Abstract
CCN1 (CYR61) is a matricellular protein that is highly expressed at sites of inflammation and wound repair. In these contexts, CCN1 can modify the activities of specific cytokines, enabling TNFα to be cytotoxic without blocking NFκB activity and enhancing the apoptotic activity of FasL and TRAIL. Here we show that CCN1 supports the adhesion of macrophages through integrin αMβ2 and syndecan-4, activates NFκB-mediated transcription, and induces a pro-inflammatory genetic program characteristic of classically activated M1 macrophages that participates in Th1 responses. The effects of CCN1 include upregulation of cytokines (TNFα, IL-1α, IL-1β, IL-6, IL-12b), chemokines (MIP-1α, MCP-3, Gro1, Gro2, IP-10), regulators of oxidative stress and complement (iNOS, C3), and downregulation of specific receptors (TLR4, IL-10rβ) and anti-inflammatory factors (TGF-β1). CCN1 regulates this genetic program through at least two distinct mechanisms: an immediate-early response resulting from direct activation of NFκB by CCN1, leading to the synthesis of cytokines including TNFα and IP-10; and a delayed response resulting from CCN1-induced TNFα, which acts as an autocrine/paracrine mediator to activate the expression of other cytokines including IL-1β and IL-6. These results identify CCN1 as a novel component of the extracellular matrix that activates pro-inflammatory genes in macrophages, implicating its role in regulating macrophage function during inflammation.
INTRODUCTION
Although the extracellular matrix (ECM) was classically viewed as an inert scaffold onto which cells are organized into organs and tissues, studies in recent decades have established the ECM as dynamic structures that can profoundly influence diverse aspects of cellular behavior and function (1). Aside from providing signals that regulate cell adhesion, shape, migration, differentiation, proliferation and survival in parenchymal cells, the ECM also affects the functions of inflammatory cells that infiltrate the parenchyma. Recent studies have recognized that fragments of ECM proteins generated due to tissue injury and inflammation, and dynamic changes in the ECM in these contexts, can significantly regulate the inflammatory response (2,3). Normally soluble matrix proteins from the blood plasma, including fibrinogen, plasma fibronectin, and vitronectin enter inflamed tissues as a result of vessel damage and become immobilized at sites of injury. Fragments of these and other ECM proteins, generated as products of elevated levels of matrix degradative enzymes, have been shown to induce chemotaxis for inflammatory cells, enhance phagocytic functions, stimulate immune responses, and induce gene expression changes (2,3). Induced expression of matricellular proteins, a group of matrix-associated signaling proteins that play diverse regulatory roles, also contribute to dynamic changes in the ECM at sites of inflammation and injury repair (4). Such matricellular proteins as osteopontin and thrombospondin are known to play regulatory roles in inflammation (5-8).
CCN1 (also named CYR61) is an angiogenic matricellular protein that is essential for cardiovascular development (9-11). Correspondingly, Ccn1-null mice are embryonic lethal and suffer from atrioventricular septal defects and vascular hemorrhage. In the adult, CCN1 is normally expressed at a low level in most tissues but becomes highly expressed as a result of bacterial or viral infections (12-15), or in tissue repair (16-21). Consistent with a role in injury repair, CCN1 regulates the expression of genes involved in wound healing in fibroblasts (19). Recent studies have shown that CCN1 cooperates with and modulates the activities of several inflammatory cytokines, including TNFα, FasL, and TRAIL, further indicating that CCN1 can play a critical role in the inflammatory response (22-24).
In this study, we show that CCN1 supports macrophage adhesion through integrin αMβ2 and the cell surface heparan sulfate proteoglycan (HSPG) syndecan-4 as coreceptors. Furthermore, CCN1 activates NFκB signaling in macrophages, leading to the expression of multiple pro-inflammatory cytokines and chemokines characteristic of classically activated M1 macrophages. These results reinforce the notion that the ECM microenvironment can play critical roles in inflammation, and highlight CCN1 as a novel player in regulating the inflammatory response of macrophages through integrin signaling.
MATERIALS AND METHODS
Cell Culture, Proteins, Antibodies, and Reagents
The non-virally immortalized splenic macrophage cell line I-13.35 (CRL-2471, ATCC) originated from the tlr4 mutant strain C3H/HeJ (25). These cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) with 1.0 mM sodium pyruvate, 10% fetal bovine serum (Intergen, Purchase, NY), and 20% LADMAC Conditioned media (CSF-1) at 37 °C with 10% CO2. The RAW 264.7 cell line, established from a tumor induced by Abelson murine leukemia virus, was cultured in DMEM containing 10% FBS at 37 °C with 5% CO2. Both cell lines were obtained from the American Type Culture Collection. Wild-type CCN1 protein was produced in a baculovirus expression system using Sf9 cells and purified from serum-free insect cell conditioned medium on Sepharose-S columns as described (26). Fibronectin, bovine serum albumin, heparin (sodium salt, from porcine intestinal mucosa), lipopolysaccharides (LPS, from E. coli 026:B6) and anisomycin were from Sigma. Biotin-11-UTP was obtained from Perkin Elmer (Waltham, MA). Chemical inhibitors U0126, SB203580, BAY11-7082, and IKK inhibitor X were from Calbiochem Corp. (San Diego, CA). Anti-CCN1 polyclonal antibodies were raised in rabbits using GST fusion proteins linked to domain II (vWC domain) of CCN1 as immunogen, and affinity-purified from a column containing GST-vWC protein cross-linked to CNBr-activated sepharose (27). mAbs recognizing mouse total and phospho (Ser536)-NFκB p65 were from Cell Signaling Technology Inc. (Danvers, MA), and function-inhibiting mAbs against integrin αM (clone M1/70) was from Abcam Inc. (Cambridge, MA), integrin αL (clone M17/4) from eBioscience (San Diego, CA), integrin α6 (clone GoH3) and integrin β2 (clone P4H9-A11) from Millipore (Billerica, MA), and TNF-R1 (clone 55R-170) from R&D Systems. Polyclonal antibodies against syndecan-4 were from Santa Cruz and antibodies neutralizing TNFα were from R&D Systems. Anisomycin (Sigma Aldrich) caused a >98% inhibition of S35-methionine labeling in our experimental conditions (data not shown), showing its effectiveness.
Adhesion assay
Adhesion assays were performed as described previously (28). Briefly, I-13.35 cells were harvested in phosphate-buffered saline by scraping and resuspended in serum-free DMEM containing 1% BSA. The protein under study was diluted to the desired concentration in phosphate-buffered saline, applied to 96-well microtiter plates (50 μl/well), incubated at 4 °C for 16 h, and blocked with 1% BSA at room temperature for 1 h. Where indicated, reagents including EDTA (2.5 mM), Ca2+ (5 mM), or heparin (1 μg/ml) were mixed with cells prior to plating. GRGDSP or GRGESP peptides (2 mM) were incubated with cells at room temperature for 30 minutes, while antibody against integrin αM, αL, α6, β2, or syndecan-4 (50 μg/ml each) for 1 hr with normal IgG as negative control before plating. To each well 5×105 macrophages were plated, and after incubation at 37°C for 15 min, wells were washed twice with PBS. Adherent cells were fixed with 10% formalin, stained with methylene blue, and quantified by dye extraction and measurement of absorbance at 620 nm.
Microarray Hybridization
The Oligo GEArray Mouse Inflammatory Cytokines and Receptors Microarray (Cat. Number OMM-011) was purchased from SuperArray Bioscience Co. (Frederick, MD). Mouse I-13.35 macrophages grown to near confluence were made quiescent by starvation in low serum (2% FBS) overnight. Cells were then washed and incubated in serum-free DMEM containing 1% BSA, and stimulated with purified recombinant CCN1 (10 μg/ml) for 6 h or as otherwise indicated. Total RNA isolation, cRNA synthesis, biotinylated-UTP probe labeling, array hybridization, and chemiluminescent detection using alkaline phosphatase conjugated with streptavidin were all performed following standard protocols. The intensity of hybridization signals was quantified by PhosphorImager and normalized against internal controls (GAPDH) on the same blot. Microarray data are available from the National Center for Biotechnology Information Gene Expression Omnibus under accession no. XXXXX.
