Abstract
Objective
The aim of this project was to assess whether any changes in the birth prevalence of cleft lip with/without cleft palate (CL[P]) occurred in Denmark during the period 1988 through 2001. In this period an official recommendation of a supplementation of folic acid to pregnant women was introduced; furthermore, smoking among pregnant women decreased considerably.
Design and Settings
There are few places in which ecological studies of oral clefts are possible. Denmark provides a particularly good setting for this kind of study because of a high ascertainment and a centralized registration of subjects with cleft over the last 65 years.
Participants
Cleft occurrence in Denmark from 1936 to 1987 has previously been reported. Here we extend the study to include all live-born children with oral clefts born in Denmark in 1988 through 2001. Among a total of 992,727 live births, 1332 children with CL(P) were born during this period.
Results and Conclusions
The birth prevalence of CL(P) in Denmark has previously been found to be constant in the period 1962 through 1987, with a frequency of 1.4 to 1.5 per 1000 live births. This study showed a similar occurrence in 1988 through 2001 (birth prevalence = 1.44 per 1000 live births, 95% confidence interval = 1.37 to 1.52). The introduction of folic acid and the decrease in smoking prevalence among pregnant women do not seem to have reduced the birth prevalence. This may be due to noncompliance with the folic acid recommendation and/or only a weak causal association between folic acid and smoking and occurrence of CL(P).
Keywords: birth prevalence, cleft lip, cleft palate, folic acid, smoking
Oral clefts are among the more common congenital malformations, with a reported prevalence between 1 and 2 per 1000 live births (Cornel et al., 1992; Croen et al., 1998).
The etiology of oral clefts is thought to be multifactorial with genes playing the major role, but still very few specific risk factors are known.
Several potential risk factors, including parental age, smoking, and alcohol, have been investigated, but no common strong risk factor has yet been identified (Leite et al., 2002; Zeiger and Beaty, 2002). There is increasing evidence that folic acid supplementation may reduce the incidence of oral clefting (Prescott and Malcolm, 2002), and smoking is the only consistent common risk factor, although the strength of the effect is very modest (Leite et al., 2002).
All medical and dental treatment of children in Denmark is free and centralized, ensuring high ascertainment of oral clefts. Furthermore, Denmark provides nationwide population and health registers, which ensure correct identification of the children (Statistics Denmark, 1995). Consequently, Denmark offers extremely good possibilities for studies of time trends in oral cleft prevalence.
Analyses of the Danish Facial Cleft Register (DFCR) have previously shown a constant birth prevalence for cleft lip with/without cleft palate (CL[P]) of 1.4 to 1.5 per 1000 from 1962 to 1987 (Christensen, 1999). The register has been updated with the 1988 through 2001 cohorts and now offers the possibility to study changes in birth prevalence of oral clefts during this period.
A decline in birth prevalence could be the result of increased folic acid supplementation or a decrease in smoking among fertile women. Folic acid supplementation for pregnant women was discussed during the 1990s but not officially recommended to pregnant Danish women before March 1997. Smoking among pregnant Danish women was found to decrease considerably during the period 1988 through 2001 (Wisborg et al., 1998).
Family studies suggest that CL(P) and cleft palate alone (CP) are two etiologically distinct malformations (Fogh-Andersen, 1967). Furthermore, the etiology of syndromic and nonsyndromic cases are at least in part different, so that analyses are done separately as well as in combination for these two groups (Mitchell et al., 2002).
This report addresses CL(P) and not CP. Because a proportion of patients with CP are diagnosed later in life (especially milder forms and submucous clefts), their inclusion would bias the estimates for the most recent years. The aim of this study was to assess whether there were any changes in birth prevalence of syndromic and nonsyndromic CL(P) during 1988 through 2001.
Methods
The present study was performed after an update of the DFCR.
The DFCR is based on linkage of nationwide ascertainment sources in Denmark and has previously been described and validated (Christensen et al., 1992; Christensen and Fogh-Andersen, 1993). The DFCR comprises the entire 1936 through 1987 birth cohorts of children born in Denmark with oral clefts. The recent update added all children born in Denmark with oral clefts during 1988 through 2001.
