Abstract
The risk for disordered oropharyngeal swallowing (dysphagia) increases with age. Loss of swallowing function can have devastating health implications including dehydration, malnutrition, and pneumonia, as well as reduced quality of life. Age-related changes place older adults at risk for dysphagia for two major reasons: One is that natural, healthy aging takes its toll on head and neck anatomy and physiologic and neural mechanisms underpinning swallowing function. This progression of change contributes to alterations in the swallowing in healthy older adults and is termed presbyphagia, naturally diminishing functional reserve. Second, disease prevalence increases with age and dysphagia is a co-morbidity of many age-related diseases and/or their treatments. Sensory changes, medication, sarcopenia and age-related diseases are discussed herein. Relatively recent findings that health complications are associated with dysphagia are presented. Nutrient requirements, fluid intake and nutritional assessment for older adults are reviewed relative to their relations to dysphagia. Dysphagia screening and the pros and cons of tube feeding as a solution are discussed. Optimal intervention strategies for elders with dysphagia ranging from compensatory interventions to more rigorous exercise approaches are presented. Compelling evidence of improved functional swallowing and eating outcomes resulting from active rehabilitation focusing on increasing strength of head and neck musculature is provided. In summary, while oropharyngeal dysphagia may be life-threatening, so are some of the traditional alternatives, particularly for frail, elderly patients. While the state of the evidence calls for more research, this review indicates the behavioral, dietary and environmental modifications emerging in this past decade are compassionate, promising and in many cases preferred alternatives to the always present option of tube feeding.
Keywords: aging, deglutition disorders, enteral nutrition, pneumonia, malnutrition, dehydration
The capacity to swallow effectively and safely is a basic human need and pleasure, yet nearly 40% of Americans over age 60 experience dysphagia. As leisure time increases with the anticipated extended retirements of America’s baby boomers, and older adults look forward to more opportunities to participate in social activities that include eating and drinking, an ultimate irony is the anatomical and physiological changes that take place, increasing the risk for disordered swallowing with increasing age. Indeed, the loss of swallowing function can have devastating health implications, including nutrition and hydration deficits, changes in health status, including pneumonia, and increased need for care provision, especially for older adults.
Both overt and silent aspiration may precipitate pulmonary complications. For the purposes of this manuscript, aspiration is defined as the entry of material into the airway below the level of the true vocal folds. Silent aspiration refers to the circumstance in which a bolus comprising saliva, food, liquid, medication or any foreign material enters the airway below the vocal folds without triggering obvious symptoms such as coughing or throat clearing. Both overt and silent aspiration may lead to pneumonitis, pneumonia, exacerbation of chronic lung disease, or even asphyxiation and death. To gain a better understanding of the effect these consequences have on older adults and the impact of dysphagia interventions, research in this area has aimed to develop more meaningful outcome measures. Assessments focusing on physiology, function, quality of life and health services and systems are now being conducted to create more evidence-based practices in dysphagia care.
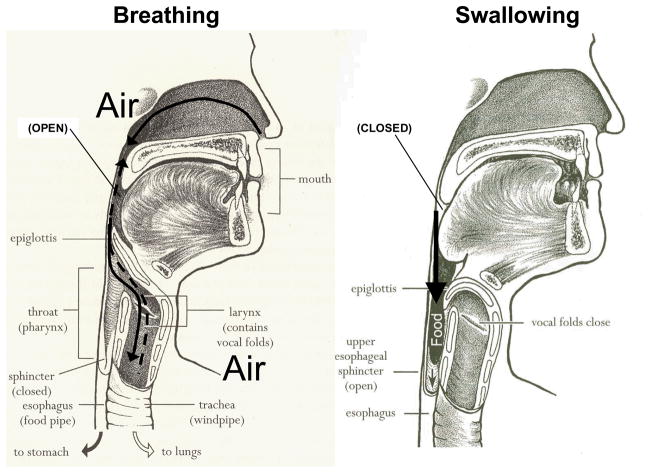
The process of swallowing requires orchestration of a complex series of psychological, sensory, and motor behaviors that are both voluntary and involuntary. Dysphagia refers to difficulty swallowing that may include oropharyngeal or esophageal problems. The upper aerodigestive tract has two primary functions: breathing and swallowing for which approximately 40 sets of bilaterally innervated muscles are activated. To safely execute swallowing, the upper aerodigestive tract must reconfigure from a system that valves and moves air for the purposes of breathing and talking, to one that ceases airflow, and protects the airway while food, fluid, secretions and medications are moved to ensure adequate nutrition and hydration (Figure 1). Normally, this complex process requires precise timing, elicitation of 6 cranial nerves (V, VII, IX, X, XI, and XII), supratentorial input, and the critical cessation of breathing until pressures are generated and material is cleared into the upper GI tract.
