Abstract
By using random PCR amplification, shotgun sequencing and sequence similarity searches, we analysed nucleic acids present in cell cultures inoculated with samples from unexplained cases of encephalitis. We identified a divergent human papillomavirus (HPV) sequence originating from a rectal swab. The full genome was amplified by inverse PCR and sequenced. The prototype of the sixth gammapapillomavirus species, HPV116, was not found in the patient's cerebrospinal fluid or respiratory secretions, nor in culture supernatants from other unexplained cases of encephalitis, indicating that its identification in an encephalitis patient was accidental.
Papillomaviruses (PVs) are small, non-enveloped, circular double-strand DNA (dsDNA) viruses with a genome size of 6.8–8.4 kb (de Villiers et al., 2005). The family Papillomaviridae comprises a large and heterogeneous group of epitheliotropic viruses, which are either present as presumed commensal viruses of the skin and mucosa or able to induce benign and malignant epithelial proliferations (Antonsson et al., 2000, 2003; Doorbar, 2005). PVs infect a wide variety of vertebrate species, including many mammalian species such as humans (HPVs), cow (bovine PVs) (Borzacchiello & Roperto, 2008), deer (DPV) (Groff & Lancaster, 1985), dog (canine oral PV) (Delius et al., 1994), horse (Equus caballus PV) (Ghim et al., 2004), hedgehog (European hedgehog PV) (Schulz et al., 2009), as well as bird and reptile species including parrot (Psittacus erithacus timneh PV) (Tachezy et al., 2002) and sea turtle (Chelonia mydas PV-1) (Herbst et al., 2009). PV infections are typically highly host species-specific, but some PV types have been reported in different mammalian species (Chambers et al., 2003; Martens et al., 2001).
So far, more than 100 HPVs and about 30 non-human PVs have been characterized fully and more than 150 have been partially sequenced; they have been classified phylogenetically into 16 genera and 44 species based on nucleotide sequence similarity (Chan et al., 1995; de Villiers et al., 2004; de Villiers & Gunst, 2009; Forslund, 2007). Members of only five genera have been identified in humans. For a PV to be recognized as a novel type, the sequence of the L1 gene should have <90 % similarity to any known HPV type, whilst a novel species should have <70 % similarity and a novel genus <60 % similarity (de Villiers et al., 2004; de Villiers & Gunst, 2009). The current members of the genus Gammapapillomavirus are all from humans and include five species, most of which cause benign skin lesions (Egawa et al., 1993; Favre et al., 1989; Matsukura et al., 1992; Muller et al., 1989). It was reported that two γ-PV types, HPV48 and HPV88, were isolated from the squamous cell carcinoma of immunosuppressed patients (Kullander et al., 2008; Muller et al., 1989).
Using sequence-independent PCR (random PCR) amplification, shotgun sequencing and sequence similarity searches (Victoria et al., 2009), we analysed the virus sequences present in the supernatants of 159 cell cultures inoculated with various clinical materials from cases of unexplained encephalitis. These cell cultures were performed to amplify known or novel viral agents associated with encephalitis. Human fetal diploid lung (HFDL) and rhesus monkey (RMK) cells were inoculated separately with the nasopharyngeal wash/throat swab, stool/rectal swab or cerebrospinal fluid (CSF) of encephalitis patients. Stools and rectal swabs were initially tested to determine whether enterovirus infections were present. No cytopathic effect was observed after 14 days in culture. Following shotgun sequencing of the supernatants, a divergent HPV sequence was identified in a 14 day culture supernatant inoculated with a rectal swab from a human immunodeficiency virus antibody-negative 38-year-old Asian male patient with encephalitis, whose CSF and respiratory swab were negative by PCR for known encephalitis-causing pathogens, including herpes simplex virus 1/2, varicella-zoster virus, human herpesvirus 6, enterovirus, influenza A/B virus, adenovirus, human metapneumovirus, human respiratory syncytial virus and mycoplasma (Glaser, et al., 2006). This study was approved by the Institute Review Board of the State of California and the University of California San Francisco Committee on Human Research.