Ascertainment was based on the same two sources as the original register:
The two National Institutes for Defects of Speech (NIDS). Since 1954, midwives in Denmark have been required to report every newborn child with oral clefts to one of the institutes. Oral clefts recognized later in a child’s life are also reported to the institutes. In addition to lifelong dental treatment, all treatment is coordinated with dentists, surgeons, and speech therapists.
The University Hospital of Copenhagen. Since the mid-1930s, all surgical treatment of oral clefts in Denmark has been centralized to this site. The hospital’s discharge register comprises information on all treated individuals. Capture-recapture methods applied to the original DFCR have indicated that nearly all (99%) live-born subjects with oral cleft without associated malformations have been ascertained (except submucous CP, which often remains asymptomatic) (Christensen et al., 1992; Christensen and Fogh-Andersen, 1994).
The update added 1332 subjects with CL(P) and 795 subjects with CP to the DFCR, which now includes a total of 9483 subjects with cleft. All but 21 subjects in the update period are registered by a unique 10-digit personal identification number (PIN). The PIN has a built-in check code disclosing invalid numbers and ensuring a highly reliable identification of cases. The PIN enables linkage with the Danish Civil Registration System (2004), which was established in 1968 and registers all individuals with a residence in Denmark on or after April 1, 1968, by means of the PIN. In addition to birth date, which is included in the PIN, birthplace is included in the CPR.
Information on birthplace is used to identify and exclude anyone born outside Denmark. Because of the incomplete ascertainment of defects of stillbirths, only live births were included in the study.
All medical records of subjects born in the update period were scrutinized by one of the authors (C.B.) to obtain information about associated anomalies. Associated anomalies were classified into the categories shown in Table 1. Malformations such as neural tube defects, monogenic traits, syndromes, and sequences were designated as major anomalies. Defects such as congenital dislocation of the hip or polydactyly of digit V were considered minor malformations. Minimal defects such as nevi and undescended testes were not considered associated malformations. When more than one major malformation, one major and two minor malformations, or three minor malformations were present, a subject was classified as cleft with multiple malformations. Chromosomal abnormalities included, among others, trisomy 13, 18, and 21, and monogenic traits included Stickler, Van der Woude, and Apert syndromes. Other syndromes included Cornelia de Lange, Goldenhar, Prader Willi, and fetal alcohol syndrome. Clefts with no or one or two minor associated malformations are referred to below as nonsyndromic subjects, and the remaining subjects are syndromic subjects.
TABLE 1.
Number of Patients With Oral Cleft in the Update Period (1988–2001) of the Danish Facial Cleft Register Sorted Into Different Categories of Associated Malformations
| Type of cleft* |
||||
|---|---|---|---|---|
| CL(P) |
CP |
|||
| Variable | n | % | n | % |
| Isolated oral clefts | 1146 | 86.0 | 513 | 64.5 |
| Major malformations | 44 | 3.3 | 31 | 3.9 |
| Minor malformations | 40 | 3.0 | 30 | 3.8 |
| Pierre Robin | — | — | 107 | 13.5 |
| Mentally retarded | 6 | 0.5 | 9 | 1.2 |
| Monogenic traits excluding Van der Woude | 13 | 1.0 | 24 | 3.0 |
| Van der Woude/fistula labii inferior | 13 | 1.0 | 7 | 0.9 |
| Chromosomal abnormalities | 26 | 1.9 | 12 | 1.5 |
| Multiple malformations | 39 | 2.9 | 41 | 5.2 |
| Syndromes with uncertain inheritance | 5 | 0.4 | 21 | 2.6 |
| Total | 1332 | 100 | 795 | 100 |
CL(P) = cleft lip with or without cleft palate; CP = cleft palate alone.
A previous analysis of time trends in the DFCR showed a delayed entry for subjects with CP, compared with subjects with CL(P) (Christensen, 1999). The later identification of subjects with CP make ecological studies unsuitable for CP in more recent years, so this report deals only with subjects with CL(P). All subjects with CL(P) were divided into nonsyndromic and syndromic subjects and into girls and boys.