β2-integrin siRNA knockdown
The siRNA against mouse integrin β2 (ACCGUGGUAGGUGUCGUACUGAUUG) was purchased from Invitrogen. Transfection was done using Lipofectamine RNAiMAX reagent (Invitrogen) with 10 nM siRNA following manufacturer’s protocol. Cells were used for experiments 48 hrs after transfection.
Real-time and semi-quantitative RT-PCR
I-13.35 and RAW 264.7 cells were starved as described above and treated as indicated, and total RNA was extracted using RNeasy Kit (Qiagen). cDNA was generated from 0.5 μg total RNA using an iScript™ cDNA Synthesis Kit. Reaction product was mixed with iQ™ SYBR Green Supermix, and real-time PCR reaction was carried out, detected, and analyzed using the iQ5 Real-Time PCR Detection System according to manufacturer’s instructions (Bio-Rad, CA). The level of target gene expression was normalized against the internal control cyclophilin and expressed as relative mRNA levels. Primer sequence and annealing temperature (Ta) were listed in Table I.
| Gene | qPCR Sense Primer | qPCR Antisense Primer | Ta (°C) |
|---|---|---|---|
| TNFα | TCT CAT GCA CCA CCA TCA AGG ACT | ACC ACT CTC CCT TTG CAG AAC TCA | 60 |
| IL-1α | ACG GCT GAG TTT CAG TGA GAC CTT | AGG TGT AAG GTG CTG ATC TGG GTT | 60 |
| IL-1β | AAG GGC TGC TTC CAA ACC TTT GAC | ATA CTG CCT GCC TGA AGC TCT TGT | 60 |
| IL-6 | ATC CAG TTG CCT TCT TGG GAC TGA | TAA GCC TCC GAC TTG TGA AGT GGT | 60 |
| MIP-1α | ACT GAC CTG GAA CTG AAT GCC TGA | ATG TGG CTA CTT GGC AGC AAA CAG | 60 |
| MCP-3 | TCT GTG CTG AAG CCC ATC AGA AGT | TTG CTT CTT GGC TCC TAG GTT GGT | 60 |
| Gro1 | TGG CTG GGA TTC ACC TCA AGA ACA | TGT GGC TAT GAC TTC GGT TTG GGT | 60 |
| Gro2 | ACA TCC CAC CCA CAC AGT GAA AGA | ACT CCT TCC ATG AAA GCC ATC CGA | 60 |
| IP-10 | TGG CTA GTC CTA ATT GCC CTT GGT | TCA GGA CCA TGG CTT GAC CAT CAT | 60 |
| iNOS | TCT TTG ACG CTC GGA ACT GTA GCA | ACC TGA TGT TGC CAT TGT TGG TGG | 60 |
| C3 | TGT TGT GAG GAT GGT ATG CGG GAT | TTC TGT GTT GTT CAC GCA GCT TGG | 60 |
| IL-10 | TGC ACT ACC AAA GCC ACA AAG CAG | AGT AAG AGC AGG CAG CAT AGC AGT | 60 |
| IL10rβ | CTT TCC AAG ATC ACT GCA AGC GCA | AAT GTT CAT CCG CCA ATT CAG CCC | 60 |
| IL-12b | GCT GTC TTC TGC TTG GTT GGC TTT | CTG GCT CTG CGG GCA TTT AAC ATT | 60 |
| TGFβ1 | TAA AGA GGT CAC CCG CGT GCT AAT | ACT GCT TCC CGA ATG TCT GAC GTA | 60 |
| TLR4 | AAC CAG CTG TAT TCC CTC AGC ACT | ACT GCT TCT GTT CCT TGA CCC ACT | 60 |
| Cyclophilin | GGC AAA TGC TGG ACC AAA CAC | TTC CTG GAC CCA AAA CGC TC | 60 |
For semi-quantitative RT-PCR, cDNA was synthesized from 1 μg total RNA using an M-MLV Reverse Transcriptase Kit (Promega, WI), and PCR was performed using a Taq DNA Polymerase Kit from Takara (Shiga, Japan). Primer sequence and annealing temperatures are listed in Table II. Pilot experiments were performed to determine appropriate number of PCR cycles so that all experiments were done in the exponential phase. Reaction products were resolved on 1% agarose gels, stained with ethidium bromide, and visualized under UV light. β-actin mRNA level was used as a loading control.
| Gene | RT-PCR sense Primer | RT-PCR Antisense Primer | Ta (°C) |
|---|---|---|---|
| TNFα | CAG CCT CTT CTC ATT CCT GCT TGT G | CTG GAA GAC TCC TCC CAG GTA TAT | 55 |
| IL-1α | GAC TGC CCT CTA TGA CAG ACT TC | GGA GTA AAA CCC ACT GAG GTA GG | 52 |
| IL-1β | CAC TAC AGG CTC CGA GAT GAA C | CTC CTA GAG ATT GAG CTG TCT GC | 52 |
| IL-6 | GTT CCT CTC TGC AAG AGA CTT CC | CTT AGC CAC TCC TTC TGT GAC TC | 52 |
| MIP-1α | GAC ACT CTG CAA CCA AGT CTT CTC | GGA ACG TGT CCT GAA GTC TTT CAG | 52 |
| MCP-3 | CCC AAT GCA TCC ACA TGC TGC TAT | AGA CCA TTC CTT AGG CGT GAC TGT | 60 |
| Gro1 | ACC CAA ACC GAA GTC ATA GCC ACA | AAT GTC CAA GGG AAG CGT CAA CAC | 52 |
| Gro2 | CTA ACT GAC CTG GAA AGG AGG AG | TGT TCT ACT CTC CTC GGT GCT TAC | 52 |
| IP-10 | CAG TGA GAA TGA GGG CCA TAG G | CTT AGA ACT GAC GAG CCT GAG CTA | 52 |
| iNOS | CTG CTG GTG GTG ACA AGC ACA TTT | CGT TCT TTG CAT GGA TGC TGC TGA | 60 |
| C3 | CCA AGC TGC GTG AAC AAC ACA GAA | TAT GCA ACA GTT CCA CCC TCA CCT | 60 |
| IL10rβ | AGT GAA CCC ATC TGT GAA CGG ACA | TTC TGA GGT CGA CGT GAG CAG TTT | 60 |
| IL-12b | ACC TGT GAC ACG CCT GAA GAA GAT | ATT CCC GCC TTT GCA TTG GAC TTC | 60 |
| TGFβ1 | TAA AGA GGT CAC CCG CGT GCT AAT | TGT ACT GTG TGT CCA GGC TCC AAA | 60 |
| IFNγ | AGT TTG AGG TCA ACA ACC CAC AGG | GGA CAG CCT GTT ACT ACC TGA CTC | 52 |
| TLR4 | TAG AAG AGC TGC AGC ACC TGG ATT | ACT GCT TCT GTT CCT TGA CCC ACT | 60 |
| β-actin | GAC TAC CTC ATG AAG ATC CTG ACC | CTC AGT AAC AGT CCG CCT AGA AG | 52 |
NFκB-dependent transcription activity assay
Reporter constructs for NFκB activity (pNFκB-Luc) and transfection efficiency (pRL-CMV) were from Promega (Madison, WI), and transfected using Lipofectamine-2000 (Invitrogen Corp., Carlsbad, CA) as described by the vendor. Luciferase activity was assayed using a dual-luciferase reporter assay kit (Promega). Activity of the firefly luciferase, which is encoded by pNFκB-Luc, was normalized against Ranilla luciferase activity, which is encoded by the transfection efficiency control vector pRL-CMV.
