Abstract
Recent years have witnessed an explosion of interest in the innate immune system. Questions about how the innate immune system senses infection and empowers a protective immune response are being answered at the molecular level. These basic science discoveries are being translated into a more complete understanding of the central role innate immunity plays in the pathogenesis of many human infectious and inflammatory diseases. It is particularly exciting that we are already seeing a return on these scientific investments with the emergence of novel therapies to harness the power of the innate immune system. In this review we explore the defining characteristics of the innate immune system, and through more detailed examples, we highlight recent breakthroughs that have advanced our understanding of the role of innate immunity in human health and disease.
Keywords: host defense, innate immunity, Toll-like receptors, NOD-like receptors
THE “NEW” SCIENCE OF INNATE IMMUNITY
The integrated human immune response has traditionally been divided into two branches: innate and adaptive (or acquired) immunity. While appreciation of innate immunity dates back to at least the 1908 Nobel Prize winning efforts of Ilya Mechnikov; until the last decade, study of innate immunity has been eclipsed by dramatic discoveries in the field of adaptive immunity. However, the recent molecular definition of how the innate immune system senses infection to empower protective immune responses has precipitated a renaissance in the field of innate immunity. Innate immunity has shed its older, disparaging title of ‘non-specific immunity’ and now stands as a proud partner with the adaptive immune system in protecting human hosts from infectious insults. For any who doubt the impressive protective capacity of the innate immune system, it is instructive to consider that only vertebrates boast the added benefits of an adaptive immune system, leaving most organisms on our planet to survive on innate immunity alone!
While innate immunity is critical for host defense against infectious challenges, the innate immune system is emerging as a critical regulator of human inflammatory disease. Indeed, innate immune responses have been implicated in the development of asthma and atopy, as well as a variety of autoimmune disorders including Type 1 diabetes, inflammatory bowel disease and systemic lupus erythematosus.
In this review we examine the basic structure of the innate immune system and how innate immunity interfaces with adaptive immune responses. We explore the role of innate immunity in human health and disease and we outline how novel therapies may harness the beneficial capacity of the innate immune system. Rather than attempting to comprehensively review this enormously broad topic, our focus is on highlighting common defining mechanisms of innate immunity and illustrating the clinical relevance of innate immunity to human health. We have deliberately avoided a detailed exploration of the complement system as a separate Primer chapter is devoted to this important aspect of innate immunity (Chapter 23: Complement Disorders and Hereditary Angioedema. Michael Frank).
ORGANIZATION OF THE HUMAN IMMUNE SYSTEM: THREE LEVELS OF HOST DEFENSE
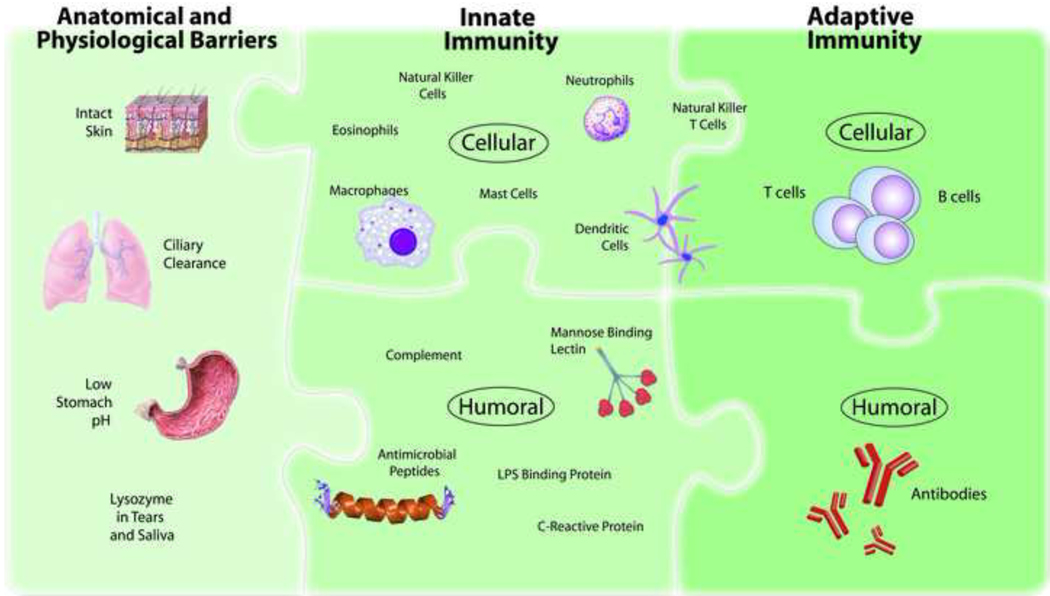
The human microbial defense system can be simplistically viewed as consisting of three levels: (i) anatomical and physiological barriers; (ii) innate immunity; and (iii) adaptive immunity (Figure 1 and Table 1). Failure in any of these systems will greatly increase susceptibility to infection.
Figure 1. Integrated Human Immune System.
The human microbial defense system can be simplistically viewed as consisting of three levels: (i) anatomical and physiological barriers; (ii) innate immunity; and (iii) adaptive immunity. In common with many classification systems, some elements are difficult to categorize. For example, NKT cells and DCs could be classified as being on the cusp of innate and adaptive immunity rather than being firmly in one camp.
Table 1. Overview of Defining Features of Innate and Adaptive Immunity.