The original 408 bp HPV sequence was obtained from the culture supernatant by a previously described method (Victoria et al., 2008). In brief, the supernatants were filtered through a 0.45 μm pore-size sterile filter (Millipore Ultrafree-MC HV). The filtrates were ultracentrifuged and the resulting pellets were resuspended. DNase and RNase were added to remove non-virus-particle-protected nucleic acid. Particle-protected nucleic acids were then extracted by using a QIAamp Viral RNA Mini kit (Qiagen). The resulting viral nucleic acid samples were converted to cDNA by reverse transcription and then amplified randomly. The DNA products of approximately 400–1500 bp were collected and cloned. Forty-eight white colonies were picked and sequenced for each sample. The remaining part of the circular DNA genome was generated by inverse nested PCR. The primers were HPV-F1 (5′-TAGATGGCTACTTGGACCCCT), HPV-R1 (5′-ATTGCTGCTTATGTCTGTGGG), HPV-F2 (5′-CTAAACCTGTGGCGACTGTTC) and HPV-R2 (5′-ACACCCTAACACCAATACCCG). A PCR product of approximately 7 kb was generated by overlapping both ends of the original fragment, and then sequenced by primer walking. The nucleotide sequence of the complete genome was covered twice and the sequence was deposited in GenBank under accession number FJ804072. Putative open reading frames (ORFs) were predicted by Vector NTI Advance 10.3 (Invitrogen), taking into consideration the organization of other PV type genomes. The complete genome sequence and DNA plasmid subclones covering the whole genome were submitted to the International Reference Centre for Papillomaviruses at the German Cancer Research Centre, Heidelberg, Germany, and designated HPV116.
The nucleotide sequence of HPV116 was 7184 bp in length, with a G+C content of 38.5 mol%. HPV116 was related closely to γ-PVs, with L1 gene nucleotide similarity ranging from 58.5 to 62.0 %, and the E1 gene shared 59.9–65.7 % nucleotide similarity with known γ-PV types (Table 1). Phylogenetic analysis of the sequence data indicated that the virus, HPV116, was a γ-PV. According to the criteria for PV classification, HPV116 therefore qualified as a novel species, with <70 % similarity of the L1 gene to any previously reported PV type.
Table 1.
Nucleotide sequence pairwise comparison of HPV116 ORFs and URR with types representing γ-HPV species
Sequences for the γ-HPV types HPV4 (X70827), HPV65 (X70829), HPV95 (AJ620210), HPV48 (U31789), HPV50 (U31790), HPV60 (U31792) and HPV88 (EF467176) were obtained from GenBank.
| HPV type (species) | Pairwise similarity with HPV116 (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| E6 | E7 | E1 | E2 | E4 | L2 | L1 | URR | |