ELISA
Measurement of TNFα and IL-6 protein levels in cell lysates and conditioned media was carried out using TNFα and IL-6 ELISA kits (eBioscience) following manufacturer protocols. Conditioned media were collected and cleared by centrifugation at 500 × g, 5 min. at 4°C. Cell lysates were prepared by first rinsing cells with PBS followed by lysis on ice for 15 min. using 0.3 ml/ 106 cells of lysis buffer (50 mM HEPES, pH7.4, 130 mM NaCl, 1% Triton X-100, 1% SDS, 1% Na Deoxycholate, 2 mM EDTA, 2 mM EGTA, 0.1M NaF, 10 mM Na Pyrophosphate, 10 mM β-Glycerolphosphate, 1 mM Na Orthovanadate, 1 mM PMSF, and a protease inhibitor cocktail from Roche).
Peritoneal macrophage isolation
Six normal 12-week old male wild-type or Itgam-null (αM/CD11b knockout) mice of the C57BL/6 background (Jackson laboratory) were housed in a barrier facility, and each was treated with 1 ml 3% Brewer thioglycollate medium by i.p. injection. Four days later mice were euthanized by CO2 asphyxiation, and peritoneal macrophages were isolated according to published protocol with minor modifications (29,30). Briefly, peritoneal cells were flushed out by ice-cold DMEM containing 5% FBS, and macrophages were collected by plating cells on plastic culture dishes at 37°C for 2 hrs followed by washing away the floaters with normal saline. The adherent macrophages were then incubated in serum-free DMEM containing 1 % BSA at 37°C and immediately used for experiments in the same day after isolation.
RESULTS
CCN1 supports macrophage adhesion through integrin αMβ2 and syndecan-4
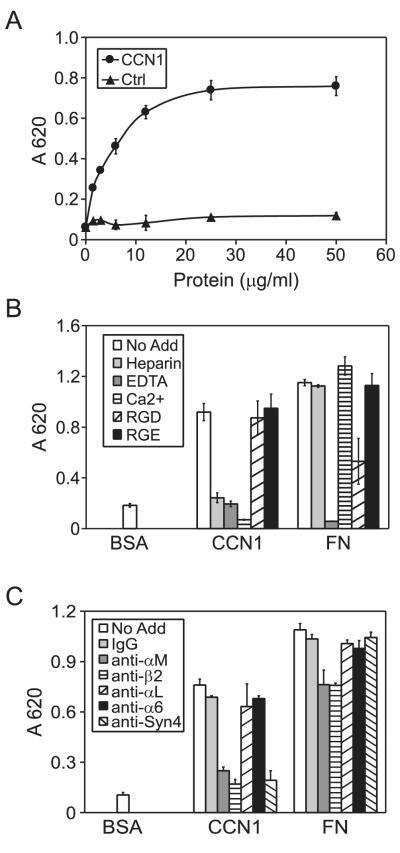
CCN1 supports cell adhesion in a variety of cell types including fibroblasts, endothelial cells, smooth muscle cells, and peripheral blood monocytes (20,21,31). However, its cell adhesive property in differentiated macrophages has not been tested. To help avoid issues with potential LPS contamination in laboratory reagents, we focused most of our studies on I-13.35 mouse macrophages. I-13.35 cells originated from the C3H/HeJ mouse, which carry a missense mutation in the tlr4 gene and express a non-functional LPS receptor TLR-4 (32-34). I-13.35 cells were plated on microtiter wells coated with purified insect cell-produced recombinant CCN1 protein in serum-free medium and incubated for 15 min. As shown in Fig. 1A, macrophage adhesion to CCN1 was dose-dependent and saturable, reaching optimal level when CCN1 was coated at 12.5 μg/ml. The presence of Ca2+ or EDTA completely blocked macrophage adhesion to CCN1, consistent with the notion that CCN1 supports cell adhesion through certain integrins that are sensitive to Ca2+ (Fig. 1B). The RGD-containing peptide had little effect on cell adhesion to CCN1 but inhibited adhesion to fibronectin (FN), indicating that RGD-binding integrins (α5β1, α8β1, and αv- and β3-integrins) are unlikely to be involved (Fig. 1B). Monoclonal antibodies (mAbs) against integrin subunits αM and β2, but not αL or α6, effectively blocked macrophage adhesion to CCN1, indicating that adhesion to CCN1 is mediated through integrin αMβ2, which is prominently expressed in macrophages (Fig. 1C). Since cell surface HSPGs are involved in CCN1-mediated cell adhesion in fibroblasts (31), we tested the role of heparin binding in CCN1-mediated cell adhesion. Soluble heparin added to the cell medium completely blocked macrophages adhesion to CCN1 (Fig. 1B), suggesting that CCN1 interaction with cell surface HSPGs may be indispensable for macrophage adhesion. Since the HSPG syndecan-4 has been found to serve as a coreceptor for CCN1 in some contexts, we tested the potential role of syndecan-4 (22,35). Indeed, antibodies against syndecan-4 effectively blocked adhesion to CCN1 but not to FN, showing that syndecan-4 is specifically required for macrophage adhesion to CCN1 (Fig. 1C).
Figure 1. CCN1 supports macrophage adhesion through integrin αMβ2 and syndecan-4.
A. Microtiter wells were coated with various concentrations of recombinant CCN1 and control protein (Ctrl) as indicated, onto which I-13.35 macrophages were plated in serum-free medium and incubated at 37 °C for 15 min. Adherent cells were stained with methylene blue and quantified by absorbance at 620 nm. B. Cells were incubated with various inhibitors as indicated (EDTA, 2.5 mM; Ca2+, 5 mM; soluble heparin, 1 μg/ml; GRGDSP and GRGESP peptides, 2 mM each) and cell adhesion assay was performed in dishes coated with CCN1 or fibronectin (FN) as adhesion substrates. C. Cells were pre-incubated with mAbs against integrins αM, αL, α6, β2, or antibodies against syndecan-4, or normal mouse IgG (50 μg/ml each) for 1 hr at room temperature, followed by assay for cell adhesion to CCN1 and FN. Data are mean ± S.D. of triplicate determinations, and all experiments were repeated three times with similar results.