Comparing and contrasting some of the defining features of the innate and adaptive immune systems. Adapted from [1].
| Innate Immune System | Adaptive Immune System | |
|---|---|---|
| Cellular elements | Hematopoietic cells: macrophages, dendritic cells, mast cells, neutrophils, eosinophils, natural killer cells & natural killer T cells. | Hematopoietic cells: T & B lymphocytes. |
| Non-hematopoietic cells: epithelial cells (e.g. skin, airways, gastrointestinal tract). | ||
| Humoral elements | Large arsenal of components: Complement proteins, LPS binding protein, C-reactive protein and other acute phase reactants, anti-microbial peptides, mannose-binding lectin. | Immunoglobulins secreted by B cells. |
| Receptor characteristics | Invariant, germline encoded. | Generated by random somatic gene-segment rearrangement. |
| All cells of a class express identical receptors (i.e. non-clonal). | All cells of a class express a single type of receptor with unique specificity (i.e. clonal). | |
| Ligands recognized | Conserved microbial components. | Specific ‘details’ or epitopes of macromolecules (e.g. proteins, peptides, carbohydrates). |
| Common metabolic or biological consequences of infection (e.g. uric acid, K+ efflux, MHC class I downregulaton). | ||
| Types of receptors | Activating: TLR, NLR, complement. | B cell receptor and T cell receptor. |
| Inhibitory: Killer cell immunoglobulin-like receptors (KIR). | ||
| Response time | Immediate | Delayed by hours to days. |
| Immunological memory | None. Responses are the same with each exposure. Non-anticipatory immunity | Responsiveness enhanced by repeated antigen exposure. Anticipatory immunity. |
| Risk of autoreactivity | Low. Self-tolerant receptors selected during evolution | High. Random gene rearrangement generates autoreactive receptors requiring presence of multiple tolerance mechanisms. |
Anatomical and physiological barriers provide the crucial first line of defense against pathogens. These barriers include intact skin, vigorous mucociliary clearance mechanisms, low stomach pH and bacteriolytic lysozyme in tears, saliva and other secretions. The extreme susceptibility to infections observed in individuals with severe cutaneous burns or primary ciliary dyskinesia demonstrates that intact innate and adaptive immune systems are not able to compensate for failure of essential anatomical and physiological barriers.
Innate immunity augments the protection offered by anatomical and physiological barriers.1 The innate immune system relies upon a limited repertoire of receptors to detect invading pathogens, but compensates for this limited number of invariant receptors by targeting conserved microbial components that are shared by large groups of pathogens. Speed is a defining characteristic of the innate immune system—within minutes of pathogen exposure the innate immune system starts generating a protective inflammatory response. Moreover, innate immunity plays a central role in activating the subsequent adaptive immune response.
T- and B-lymphocytes are the main self-defense weapons of the adaptive immune system, so-named because this system is shaped by antigen exposure. In contrast to the limited number of pathogen receptors utilized by the innate immune system, the adaptive immune system boasts an extremely diverse, randomly-generated repertoire of receptors. The benefit of this receptor diversity is that the adaptive immune system can recognize virtually any antigen, but there is a price for this diversity. First is the risk of autoimmune disease. Receptors specific for self proteins (such as insulin and myelin) are created by the random process of gene rearrangement that generates receptors expressed by T cells and B cells. Consequently, elaborate tolerance mechanisms have evolved to eliminate or regulate self-reactive cells. Second is the time delay required to generate a protective adaptive immune response following the first exposure to a pathogen. Adaptive immunity relies upon a clonal system with each T cell and B cell expressing its own unique receptor and following the initial encounter with a pathogen, it takes up to five days for clonal expansion of these rare antigen-specific T and B cells to occur before the adaptive immune response is sufficiently robust to clear the pathogen.
ELEMENTS OF THE INNATE IMMUNE SYSTEM
In contrast to the adaptive immune system which depends upon T and B lymphocytes, innate immune protection is a task performed by cells of both hematopoietic and non-hematopoietic origin (Figure 1 and Table 1). Hematopoietic cells involved in innate immune responses include macrophages, dendritic cells, mast cell, neutrophils, eosinophils, natural killer (NK) cells and natural killer T cells. In addition to hematopoietic cells, innate immune responsiveness is a property of the skin and the epithelial cells lining the respiratory, gastrointestinal and genitourinary tracts.
To augment these cellular defenses, innate immunity also has a humoral component that includes well characterized components such as complement proteins, LPS binding protein (LBP), C-reactive protein and other pentraxins, collectins, and anti-microbial peptides including defensins. Circulating innate immune proteins are involved in both in sensing microbes and effector mechanisms to facilitate clearance of the infection. For example, mannose-binding lectin (MBL), a member of the collectin family of receptors, binds mannose-containing carbohydrates on microbes triggering activation of the complement cascade which enhances clearance of the pathogen.
HOST DEFENSE IS ACHIEVED THROUGH INTEGRATION OF INNATE AND ADAPTIVE IMMUNITY
Innate immunity, an evolutionarily ancient component of host defense, is present in all multicellular organisms while adaptive immunity evolved much later and is only found in jawed fish and all ‘higher' vertebrates.2 During evolution, adaptive immunity developed in the context of a functioning innate immune system. Consequently, the classic demarcation between innate and adaptive immunity is overly simplistic as many adaptive immune responses build on the foundation of innate immunity. For example, the capacity of neutrophils to kill bacteria is enhanced when the bacteria are opsonized by antibodies produced through the coordinated efforts of T and B cells. In a similar fashion, the C3d fragment that is generated in the course of complement activation acts as a molecular adjuvant to profoundly influence the subsequent adaptive immune response. Specifically, Cd3 fragments act to bridge innate and adaptive immunity as covalent binding of single or multiple copies of C3d to a foreign antigen generally enhances B cell effector and memory function.3 Another illustrative example of the interdependence of innate and adaptive immunity is the critical role played by antigen-presenting cells of the innate immune system (e.g. dendritic cells) to empower full activation of the T and B cells of the adaptive immune system. Further blurring of the distinction between innate and adaptive immunity is highlighted by the fact that cells of the adaptive immune system, including regulatory T lymphocytes, express Toll-like receptors (TLRs) and other innate immune receptors.4 The inter-relatedness of innate and adaptive immunity is most eloquently articulated by Beutler in his observation that “…the roots of adaptive immunity are buried deep in the soil of the innate immune system”.5
INNATE IMMUNE RECOGNITION STRATEGIES
The innate immune system serves as the initial immune defense against foreign and dangerous material. In the most simplistic view, the innate immune system is hardwired with germline-encoded receptors for immediate responsiveness. In contrast to adaptive immunity, innate immune responses do not require genetic recombination events or a developmental phase to mediate function.