| HPV4 (γ1) | 55.6 | 52.9 | 64.4 | 58.8 | 53.1 | 51.1 | 60.7 | 46.2 |
| HPV65 (γ1) | 57.7 | 54.4 | 65.7 | 57.8 | 50.3 | 50.8 | 59.6 | 45.2 |
| HPV95 (γ1) | 56.0 | 53.5 | 65.4 | 59.1 | 50.9 | 50.7 | 61.5 | 44.7 |
| HPV48 (γ2) | 60.0 | 55.0 | 60.1 | 52.6 | 46.3 | 49.9 | 59.6 | 37.1 |
| HPV50 (γ3) | 57.2 | 55.3 | 59.9 | 53.6 | 46.9 | 51.9 | 58.5 | 40.7 |
| HPV60 (γ4) | 51.7 | 53.1 | 63.7 | 56.6 | 51.7 | 51.0 | 61.5 | 33.3 |
| HPV88 (γ5) | 48.9 | 58.4 | 62.5 | 56.8 | 49.6 | 54.7 | 62.0 | 41.3 |
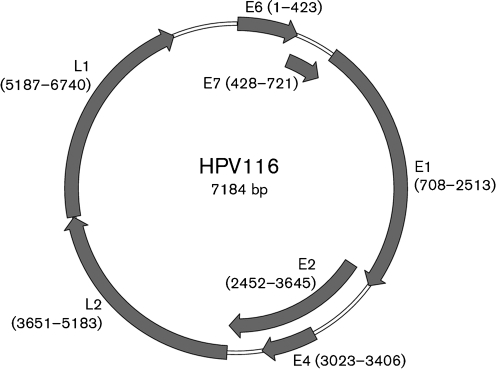
The genome of HPV116 has seven ORFs, potentially encoding the genes E6, E7, E1, E2, E4, L2 and L1 (Fig. 1). None of the small ORFs (<100 aa) or the ORFs with opposite orientation in the genome showed any similarity to known E5 genes (Leechanachai et al., 1992). The putative E6 and E7 ORFs contained respectively two and one conserved zinc-binding domains, Cys-Xaa2-Cys-Xaa29–30-Cys-Xaa2-Cys, spaced 36 resides apart (Ullman et al., 1996). The conserved ATP-binding site of the ATP-dependent helicase (GPPDTGKS) was also found in the E1 ORF. The putative E4 ORF located within the E2 ORF was the smallest (128 aa) among all known γ-PV types. A non-coding region, the upstream regulatory region (URR), was located between the L1 and E6 ORFs and was 441 bp long. No non-coding region existed between the E2 and L2 ORFs. The URR contained seven typical E2-binding sites (ACCN6GGT), a TATA box (TATAA) and multiple previously reported transcription factor-binding sites for AP-1, GATA and USF, which might be involved in the regulation of virus transcription and replication (Chen et al., 2007a, b).
Fig. 1.
Organization of the HPV116 genome. Predicted ORFs are indicated by arrows proportional to the gene length, with start and end nucleotide positions marked. The first ATG of the E6 ORF was defined as the starting position of the genome.
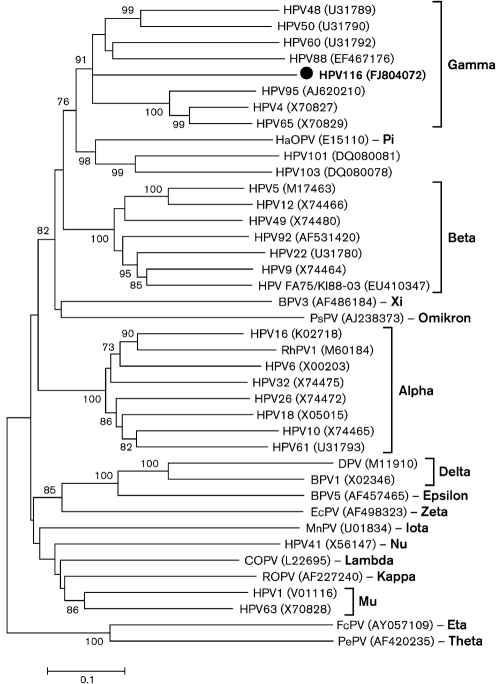
Phylogenetic analysis with complete L1 nucleotide sequences of representative PV types confirmed the existence of the genera and species defined by previous studies and demonstrated that HPV116 was related most closely to γ-PV types HPV95, HPV65 and HPV88 (Fig. 2) (de Villiers et al., 2004; de Villiers & Gunst, 2009). The phylogenetic tree constructed from the E1 region showed a similar relationship (data not shown). The identity of L1 nucleotide sequences between HPV116 and γ-PV types was <62.0 %, which is lower than some γ-PV interspecies identities, such as 70.2 % between HPV48 (γ2) and HPV50 (γ3) and 65.5 % between HPV60 (γ4) and HPV88 (γ5) (data not shown).
Fig. 2.