CCN1 regulates the expression of pro-inflammatory genes in macrophages
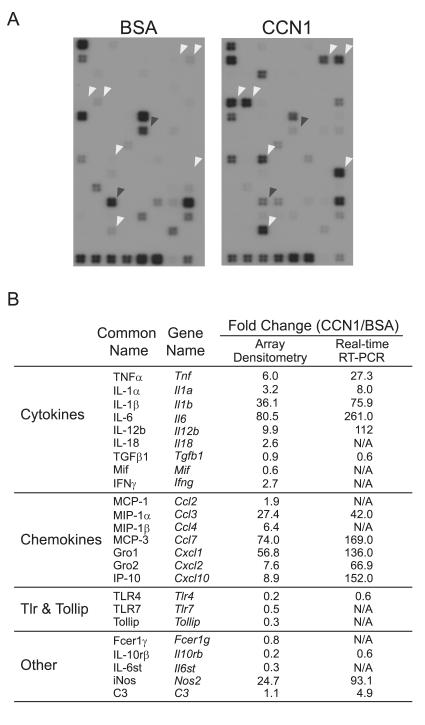
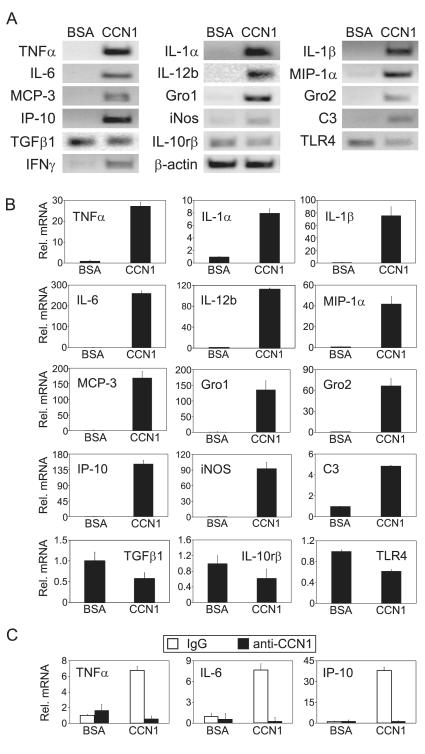
The engagement of integrin αMβ2 is known to regulate gene expression (36-38). To assess whether CCN1 can regulate gene expression in macrophages, I-13.35 cells were treated with BSA or soluble CCN1 protein in serum-free medium for 6 hrs, after which cellular RNAs were collected and used as probes to interrogate a microarray containing 113 key genes involved in the inflammatory response (Fig. 2). A total of 24 genes were identified as significantly regulated by CCN1, including those encoding inflammatory cytokines (TNFα, IL-1α, IL-1β, IL-6, IL-12b, and IFN-γ) and chemokines (MCP-1, MCP-3, MIP-1α, MIP-1β, Gro1, Gro2, and IP-10). Other CCN1-regulated genes encode the anti-inflammatory cytokine TGFβ1, the inducible nitric oxide synthase (iNOS), the complement protein C3, and signaling receptors involved in host defense and inflammation, including TLR4 and TLR7, the TLR-interacting protein (Tollip), the IL-10 receptor β-subunit (IL-10rβ), the IL-6 receptor complex signaling subunit IL6st (also known as gp130), and the high affinity IgE receptor γ (FcεR1γ). To confirm the microarray hybridization results, expression of 15 genes was verified by both semi-quantitative (Fig. 3A) and quantitative real-time RT-PCR (Fig. 3B) analyses. CCN1-regulated gene expression was annihilated by neutralizing anti-CCN1 antibodies (Fig. 3C), indicating that induced gene expression is an activity specific to the CCN1 polypeptide. Many of the genes, including TNFα, IL-1β, IL-6, IL-12b, MIP-1α, MCP-3, Gro1, Gro2, IP-10, and iNOS, were up-regulated by CCN1 by >30 fold compared to BSA-treated controls. Several other genes, such as TLR4, TGF-β1, and IL-10rβ, were down-regulated by ~40%. These CCN1-regulated genes are known to play critical roles in the inflammatory response, suggesting that the presence of CCN1 in the ECM microenvironment may significantly regulate the genetic program for inflammation in macrophages.
Figure 2. Microarray hybridization.
A. Serum-starved I-13.35 macrophages were incubated with 10 μg/ml CCN1 or BSA at 37°C for 6 hr. Total RNA was used to prepare cRNA probes and hybridized to microarrays containing oligodeoxynucleotides corresponding to 113 mouse inflammatory genes. White arrowheads point to representative genes whose expression is elevated in CCN1-treated cells, and black arrowheads point to CCN1 down-regulated genes. B. A list of CCN1 regulated genes. Fold changes were calculated by densitometry scanning of the x-ray films or from results of real-time PCR (see Fig. 3), and was defined as expression levels in CCN1-treated samples divided by BSA-treated controls.
Figure 3. CCN1-regulated inflammatory gene expression.
A. Cells were treated with CCN1 or BSA for 6 hr and mRNA levels of indicated genes were analyzed by semi-quantitative RT-PCR. B. Effects of CCN1 on mRNA levels of indicated genes were analyzed by quantitative real-time PCR. mRNA levels in BSA-treated controls were set as one. C. CCN1 protein (10 μg/ml) was incubated with affinity-purified rabbit anti-CCN1 polyclonal antibodies (anti-CCN1) or normal rabbit IgG (100 μg/ml each) at 4°C overnight before being added to cells and incubated for 3 hrs. The mRNA levels of indicated genes were analyzed by quantitative real-time PCR.
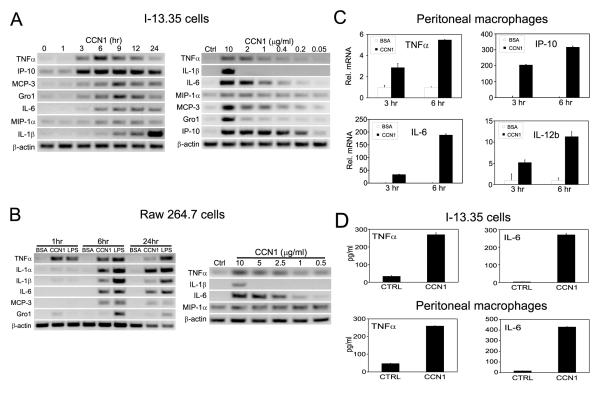
To characterize the regulation of macrophage gene expression by CCN1, we monitored the time course and dose-dependence of the response. Upregulation of pro-inflammatory cytokines and chemokines was noticeable by 3-6 hrs after incubation with CCN1, and sustained for >12 hrs (Fig. 4A, left panel). Among the genes studied, maximal TNFα and IP-10 up-regulation occurred at ~6 hrs, while other genes peaked around or after 9 hrs. IL-1β expression reached the highest level by 24 hrs (Fig. 4A). The upregulation of TNFα, IL-6, MIP-1α, MCP-3 and IP-10 was observed at ~0.4 μg/ml (10 nM) of CCN1, while IL-1β and Gro1 induction required a higher concentration of CCN1 (2-10 μg/ml)(Fig. 4A, right panel). To examine whether the regulation of inflammatory genes by CCN1 occurs in macrophages generally, we tested the effects of CCN1 on gene expression in RAW264.7 cells, another macrophage cell line derived from Abelson murine leukemia virus (A-MuLV)-induced tumors in mice, and in freshly isolated peritoneal macrophages from wild type C57BL/6 mice. CCN1 treatment upregulated the expression of a number of representative cytokines and chemokines in RAW264.7 cells with similar time course and dosage requirement as in I-13.35 cells (Fig. 4B), and increased TNFα, IL-6, IP-10, and IL-12b expression in peritoneal macrophages (Fig. 4C). These results show that CCN1 can regulate inflammatory gene expression in macrophages generally. Next we measured the levels of proteins produced by I-13.35 cells and peritoneal macrophages in response to CCN1 treatment. After incubation with CCN1 for 6 hrs, TNFα protein in I-13.35 cell lysates was elevated by >7 fold (basal: 35 pg/ml, CCN1: 270 pg/ml), and IL-6 in conditioned media increased >65 fold (basal: 4 pg/ml, CCN1: 270 pg/ml) as determined by ELISA (Fig. 4D). In peritoneal macrophage conditioned media, CCN1 increased TNFα by >5 fold (basal: 47 pg/ml, CCN1: 260 pg/ml) and IL-6 by >25 fold (basal: 17 pg/ml, CCN1: 430 pg/ml) (Fig. 4D). These results confirmed that CCN1 increased cytokine production on the protein level.
Figure 4. Time course and dosage dependence of CCN1-regulated inflammatory gene expression.
CCN1 regulated gene expression was tested in two macrophage cell lines: I-13.35 (panels in A), and Raw 264.7 (panels in B). Serum-starved cells were incubated with CCN1 (10 μg/ml) for various times from 0 ~ 24 hr as indicated (left panels in A, B), or cells were treated with various concentrations of CCN1 as indicated for 6 hr, and gene expression was assessed using semi-quantitative RT-PCR. C. Expression of TNFα, IP-10, IL-6, and IL-12b in freshly isolated peritoneal macrophages from wild type C57BL/6 mice treated with BSA or CCN1 for 3 or 6 hrs was assayed by real-time RT-PCR. D. The amount of TNFα and IL-6 proteins detected in I-13.35 cells or conditioned medium, respectively (upper panel) and in conditioned medium of freshly isolated peritoneal macrophages (lower panel) was detected by ELISA.