Statistics
The overall birth prevalence as well as the birth prevalence of each subgroup was calculated using data on number of live births in Denmark from Statistics Denmark (Danmarks Statistik, 2004). All prevalences were graphed by year of birth for the period 1962 through 2001. A smoothing procedure was used (5-year moving average). For the first two and last two cohorts in the period, the smoothed birth prevalence was calculated as the mean of the obtainable 3 or 4 observation years.
Birth prevalences for nonsyndromic subjects in addition to sex-specific curves were graphed. Curves for cleft lip alone were made but did not differ substantially from the CL(P) curves except level and are not shown.
Logistic regressions were used to test for any changes in birth prevalence of CL(P) during the periods 1962 through 2001 and 1988 through 2001. The tendencies for an un-smoothed overall birth prevalence of CL(P) were further visualized by a regression line for the period 1962 through 2001 (see Fig. 2).
FIGURE 2.
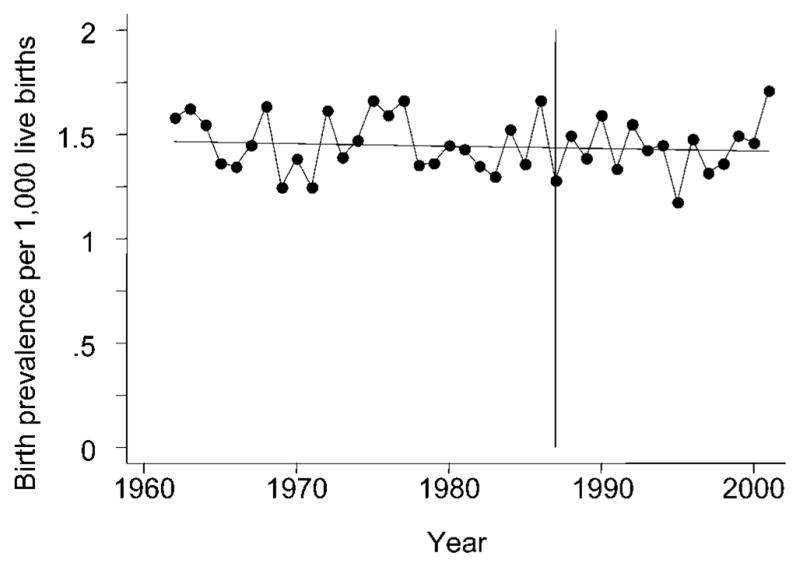
Overall birth prevalence of cleft lip with/without cleft palate per 1000 live births in Denmark during 1962 through 2001 with fitted regression line. The vertical line indicates the beginning of the update period, January 1988.
Confounder Control
Maternal and paternal ages have increased over the last decades. Previous unpublished work has shown that the joint effect of maternal and paternal ages is associated with the risk of CL(P), but the contribution depends on the age of the other parent. Although the increase in birth prevalence of CL(P) because of increased parental age is small, an analysis of confounding by parental age was necessary. Parental age distribution for every single live birth in Denmark was obtainable for the period 1973 through 1996 (Knudsen, 1998). The distribution of maternal and paternal ages in 5-year intervals (15 through 19 years, …, 45 through 49 years, 50+ years) was calculated for the period 1973 through 1996. The corresponding parental age distribution among subjects with CL(P) was retrieved from the DFCR. Parental age-specific case rates were calculated for the whole period 1973 through 1996 and then applied to the overall parental age distribution in the years 1987 and 1996. The expected number of cases was calculated for the 2 years, 1987 and 1996, and the difference because of increased parental age through 1987 to 1996 was calculated.
Results
A total of 2127 subjects with oral cleft were identified among 922,727 live births in the period 1988 through 2001. As shown in Table 1, there was a total of 1332 subjects with CL(P) and 795 subjects with CP. Associated malformations were found in 14.0% of the subjects with CL(P) and in 35.5% of the subjects with CP, of which 3.0% and 3.8%, respectively, had only minor malformations. Thus, syndromic cases constituted 11.0% of the subjects with CL(P) and 31.7% of the subjects with CP.