The strategy used for immune recognition is the main feature distinguishing innate and adaptive immunity. In contrast to the massive, randomly-generated repertoire of antigen receptors expressed by T and B lymphocytes, the innate immune system relies upon a limited number of genetically predetermined germline-encoded receptors that recognize either highly conserved structures expressed by large groups of microbes or common biological consequences of infection. Pathogens can rapidly evolve and, in principle, could avoid detection by the innate immune system by simply altering the targeted microbial molecules. However, the innate immune system has evolved to recognize either microbial components that are essential for the viability and virulence of microbes and are thus less prone to modifications, or common biological consequences of infection.
At least three broad strategies are used by the innate immune system to recognize invading microorganisms (Table 2). In the first, innate immunity relies upon a limited repertoire of germline-encoded receptors to recognize ‘microbial non-self’ – conserved molecular structures that are expressed by a large variety of microbes. Charles Janeway coined the terms ‘pattern recognition receptors’ (PRRs) to collectively describe these receptors and ‘pathogen-associated molecular patterns’ (PAMPs) to denote the microbial structures recognized by the PRRs.6 However, this terminology has been criticized as being vague5; therefore in this review we will focus on naming specific receptors and their microbial ligands. A second approach used by the innate immune system is to detect immunological ‘danger’ in the form of ‘damage-associated molecular patterns’ (DAMPs). DAMPs represents common metabolic consequences of infection and inflammation.7 DAMPs are molecules that are upregulated and released during the cell lysis and tissue damage that occurs in the context of both infectious and sterile inflammation. Well characterized DAMPs include high mobility group box 1 protein (HMGB-1) and other endogenous alarmins, heat-shock proteins (HSPs) and uric acid. In the third innate immune recognition strategy, innate immune receptors detect “missing self” – molecules expressed by normal, healthy cells but not expressed by infected cells or microbes. Recognition of these signals indicates that ‘all-is-well’ and an inhibitory signal is delivered to prevent activation of the immune response against host tissues. This inhibitory system is well illustrated by NK cells. Inhibitory receptors specific for self-MHC class I molecules play a central role in missing-self recognition by NK cells, ensuring NK cells preferentially attack infected cells that downregulate their MHC class I proteins.8
Table 2. Common Innate Immune Recognition Strategies.
| Innate Immune Recognition Strategy | Receptor Families | Specific examples | |
|---|---|---|---|
| Receptor | Ligand | ||
|
1. Detecting ‘microbial non-self’ (i.e. pathogen-associated molecular patterns (PAMPs)) |
Toll-like receptors | TLR4 | Lipopolysaccharide |
| TLR5 | Flagellin (extracellular) | ||
| NOD-like receptors | NOD2 | Muramyl dipeptide | |
| IPAF | Flagellin (intracellular) | ||
| Collectin family | MBP | Microbial terminal mannose residues | |
|
2. Detecting common metabolic consequences of cell infection or injury (i.e. damage-associated molecular patterns (DAMPs)) |
NOD-like receptors | NLRP3 (or NALP3) | Uric acid, K+ efflux, ATP |
| RAGE (receptor of advance glycation end product) family | RAGE | HMGB1, S100 | |
| 3. Detecting ‘missing self’ | MHC-class-I-specific inhibitory receptors | KIR | Self MHC class I (inhibitory signal) |
| CD94-NKG2A heterodimers |
Self MHC class I (inhibitory signal) | ||
ROLE OF THE INNATE IMMUNE SYSTEM IN HEALTH AND DISEASE
We will now turn our attention to specific components of the innate immune system. We deliberately selected two illustrative examples – TLRs and NLRs – where our mechanistic understanding has increased considerably in the past 5 years and where the clinical relevance of these systems is beginning to emerge.
1. TOLL-LIKE RECEPTORS (TLRS)
Overview of TLR Structure and Function
The recent explosion of interest in innate immunity was catalyzed in the mid-1990s when the Drosophila protein Toll was shown to be critical for defending fruit flies against fungal infections.9 This observation opened the way for the subsequent description of similar proteins, called Toll-like receptors (TLRs), in mammalian cells. The human TLR family consists of 10 receptors that are critically important for innate immunity.10, 11 TLRs allow for recognition and response to diverse microbial epitopes on pathogens enabling the innate immune system to discriminate among groups of pathogens and to induce an appropriate cascade of effector adaptive responses.