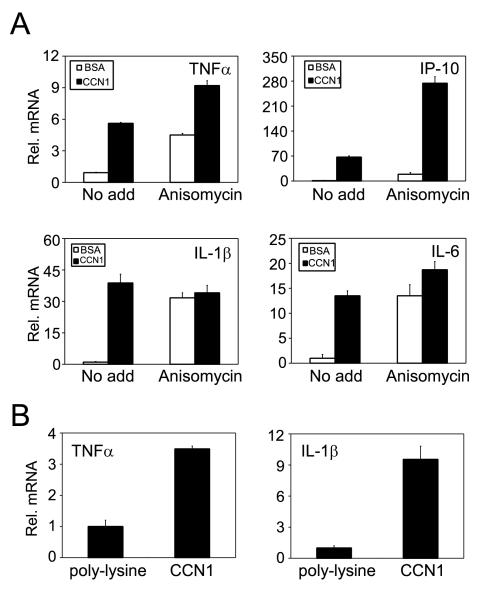
Real-time RT-PCR analysis showed that CCN1 upregulated TNFα and IP-10 expression even in the presence of the protein synthesis inhibitor anisomycin, indicating that this process occurs without requiring de novo protein synthesis (Fig. 5A). By contrast, even though anisomycin alone elevated IL-1β and IL-6 mRNA levels, CCN1 did not cause a further increase, suggesting that de novo protein synthesis is necessary for their upregulation by CCN1 (Fig. 5A). These results indicate that CCN1 can induce TNFα and IP-10 gene expression directly through activation of intracellular signaling, but upregulation of IL-1β and IL-6 may require the synthesis of intermediary protein factors.
Figure 5. Characterization of CCN1-regulated inflammatory gene expression.
A. Cells were incubated with CCN1 protein either with or without anisomycin (10 μM) and treated with BSA or CCN1 for 6 hrs. Relative levels of TNFα, IP-10, IL-1β, and IL-6 mRNA were analyzed by real-time RT-PCR. B. Cells were adhered for 3 hr on culture dishes coated with CCN1 (10 μg/ml) or the inert substrate poly-L-lysine (5 μg/ml). Relative TNFα and IL-1β mRNAs levels were determined by real-time RT-PCR.
Since CCN1 can support macrophage cell adhesion, we tested whether CCN1 can regulate gene expression as a cell adhesion substrate. Thus, we allowed I-13.35 cells to adhere for 3 hrs to surfaces coated with poly-L-lysine or recombinant CCN1 protein in serum-free medium, and tested the expression of TNFα and IL-1β by real-time RT-PCR. Cell adhesion to CCN1 caused a 4-fold higher TNFα expression and a 10-fold higher IL-1β expression when compared to cells plated on the inert substrate poly-L-lysine (Fig. 5B). These results show that CCN1 can regulate gene expression both as a soluble and an immobilized adhesive factor.
CCN1 regulates inflammatory gene expression through integrin αMβ2 and syndecan-4
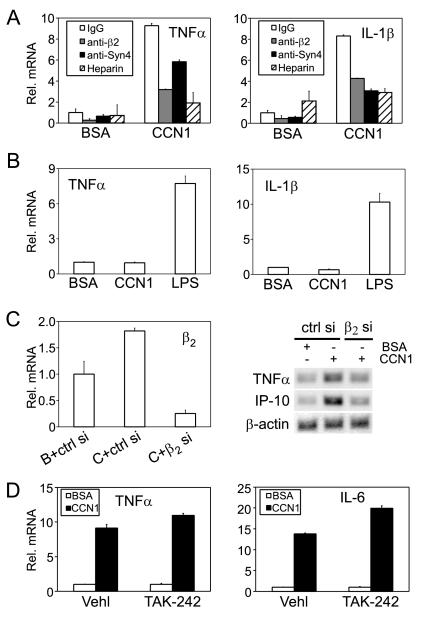
Since CCN1 can act as a macrophage adhesion substrate via αMβ2 and syndecan-4 (Fig. 1), we hypothesized that the same receptors may mediate CCN1 effects on inflammatory gene expression. Pre-incubation of I-13.35 cells with function-blocking mAb against β2-integrin significantly reduced TNFα and IL-1β upregulation by >50% but non-specific murine IgG had no effect, indicating a critical role of β2-integrin in mediating CCN1 regulation of gene expression (Fig. 6A). The addition of soluble heparin in the culture medium effectively inhibited TNFα and IL-1β upregulation by CCN1 (Fig. 6A), consistent with the interpretation that heparin can bind to CCN1 and prevent CCN1 interaction with cell surface HSPGs to regulate gene expression. Indeed, pre-treatment of cells with anti-syndecan-4 antibodies reduced the upregulation of TNFα and IL-1β expression by CCN1 by 35% and 60%, respectively, indicating that syndecan-4 plays an important role in CCN1-regulated gene expression (Fig. 6A). To pinpoint the role of integrin αMβ2 in CCN1 function further, we isolated and examined peritoneal macrophages from αM knockout mice in the C57BL/6 background. These αM-null macrophages were completely refractory to CCN1-induced gene expression (Fig. 6B), whereas peritoneal macrophages from the syngeneic wild type C57BL/6 mice were highly responsive to CCN1 (Fig. 4C), indicating that αM is critical for CCN1-regulated gene expression. Likewise, siRNA against β2 integrin effectively knocked down β2 expression in I-13.35 cells (Fig. 6C, left panel) and resulted in inhibition of CCN1-regulated TNFα and IP-10 expression (Fig. 6C, right panel). Control siRNA, by contrast, had no effect. These results show that CCN regulates macrophage gene expression through integrin αMβ2, and syndecan-4 plays an important role in this process.
Figure 6. Integrin β2 and syndecan-4, but not TLR4, are critical for CCN1-regulated gene expression.
A. I-13.35 cells were pre-incubated for 30 min with an anti-β2 mAb (100 μg/ml), anti-syndecan-4 antibodies (anti-Syn 4, 40 μg/ml) or nonspecific IgG (100 μg/ml) before being treated with CCN1 for 6 hr. Where indicated, cells were treated with CCN1 in the presence of soluble heparin (2 μg/ml) in the culture media. The levels of TNFα and IL-1β mRNA were analyzed by real-time RT-PCR. B. Peritoneal macrophages isolated from integrin αM knock-out mice were treated for 6 hrs with CCN1 or LPS. Relative levels of TNFα and IL-1β mRNA were analyzed by real-time RT-PCR. C. I-13.35 cells were transfected with siRNA against integrin β2 (β2 si) or a control non-targeting siRNA (ctrl si) for 48 hrs. Cells were then incubated with CCN1 or BSA for 3 hrs. Down-regulation of β2 mRNA was assessed by real-time RT-PCR (left panel). Expression of TNFα and IP-10 was evaluated by semi-quantitative RT-PCR. D. Cells were incubated with the TLR4 inhibitor TAK-242 (0.5 μM) or vehicle DMSO (Vehl) for 30 min. prior to CCN1 treatment. TNFα and IL-1β mRNA levels were measured by real-time RT-PCR.
Since I-13.35 cells express a non-functional TLR-4 (32-34), it is unlikely that effects of CCN1 are mediated through TLR4. Furthermore, inhibition of TLR4 signaling by the selective inhibitor TAK-242 (39) had no effect on CCN1 upregulation of TNFα and IL-6 mRNA (Fig. 6D), indicating that CCN1 signaling does not involve TLR4. Taken together, our results show that CCN1-regulated gene expression is an activity specific to the CCN1 polypeptide (Fig. 3C) and is mediated through αMβ2 and syndecan-4, whereas TLR4 plays no role in this process.
