sec apart) | single |
| stimulus delivery began 1.5 sec from start of stimulation trial | stimulus delivery began 1.5 sec from start of stimulation trial | stimulus delivery began 1.5 sec from start of stimulation trial | 4-stimuli series began 1.5 sec from start of stimulation trial | stimulus delivery began 1.5 sec from start of stimulation trial | |
| interval between end of one trial and start of next trial | Average of 6 sec, ranging randomly between 1–11 sec | Exactly 1 sec | Average of 6 sec, ranging randomly between 1–11 sec | Average of 6 sec, ranging randomly between 1–11 sec | Average of 2 sec, ranging randomly between 1–3 sec |
| stimulus delivery interval (SDI) | Centered at 42 sec | Exactly 4 sec | Centered at 21 sec | (not applicable) | Centered at 4 sec |
| SDI jitter range | >20 sec | 0 sec (no jitter) | 10 sec | 2 sec | |
| data collection duration | 100 minutes to complete 128 stim trials | 10 minutes to complete 128 stim trials | 50 minutes to complete 128 stim trials | (not relevant) | 5 minutes to complete 64 stim trials |
In all experiments, trials were collected with intrinsic signal optical imaging (ISOI) of rat barrel cortex (~6.5×5mm) using a red (635nm wavelength) illumination source. A CCD camera was used to capture frames at 10 Hz rate (i.e., 100-msec frames). Trials were summed to achieve a total of 128 trials per experimental condition (except 64 trials for Column 5), and the summed data collapsed into 500-msec frames to increase the signal-to-noise ratio. Note the difference in the SDI and SDI jitter range between the different experimental conditions (shaded row). For all experiments, data analysis entailed the conversion of post-stimulus 500-msec frames into fractional change values relative to pre-stimulus data (see Fig. 1B). See text for details.
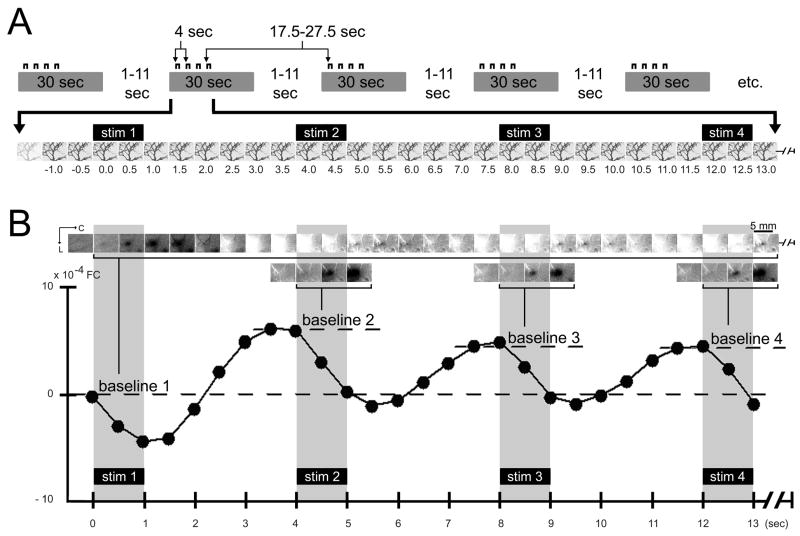
Fig. 3.
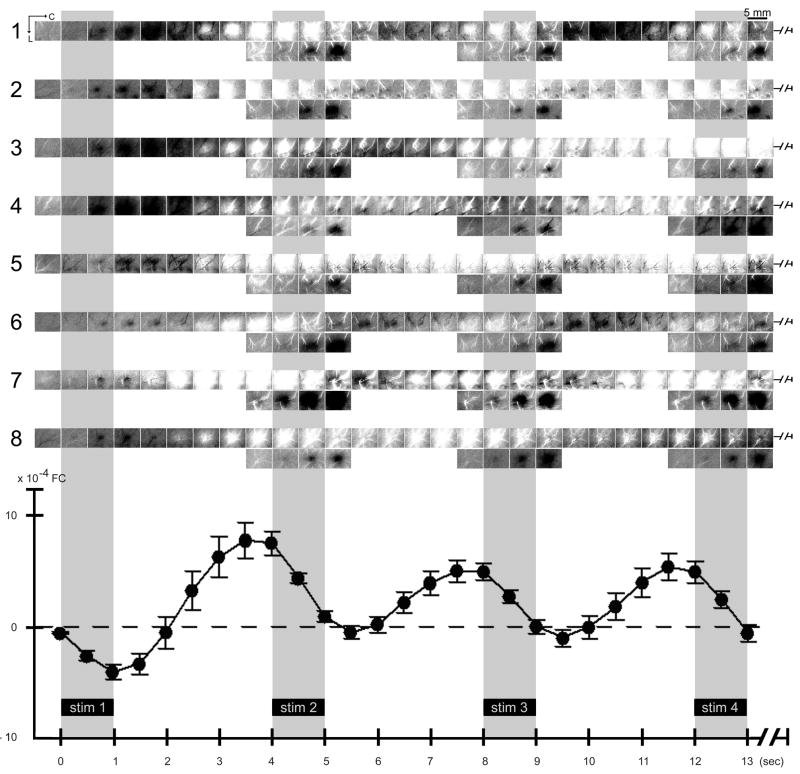
Activity profile for multiple stimuli delivered at 4 sec intervals. (A) Schematic of trial accumulation. Trial collection parameters are detailed in Table 1, Column 4. (B) Results for a representative rat. Plot. The post-stimulus intrinsic signal time course was extracted and plotted at the location of peak initial dip evoked by stim 1 delivery. Note that stim 1 of the 4-stimuli series evoked an initial dip plus onset of overshoot as typically observed for a single stimulus (compare to Figs. 1C plot and 2 plot). Upon delivery of stim 2–4, the signal was observed to reliably dip for each of stim 2–4 despite a strong rise in signal immediately prior to stimulus onset. Images. The same post-stimulus frames are visualized in reference to either the frame collected immediately prior to stim 1 (baseline 1; top row images) or the frame collected immediately prior to a particular stimulus delivery (stim 2, 3, or 4; baseline 2, 3, or 4, respectively; bottom row images). Note that an evoked dip in signal coinciding with a strong signal rise (as is the case for stim 2–4) can be effectively visualized as a dark activity area only when the frame immediately prior to each of stim 2–4 deliveries is used as baseline (bottom row images).