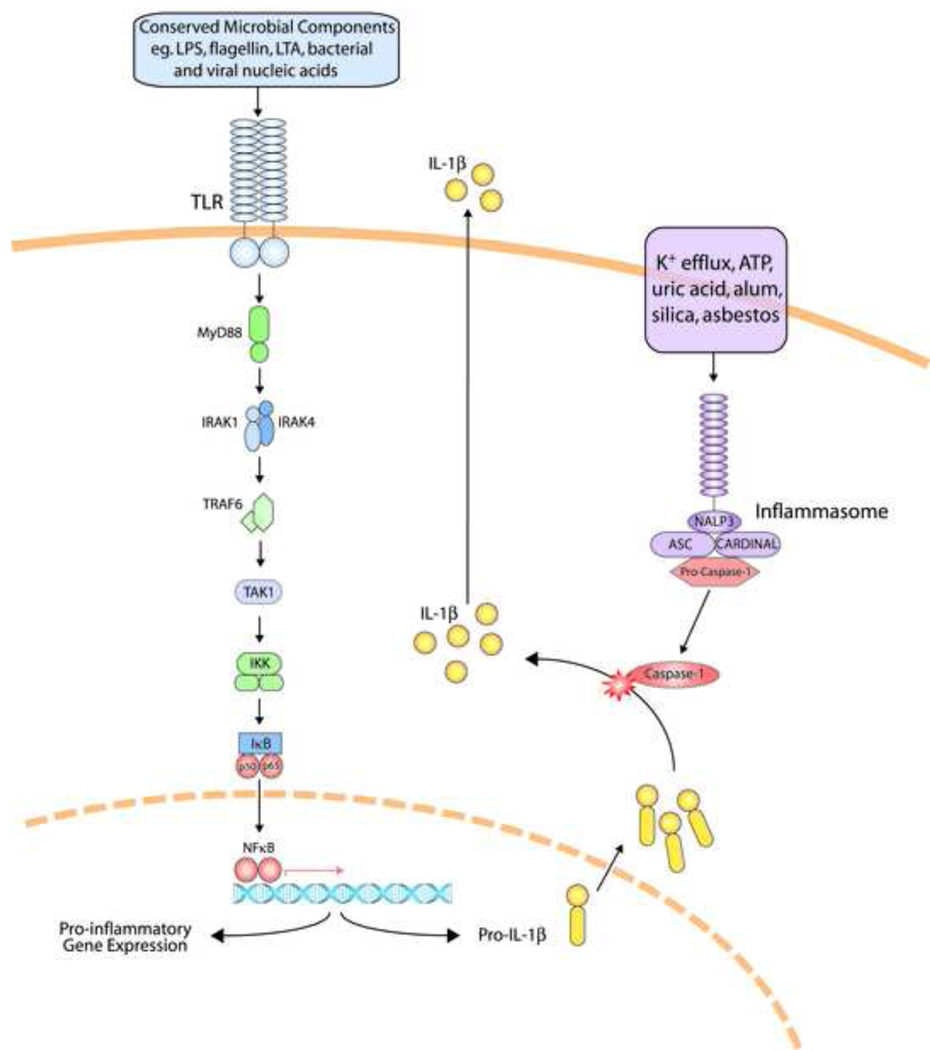
TLRs exist as dimeric proteins (either heterodimers or homodimers). The ectodomains of TLRs are composed of leucine-rich repeat motifs while the cytosolic component, called a Toll/interleukin-1 receptor (TIR) domain, is involved in signaling. Individual TLRs recognize a distinct, but limited, repertoire of conserved microbial products; for example, well characterized receptor-ligand pairs include TLR4 and lipopolysaccharide (LPS), TLR5 and flagellin, TLRs1/2/6 and lipoproteins. Collectively, the complete TLR family allows the host to detect infection by most (if not all) types of microbial pathogens. For example, Gram positive organisms, such as Streptococcus pneumoniae, are initially recognized by TLR1, 2, 4, 6 and 9, which in turn interact with a range of downstream signaling molecules to activate an inflammatory cascade. TLR signaling pathways have been the focus of considerable attention (reviewed in 11, 12 and depicted in Figure 2). The emerging model has ligation of microbial products by TLRs culminating in the activation of nuclear factor kappa-B (NF-κB), activator protein-1 (AP-1), interferon-regulatory factor (IRF)-3 and other transcription factors, driving the production of proinflammatory cytokines, maturation of dendritic cells and other immunological responses.
Figure 2. Overview of TLR Signaling and the NLRP3 Inflammasome.
TLR ligation initiates a signaling cascade that culminates in the translocation of the transcription factors, NF-κB and others, to the nucleus generating an acute inflammatory response. The NLRP3 (or NALP3) inflammasome is triggered by a wide variety of stimuli culminating in the activation of caspase 1 which will then cleave pro-IL1β and pro-IL18 to drive an inflammatory response. Human mutations and polymorphisms in many of the genes encoding elements of these pathways appear to alter susceptibility to infectious and inflammatory diseases.
Human Disease Resulting from TLR Defects
Naturally-occurring genetic mutations in humans, causing extreme immunodeficiency phenotypes, present powerful opportunities to determine the relationship between specific immunological defects and human disease processes in vivo. Recent description of human primary immunodeficiencies associated with abnormal TLR signaling demonstrate that this pathway is critical for human defense against infection. Empowered by technological advances in genotyping and bioinformatics, we are now beginning to appreciate how common genetic variation and polymorphisms in genes controlling the innate immune response alters infectious susceptibility in a subtle but specific fashion. Importantly, human primary immunodeficiencies associated with abnormal TLR signaling provide unique insights into the immunological pathways vital for host defense and identify candidate genes that may cause subtle immunodeficiencies in the broader population of apparently healthy people13.
a) Monogenic primary immunodeficiencies
Interleukin-1 receptor associated kinase 4 (IRAK4) deficiency (OMIM #607676)14 and myeloid differentiation primary response gene 88 (MyD88) deficiency (OMIM #612260)15 are novel primary immunodeficiencies specifically affecting TLR function. MyD88 and IRAK4 are binding partners involved in downstream signaling from most TLRs (Figure 2); hence, the clinical and laboratory phenotypes of IRAK4- and MyD88-deficiencies are identical. The narrow spectrum of infections experienced by affected individuals is striking in light of their profound impairment of TLR function and pathogen sensing. IRAK4- and MyD88-deficient patients predominantly suffer from recurrent infections caused by pyogenic Gram-positive bacteria, with Streptococcus pneumoniae causing invasive infection in all reported cases while Staphylococcus aureus and Pseudomonas aeruginosa caused infections in about half the patients. The surprising clinical observation that IRAK4-deficient patients are resistant to viral infections was recently explained at a molecular level as IRAK4-deficient patients are able to control viral infections by TLR3- and TLR4-dependent production of IFNs.16
Arguably one of the most powerful messages to arise from the recognition of IRAK4- and MyD88-deficiency is the value of studying humans to understand human immune function! While MyD88-deficient patients are susceptible to Streptococcus pneumoniae and a limited number of pyogenic bacteria, they are able to resist infection by most common bacteria, viruses, fungi, and parasites. In contrast, MyD88-deficiency renders mice profoundly susceptible to most pathogens tested.
b) Contribution of TLR polymorphisms to human disease
At the population level susceptibility to common diseases, such as infections, seldom follows the simple pattern of Mendelian inheritance seen in IRAK4- and MyD88-deficiency.17 Most infections follow a complex mode of inheritance, with disease arising from an intricate interplay between environmental and genetic factors. The complexity of common infectious diseases has made them, until very recently, largely impervious to genetic analysis. However, advances in high throughput genotyping techniques and bioinformatics is now allowing us to understand how common genetic variants alter human susceptibility to infection.