Phylogenetic analysis of HPV116 and representative HPV types based on the complete nucleotide sequence of the L1 region, using the neighbour-joining method with 1000 bootstrap replicates. All HPV sequences were obtained from GenBank; accession numbers are shown in parentheses. Abbreviations: BPV, bovine papillomavirus; COPV, canine oral papillomavirus; DPV, deer papillomavirus; EcPV, Equus caballus papillomavirus; FcPV, Fringilla coelebs papillomavirus; HaOPV, hamster oral papillomavirus; MnPV, Mastomys natalensis papillomavirus; PePV, Psittacus erithacus timneh papillomavirus; PsPV, Phocoena spinipinnis papillomavirus; RhPV1, rhesus papillomavirus type 1; ROPV, rabbit oral papillomavirus.
As HPV116 was detected in the 14 day cell-culture supernatants (passage 1) of primary HFDL and RMK cells, we investigated its potential replication in vitro by using real-time PCR. For the establishment of standard curves, serial dilutions of a plasmid containing 500 bp of the E1 region of HPV116 ranging from 106 copies to 10 copies per reaction were employed in SYBR Green real-time PCR (Applied Biosystems). The viral loads in the original rectal-swab specimen used to inoculate the cells and the supernatants of passage 1 and of three subsequent serial passages in HFDL and RMK cells were measured. The rectal-swab suspension had the highest HPV116 DNA copy number of 104 μl−1; the viral copy number decreased by approximately one order of magnitude at each in vitro passage, becoming undetectable at passage 4 in both cell lines. Considering the 10-fold dilutions of the original swab suspension for the first cell-culture inoculations and upon each successive passage, it appeared that no virus replication took place in vitro, although HPV116 DNA was still detected after 6 weeks in culture, reflecting the robustness of the small, circular dsDNA PV genomes.
All known PV types require terminally differentiated squamous epithelium cells of skin or mucosa for replication and virion production, making it difficult to use a reproducible tissue-culture system to study the virus life cycle. Researchers have launched intensive efforts to search for an effective culture system for PV, but have had only limited success using organotypic raft cultures of keratinocytes, oral cavity epithelial cells, the athymic mouse xenograft system or related systems for human cutaneous or mucosal membranes (Aaltonen et al., 1998; Bryan et al., 2000; Chen et al., 2003; Taichman et al., 1984). Our results showing the lack of replication in HFDL and RMK cells are consistent with these previous studies.
To determine the prevalence of HPV116, 161 additional 14 day cell-culture supernatants inoculated with nasopharyngeal/throat swab, rectal/stool swab or CSF samples from 100 cases of unexplained encephalitis were screened by HPV116-specific PCR. The CSF and respiratory fluids collected from the index patient were also tested. No further PCR-positive sample was detected, indicating that the occurrence of this virus in encephalitis patients is not a common event and that the virus was not present in the CSF or respiratory secretions of the rectally infected patient. The detection of HPV116 in an encephalitis patient therefore appears to be a fortuitous occurrence and may not be related to his clinical condition.
We therefore report the identification of a novel HPV type, which has <70 % similarity of the L1 gene to any known PV type and was present in a rectal-swab suspension at a viral load of 104 μl−1. Previous studies have shown that PVs are commonly present on the healthy skin or mucosa of healthy humans, presumably as a commensal agent (Antonsson et al., 2000, 2003; Weissenborn et al., 2009). The characterization of HPV116 enlarges our knowledge of the diversity of γ-PVs. The medical significance of this virus will require further study.
Acknowledgments
We thank Drs Flavien Bernardin, Elisabeth Slikas and Tzong-Hae Lee for helpful advice with the real-time PCR and automated robotic-extraction techniques. The work was supported by the Blood Systems Research Institute and NIH grant R01 HL083254 to E. D.
Footnotes
The GenBank/EMBL/DDBJ accession number for the complete genome sequence of HPV116 is FJ804072.