Fig. 2.
Single stimulus delivery and trial collection using a long SDI (stim+control trials) - summary of 10 rats. Visualization and average plot (means and standard errors) of the triphasic intrinsic signal are provided for ten rats. First rat is the same as in Fig. 1C. Note that on average the overshoot signal peaked approximately 4 sec after stimulus onset.
Experimental Design 2– activity map from trial collection using a long versus short SDI
The imaging of activity evoked by a single stimulus delivery as described above for the first group of rats (n = 10) involved a long SDI (Fig. 1A; Table 1, Column 1) and thus the data were also used to provide mapping results from trials collected using a long SDI. Additional imaging was performed on this group of rats as detailed in Table 1, Column 2 (Fig. 7A) to provide mapping results from trials collected using a short SDI. The long SDI was in essence centered at 42 sec with a > 20 sec jitter range because an equal number of control trials were interleaved with stimulation trials; ~ 100 minutes were required to complete 128 stimulation trials. It should be noted that employment of a long SDI with jittering, where both stimulation and control trials are collected, is an example of what is commonly practiced in ISOI experiments. The short SDI was set to exactly 4 sec (hence, no jitter) to be congruent to the 4-stimuli series used in the experiments described above; < 10 minutes were required to complete 128 stimulation trials. Trial blocks using a long versus short SDI were interleaved in random order throughout the experiment.
Data analysis
Data files were analyzed for visualization, quantification, and plotting of pre- and post-stimulus intrinsic signal activity.
VISUALIZATION
Post-stimulus activity was visualized as described in detail elsewhere (Chen-Bee et al., 1996); (Chen-Bee et al., 2000); (Chen-Bee et al., 2007). Briefly, fractional change (FC) values for a given post-stimulus 500-msec frame were calculated relative to the 500-msec frame collected immediately prior to stimulus onset (baseline frame) on a pixel-by-pixel basis (Fig. 1B). For the 4-stimuli series data files, the 500-msec frame immediately prior to stim 1 of the series served as the baseline frame. Then, to generate images of post-stimulus activity, an 8-bit linear grayscale mapping function was applied to the FC values, with an FC value of 0 (no change from baseline) mapped to middle gray shade and an arbitrary threshold of ±2.5×10−4 FC (±0.025%) from 0 used such that evoked ISOI initial dip (also ISOI undershoot) and ISOI overshoot signal phase would appear as black or white, respectively, in generated images (Fig. 1B). The same method was applied for visualizing pre-stimulus activity except that FC values were calculated for the 500-msec frame immediately prior to stimulus delivery relative to the frame immediately prior to it.
QUANTIFICATION AND PLOTTING
For the pre-stimulus data, the median FC value was used as a measure of average pre-stimulus activity. For the post-stimulus data, the FC values obtained from averaging the 2nd and 3rd 500-msec post-stimulus frames were used for quantifying the areal extent, absolute peak magnitude, and peak location of the ISOI initial dip. Prior to quantification, the post-stimulus FC values were first processed with a two-pass Gaussian filter (half-width = 5) to remove high frequency spatial noise. Then, the areal extent of the ISOI initial dip was quantified by applying an arbitrary threshold of 2.5×10−4 from 0 to the filtered FC values, and the magnitude and location of peak value within the quantified areal extent were determined. Lastly, after determination of peak value location, the time course of FC values from that location was extracted and plotted for the entire post-stimulus trial duration. For specifically the 4-stimuli series data files, peak location of ISOI initial dip activity was determined for stim 1 of the 4-stimuli series, and the time course of FC values over the entire post-stimulus trial duration was extracted and plotted for that location.
PEAK LOCATION COMPARISON
Because the ISOI initial dip imaged in the present study was evoked by stimulation of a single whisker in the rat and its location of peak activity has been found to correctly register with the appropriate anatomical location when trials were collected using a long SDI (i.e., (Masino et al., 1993); (Brett-Green et al., 2001); (Frostig et al., 2008)), we also investigated whether there were potential shifts in peak location when trials were collected using a short SDI. For each rat, the x-y coordinates of peak location obtained using a long SDI were re-expressed relative to those obtained using a short SDI such that a relative coordinate of (0,0) would indicate perfect co-localization, and shifts along the x-axis or y-axis would indicate shifts in peak location along the rostral-caudal or medial-lateral axis, respectively (see Fig. 7G). It should be noted that the radius of a large whisker’s anatomical representation (or barrel) in rat primary somatosensory cortex is ~0.2 mm, distance between the centers of adjacent representations is ~0.5 mm (Jensen and Killackey, 1987); (Land and Simons, 1985).
Statistics
All graphing and statistics were performed using Systat 11 software. Prior to performing inferential statistics, areal extent and absolute peak magnitude values were transformed with the square root and natural log function, respectively, to better satisfy assumptions of the inferential statistics (no data transformation was needed for the pre-stimulus median values). The FC values of the signal time course extracted at the location of peak initial dip activity also underwent a natural log transformation before inferential statistics were performed. Alpha level was set to 0.05.