Although humans are identical at most of the 3 billion base pairs in their genome, inter-individual variation is present in approximately 3 million nucleotides (i.e. 0.1% of the genome).18 A common type of human genetic variation is the single nucleotide polymorphism (SNP), where two alternative bases occur at appreciable frequency (>1%) in the population. There is convincing evidence that common TLR SNPs regulate cellular signaling events, cytokine production and susceptibility to infection based on the specific pathogens recognized by the TLR. Arguably the best evidence implicates amino acid changing (i.e. non-synonymous) SNPs in TLRs 1, 2 and 5, as well as variants in the adaptor molecule TIR domain-containing adaptor protein (TIRAP, also know as MAL). This genetic variation in the population results in some individuals having a ‘subtle’ but specific immunodeficiency. For example, a common TLR5 polymorphism in the ligand-binding domain of TLR5 (392STOP) abolishes flagellin signaling and is associated with increased susceptibility to Legionnaire’s disease caused by the flagellated bacterium, Legionella pneumophila.19 In a similar fashion, polymorphisms in the adaptor molecule MAL/TIRAP which mediates signaling through TLR1, 2, 4, and 6, have been associated with susceptibility to tuberculosis, malaria and pneumococcal disease.20
Given the role of TLRs in sensing the extracellular environment and shaping inflammatory response, the TLR pathway has been hypothesized to influence the development of atopy and asthma. The best studied example is CD14. CD14 is encoded on chromosome 5q31.1 in a region linked to atopy and asthma, and CD14 partners with TLR4 to recognize LPS. Therefore, a SNP in this gene (CD14/–159 C to T) which appeared to alter the functional production of CD14, made an excellent candidate to influence susceptibility to asthma and atopy. Initial investigations showed remarkable variation with some studies indicating the T-allele as a risk factor, others the C-allele, and others finding no association.21 However, when the level of LPS (or endotoxin) exposure was considered a biologically plausible gene-by-environment interaction was revealed with data suggesting that the C-allele is a risk factor for allergic phenotypes at low levels of exposure, whereas the T-allele is a risk factor at high levels of exposure.22 Through this informative example it is clear that complex interactions between genes and environment determine asthma-related outcomes. Consequently, if we fail to integrate genetic and environmental factors in our study of asthma and allergy, we will only generate an impoverished appreciation of the etiology of atopic disease.
While a rapidly growing number of genetic association studies suggest that TLR polymorphisms may be associated with susceptibility to different infectious and immunologically-mediated diseases, very few of these studies have been replicated in a convincing fashion. For example, the initial association reported between MAL/TIRAP and susceptibility to tuberculosis was not replicated in another large study.23 As this field advances and expands to include genome-wide association studies, it is essential to appreciate that the best studies will include large sample sizes, statistical adjustments for multiple comparison, replication of findings with independent cohorts, multiple study designs (including case-control and family-based studies), adjustment of the analysis for population admixture, consideration of environmental variables and detailed molecular and cellular analyses to determine whether a polymorphism alters function.
2. NUCLEOTIDE OLIGOMERIZATION DOMAIN (NOD)-LIKE RECEPTORS (NLRS)
Overview of NLR Structure and Function
While TLRs are outward looking innate immune receptors detecting microbial signatures either in the extracellular milieu or engulfed in the lumen of endocytic vesicles, nucleotide oligomerization domain (NOD)-like receptors (or NLRs) are a recently appreciated family of receptors that survey the intracellular environment.24, 25 In common with other innate immune receptor systems, the NLRs have ancient origins being structurally reminiscent of plant R-proteins that mediate plant cell defense against pathogenic bacteria. NLRs sense microbial products and metabolic stress driving inflammation through the formation of an inflammasome—a large cytoplasmic complex that activates inflammatory caspases and the production of the cytokines IL-1β and IL-18.26
The human NLR family consists of at least 23 members and can be structurally divided into four subfamily designations N-terminal effector domains.27 The first NLRs reported to have a direct function as intracellular pathogen detectors were NOD1 and NOD2.25 Both NOD proteins detect distinct substructures generated during the synthesis, degradation and remodeling of bacterial peptidoglycan, ensuring the recognition of peptidoglycan from both Gram-positive and Gram-negative bacteria. IPAF (for ICE protease-activating factor) is another member of the NLR family known to detect bacterial pathogens.28 IPAF partners with TLR5 to detect infection by flagellated bacteria—TLR5 senses extracellular flagellin while IPAF focuses on intracellular flagellin. In addition to sensing microbial products, NLRs can sense metabolic stress related to infection and sterile inflammation. This sensing capacity is best demonstrated by NLRP3 (NLR family, pyrin domain-containing 3).29 When triggered NLRP3 (also called NALP3 or cryopyrin) activates the caspase-1 'inflammasome' leading to interleukin 1β (IL-1β) and IL-18 processing (Figure 2). The NLRP3 inflammasome appears to be activated by common metabolic ‘danger signals’ such as potassium efflux which occurs during inflammation due to disruption of the plasma membrane or increased extracellular ATP released by injured cells. Other clinically relevant NLRP3 activators include uric acid, asbestos, silica and alum.