Figure 1.
The upper aerodigestive tract has two primary functions: breathing and swallowing. (Reprinted from Easy to Swallow, Easy to Chew Cookbook: Over 150 Tasty and Nutritious Recipes for People Who Have Difficulty Swallowing by Weihofen D, Robbins J, and Sullivan PA. 2002, with permission of John Wiley & Sons, Inc.
Eating and drinking are social events that relate to friendship, acceptance, entertainment, and communication. As such, major adjustments in the process of swallowing, eating and dining can lead to distressing responses such as shame, anxiety, depression, and isolation. Dysphagia profoundly influences quality of life (QOL). Practical dysphagia-specific, comprehensive, QOL measures have been developed that monitor functional outcomes in clinical practice allowing health care providers to better assess and adjust their treatment of dysphagia (1–3). The multidisciplinary swallowing team, which may comprise the swallowing clinical specialist (usually a speech language pathologist), dietician, nurse, radiologist, geriatrician and other professionals such as dentist or hygienist, gastroenterologist, otolaryngologist, oncologist, neurologist, depending on underlying etiology for the dysphagia and resources available, determine the optimal strategies to increase safety and enjoyment (designed relative to the sensorimotor aspects of an individual’s swallowing mechanism) by older adults who are most at risk for dysphagia.
Presbyphagia vs. Dysphagia
Age-related changes place older adults at risk for dysphagia for two major reasons. One reason is that natural healthy aging takes its toll on the head and neck anatomy, as well as the physiologic and neural mechanisms underpinning swallowing function. These changes, termed presbyphagia, refer to characteristic alterations in the swallowing mechanism of otherwise healthy older adults (4). This progression of change contributes, in part, to a more pervasive naturally diminished functional reserve (the resilient ability of the body to adapt to physiological stress), making the older population more susceptible to dysphagia. Another reason for age-related associations with swallow dysfunction is that disease prevalence increases with age, and dysphagia is a co-morbidity of many age-related diseases and/or their treatments.
With the aging of America, it is increasingly critical that clinicians be able to distinguish between dysphagia and presbyphagia (an aged but healthy swallow) or other related conditions in order to avoid over diagnosing and over treating dysphagia. Older adults can be more vulnerable to dysphagia, primarily with additional stressors such as acute illnesses and certain medications. Presence of such stressors can result in crossing over the line from having a healthy older swallow (presbyphagia) to experiencing dysphagia. It is essential for health care professionals to be alert in order for dysphagia to be correctly diagnosed and treated in a timely manner.
What are the affects of age?
Age-Related Change in Lingual Pressure
The tongue is the primary propulsive agent for pumping food through the mouth, into the pharynx while bypassing the airway, and through to the esophagus. Recent findings clearly reveal that age-related change in lingual pressure generation is another contributing factor to presbyphagia. Healthy older individuals demonstrate significantly reduced isometric (i.e., static) tongue pressures compared with younger counterparts. In contrast, maximal tongue pressures generated during swallowing (i.e., dynamic) remain normal in magnitude (5, 6) because swallowing is a submaximal pressure-demanding activity. Nonetheless, although older individuals manage to achieve pressures necessary to affect an adequate swallow despite a reduction in overall maximum tongue strength, they achieve these pressures more slowly than young swallowers.