Activity profile for multiple stimuli delivered at 4 sec intervals
For the between-subjects comparison between delivering a single stimulus (Fig. 1A) versus the 4-stimuli series (Fig. 3A), a repeated measures ANOVA with one within-subjects variable (time) and one between-subjects variable (type of stimulus) was performed to compare the FC values extracted at the location of peak ISOI initial dip evoked by the single stimulus versus the 4-stimuli series (Fig. 4). One rat (case 10 for the long SDI, stim+control) was identified as an outlier (exhibiting particularly strong overshoot and undershoot) and excluded from statistical analysis. The ANOVA was followed by two specific contrasts to compare the two types of stimuli for the collective time points starting (i) at the 0 sec time point, corresponding to the delivery onset of both the single stimulus and the 4-stimuli series, up until the time point corresponding to the delivery onset of stim 2 of the 4-stimuli series; these collective time points spanned a time epoch when there was no difference in stimulation between the two types of stimuli; or (ii) 4.5 up through 6.0 sec, a time epoch where the greatest difference in ISOI overshoot signal was observed in response to delivery of stim 2 of the 4-stimuli series as compared to a single stimulus. Bonferroni correction was applied to account for multiple contrasts, for an adjusted alpha level of 0.05/2 = 0.025.
Fig. 4.
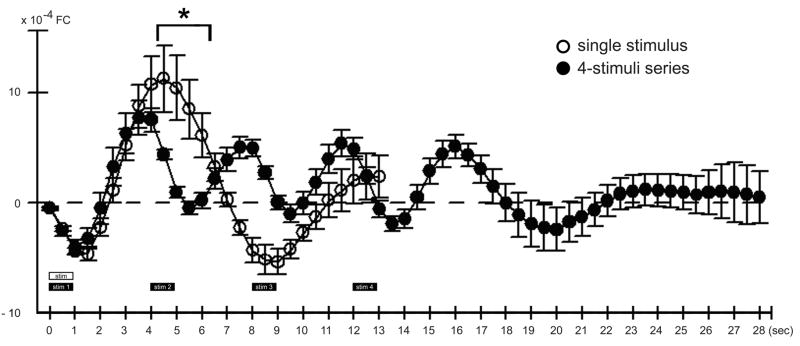
Activity profile evoked by a single stimulus using a long SDI versus the 4-stimuli series. Plotted here is the average signal time course (means and standard error bars) for a single stimulus delivery using a long SDI (open circle; same plot as in Fig. 2) versus the 4-stimuli series (filled circle). For the collective time points of 0 up until the 4 sec time point (stim 2 onset), no significant difference in signal time course was found between the two types of stimulus deliveries (F(1,15)=0.21, p=0.656), indicating that stim 1 of the 4-stimuli series evoked an initial dip and beginning portion of the overshoot comparable to that for a single stimulus using a long SDI. In contrast, a significant difference was found for the collective time points starting at 4.5 sec up through 6.0 sec (F(1,15)=9.83, p=0.007; asterisk), where compared to the overshoot evoked by a single stimulus using a long SDI (open circle) stim 2 delivery induced a dip in signal (filled circle). Note that stim 3–4 deliveries also each induced a dip in signal (filled circle).
Activity map from trial collection using a long versus short SDI
For the within-subjects comparison of mapping results for trial collection using a long versus short SDI, two-tailed paired t-tests were performed on the pre-stimulus median, transformed areal extent, and transformed peak magnitude values (Fig. 7D–F).
Results
Experimental Design 1 – Activity profile for multiple stimuli delivered at 4 sec intervals
As expected based on our previous findings (Chen-Bee et al., 2007), a single stimulus (Fig. 1A; Table 1, Column 1) reliably evoked a triphasic signal spanning 10+ sec (plots in Figs. 1–2) consisting of an ISOI initial dip (visualized as a black coherent area in generated images) followed by an ISOI overshoot (white area) and then an ISOI undershoot (second black area). In contrast, delivery of the 4-stimuli series (Fig. 3A; Table 1, Column 4) evoked a signal profile with markedly different temporal properties. Results from a representative case are provided in Fig. 3B. Stim 1 of the 4-stimuli series evoked an ISOI initial dip similarly to that for a single stimulus using a long SDI (compare stim 1 of Fig. 3B plot, with Fig. 2 plot), presumably due to the long interval (17.5–27.5 sec) between stim 1 of one series and stim 4 of the preceding series (see Fig. 3A). Furthermore, initiation of stim 1’s ISOI overshoot signal was confirmed along with the coinciding of this strong overshoot with stim 2 delivery at the 4.0 sec time point (Fig. 3B plot).
Upon stim 2 delivery, the signal was observed to dip (Fig. 3B plot) in a manner similar for stim 1 and for a single stimulus using a long SDI (Fig. 2 plot), despite a strong rise in signal just prior to stim 2 onset. A repeated measures ANOVA performed on the signal time course (Fig. 4) found a significant interaction (F(26,390)=11.95, p= 1×10−15) between type of stimulus (4-stimuli series vs. single) and trial time point. Specifically, no significant difference between stimulus type was found for the collective time points starting at 0 sec up until onset of stim 2 delivery (F(1,15)=0.21, p=0.656). In contrast, a significant difference was found for the collective time points starting at 4.5 sec up through 6.0 sec, corresponding to those time points with the greatest dip in signal upon stim 2 delivery (F(1,15)=9.83, p=0.007), indicating that the signal dip observed just after stim 2 onset was due specifically to stim 2 delivery. Complementary results were obtained for stim 3–4: a strong rise in signal prior to stimulus delivery followed by a dip in signal after stimulus delivery (Fig. 4, filled circles). The collective results demonstrated the ability of stim 2–4 to exert a dip in signal despite a rise in signal prior to their deliveries. Interestingly, another dip in signal occurred 4 sec after stim 4 onset even though no more stimuli were being delivered (Fig. 4, filled circles). In summary, multiple stimuli delivered at short intervals (as is the case for the 4-stimuli series) each successfully evoked a signal dip, for a final activity profile that consisted of multiple signal dips occurring in a series at the same frequency as the stimulus deliveries (Fig. 4, filled circles) and that collectively constitute an activity profile different from the triphasic activity profile associated with a single stimulus (Fig. 4, open circles).