CCN1 activates NFκB through integrin β2 and syndecan-4
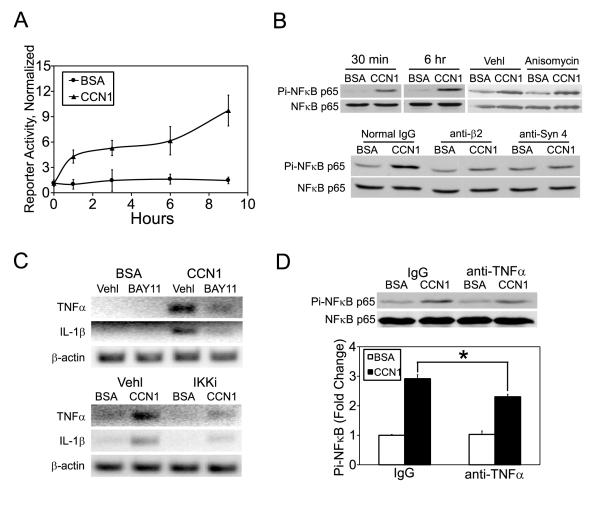
Engagement of integrin αMβ2 can lead to the activation of NFκB, a transcription factor capable of regulating a multitude of genes involved in inflammation, cell survival and cell proliferation. (36-38,40,41). CCN1 may thus activate NFκB signaling through binding to αMβ2. Since CCN1 can induce the expression of TNFα, which is a potent activator of NFκB, it may further activate NFκB through the induction of TNFα. To evaluate whether CCN1 can activate NFκB signaling, we monitored NFκB-dependent transcription and phosphorylation of p65 NFκB in I-13.35 cells. CCN1 treatment enhanced NFκB-dependent transcription by ~5 fold within 3 hours as judged by luciferase activity in cells transiently transfected with an NFκB-luciferase reporter construct (Fig. 7A), and rapidly upregulated p65 NFκB phosphorylation within 30 min. (Fig. 7B, upper left). Effects of CCN1 on p65 phosphorylation and NFκB-dependent transcription lasted for at least 6 hrs (Fig. 7A,B). Consistent with a direct role of CCN1 on NFκB activation, the protein synthesis inhibitor anisomycin did not prevent p65 phosphorylation (Fig. 7B, upper right). Pretreatment of cells with antibodies blocking integrin β2 or syndecan-4 effectively annihilated NFκB p65 phosphorylation (Fig. 7B, lower panel), showing that CCN1 acts through these receptors to activate NFκB. Addition of the IKK complex inhibitor BAY-117082 or an IκB kinase inhibitor (IKKi, N-(6-chloro-9H-β-carbolin-8-yl) nicotinamide) significantly attenuated CCN1-induced TNFα and IL-1β mRNA, indicating that their upregulation is dependent on NFκB (Fig. 7C). To test whether CCN1-induced TNFα may further enhance NFκB activation, we treated cells with CCN1 for 6 hours in the presence of polyclonal antibodies that neutralize TNFα activity and examined NFκB p65 phosphorylation. Results show that the antibodies attenuated p65 phosphorylation by ~30% compared to control IgG (Fig. 7D). Taken together, we demonstrate that CCN1 can bind to β2-integrin and syndecan-4 to activate NFκB to induce the synthesis of TNFα and IL-1β. TNFα in turn acts in an autocrine/paracrine mechanism to further enhance NFκB activation.
Figure 7. CCN1 activates NFκB in macrophages.
A. I-13.35 cells were co-transfected the luciferase reporter pNFκB-Luc driven by multiple NFκB-responsive elements (57), and pRL-CMV, a construct for transfection efficiency controls. Cells were treated with CCN1 or BSA one day later for the indicated times, and luciferase activity in cell lysates determined and normalized against transfection efficiency controls. B. NFκB activation was assessed by immunoblotting of cell lysates resolved on SDS-PAGE with antibodies against total and phosphorylated NFκB-p65. Upper panel: cells were treated with CCN1 for 30 min. or 6 hrs, or for 3 hrs with and without anisomycin (10 μM) or its vehicle DMSO. Lower panel, cells were preincubated with antibodies against integrin β2 (100 μg/ml) or syndecan-4 (40 μg/ml) for 30 min. before being treated with CCN1 for 1.5 hr. C. Cells were pre-incubated with NFκB signaling inhibitors for 30 min (BAY11, 5 μM IκB complex inhibitor BAY11-7082; IKKi, 50 μM IκB kinase inhibitor) before being treated with CCN1. TNFα and IL-1β mRNA expression was analyzed by semi quantitative RT-PCR. D. Cells were incubated with anti-TNFα (100 μg/ml) antibodies or normal IgG for 30 min and treated with CCN1 for 6 hrs. Phosphorylation on NFκB-p65 was analyzed by immunoblotting and quantified by densitometry. *: p<0.05.
CCN1 upregulates IL-1β and IL-6 expression through induction of TNFα
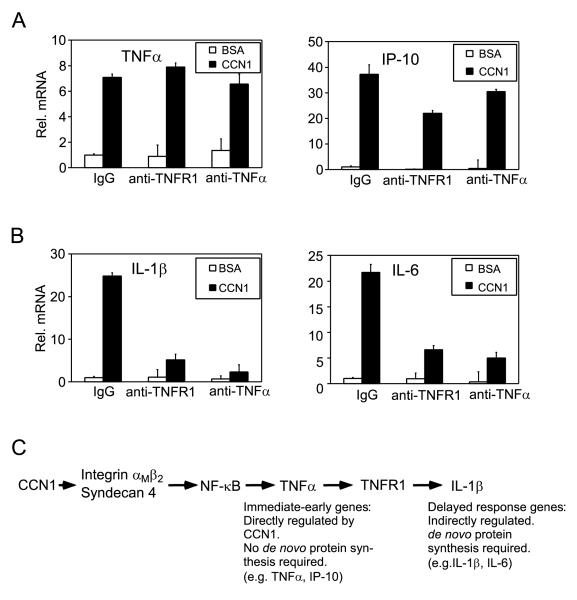
Since CCN1 effect on gene expression is dependent on NFκB activation and the upregulation of IL-1β and IL-6 requires de novo protein synthesis (Figs. 7, 5A), we tested whether CCN1-induced TNFα may be a necessary mediator for IL-1β and IL-6 induction. Pre-incubation of cells with either a mAb that inhibits TNFR1 function or polyclonal antibodies that neutralize TNFα had no effect on TNFα induction and only modest effects on IP-10 expression, consistent with the idea that CCN1 can directly activate TNFα and IP-10 expression without requiring de novo protein synthesis (Fig. 5A, 8A). In contrast, both antibodies severely reduced IL-1β and IL-6 expression, showing that TNFα is a critical mediator for the expression of these genes (Fig. 8B). Taken together, these results demonstrate that CCN1 can directly induce the expression of certain inflammatory genes such as TNFα and IP-10, whereas upregulation of other inflammatory genes, including those encoding IL-1β and IL-6, requires the induction of TNFα as a mediator (Fig. 8C).
Figure 8. A role of CCN1-induced TNFα as mediator for IL-1β and IL-6 induction.
Macrophages were pre-incubated for 30 min with a monoclonal antibody blocking TNF-R1, ployclonal antibodies neutralizing TNFα, or a normal IgG (100 μg/ml each), before being treated with CCN1 for 6 hr. and total RNA isolated for gene expression study using real-time RT-PCR. Expression of TNFα and IP-10 (A), and of IL-1β and IL-6 (B) is shown. C. CCN1 activates NFκB (Fig. 7A, B) through β2 integrin and syndecan-4 (Fig. 6), leading to the expression of NFκB-inducible genes such as TNFα and IP-10. Other genes, exemplified by IL-1β, are upregulated through de novo protein synthesis (Fig. 5A) of CCN1-induced TNFα (Fig. 8B).