Role of NLRs in human health and disease
Although our molecular appreciation of NLRs is very recent, this class of innate immune receptors plays a central role in several human inflammatory diseases and mediates the adjuvant effect of a common vaccine component, alum.
a) NLR defects associated with inflammatory diseases
The convergence of clinically defined autoinflammatory disease with the biology of innate immunity and NLRs came with the discovery that three well-established autoinflammatory diseases are all caused by activating, gain-of-function mutations in NLRP3.30 These diseases, collectively known as the cryopyrinopathies, are: (i) familial cold autoinflammatory syndrome (OMIM #120100), which presents with cold-induced fevers, urticaria-like rash, and constitutional symptoms; (ii) Muckle-Wells syndrome (OMIM #191900), which is characterized by fevers, hives, sensorineural hearing loss, and arthritis unrelated to cold exposure; and (iii) neonatal-onset multisystem inflammatory disease (NOMID) [or chronic infantile neurologic cutaneous articular (CINCA) syndrome] (OMIM #607115), which is a devastating neonatal disease presenting with fever, urticaria and chronic aseptic meningitis. In these disorders NLRP3 mutations affect IL-1β production, and IL-1β is upregulated in these diseases.31 Appreciation of the role of the IL-1β axis in these diseases associated with NLRP3 mutations has allowed the rational use of targeted anti-inflammatory therapy.32 Strikingly, even the most clinically severe cryopyrinopathy, NOMID/CINCA, appears to respond well to the IL-1 receptor antagonist, anakinra.33
More insight into the clinical relevance of NLRs arose when it was recognized that 30–50% of patients with Crohn's disease in the Western hemisphere carry NOD2 mutations on at least one allele.34, 35 The most common mutations are located in or near the leucine-rich repeat (LRR) domain of NOD2 and patients homozygous for the 3020insC mutation, resulting in partial truncation of the LRR, demonstrate a much more severe disease phenotype. It seems paradoxical that while Crohn's disease results in overt inflammation that probably is triggered by normal bacterial flora, the NOD2 mutations associated with Crohn’s disease result in a protein product less capable of responding to the bacterial ligand, muramyl dipeptide (MDP) which is a component of peptidoglycan. A unifying paradigm addressing this paradox is that NOD2 appears to provide homeostatic signals to maintain the gut environment in a state that is tolerant of its flora and cells with NOD2 mutations are deficient in their production of IL-10, an immunomodulatory and tolerogenic cytokine.36 Other evidence suggests that NOD2 variants are associated with Crohn's disease because they lead to a decrease in the negative regulation of TLR responses occurring in the normal gut, and thus a pathologic increase in responses to the normal flora.37 Nevertheless, the genetic polymorphisms that show a well-established association with Crohn's disease (including NOD2) account for only approximately 20% of the genetic variance observed in Crohn's disease, suggesting that significant additional genetic contributions have yet to be discovered.
b) NLR contribution to vaccine responsiveness
Increased understanding of NLRs has allowed us to shed light on the mechanism of action of vaccine adjuvants.6 Aluminum-containing adjuvants (alum) have historically served as immunopotentiators in vaccines and continue to be the most widely used clinical adjuvants. Despite the fact that most people reading this review have received vaccines containing alum, it is only very recently that we have begun to fully appreciate the molecular mechanism of alum adjuvancy. Studies published in 2008 demonstrated that the NLRP3 (NALP3) inflammasome is involved in mediating the adjuvant effects of alum.38–40 This adjuvancy may occur directly via the triggering of the NALP3 inflammasome by alum crystals, or indirectly through release of the endogenous danger signal, uric acid, which subsequently activates NLRP3.
THERAPEUTIC MODULATION OF INNATE IMMUNITY
With increased appreciation of the contribution of innate immunity to human health and disease, attention quickly shifted to the possibility of therapeutic modulation of innate immunity. This is an area of active investigation, so rather than attempting to survey the field broadly, we will focus our review on recent attempts to harness the TLR system to modulate infectious and allergic diseases.
1. ACTIVATION OF TLRS AND MODULATION OF ALLERGIC IMMUNE RESPONSE
The interaction of two fields of research in the 1990s--epidemiologic investigations of the “hygiene hypothesis” in allergy and asthma, and basic research in the field of TLRs--provided the impetus to investigate whether activating TLRs might represent a novel therapeutic option for the treatment and prevention of allergy and asthma.41 TLR based therapies in allergy target in particular the dendritic cell interaction with T cells, which is a critical component in shaping the Th2 immune response associated with allergic inflammation. As TLRs are highly expressed on dendritic cells but not on T cells, the goal of TLR based therapies in allergy and asthma is to activate dendritic cells to produce a cytokine milieu (IL-12, IFNs, etc) that favors inhibition of Th2 immune response. Thus, TLR based therapies target the innate immune response to consequently inhibit the adaptive Th2 immune response and do not directly target T cells.
Studies have examined whether activation of TLRs can modulate allergic immune responses in pre-clinical animal models of allergy and asthma as well as in more limited studies in human subjects. The majority of studies have evaluated TLR9 agonists, but additional studies have also examined TLR4 agonists and a TLR7/8 agonist. Studies of the TLR9 agonist CpG DNA have demonstrated that it inhibits eosinophilic airway inflammation, Th2 cytokine responses, mucus expression, airway remodeling, and airway responsiveness in a mouse model.41, 42 Administration of an inhaled TLR9 agonist for approximately 8 months to monkeys allergic to dust mite demonstrated that they had reduced eosinophilic airway inflammation, mucus, airway remodeling, and reduced airway responsiveness.43 The only published studies in human asthmatics were performed in mild asymptomatic asthmatics treated with an inhaled TLR9 agonist prior to allergen challenge.44 Although treatment with the inhaled TLR9 agonist increased expression of IFN-inducible genes, there was no inhibition of the early or late phase decrease in forced expiratory volume in one second (FEV1), nor a reduction in sputum eosinophils. These studies suggest that either TLR9 based therapies will not be effective in human subjects with asthma, or that different doses, routes of administration (i.e. systemic vs local), or different study populations (symptomatic asthmatics as opposed to allergen challenged asymptomatic asthmatics) need to be evaluated.