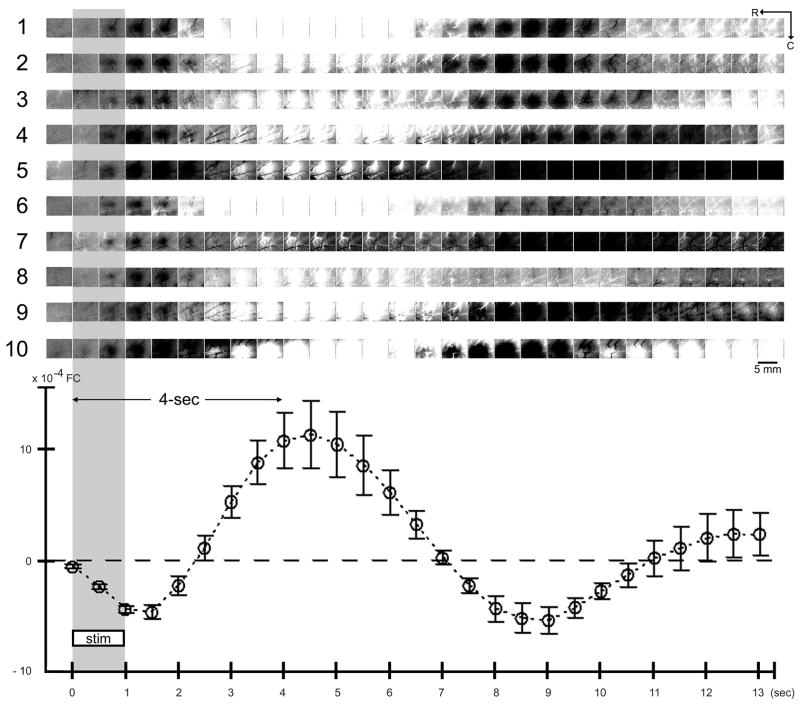
The reliable signal dips described above must be due to successful evoking of ISOI initial dips. We found that successful visualization of such signal dips was dependent on the baseline frame used for generating images. When the frame immediately prior to stim 1 onset was used as baseline (baseline 1), the generated images (Fig. 3B, top row images) were able to visualize stim 1’s signal dip (dark activity area) and onset of overshoot (white activity area) similarly to that for a single stimulus delivery (Fig. 2 images), but were unable to effectively visualize signal dips superimposed on signal rises as was the case for stim 2–4. In contrast, by using as baseline the frame captured immediately prior to each delivery of stim 2–4 (i.e., baseline 2 for stim 2, baseline 3 for stim 3, etc.; see Fig. 3B plot), the generated images were finally able to visualize the signal dips of stim 2–4 as dark activity areas in a manner similar to stim 1 and to a single stimulus delivery, areas that exhibited comparable peak magnitude and areal extent except with increased ‘white’ vessel activity. (Also, the frame immediately prior to stim 2–4 appeared relatively homogeneous with some increased ‘white’ vessel activity.) Note the use of a baseline prior to each of stim 2–4 was analogous to baseline 1 for stim 1, as well as for the baseline frame used for single stimulus delivery. See Fig. 5 for summary of all cases.
Fig. 5.
Activity profile for multiple stimuli delivered at 4 sec intervals - summary of 10 rats. Average plot (means and standard errors) and visualization of the activity profile evoked by the 4-stimuli series are provided for 10 rats. First rat is the same as in Fig. 3B. For details see Fig. 3 legend.
The results of Figs. 3–5 collectively suggested that trial collection using a short SDI of 4 sec can be successfully employed to map brain activity, as long as data analysis utilized a baseline captured immediately prior to each stimulus delivery. Results from the next set of experiments were obtained to explicitly confirm the implementation of such mapping.
Experimental Design 2 – Activity maps from trial collection using a long versus short SDI
The ISOI initial dip results for a single stimulus delivery as described above were used to provide mapping results from trial collection using a long SDI (centered at 42 sec with a >20-sec jitter range; Fig. 1A; Table 1, Column 1). Besides the reliable evoking of a triphasic signal (Fig. 2 images), pre-stimulus data were relatively homogeneous across the imaged region, as supported by minimal activity observed from large surface blood vessels (subtle streaks in images) and an average FC value of 0.2 × 10−4 (or ~10× weaker than evoked signal; compare Fig. 7D and E). (The ISOI initial dip exhibited the same signal properties when control trials were excluded, which entailed the use of an SDI that was sufficiently long but reduced by half and therefore provided a 2× increase in efficiency; Fig. 6). Mapping results from trial collection using a short SDI (centered at 4 sec with no jitter; Table 1, Column 2) were also obtained from the same animals (Fig. 7A). Except for some increased ‘white’ vessel presence in images, pre-stimulus data appeared quite similar for the short SDI (Fig. 7B–C) and no significant difference was found for the average pre-stimulus FC values (Fig. 7D; two-tailed paired t(9) = −0.20, p = 0.847). In contrast, differences in the evoked ISOI initial dip were found. The ISOI initial dip images for the short SDI contained some increased ‘white’ vessel presence (Fig. 7B–C) similarly observed for the pre-stimulus data, and the peak magnitude (Fig. 7E; two-tailed paired t(9) = 3.50, p = 0.007) and areal extent (Fig. 7F; two-tailed paired t(9) = 3.23, p = 0.010) of the ISOI initial dip were significantly increased. The ISOI initial dip peak locations, however, were comparable as indicated by co-registry within 0.2 mm (approximate radius of a large whisker barrel) in half of the cases and 0.2–0.5 mm for the remaining cases (Fig. 7G).