Figure 1 presents the overall smoothed birth prevalence for CL(P) for the entire 1962 through 2001 cohorts. Both overall and sex-specific birth prevalence of CL(P) in the update period 1988 through 2001 appears to be rather constant and at the same level as the birth prevalence in the period 1962 through 1987. The gap between the curves representing all subjects and nonsyndromic subjects only is greater and slightly increasing throughout the update period (1988 through 2001), compared with the period 1962 through 1987.
FIGURE 1.

Smoothed prevalence (5-year moving average) of subgroups of cleft lip with/without cleft palate per 1000 live births in Denmark during 1962 through 2001. The vertical line indicates the beginning of the update period, January 1988. (all,———; nonsyndromic,–··–; male,– – –; female,— — —).
The constant overall birth prevalence of CL(P) is visualized in Figure 2 by the fitted logistic regression line for the 1962 through 2001 cohorts. The regression line is horizontal (β = −0.001, 95% confidence interval [CI] = −0.005 to 0.002), indicating no changes in birth prevalence over time. Furthermore, simple logistic regression shows changes in the birth prevalence of CL(P) in the update period 1988 through 2001 when analyzed separately (β = −0.003, 95% CI = −0.018 to 0.023). Mean birth prevalence in this period was estimated to be 1.44 per 1000 live births.
Discussion
The birth prevalence for CL(P) in Denmark in the update period 1988 through 2001 was rather constant and equal to the constant prevalence of 1.4 to 1.5 per 1000 births in the preceding period 1962 through 1987 (Christensen, 1999). An increase in birth prevalence during 1936 through 1961 was previously explained by a combination of improvement of survival among newborn subjects with cleft and a better ascertainment of syndromic cases and small clefts (Christensen, 1999). This suggests that no major changes in birth prevalence of CL(P) have occurred during the last 65 years.
The ascertainment, made through multiple information sources, is one of the strengths of this study. It has been shown for the 1936 through 1987 cohorts that when using the two NIDS and the surgical records an ascertainment of 99% of subjects without associated malformations were achieved. It is, however, possible that the births of severely malformed children with clefts are not reported immediately and then only if they survive the first weeks of life. The true number of subjects with cleft may therefore be slightly higher and biased toward an increasing ascertainment over time because of increased survival of syndromic clefts. The number of such subjects is probably small and the ascertainment of subjects with nonsyndromic cleft is unaffected by this increased ascertainment. The ascertainment of nonsyndromic clefts is, however, affected by increased detection of accompanying defects in subjects formerly thought to be nonsyndromic. An increased detection rate of associated anomalies is seen for the update period. This probably is due to the thorough scrutinizing of medical records but also to the enhanced focus and knowledge of associated anomalies and syndromes during the last decades. The increase is seen in Figure 1 in which the constant overall birth prevalence and the decreasing birth prevalence of nonsyndromic clefts result in an increasing gap between the two curves.
Definition and thoroughness of classification of associated malformations differ widely between studies. The percentage of syndromic cases with CL(P) for the 1988 through 2001 cohorts in the present study was 11%. Compared with other studies that have found associated anomalies in as many as 26.4%, this number is small (Croen et al., 1998; Tolarova and Cervenka, 1998). The occurrence of associated anomalies is therefore probably underreported in our register. An examination of every single case by a trained clinical geneticist would have been preferable. However, the most severe cases are identified at surgery, leaving only the milder forms un-identified and mixed with the study group of nonsyndromic clefts. Additionally, the proportion of subjects with cleft with Van der Woude syndrome of 1% for CL(P) and 0.9% for CP is in agreement with other studies in which each individual was examined by a clinical geneticist (Murray et al., 1997), indicating a rather good detection rate.
During the update period, changes in intensity of several risk factors might have influenced the birth prevalence of CL(P). On the one hand, a decreasing tendency is expected because of a decrease in smoking among pregnant women and an increase in supplementation of folic acid. On the other hand, the improved treatment of subjects with cleft possibly leading to a higher fertility for these together with the substantial increased recurrence risk among clefts result in an increase in birth prevalence of CL(P). Another increasing factor for the birth prevalence of CL(P) might be the increased parental age.