References
- Aaltonen, L. M., Wahlstrom, T., Rihkanen, H. & Vaheri, A. (1998). A novel method to culture laryngeal human papillomavirus-positive epithelial cells produces papilloma-type cytology on collagen rafts. Eur J Cancer 34, 1111–1116. [DOI] [PubMed] [Google Scholar]
- Antonsson, A., Forslund, O., Ekberg, H., Sterner, G. & Hansson, B. G. (2000). The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol 74, 11636–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson, A., Erfurt, C., Hazard, K., Holmgren, V., Simon, M., Kataoka, A., Hossain, S., Hakangard, C. & Hansson, B. G. (2003). Prevalence and type spectrum of human papillomaviruses in healthy skin samples collected in three continents. J Gen Virol 84, 1881–1886. [DOI] [PubMed] [Google Scholar]
- Borzacchiello, G. & Roperto, F. (2008). Bovine papillomaviruses, papillomas and cancer in cattle. Vet Res 39, 45. [DOI] [PubMed] [Google Scholar]
- Bryan, J. T., Tekchandani, J., Schroeder, J. M. & Brown, D. R. (2000). Propagation of human papillomavirus type 59 in the athymic mouse xenograft system. Intervirology 43, 112–118. [DOI] [PubMed] [Google Scholar]
- Chambers, G., Ellsmore, V. A., O'Brien, P. M., Reid, S. W., Love, S., Campo, M. S. & Nasir, L. (2003). Association of bovine papillomavirus with the equine sarcoid. J Gen Virol 84, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Chan, S. Y., Delius, H., Halpern, A. L. & Bernard, H. U. (1995). Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J Virol 69, 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. W., Aalto, Y., Teesalu, T., Durst, M., Knuutila, S., Aaltonen, L. M. & Vaheri, A. (2003). Establishment and characterisation of human papillomavirus type 16 DNA immortalised human tonsillar epithelial cell lines. Eur J Cancer 39, 698–707. [DOI] [PubMed] [Google Scholar]
- Chen, Z., Schiffman, M., Herrero, R. & Burk, R. D. (2007a). Identification and characterization of two novel human papillomaviruses (HPVs) by overlapping PCR: HPV102 and HPV106. J Gen Virol 88, 2952–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Schiffman, M., Herrero, R., Desalle, R. & Burk, R. D. (2007b). Human papillomavirus (HPV) types 101 and 103 isolated from cervicovaginal cells lack an E6 open reading frame (ORF) and are related to gamma-papillomaviruses. Virology 360, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius, H., Van Ranst, M. A., Jenson, A. B., zur Hausen, H. & Sundberg, J. P. (1994). Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology 204, 447–452. [DOI] [PubMed] [Google Scholar]
- de Villiers, E.-M. & Gunst, K. (2009). Characterization of seven novel human papillomavirus types isolated from cutaneous tissue, but also present in mucosal lesions. J Gen Virol 90, 1999–2004. [DOI] [PubMed] [Google Scholar]
- de Villiers, E. M., Fauquet, C., Broker, T. R., Bernard, H. U. & zur Hausen, H. (2004). Classification of papillomaviruses. Virology 324, 17–27. [DOI] [PubMed] [Google Scholar]
- de Villiers, E.-M., Bernard, H.-U., Broker, T., Delius, H. & zur Hausen, H. (2005). Papillomaviridae. In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Edited by C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger & L. A. Ball. San Diego, CA: Elsevier Academic Press.