Fig. 6.
Single stimulus delivery and trial collection using a long SDI – stim+control versus stim only. Trial collection parameters are detailed in Table 1, Columns 1 and 3. A between-subjects comparison was performed between the two types of long SDI (stim+control, see Fig. 2, versus stim only, see panel A in present figure). Two-tailed unpaired t-tests were performed on the pre-stimulus median, transformed areal extent, and transformed peak magnitude values. Also, a repeated measures ANOVA with one within-subjects variable (time point) and one between-subjects variable (type of long SDI) was performed on the FC values of the signal time course extracted at the location of peak ISOI initial dip activity. Prior to the ANOVA, one rat (case 10 for stim+control group) was excluded from statistical analysis (images and average plots for all 10 cases are provided in Fig. 2). (A) As with stim+control trials (see Fig. 2), a triphasic signal can still be obtained when only stimulation trials are collected – i.e., SDI is reduced by half, thereby achieving 2x increase in efficiency, but is still sufficiently long to avoid any overlap between consecutively evoked signals; also, pre-stimulus activity remained relatively homogeneous. In particular, note the similarity in the visualization and plotting of the initial dip compared to that in which stim+control trials were collected (compare to Fig. 2). No significant difference in the average signal time course was found between stim+control versus stim only when collapsing across all trial time points (repeated measures ANOVA, F(1,17)=3.73, p=0.07), nor was a significant interaction with trial time point obtained (repeated measures ANOVA, F(26,442)=0.77, p=0.79). (B–D) Means and standard errors are plotted to illustrate that no significant difference was found between stim+control versus stim only for the average pre-stimulus FC value (B; two-tailed two-sample t(18) = 1.88, p = 0.077), nor the peak magnitude (C; two-tailed two-sample t(18) = 0.13, p = 0.898) or areal extent (D; two-tailed two-sample t(18) = −0.27, p = 0.788) of the initial dip.
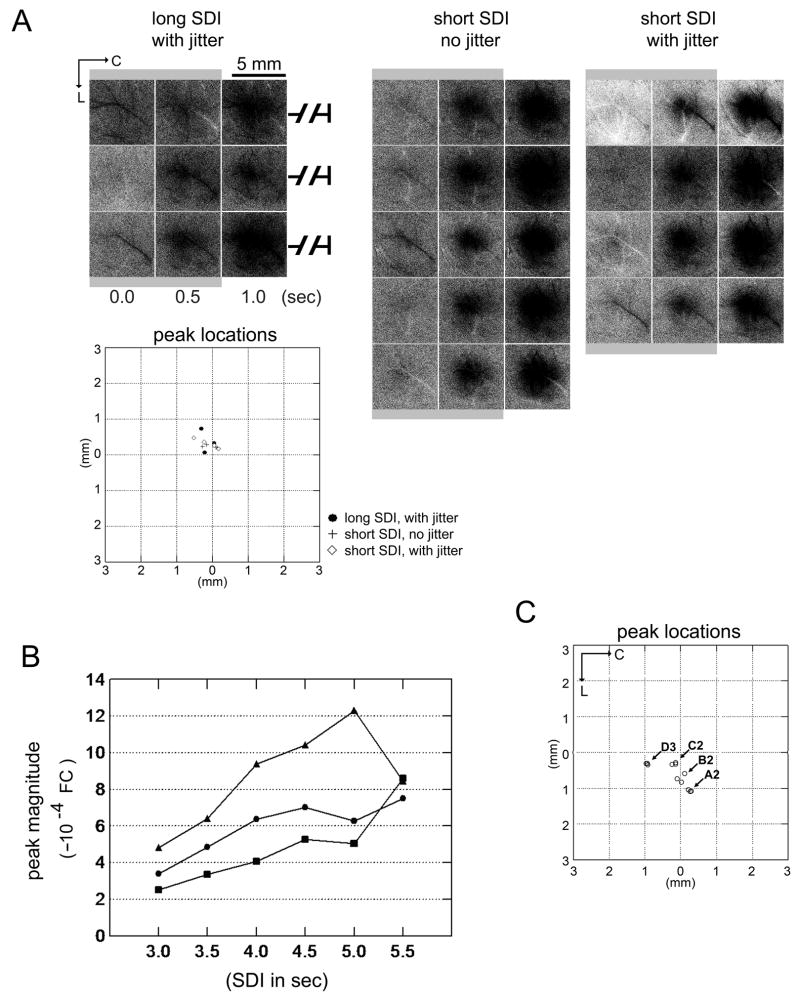
In additional experiments, we began to tackle the following questions regarding the use of short SDIs for ISOI mapping of brain function: 1) is the increase in the initial dip peak magnitude and areal extent observed for the short SDI due to a lack of temporal jittering?; 2) how does the response amplitude change across a range of short SDIs?; and 3) can a short SDI be used to successfully fulfill the mapping needs of an imaging study? Regarding the first question, within the same rat (n = 3) mapping results were obtained from collecting trials using three SDIs: short SDI with jitter (Table 1, Column 5); short SDI no jitter (Table 1, Column 2); and long SDI with jitter (Table 1, Column 3). Results from a representative case are provided in Fig. 8A. While the mapping results exhibited some degree of variability throughout the experiment day for all three SDIs, compared to using a long SDI with jitter (Fig. 8A, left column) similar if not stronger mapping results were obtained when using a short SDI irrespective of whether the short SDI was jittered (Fig. 8A, right column) or not (Fig. 8A, middle column): peak magnitude means±SEs were 5.54±0.48 (long SDI), 8.7±0.42 (short SDI no jitter), and 8.35±1.04 (short SDI with jitter) × 10−4 FC, and the areal extent means±SEs were 7.51±2.15, 10.33±1.85, and 8.26±2.30 mm2 respectively. More importantly, no apparent difference in mapping was observed between trials with jittering or no jittering of the short SDI (Fig. 8A, right versus middle column, respectively; graph). Regarding the second question, within the same rat (n = 3) mapping results were obtained using six different SDIs (no jitter): 3.0, 3.5, 4.0, 4.5, 5.0, and 5.5 sec. As illustrated in Fig. 8B, across the 3 rats the peak magnitude was observed to increase as the SDI increased from 3.0 to 4.5 sec, with the trend becoming less predictable across the SDI range of 5.0–5.5 sec (increased contribution from large surface blood vessels also seemed to increase with increasing SDI). Regarding the third question, within the same rat (n = 1) mapping results using a short SDI of 4 sec (no jitter) were obtained from individually stimulating four different whiskers (A2, B2, C2, and D3). As illustrated in Fig. 8C, the relative locations of peak responses evoked by the stimulated whiskers were consistent with the known anatomical topography for those whiskers, indicating that the use of a short SDI can successfully fulfill the mapping needs of an imaging study such as providing the topographical map of barrel cortex.