DISCUSSION
This study shows that the matricellular protein CCN1 can induce a pro-inflammatory genetic program in macrophages, implicating CCN1 as a novel regulator of macrophage function. Upon inflammatory stimuli, IFNγ in combination with the bacterial component LPS can activate macrophages in a process known as “classical activation,” resulting in macrophages that participate in T helper cell type 1 (Th1) responses (42-44). CCN1 induces robust expression of pro-inflammatory cytokines characteristic of classically activated M1 macrophages, including upregulation of inflammatory cytokines and chemokines, and the microbicidal iNOS and complement component C3. Consistent with a pro-inflammatory role, CCN1 down-regulates the anti-inflammatory cytokine TGF-β1 and the receptor for IL-10, IL-10rβ. CCN1 regulates pro-inflammatory genes in two macrophage cell lines of distinct origins as well as in mouse peritoneal macrophages, indicating that these effects are not cell line-specific. In contrast to this pro-inflammatory M1 response, macrophages may also undergo “alternative activation” by cytokines involved in Th2-type response, such as IL-4 and IL-13, or other anti-inflammatory factors such as glucocorticoids, leading to various forms of M2 macrophages with phenotypic changes important for humoral immunity, immune suppression, allergic and anti-parasitic responses, or immune regulation (42-44). CCN1 does not induce gene expression changes consistent with the M2 phenotype, suggesting that CCN1 may help to polarize macrophages to participate as inducers and effectors in polarized Th1 responses.
Several lines of evidence show that the CCN1 polypeptide acts through integrin αMβ2 to activate this pro-inflammatory genetic program. First, most of the experiments in this study were conducted with I-13.35 cells isolated from the TLR-4 mutant C3H/HeJ strain, which are unresponsive to signaling through the LPS receptor TLR-4 (32-34). Furthermore, the TLR inhibitor TAK-242 was unable to curtail CCN1-regulated gene expression (Fig. 6D), indicating that TLR-4 does not mediate CCN1 action. Second, neutralizing anti-CCN1 antibodies abrogated CCN1-induced gene expression (Fig. 3C), showing that the activity is specific to the CCN1 polypeptide. Third, CCN1-induced gene expression was annihilated in peritoneal macrophages isolated from integrin αM knockout mice, whereas macrophages from syngeneic wild type mice were highly responsive (Figs. 4C, 6B). Moreover, both siRNA knockdown and antibody blockade of β2 integrin greatly blunted CCN1-induced gene expression (Figs. 6A, 6C). Together, these results show that the CCN1 protein acts through integrin αMβ2 to induce a pro-inflammatory program in macrophages, and TLR4 signaling does not play a role in this process.
The leukocyte-specific integrin receptor αMβ2 is broadly expressed in monocytes, granulocytes, macrophages, and natural killer cells, and plays a critical role in regulating leukocyte adhesion, migration, phagocytosis and cell-mediated cytotoxicity. Individuals with αMβ2 deficiency suffer recurrent and even fatal bacterial and fungal infections (45). CCN1 supports macrophage adhesion through integrin αMβ2 and the HSPG syndecan-4, and induces macrophage gene expression by activating NFκB in a β2- and syndecan-4-dependent manner (Figs. 1, 6). Engagement of αMβ2 through extracellular ligands or cell adhesion to certain ECM proteins is known to activate the transcription factor NFκB, thereby inducing the expression of inflammatory cytokines (37,46-48). Whereas activation of NFκB through engagement of integrin αMβ2 has been previously reported (46,49), here we show evidence that syndecan-4 is also involved, suggesting that syndecan-4 is a coreceptor of αMβ2 that can mediate NFκB activation. Syndecan-4-null mice are more susceptible to endotoxin shock, suggesting a role in the inflammatory response (50). It is interesting to note that the αMβ2 binding site of CCN1 is closely juxtaposed to a HSPG binding site located in the carboxyl domain (27,31), although it is currently unclear whether a single CCN1 molecule must simultaneously bind both αMβ2 and syndecan-4 to activate NFκB, or separate CCN1 molecules can bind αMβ2 and syndecan-4 independently to induce signaling in the same cell.
Mechanistically, genes regulated by CCN1 in I.13-35 cells fall into two categories: immediate-early genes that are induced with rapid kinetics (peaking at 3-6 hrs) without requiring de novo protein synthesis, including TNFα and IP-10; and delayed response genes that are upregulated with delayed kinetics (peaking at 12-24 hrs) through the synthesis of protein mediators, represented by IL-1β and IL-6 (Fig. 4A, 5A). We show that CCN1 induces the synthesis of TNFα as an immediate-early gene product (Fig. 4D), which in turn acts as the mediator for the upregulation of delayed response genes such as IL-1β and IL-6, since function-blocking antibodies against TNFα or TNFR1 obliterated their response to CCN1 (Fig. 8). The different responses of immediate-early and delayed response genes may be due to their differential sensitivity to NFκB activation. Both TNFα and IP-10 promoters contain two or more copies of functional κB-like elements (51,52), whereas the IL-1β and IL-6 promoters have only a single potential κB site (53,54), suggesting a differential responsiveness to NFκB activation among these genes. Since TNFα is a potent activator of NFκB, it may enhance IL-1β and IL-6 gene transcription by amplifying the activation of NFκB (Fig 7D). Alternatively, TNFα may also enhance IL-1β and IL-6 transcription through a p38 MAPK/AP-1-dependent mechanism (47,55).
Although our studies establish the role of CCN1 in modulating pro-inflammatory gene expression in macrophage, the significance of this activity in inflammation and injury repair in vivo remains to be determined. CCN1 is normally expressed at low levels in most adult tissues but is re-deployed at a high level at sites of inflammation and injury repair (56), where its ability to regulate gene expression in macrophages may play an important role. Recent studies have shown that CCN1 enables the cytotoxicity of the inflammatory cytokine TNFα and enhances the apoptotic activity of FasL and TRAIL (22-24). Further, TNFα- or Fas-mediated apoptosis is blunted in mice expressing an apoptosis-defective CCN1, indicating that CCN1 is an important physiologic modulator of the biological responses to inflammatory cytokines in vivo. Based on its induction of Th1 type responses in macrophages, it is tempting to speculate that CCN1 might play a role in M1/M2 polarization to enhance the inflammatory response (42-44). In wound healing, CCN1 may provide a provisional matrix and may serve as a localized regulator of inflammatory cell recruitment and function (18,19). Further investigations using models of infection and tissue injury repair will be required to elucidate the role of CCN1 in these biological contexts.
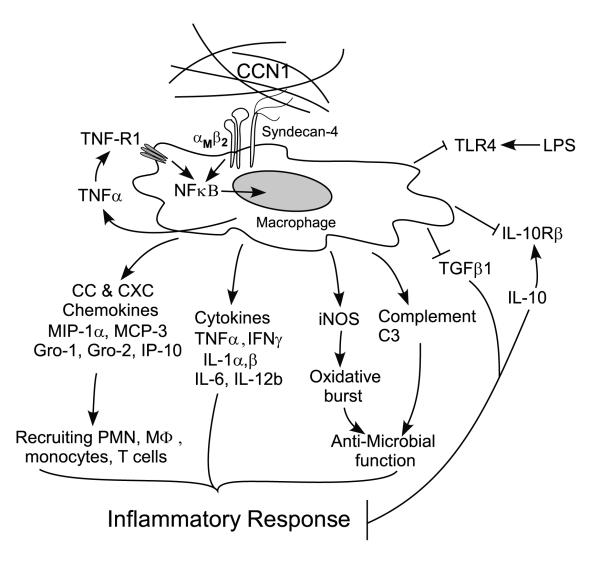
Figure 9. A model for CCN1 regulation of inflammatory genes in macrophages.
The matricellular protein CCN1 induces gene expression changes characteristic of the classically activated M1-type response in macrophages through its cell surface receptors integrin αMβ2 and syndecan-4, resulting in elevated expression of pro-inflammatory cytokines like TNFα, IL-1β, IL-6, and IFNγ, and chemokines including MIP-1α, MCP-3, Gro1, Gro2, and IP-10. These factors may recruit and activate more inflammatory cells including polymorphonuclear neutrophils, monocyte/macrophages, and T-lymphocytes. Simultaneously, expression of anti-inflammatory effectors such as TGF-β1 and IL-10rβ are attenuated, resulting in a further amplification of inflammatory response. CCN1 may enhance host defense against invading bacteria by upregulating macrophage expressions of iNOS, which is critical for suppressing bacterial replication (58), and complement C3 protein, which is required for activation of the complement system. On the other hand, CCN1 may also confine the host response to invading microbes by down-regulating the LPS receptor TLR4. Collectively, our data support the hypothesis that CCN1 exerts strong pro-inflammation response on macrophages by promoting diverse inflammatory gene expressions.