Age Associated Changes in Oropharyngeal Swallowing
Various age-related changes in oropharyngeal swallow function have been observed among older adults. As indicated previously, the healthy older swallow is slow (4, 7–9). The longer duration occurs largely before the more automatic pharyngeal phase of the swallow is initiated. In those over age 65, the initiation of laryngeal and pharyngeal events, including laryngeal vestibule (and hence, airway) closure, are delayed significantly longer than in adults younger than 45 years of age (7). Although the specific neural underpinning is not confirmed, it might be hypothesized that the more voluntary oral events become “uncoupled” from the more “neurally hardwired” brainstem pharyngeal response which includes airway protection. Thus, in older healthy adults it is not uncommon for the bolus to be adjacent to an open airway by pooling or pocketing in the pharyngeal recesses, for more time than in younger adults, increasing the risk of adverse consequences due to ineffective deglutition (Figures 2a, b).
Figure 2.
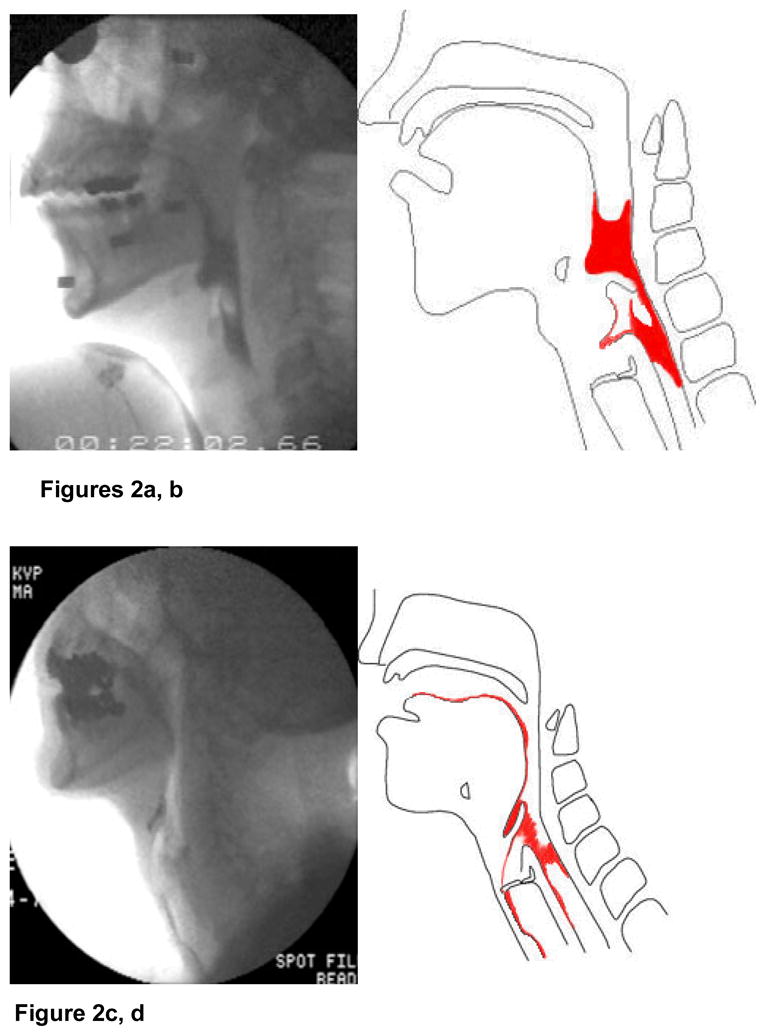
Figures 2a, b. Airway penetration, defined as entry of material into the laryngeal vestibule without passing below the level of the vocal cords.
Figures 2c, d. Aspiration, defined as entry of material into the airway (trachea). Reprinted from Robbins J, Kays S, McCallum S. Team Management of dysphagia in the institutional setting. J Nutr Elderly 2007; 26: 59–104, with permission from Taylor & Francis.