Fig. 8.
Activity maps using a short SDI: temporal jittering, response magnitude versus SDI, and topographical mapping. (A) Temporal jittering of the 4-sec SDI. Within the same rat, multiple trial blocks were collected for each of three SDIs: long SDI with jitter (Table 1, Column 3); short SDI no jitter (Table 1, Column 2); short SDI with jitter (Table 1, Column 5). Post-stimulus images of evoked initial dip from a representative rat are provided here for each trial block (stimulus delivery indicated with a gray background bar) along with a plot of their peak locations. Compared to using a long SDI with jitter (left column), the initial dip for using a short SDI (middle and right columns) was readily observed to be comparable if not stronger whether there was jittering (right column) or not (middle column). In addition, for the short SDI, no apparent difference was observed whether jitter (right column) or no jitter (middle column) was used (see plot). (B) Response magnitude as a function of SDI. Three trial blocks were collected and summed together for each of six SDIs (no jitter): 3.0, 3.5, 4.0, 4.5, 5.0, and 5.5 sec within each of 3 rats. The initial dip peak magnitude was then determined and plotted as a function of SDI. An increase in magnitude was observed with increasing SDI in the range of 3.0–4.5 sec across all rats, with the trend less predictable for the SDI range of 5.0–5.5 sec. (C) Topographical mapping of activity evoked by individually stimulating several whiskers. Within the same rat, three trial blocks were collected for each of four whiskers: A2, B2, C2, and D2. All whiskers were individually stimulated in the same manner as described in the Materials and Methods section. Initial dip peak location was determined for each trial block and plotted. Note that the peak locations clustered according to whisker type and that the relative locations of the peak location clusters were consistent with the known anatomical topography of barrel cortex. Additional experiment details: Imaging was conducted as described in the Materials and Methods section except a 16-bit CCD camera (Cascade 512B II; Photometrics, Tucson, AZ) was used. Each trial block consisted of 64 trials in order to increase the total number of trial blocks collected per animal. We previously found that imaging results were comparable between 64 and 128 trials (Chen-Bee et al., 2007) and thus data analysis was performed on each 64-trial block for panels A and C. Care was given to collect different types of trial blocks in random order throughout the experiment day. Visualization and quantification was achieved as described in the Materials and Methods section.
Discussion
Our findings indicate that the activity profile evoked by multiple stimuli delivered at short intervals (4 sec apart) is different from the triphasic signal associated with a single stimulus delivery, consisting of multiple signal dips occurring at the same frequency as the stimulus deliveries (Fig. 4). These successive signal dips occur despite the fact that they coincide with a strong rise in signal beginning with the second stimulus delivery (Figs. 3–5). They can be effectively visualized as dark activity areas as long as images are generated using a baseline temporally local to each stimulus delivery (Figs. 3B and 5), which in principle is analogous to how ISOI initial dip visualization is achieved when a single stimulus delivery is used (Figs. 2 and 6). Trial collection using a short SDI is explicitly confirmed as a viable option for mapping of ISOI initial dip. It can be successfully used to target the appropriate anatomical location (Fig. 7G), and to map the peak magnitude (Fig. 7E) and areal extent (Fig. 7F). Mapping using a short SDI offers the advantage of substantially increasing data collection efficiency (> 10×). Furthermore, for the same number of collected trials (128), the mapping results exhibit an increased peak magnitude and areal extent (Fig. 7C, E–F), thus offering the opportunity to reduce the number of trials needed for effective brain mapping and thereby further increasing efficiency. Indeed, the present study provides several examples of successful mapping achieved by reducing the total number of collected trials by half (down to 64 trials) while using an SDI of 4 sec (a mere five minutes to complete) to address such questions as the effect of temporal jittering of the SDI (Fig. 8A), the response curve as a function of SDI interval (Fig. 8B), and the topographical activity map of several whiskers (Fig. 8C). Lastly, from a behavioral perspective, the use of a short SDI offers progress towards employing a regimen of stimulus deliveries more akin to stimulation experienced by awake and behaving subjects.