Our analysis of parental age showed an expected increase in birth prevalence of nonsyndromic CL(P) of 1.1% in the period 1987 through 1996 because of an increase in parental age. The increase in maternal age during the period 1996 through 2001 is only 0.4 years, compared with 1.2 years for the period 1987 through 1996. As such, the assembled influence of parental age during the whole study period is estimated to be around 1.5%.
The increase in syndromic CL(P) could also reflect a true increase because of increased maternal age, resulting in an increased occurrence of chromosomal abnormalities in which CL(P) is included. Parental age has, however, been increasing throughout the 1970s and 1980s without any visible increase in syndromic CL(P), as seen in Figure 1.
A decrease in birth prevalence of neural tube defects has been found after folic acid fortification of cereal grain products in both the United States and Canada (Ray et al., 2002; Williams et al., 2002). Animal studies and studies of folate antagonists (Paros and Beck, 1999; Hernandez-Diaz et al., 2000; Schubert et al., 2002) suggest a similar correlation between folate metabolism and orofacial morphogenesis. Recent case-control studies and clinical trials (Shaw et al., 1995; Tolarova and Harris, 1995; Czeizel et al., 1999; Hartridge et al., 1999) have presented conflicting results with regard to the beneficial effects of folic acid supplementation during pregnancy for the prevention of oral clefts.
Official recommendations for supplementation of 400 μg of folic acid per day started in 1997 in Denmark (Rasmussen et al., 1997). However, in 2001 only 13% of pregnant Danish woman used a supplement of folic acid corresponding to the recommendations (Olsen et al., 2003). A preventive effect of folic acid is difficult to detect when only a low percentage of pregnant women are following the recommendations.
Studies concerning the risk of oral clefts and maternal smoking are more consistent (Kallen, 1997; Lieff et al., 1999; Chung et al., 2000). The majority, including a meta-analysis, have shown that periconceptional smoking is a risk factor, although the excess risk is modest (odds ratio = 1.2; Kallen, 1997; Wyszynski et al., 1997; Leite et al., 2002; Wyszynski and Wu, 2002).
Thirty-four percent of pregnant Danish women were smokers in 1989 (Wisborg et al., 1998). With a relative risk for CL(P) of 1.4 for smokers (Christensen et al., 1999), the attributable fraction of smoking is 12%, assuming that all of the excess risk is attributable to smoking. A complete elimination of smoking among pregnant women would therefore result in a 12% decrease in occurrence of CL(P). The corresponding proportion of smokers in 2001 was 24% (National Health Service of Denmark, 2004). A 10% decrease in smoking (from 34% in 1989 to 24% in 2001) would result in a 4% decrease in occurrence of CL(P).
We found no decrease in birth prevalence of CL(P) in Denmark during the update period (1988 through 2001). There can be several reasons that neither reduced smoking nor the introduction of folic acid seems to have reduced the occurrence of CL(P). One reason could be that only few women followed the folic acid supplement recommendation. Another could be a masked effect because of an increase in other still-unknown risk factors. A third reason could be that there was only a weak causal association between folic acid and smoking and the occurrence of CL(P).
Acknowledgments
We thank Jeff Murray for useful comments on the draft version of this work.
Funding and other support for this project was provided by the Egmont Foundation; the National Institute of Dental Research (R01 DE 11948); the Faculty of Health, University of Southern Denmark; the two National Institutes of Defects of Speech; the University Hospital of Copenhagen; and Statistics Denmark.
Contributor Information
Dr. Camilla Bille, Institute of Public Health, University of Southern Denmark, Odense, Denmark.
Mrs. Lisbeth B. Knudsen, Department of Social Studies and Organization, Aalborg University, Aalborg, Denmark
Dr. Kaare Christensen, Institute of Public Health, University of Southern Denmark, Odense, Denmark.