Footnotes
This work was supported by a grant from the NIH (GM078492) to L.F.L. and an American Heart Association predoctoral fellowship from to T.B.
Reference List
- 1.Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006;22:591. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 2.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008;40:1101. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Reilly PJ, Gaggar A, Blalock JE. Interfering with extracellular matrix degradation to blunt inflammation. Curr. Opin. Pharmacol. 2008;8:242. doi: 10.1016/j.coph.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002;14:608. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 5.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007;27:2302. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 6.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J. Immunol. 2007;178:5930. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 7.Sangaletti S, Colombo MP. Matricellular proteins at the crossroad of inflammation and cancer. Cancer Lett. 2008;267:245. doi: 10.1016/j.canlet.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat. Rev. Cancer. 2009;9:182. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6355. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity. Mol. Cell Biol. 2002;22:8709. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo F-E, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circulation Research. 2006;99:961. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13118. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SM, Park JH, Chung SK, Kim JY, Hwang HY, Chung KC, Jo I, Park SI, Nam JH. Coxsackievirus B3 infection induces cyr61 activation via JNK to mediate cell death. J. Virol. 2004;78:13479. doi: 10.1128/JVI.78.24.13479-13488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A, Chiocca EA, Kaur B. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol. Ther. 2008;16:1382. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiedmaier N, Muller S, Koberle M, Manncke B, Krejci J, Autenrieth IB, Bohn E. Bacteria induce CTGF and CYR61 expression in epithelial cells in a lysophosphatidic acid receptor-dependent manner. Int. J. Med. Microbiol. 2008;298:231. doi: 10.1016/j.ijmm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J. Bone Miner. Res. 2000;15:1014. doi: 10.1359/jbmr.2000.15.6.1014. [DOI] [PubMed] [Google Scholar]
- 17.Wu KJ, Yee A, Zhu NL, Gordon EM, Hall FL. Characterization of differential gene expression in monkey arterial neointima following balloon catheter injury. Int. J Mol. Med. 2000;6:433. [PubMed] [Google Scholar]
- 18.Latinkic BV, Mo F-E, Greenspan JA, Copeland NG, Gilbert DJ, Jenkins NA, Lau LF. Promoter function of the angiogenic inducer Cyr61 gene in transgenic mice: tissue specificity, inducibility during wound healing, and role of the serum response element. Endocrinology. 2001;142:2549. doi: 10.1210/endo.142.6.8208. [DOI] [PubMed] [Google Scholar]
- 19.Chen C-C, Mo F-E, Lau LF. The angiogenic inducer Cyr61 induces a genetic program for wound healing in human skin fibroblasts. J. Biol. Chem. 2001;276:47329. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- 20.Schober JM, Chen N, Grzeszkiewicz TM, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SCT. Identification of integrin αMβ2 as an adhesion receptor on peripheral blood moncytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2), immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- 21.Grzeszkiewicz TM, Lindner V, Chen N, Lam SCT, Lau LF. The angiogenic factor CYR61 supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143:1441. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 22.Chen C-C, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFα is regulated by Integrin-Mediated Matrix Signaling. EMBO J. 2007;26:1257. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franzen CA, Chen CC, Todorovic V, Juric V, Monzon RI, Lau LF. The Matrix Protein CCN1 is Critical for Prostate Carcinoma Cell Proliferation and TRAIL-Induced Apoptosis. Mol. Cancer Res. 2009;7:1045. doi: 10.1158/1541-7786.MCR-09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juric V, Chen CC, Lau LF. Fas-Mediated Apoptosis is Regulated by the Extracellular Matrix Protein CCN1 (CYR61) in vitro and in vivo. Mol. Cell Biol. 2009;29:3266. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simental JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J. Biol. Chem. 1991;266:510. [PubMed] [Google Scholar]
- 26.Kireeva ML, Mo F-E, Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol. Cell. Biol. 1996;16:1326. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schober JM, Lau LF, Ugarova TP, Lam SC. Identification of a novel integrin αMβ2 binding site in CCN1 (CYR61), a matricellular protein expressed in healing wounds and atherosclerotic lesions. J. Biol. Chem. 2003;278:25808. doi: 10.1074/jbc.M301534200. [DOI] [PubMed] [Google Scholar]
- 28.Kireeva ML, Lam SCT, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J. Biol. Chem. 1998;273:3090. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- 29.Ding AH, Nathan CF. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J. Immunol. 1987;139:1971. [PubMed] [Google Scholar]
- 30.Coligan JE, Kruisbeek AM, Marguliees DH, Shevach EM, Strober W. Macrophages and Monocytes. In: Coligan JE, Kruisbeek AM, Marguliees DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Vol. 3. John Wiley and Sons, Inc.; 1995. pp. 14.0.3–14.9.5. [Google Scholar]
- 31.Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2000;275:24953. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- 32.Wilson CM, Gatewood JW, McCormack JM, Walker WS. Immortalization of growth factor-dependent mouse splenic macrophages derived from cloned progenitors. J. Immunol. Methods. 1991;137:17. doi: 10.1016/0022-1759(91)90389-w. [DOI] [PubMed] [Google Scholar]
- 33.Poltorak A, He X, Smirnova I, Liu MY, Van HC, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749. [PubMed] [Google Scholar]
- 35.Todorovic V, Chen C-C, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J. Cell Biol. 2005;171:559. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez RL, Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J. Immunol. 1995;154:1879. [PubMed] [Google Scholar]
- 37.Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001;97:2932. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- 38.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 2004;113:1596. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008;584:40. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 40.Fan ST, Edgington TS. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-alpha responses of monocytes. J. Immunol. 1993;150:2972. [PubMed] [Google Scholar]
- 41.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868. [PubMed] [Google Scholar]
- 42.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 1987;38:175. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 46.Sitrin RG, Pan PM, Srikanth S, Todd RF., III Fibrinogen activates NF-kappa B transcription factors in mononuclear phagocytes. J. Immunol. 1998;161:1462. [PubMed] [Google Scholar]
- 47.Perez RL, Ritzenthaler JD, Roman J. Transcriptional regulation of the interleukin-1beta promoter via fibrinogen engagement of the CD18 integrin receptor. Am. J. Respir. Cell Mol. Biol. 1999;20:1059. doi: 10.1165/ajrcmb.20.5.3281. [DOI] [PubMed] [Google Scholar]
- 48.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 49.Shi C, Zhang X, Chen Z, Robinson MK, Simon DI. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-kappaB activity. Circ. Res. 2001;89:859. doi: 10.1161/hh2201.099166. [DOI] [PubMed] [Google Scholar]
- 50.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J. Biol. Chem. 2001;276:47483. doi: 10.1074/jbc.M106268200. [DOI] [PubMed] [Google Scholar]
- 51.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell Biol. 1990;10:1498. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med. 1990;171:35. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiscott J, Marois J, Garoufalis J, D’Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol. Cell Biol. 1993;13:6231. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10:2327. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beyaert R, Cuenda A, Vanden BW, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914. [PMC free article] [PubMed] [Google Scholar]
- 56.Chen C-C, Lau LF. Functions and Mechanisms of Action of CCN Matricellular Proteins. Int. J. Biochem. Cell Biol. 2009;41:771. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J. Biol. Chem. 2004;279:39541. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 58.Vazquez-Torres A, Fang FC. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 2001;9:29. doi: 10.1016/s0966-842x(00)01897-7. [DOI] [PubMed] [Google Scholar]