Evoking and imaging of ISOI initial dip is possible when using a short SDI under a condition as adverse as coinciding stimulus delivery with a strong rise in signal (Figs. 3B, 4, and 5). To effectively visualize evoked ISOI initial dip as a dark activity area, however, requires the use of a baseline frame captured immediately prior to stimulus onset (e.g., baseline 2, 3 or 4) rather than a more temporally remote baseline (e.g., baseline 1; Fig. 3B), analogous to when a long SDI is used. It should be noted that the same post-stimulus data can provide different information (attenuation in signal rise visualized as a white activity area versus successful evoking of a signal dip visualized as a dark activity area that coincides with a signal rise) depending on the baseline frame used for visualization (baseline 1 versus baseline 2, 3, or 4; Fig. 3B). The effective visualization of evoked ISOI initial dip despite such an adverse condition bodes well for ISOI mapping of brain function; if an ISOI initial dip can be successfully evoked and mapped when it coincides with a rise in signal, then it should also be the case when an ISOI initial dip is evoked during spontaneous fluctuations in activity known to occur throughout the course of an experiment ((Mayhew et al., 1996); (Ferezou et al., 2006); (Chen-Bee et al., 2007)), as long as baseline used for analysis is captured immediately prior to stimulus onset. It remains to be determined whether SDIs other than 4 sec can provide comparable mapping of brain activity.
Successful ISOI mapping of brain activity does not depend on the periodic nature of the short SDI. As illustrated in Fig. 8, comparable results are obtained irrespective of whether or not the short SDI is jittered. At first glance, these results appear contradictory to results of Dale, Bandettini, and their colleagues (Dale and Buckner, 1997); (Bandettini and Cox, 2000) using BOLD fMRI, albeit for the BOLD fMRI overshoot signal phase. As with ISOI, BOLD fMRI is a hemodynamic-based imaging technique that can be used to map brain function. Their collective results indicate SDI jitter is required for successful mapping and it is argued that jittering is a means to overcome complications arising from overlapping of consecutive overshoot signals. More specifically, because the overlap of BOLD fMRI overshoot signals increases with decreasing SDI duration, jittering is necessary for SDIs lasting just a few seconds but becomes less critical with longer SDIs (Dale, 1999); (Bandettini and Cox, 2000); see also Chapter 9 by (Huettel et al., 2008) on issues related to SDI jittering for successful BOLD fMRI detection and estimation of overshoot signal). Our results (Fig. 8) can be explained along this line of reasoning by taking into consideration that the ISOI initial dip is under investigation here. Specifically, compared to the overshoot, the ISOI initial dip terminates several seconds sooner (Fig. 2) and thus to avoid overlap of consecutive signals should not require as long of an SDI. In other words, an SDI of 4 sec is sufficiently long and thus jittering is no longer necessary because overlap of ISOI initial dip signals has already been avoided. For mapping of specifically the ISOI initial dip, it would be interesting to see to what extent the SDI needs to be shortened in order to necessitate jittering.
Our similarity in results irrespective of whether the short SDI is jittered (Fig. 8) offers two other implications. First, it specifically confirms that a short SDI can be implemented in a manner more comparable to a long SDI (where jittering is commonly employed). Second, the greater peak magnitude and areal extent of ISOI initial dip signal observed when using a short SDI (Fig. 7B–C, E–F) cannot be easily explained as a strengthening of evoked activity induced by the periodic evoking of signals because comparable results are obtained with jittering (Fig. 8). Thus, for mapping of specifically the ISOI initial dip, unexpected strengthening of brain activity need not be a concern when the short SDI is not jittered. If not due to the periodicity of evoked signals, then it remains to be determined how to explain the observed increase in ISOI initial dip peak magnitude and areal extent. One possibility is that the increase is simply indicative of the brain’s increased response to an increased degree of stimulation (as achieved with an increased density of stimulus deliveries per unit time), analogous to increasing magnitude and areal extent of a single whisker functional representation with increasing stimulus amplitude (Petersen, 2003). Another possibility is that the increase is in some way benefitting from an elevated level of oxygenated blood present during stimulus deliveries, as suggested by the rise in signal prior to each delivery of stim 2–4 as compared to stim 1 (Figs. 3B and 4). Future research is needed to understand the underlying neuronal and hemodynamic mechanisms contributing to our observed increase in ISOI initial dip signal when using short SDI, as well as how the ISOI initial dip properties may change with changes in underlying mechanisms across a range of short SDIs. Nevertheless, such future elucidation need not preclude the use of a short SDI as a viable option for ISOI mapping of brain function.
Finally, the increase in ISOI initial dip peak magnitude (Fig. 7E) and areal extent (Fig. 7F) indicates that these activity attributes are dependent on the interval of the SDI, but may not be the case for location of peak activity (Fig. 7G). Based on a small sample size of 3 rats, it would appear that the signal strength increases as the short SDI increases from 3.0–4.5 sec (results were less predictable for 5.0–5.5 sec; Fig. 8B). Further research is needed to determine whether this trend is upheld. Therefore, accounting for the exact interval of the short SDI may be less critical when using ISOI mapping for localization purposes (i.e., targeting of peak or overall activity location). The short SDI should be taken into account, however, when detailed mapping of activity involves direct comparison in peak magnitude and/or areal extent, especially as the underlying mechanisms of the imaged signal have the potential to differ in their dynamics. Interestingly, when evoked signals are sufficiently far apart, no difference is observed in peak magnitude or areal extent of the ISOI initial dip even when the interval between evoked signals is reduced by half (Fig. 6B). Thus, beyond some maximum value, the peak magnitude and areal extent of the ISOI initial dip is likely no longer dependent on the SDI interval.