References
- Christensen K. The 20th century Danish facial cleft population—epidemiological and genetic-epidemiological studies. Cleft Palate Craniofac J. 1999;36:96–104. doi: 10.1597/1545-1569_1999_036_0096_tcdfcp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Christensen K, Fogh-Andersen P. Etiological subgroups in non-syndromic isolated cleft palate. A genetic-epidemiological study of 52 Danish birth cohorts. Clin Genet. 1994;46:329–335. doi: 10.1111/j.1399-0004.1994.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Christensen K, Fogh-Andersen P. Isolated cleft palate in Danish multiple births, 1970–1990. Cleft Palate Craniofac J. 1993;30:469–474. doi: 10.1597/1545-1569_1993_030_0469_icpidm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Christensen K, Holm NV, Olsen J, Kock K, Fogh-Andersen P. Selection bias in genetic-epidemiological studies of cleft lip and palate. Am J Hum Genet. 1992;51:654–659. [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Olsen J, Norgaard-Pedersen B, Basso O, Stovring H, Milhollin-Johnson L, Murray JC. Oral clefts, transforming growth factor alpha gene variants, and maternal smoking: a population-based case-control study in Denmark, 1991–1994. Am J Epidemiol. 1999;149:248–255. doi: 10.1093/oxfordjournals.aje.a009799. [DOI] [PubMed] [Google Scholar]
- Chung KC, Kowalski CP, Kim HM, Buchman SR. Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg. 2000;105:485–491. doi: 10.1097/00006534-200002000-00001. [DOI] [PubMed] [Google Scholar]
- [Accessed March 2004];Civil Registration System in Denmark. Available at: http://www.cpr.dk/
- Cornel MC, Spreen JA, Meijer I, Spauwen PH, Dhar BK, ten Kate LP. Some epidemiological data on oral clefts in the northern Netherlands, 1981–1988. J Craniomaxillofac Surg. 1992;20:147–152. doi: 10.1016/s1010-5182(05)80389-0. [DOI] [PubMed] [Google Scholar]
- Croen LA, Shaw GM, Wasserman CR, Tolarova MM. Racial and ethnic variations in the prevalence of orofacial clefts in California, 1983–1992. Am J Med Genet. 1998;79:42–47. doi: 10.1002/(sici)1096-8628(19980827)79:1<42::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Timar L, Sarkozi A. Dose-dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics. 1999;104:e66. doi: 10.1542/peds.104.6.e66. [DOI] [PubMed] [Google Scholar]
- [Accessed March 2004];Danmarks Statistik. Available at: http://www.statistikbanken.dk.
- Fogh-Andersen P. Genetic and non-genetic factors in the etiology of facial clefts. Scand J Plast Reconstr Surg Hand Surg. 1967;1:22–29. [Google Scholar]
- Hartridge T, Illing HM, Sandy JR. The role of folic acid in oral clefting. Br J Orthod. 1999;26:115–120. doi: 10.1093/ortho/26.2.115. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- Kallen K. Maternal smoking and orofacial clefts. Cleft Palate Craniofac J. 1997;34:11–16. doi: 10.1597/1545-1569_1997_034_0011_msaoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Knudsen LB. The Danish Fertility Database. Dan Med Bull. 1998;45:221–225. [PubMed] [Google Scholar]
- Leite IC, Paumgartten FJ, Koifman S. Chemical exposure during pregnancy and oral clefts in newborns. Cad Saude Publica. 2002;18:17–31. doi: 10.1590/s0102-311x2002000100003. [DOI] [PubMed] [Google Scholar]
- Lieff S, Olshan AF, Werler M, Strauss RP, Smith J, Mitchell A. Maternal cigarette smoking during pregnancy and risk of oral clefts in newborns. Am J Epidemiol. 1999;150:683–694. doi: 10.1093/oxfordjournals.aje.a010071. [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Beaty TH, Lidral AC, Munger RG, Murray JC, Saal HM, Wyszynski DF. Guidelines for the design and analysis of studies on nonsyndromic cleft lip and cleft palate in humans: summary report from a Workshop of the International Consortium for Oral Clefts Genetics. Cleft Palate Craniofac J. 2002;39:93–100. doi: 10.1597/1545-1569_2002_039_0093_gftdaa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Murray JC, Daack-Hirsch S, Buetow KH, Munger R, Espina L, Paglinawan N, Villanueva E, Rary J, Magee K, Magee W. Clinical and epidemiologic studies of cleft lip and palate in the Philippines. Cleft Palate Craniofac J. 1997;34:7–10. doi: 10.1597/1545-1569_1997_034_0007_caesoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- National Health Service of Denmark. [Accessed March 2004];(Sundhedsstyrelsen). Trends in smoking among the Danes [in Danish] Available at: http://www.sst.dk/Borgerinfo/Tobak/Fakta_stat/Udvikling_i_danskernes_rygevaner.aspx?lang=da.