- Doorbar, J. (2005). The papillomavirus life cycle. J Clin Virol 32 (Suppl. 1), S7–S15. [DOI] [PubMed] [Google Scholar]
- Egawa, K., Delius, H., Matsukura, T., Kawashima, M. & de Villiers, E. M. (1993). Two novel types of human papillomavirus, HPV 63 and HPV 65: comparisons of their clinical and histological features and DNA sequences to other HPV types. Virology 194, 789–799. [DOI] [PubMed] [Google Scholar]
- Favre, M., Obalek, S., Jablonska, S. & Orth, G. (1989). Human papillomavirus (HPV) type 50, a type associated with epidermodysplasia verruciformis (EV) and only weakly related to other EV-specific HPVs. J Virol 63, 4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund, O. (2007). Genetic diversity of cutaneous human papillomaviruses. J Gen Virol 88, 2662–2669. [DOI] [PubMed] [Google Scholar]
- Ghim, S. J., Rector, A., Delius, H., Sundberg, J. P., Jenson, A. B. & Van Ranst, M. (2004). Equine papillomavirus type 1: complete nucleotide sequence and characterization of recombinant virus-like particles composed of the EcPV-1 L1 major capsid protein. Biochem Biophys Res Commun 324, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Glaser, C. A., Honarmand, S., Anderson, L. J., Schnurr, D. P., Forghani, B., Cossen, C. K., Schuster, F. L., Christie, L. J. & Tureen, J. H. (2006). Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis 43, 1565–1577. [DOI] [PubMed] [Google Scholar]
- Groff, D. E. & Lancaster, W. D. (1985). Molecular cloning and nucleotide sequence of deer papillomavirus. J Virol 56, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst, L. H., Lenz, J., Van Doorslaer, K., Chen, Z., Stacy, B. A., Wellehan, J. F., Jr, Manire, C. A. & Burk, R. D. (2009). Genomic characterization of two novel reptilian papillomaviruses, Chelonia mydas papillomavirus 1 and Caretta caretta papillomavirus 1. Virology 383, 131–135. [DOI] [PubMed] [Google Scholar]
- Kullander, J., Handisurya, A., Forslund, O., Geusau, A., Kirnbauer, R. & Dillner, J. (2008). Cutaneous human papillomavirus 88: remarkable differences in viral load. Int J Cancer 122, 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leechanachai, P., Banks, L., Moreau, F. & Matlashewski, G. (1992). The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene 7, 19–25. [PubMed] [Google Scholar]
- Martens, A., De Moor, A. & Ducatelle, R. (2001). PCR detection of bovine papilloma virus DNA in superficial swabs and scrapings from equine sarcoids. Vet J 161, 280–286. [DOI] [PubMed] [Google Scholar]
- Matsukura, T., Iwasaki, T. & Kawashima, M. (1992). Molecular cloning of a novel human papillomavirus (type 60) from a plantar cyst with characteristic pathological changes. Virology 190, 561–564. [DOI] [PubMed] [Google Scholar]
- Muller, M., Kelly, G., Fiedler, M. & Gissmann, L. (1989). Human papillomavirus type 48. J Virol 63, 4907–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, E., Gottschling, M., Bravo, I. G., Wittstatt, U., Stockfleth, E. & Nindl, I. (2009). Genomic characterization of the first insectivoran papillomavirus reveals an unusually long, second non-coding region and indicates a close relationship to Betapapillomavirus. J Gen Virol 90, 626–633. [DOI] [PubMed] [Google Scholar]
- Tachezy, R., Rector, A., Havelkova, M., Wollants, E., Fiten, P., Opdenakker, G., Jenson, B., Sundberg, J. & Van Ranst, M. (2002). Avian papillomaviruses: the parrot Psittacus erithacus papillomavirus (PePV) genome has a unique organization of the early protein region and is phylogenetically related to the chaffinch papillomavirus. BMC Microbiol 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman, L. B., Breitburd, F., Croissant, O. & Orth, G. (1984). The search for a culture system for papillomavirus. J Invest Dermatol 83, 2s–6s. [DOI] [PubMed] [Google Scholar]
- Ullman, C. G., Haris, P. I., Galloway, D. A., Emery, V. C. & Perkins, S. J. (1996). Predicted alpha-helix/beta-sheet secondary structures for the zinc-binding motifs of human papillomavirus E7 and E6 proteins by consensus prediction averaging and spectroscopic studies of E7. Biochem J 319, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria, J. G., Kapoor, A., Li, L., Blinkova, O., Slikas, B., Wang, C., Naeem, A., Zaidi, S. & Delwart, E. (2009). Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol 83, 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn, S. J., De Koning, M. N., Wieland, U., Quint, W. G. & Pfister, H. J. (2009). Intrafamilial transmission and family-specific spectra of cutaneous betapapillomaviruses. J Virol 83, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]