General Implications
In the present study, the reliable evoking of signal dips in series at 4 sec intervals is explicitly confirmed, as well as the collecting of trials using a short SDI for successful ISOI mapping of brain function with substantially faster acquisition time. A Fourier-based approach is another means for achieving faster acquisition time, which improves the signal-to-noise ratio by effectively filtering out biological noise, and is optimal for imaging cortical functional systems that are innately cyclic and continuous such as orientation and ocular dominance columns but is less suitable for non-cyclic and non-continuous systems such as whisker representations or more complex and abstract systems represented in higher cortical areas (see Kalatsky and Stryker, 2003, recently reviewed by Kalatsky, 2009). Needless to say, deciding on the interval of the SDI for a particular ISOI study requires careful consideration of the many parameters relevant to that study (e.g., type and duration of stimulus delivery; animal model; spatiotemporal properties of signal evoked by a single stimulus delivery; signal phase of interest; data analysis algorithms; etc.). Our results suggest, however, that irrespective of the parameter space, shorter SDIs (not necessarily 4 sec) are an option worth considering for ISOI mapping of brain function in vivo, even at the risk of overlapping consecutively evoked signals to such a degree as coinciding stimulus delivery with a segment of the previously evoked signal that is strong and in the opposite direction as the signal of interest. Of course, the SDI can be shortened to the point that it prevents the simultaneous imaging of multiple ISOI signal phases and thus would not be useful for studies interested in characterizing the entire ISOI triphasic signal. Effective visualization of evoked signal – especially when the signal of interest is a transient change occurring on top of a larger signal – requires the use of a baseline that is temporally local to stimulus delivery (e.g., immediately prior to stimulus onset). The use of a more temporally remote baseline, however, can also provide useful information such as confirming whether a change in signal is present during stimulus deliveries. Determination of the optimal SDI should begin with the characterization of the entire evoked signal in response to a single stimulus delivery using a long SDI (as is the case in the present study). Depending on the total duration of the evoked signal of interest, the optimal SDI may require jittering. When multiple SDIs are being considered, standardizing the SDI may be less relevant when mapping is performed for localization purposes, but becomes more critical when shorter SDIs are used for characterizing peak magnitude and areal extent of maps. The increased peak magnitude and areal extent observed in the present study introduces the possibility that short SDIs might offer some benefit for BOLD fMRI mapping of its initial dip (Menon et al., 1995).
In conclusion, signal dips can be reliably evoked in a series at 4 sec intervals and trials can be collected using a 4-sec SDI for successful ISOI mapping of brain function, providing a substantial improvement in data collection efficiency and thus offering the opportunity to dramatically increase the number of research questions that can be addressed within the same imaging experiment (case in point, our mapping of activity evoked by individually stimulating four different whiskers or using six different SDIs within the same rat).
Acknowledgments
We thank S. Soo and C. Wah for assistance with data analysis, and M. Davis for proofreading a previous version of the manuscript. This work was supported by the NIH-NINDS NS-43165, NS-48350, and NS-055832.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini PA, Cox RW. Event-related fMRI contrast when using constant interstimulus interval: theory and experiment. Magn Reson Med. 2000;43:540–548. doi: 10.1002/(sici)1522-2594(200004)43:4<540::aid-mrm8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Brett-Green BA, Chen-Bee CH, Frostig RD. Comparing the functional representations of central and border whiskers in rat primary somatosensory cortex. J Neurosci. 2001;21:9944–9954. doi: 10.1523/JNEUROSCI.21-24-09944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bee CH, Agoncillo T, Xiong Y, Frostig RD. The triphasic intrinsic signal: implications for functional imaging. J Neurosci. 2007;27:4572–4586. doi: 10.1523/JNEUROSCI.0326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bee CH, Kwon MC, Masino SA, Frostig RD. Areal extent quantification of functional representations using intrinsic signal optical imaging. J Neurosci Methods. 1996;68:27–37. [PubMed] [Google Scholar]
- Chen-Bee CH, Polley DB, Brett-Green B, Prakash N, Kwon MC, Frostig RD. Visualizing and quantifying evoked cortical activity assessed with intrinsic signal imaging. J Neurosci Methods. 2000;97:157–173. doi: 10.1016/s0165-0270(00)00180-1. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective aceraging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Chen-Bee CH. Visualizing adult cortical plasticity using intrinsic signal optical imaging. In: Frostig RD, editor. In vivo optical imaging of brain function. 2. CRC Press; Boca Raton: 2009. [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig RD, Xiong Y, Chen-Bee CH, Kvasnak E, Stehberg J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci. 2008;28:13274–13284. doi: 10.1523/JNEUROSCI.4074-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCartny G. Functional magnetic resonance imaging. 2. Sinauer Associates; Sunderland, Massachusetts: 2008. [Google Scholar]
- Jensen KF, Killackey HP. Terminal arbors of axons projecting to the somatosensory cortex of the adult rat. II. The altered morphology of thalamocortical afferents following neonatal infraorbital nerve cut. Journal of Neuroscience. 1987;7:3544–3553. doi: 10.1523/JNEUROSCI.07-11-03544.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatsky VA. Fourier approach to optical imaging. In: Frostig RD, editor. In vivo optical imaging of brain function. CRC Press; Boca Raton: 2009. [Google Scholar]
- Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- Land PW, Simons DJ. Cytochrome oxidase staining in the rat SmI barrel cortex. Journal of Comparative Neurology. 1985;238:225–235. doi: 10.1002/cne.902380209. [DOI] [PubMed] [Google Scholar]
- Masino SA, Kwon MC, Dory Y, Frostig RD. Characterization of functional organization within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc Natl Acad Sci U S A. 1993;90:9998–10002. doi: 10.1073/pnas.90.21.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew JE, Askew S, Zheng Y, Porrill J, Westby GW, Redgrave P, Rector DM, Harper RM. Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. Neuroimage. 1996;4:183–193. doi: 10.1006/nimg.1996.0069. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JP, Anderson P, Ugurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Petersen CC. The barrel cortex--integrating molecular, cellular and systems physiology. Pflugers Arch. 2003;447:126–134. doi: 10.1007/s00424-003-1167-z. [DOI] [PubMed] [Google Scholar]
- Ts’o DY, Frostig RD, Lieke EE, Grinvald A. Functional organization of primate visual cortex revealed by high resolution optical imaging. Science. 1990;249:417–420. doi: 10.1126/science.2165630. [DOI] [PubMed] [Google Scholar]