In addition to TLR9 agonists, studies predominantly in mouse models have also evaluated the ability of TLR4 and TLR7/8 based therapies to modulate allergic responses. In mouse models of asthma TLR4 ligands either inhibit or potentiate allergic responses depending upon the timing of administration of the TLR4 ligand and associated allergen sensitization or challenge. In human studies in ragweed allergic rhinitis subjects, administration of a topical intranasal TLR4 ligand was safe but did not inhibit allergic responses in asymptomatic subjects challenged intranasally with ragweed allergen.45 Studies have also investigated whether administration of a TLR7/8 agonist imiquimod would inhibit asthma responses in pre-clinical models. Imiquimod is an FDA-approved therapy which is used as a topical treatment for genital warts, actinic keratoses, and superficial basal cell cancer. In pre-clinical mouse models the TLR7/8 agonist inhibits asthma responses. At present no human studies in allergy or asthma have been reported with the TLR 7/8 agonist.
2. TLR-BASED VACCINE ADJUVANTS IN ALLERGIC DISEASE
Studies have also examined whether administering a TLR9 agonist conjugated to an allergen would enhance the immunogenicity of the allergen when used as a TLR9 conjugated allergen vaccine in allergic rhinitis or asthma. Studies in mouse models have demonstrated that a conjugate of a TLR9 agonist and an allergen had a 100-fold enhanced uptake by antigen presenting cells compared to TLR9 ligand alone.41, 46 The ability of a TLR9 ligand to induce a Th1 immune response is also approximately 100 fold greater than that induced by equivalent amounts of a non-conjugated mixture of the TLR9 ligand and allergen. In mouse models, the TLR9 allergen conjugate significantly reduces rhinitis and asthma responses.41
Thus, based on this enhanced immunogenicity of the TLR9 allergen conjugate, studies have examined whether a TLR9 ragweed allergen conjugate would reduce allergic responses in human subjects with allergic rhinitis. Studies in humans have demonstrated mixed results in terms of the effectiveness of the TLR9 ragweed allergen vaccine. Studies in ragweed allergic rhinitis subjects in Canada demonstrated that administration of the TLR9 ragweed allergen vaccine reduced nasal mucosal biopsy eosinophil counts and Th2 cytokines, but did not reduce nasal symptom scores during the ragweed season.47 A second study in Baltimore demonstrated that administration of the same TLR9 ragweed allergen vaccine significantly reduced rhinitis symptom scores in subjects with ragweed induced allergic rhinitis during the ragweed season.48 Subjects treated with the TLR9 ragweed allergy vaccine also used fewer doses of allergy rescue medications during the ragweed season compared to placebo treated subjects. Interestingly, although the study subjects immunized with the TLR9 ragweed vaccine only received six injections of the vaccine prior to the first ragweed season, the beneficial reduction in symptoms persisted through the second ragweed season without administration of additional vaccine.
At present there are limited numbers of published human studies with either administration of TLRs alone or with TLRs conjugated to allergens. Further studies are thus needed to determine whether the interesting observations regarding TLRs in pre-clinical models will, or will not, translate into safe and effective therapeutic advances in allergy and asthma. Potential safety concerns of TLR based therapies in allergy and asthma include the induction of autoimmune disease. However, induction of autoimmune disease has not been observed in the limited number of clinical trials with TLR-9 based therapies.
3. TLR-BASED VACCINE ADJUVANTS IN INFECTIOUS DISEASE
Vaccination has proved extremely effective in preventing infectious diseases, but knowledge of the immunological mechanisms that allow vaccines to be so successful is rather limited. In contrast to live vaccines, subunit vaccines which consist of specific components of pathogens have little inherent immunogenicity and need to be supplemented with adjuvants to promote a protective immune response. However, there is a paucity of licensed adjuvants for clinical use and, thus, there is a critical need to develop safe and effective adjuvants. The renaissance in innate immune biology is facilitating the rational design of novel vaccine adjuvants.49 Characterization of the NLR system has shed light on the mechanism of action of alum adjuvancy, while our understanding of TLR function is accelerating the discovery of safe and effective vaccine adjuvants.
An illustrative example is development of the novel adjuvant, monophosphoryl lipid A (MPL).50 The TLR4 ligand LPS is a potent adjuvant, but its toxicity prevents use in humans. However, MPL comes from the cell wall LPS of Gram-negative Salmonella minnesota R595 and is detoxified by mild hydrolytic treatment and purification. MPL lacks the toxicity of LPS but retains the beneficial adjuvant properties. MPL combined with aluminum salt (referred to as the AS04 adjuvant system) shows efficacy in a vaccine against human papilloma virus51, and as a hepatitis B vaccine for patients with advanced renal disease.52 Interestingly, this adjuvant combination likely benefits from the immune enhancing capacity of both the TLR pathway (triggered by MPL) and the NALP3 inflammasome (triggered by alum crystals). Further advances in this area are almost certain as many other TLR ligands are being developed as potential vaccine adjuvants.
CONCLUSIONS
In the last decade we have witnessed exhilarating advances in our understanding of the molecular mechanisms used by the innate immune system to sense infection and trigger a protective immune response. For clinicians and scientists alike, the challenge is to now translate this basic mechanistic understanding into a more complete appreciation of the role of innate immunity in health and disease.