- Olsen SF, Michaelsen KF, Rasmussen LB, Knudsen VK. Folic acid to pregnant women [Report in Danish] Søborg, Denmark: The Danish Nutrition Council; 2003. [Google Scholar]
- Paros A, Beck SL. Folinic acid reduces cleft lip [CL(P)] in A/WySn mice. Teratology. 1999;60:344–347. doi: 10.1002/(SICI)1096-9926(199912)60:6<344::AID-TERA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Prescott NJ, Malcolm S. Folate and the face: evaluating the evidence for the influence of folate genes on craniofacial development. Cleft Palate Craniofac J. 2002;39:327–331. doi: 10.1597/1545-1569_2002_039_0327_fatfet_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Rasmussen LB, Andersen NL, Andersson G, Lange AP, Rasmussen K, Skovby F, Ovesen L. Folate and neural tube defects. Søborg, Denmark: The Danish Nutrition Council; 1997. [Report in Danish] [PubMed] [Google Scholar]
- Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet. 2002;360:2047–2048. doi: 10.1016/S0140-6736(02)11994-5. [DOI] [PubMed] [Google Scholar]
- Schubert J, Schmidt R, Syska E. B group vitamins and cleft lip and cleft palate. Int J Oral Maxillofac Surg. 2002;31:410–413. doi: 10.1054/ijom.2001.0212. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Lammer EJ, Wasserman CR, O’Malley CD, Tolarova MM. Risks of orofacial clefts in children born to women using multivitamins containing folic acid periconceptionally. Lancet. 1995;346:393–396. doi: 10.1016/s0140-6736(95)92778-6. [DOI] [PubMed] [Google Scholar]
- Statistics Denmark. Eurostat Statistics Denmark: statistics on persons, a register-based statistical system. Copenhagen: Statistics Denmark; 1995. [Google Scholar]
- Tolarova MM, Cervenka J. Classification and birth prevalence of orofacial clefts. Am J Med Genet. 1998;75:126–137. [PubMed] [Google Scholar]
- Tolarova M, Harris J. Reduced recurrence of orofacial clefts after periconceptional supplementation with high-dose folic acid and multivitamins. Teratology. 1995;51:71–78. doi: 10.1002/tera.1420510205. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LE, Miller LA, Meaney FJ, Levitt M. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–39. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Henriksen TB, Hedegaard M, Secher NJ. Smoking habits among Danish pregnant women from 1989 to 1996 in relation to sociodemographic and lifestyle factors. Acta Obstet Gynecol Scand. 1998;77:836–840. [PubMed] [Google Scholar]
- Wyszynski DF, Duffy DL, Beaty TH. Maternal cigarette smoking and oral clefts: a meta-analysis. Cleft Palate Craniofac J. 1997;34:206–210. doi: 10.1597/1545-1569_1997_034_0206_mcsaoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Wyszynski DF, Wu T. Use of U.S. birth certificate data to estimate the risk of maternal cigarette smoking for oral clefting. Cleft Palate Craniofac J. 2002;39:188–192. doi: 10.1597/1545-1569_2002_039_0188_uousbc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Zeiger JS, Beaty TH. Is there a relationship between risk factors for oral clefts? Teratology. 2002;66:205–208. doi: 10.1002/tera.10104. [DOI] [PubMed] [Google Scholar]