ACKNOWLEDGMENTS
We wish to acknowledge members of the UBC Center for Understanding and Preventing Infections in Children for constructive input and Rachel Victor for creating our high quality figures. SET is supported by a Chaim Roifman Scholar Award from the Canadian Immunodeficiency Society and a Career Development Award from the Canadian Child Health Clinician Scientist Program (CCHCSP)-a CIHR Strategic Training Program, and operating grants from the Canadian Cystic Fibrosis Foundation and the CIHR Team in Mutagenesis and Infectious Diseases.
ABBREVIATIONS
- CINCA
Chronic infantile neurological, cutaneous, and articular syndrome
- DAMP
Damage-associated molecular pattern
- HMGB-1
High mobility group box 1
- HSP
Heat-shock protein
- IPAF
ICE protease-activating factor
- IRAK4
Interleukin-1 receptor associated kinase 4
- KIR
Killer-cell immunoglobulin-like receptors
- LBP
LPS binding protein
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat
- MAL
MyD88 adapter-like
- MBL
Mannose-binding lectin
- MDP
Muramyl dipeptide
- MPL
Monophosphoryl lipid A
- MyD88
Myeloid differentiation primary response gene 88
- NLR
nucleotide oligomerization domain (NOD)-like receptors
- NOMID
Neonatal-onset multisystem inflammatory disease
- PAMP
Pathogen-associated molecular pattern
- PRR
Pattern recognition receptor
- RAGE
Receptor of advance glycation end product
- SNP
Single nucleotide polymorphism
- TIR
Toll/interleukin-1 receptor-like domain
- TIRAP
Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 4.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 8.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 12.Ho J, Li Y, Hirschfeld AF, Mansouri D, Turvey SE. Advances in Innate Immunity: The Role of Toll-Like Receptor Signaling in Human Disease. Journal of Respiratory Disease, Thoracic Surgery, Intensive Care, and Tuberculosis. 2004;3:7–14. [Google Scholar]
- 13.Turvey SE, Hawn TR. Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. Clin Immunol. 2006;120:1–9. doi: 10.1016/j.clim.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 15.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, et al. Human TLR-7-, -8-, and -9-Mediated Induction of IFN-alpha/beta and -lambda Is IRAK-4 Dependent and Redundant for Protective Immunity to Viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notarangelo L, Casanova JL, Fischer A, Puck J, Rosen F, Seger R, et al. Primary immunodeficiency diseases: an update. J Allergy Clin Immunol. 2004;114:677–687. doi: 10.1016/j.jaci.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DB, Cavalleri GL. Genomics: understanding human diversity. Nature. 2005;437:1241–1242. doi: 10.1038/4371241a. [DOI] [PubMed] [Google Scholar]
- 19.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez FD. CD14, endotoxin, and asthma risk: actions and interactions. Proc Am Thorac Soc. 2007;4:221–225. doi: 10.1513/pats.200702-035AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174:386–392. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 23.Nejentsev S, Thye T, Szeszko JS, Stevens H, Balabanova Y, Chinbuah AM, et al. Analysis of association of the TIRAP (MAL) S180L variant and tuberculosis in three populations. Nat Genet. 2008;40:261–262. doi: 10.1038/ng0308-261. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 24.Chen G, Shaw MH, Kim Y-G, Nuñez G. NOD-Like Receptors: Role in Innate Immunity and Inflammatory Disease. Annual Review of Pathology: Mechanisms of Disease. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 25.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine. 2008;43:368–373. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Mayor A, Tschopp The Inflammasomes: Guardians of the Body. Annual Review of Immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 27.Ting JPY, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, et al. The NLR Gene Family: A Standard Nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 29.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 35.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 36.Noguchi E, Homma Y, Kang X, Netea MG, Ma X. A Crohn's disease-associated NOD2 mutation suppresses transcription of human IL10 by inhibiting activity of the nuclear ribonucleoprotein hnRNP-A1. Nat Immunol. 2009;10:471–479. doi: 10.1038/ni.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn's disease. Mucosal Immunol. 2008;1 Suppl 1:S5–S9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horner AA, Redecke V, Raz E. Toll-like receptor ligands: hygiene, atopy and therapeutic implications. Curr Opin Allergy Clin Immunol. 2004;4:555–561. doi: 10.1097/00130832-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, et al. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 43.Fanucchi MV, Schelegle ES, Baker GL, Evans MJ, McDonald RJ, Gershwin LJ, et al. Immunostimulatory oligonucleotides attenuate airways remodeling in allergic monkeys. Am J Respir Crit Care Med. 2004;170:1153–1157. doi: 10.1164/rccm.200404-533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauvreau GM, Hessel EM, Boulet LP, Coffman RL, O'Byrne PM. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am J Respir Crit Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- 45.Casale TB, Kessler J, Romero FA. Safety of the intranasal toll-like receptor 4 agonist CRX-675 in allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97:454–456. doi: 10.1016/S1081-1206(10)60934-9. [DOI] [PubMed] [Google Scholar]
- 46.Shirota H, Sano K, Hirasawa N, Terui T, Ohuchi K, Hattori T, et al. Novel roles of CpG oligodeoxynucleotides as a leader for the sampling and presentation of CpG-tagged antigen by dendritic cells. J Immunol. 2001;167:66–74. doi: 10.4049/jimmunol.167.1.66. [DOI] [PubMed] [Google Scholar]
- 47.Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113:235–241. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, et al. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 49.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz TF. AS04-adjuvanted human papillomavirus-16/18 vaccination: recent advances in cervical cancer prevention. Expert Rev Vaccines. 2008;7:1465–1473. doi: 10.1586/14760584.7.10.1465. [DOI] [PubMed] [Google Scholar]
- 52.Kong NCT, Beran J, Kee SA, Miguel JL, Sanchez C, Bayas JM, et al. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int. 2007;73:856–862. doi: 10.1038/sj.ki.5002725. [DOI] [PubMed] [Google Scholar]