Aspiration and airway penetration (entry of material into the laryngeal vestibule above the vocal folds) (Figures 3a–d) are significant adverse clinical outcomes of misdirected bolus flow. In older adults, penetration of the bolus into the airway occurs more often and to a deeper and more severe level than in younger adults (10). When the swallowing mechanism is functionally altered, airway penetration can be even more pronounced. A study examining this issue found that when a nasogastric tube was in place in men and women older than 70 years, liquid penetrated the airway significantly more frequently (4), indicating that under stressful conditions or system perturbations, older individuals are less able to compensate due to the age-related reduction in reserve capacity (11).
Figure 3.
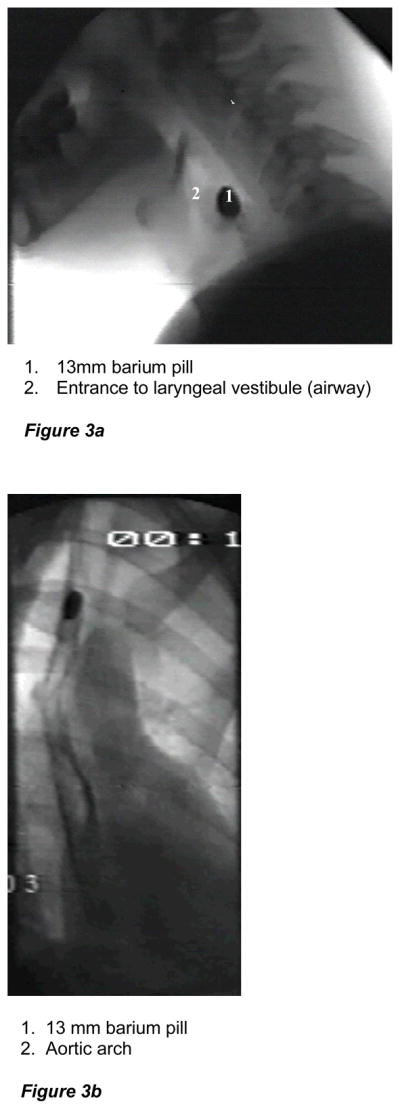
Figure 3a. Fluoroscopic image of a barium tablet (simulating a pill) lodged in pharynx, precariously located next to an open airway.
Figure 3b. Fluoroscopic image of a barium tablet (simulating a pill) lodged in mid-esophagus. Reprinted from Robbins J, Kays S, McCallum S. Team Management of dysphagia in the institutional setting. J Nutr Elderly 2007; 26: 59–104, with permission from Taylor & Francis.
Swallowing Co-morbidities
Xerostomia
Dryness, which often extends from the mouth to the pharynx and esophagus, can hinder bolus flow and result in the retention of material along the swallowing tract. Functional salivary production has been shown to remain stable throughout the age spectrum, however, older adults demonstrate decreased salivary reserve due to a loss of saliva-producing acinar cells. As a result, the drying effects of medications (discussed in later sections) are more pronounced in older adults (12). Dry mouth may also be a symptom of underlying diseases, particularly autoimmune diseases such as Sjogren’s syndrome or scleroderma. Oral residue can increase the risk for bacterial growth if careful oral care is not provided post meal, while residue more inferiorly can be a critical risk factor for aspiration. That is, material retained in the pharyngeal recesses can be inhaled into the trachea post-swallow.
Esophageal Motility
Equally threatening is the risk of residue within the esophagus traveling retrograde, or refluxing from the esophagus into the pharynx and potentially the trachea, when a patient reclines after a meal. The retention of material within the esophagus, termed intraesophageal stasis, and its potential to flow retrograde toward the pharynx, known as intraesophageal reflux, (Jou et al, submitted, 2008; Robbins et al, submitted, 2008) are distinct entities from the more commonly recognized gastroesophageal reflux and once identified can be treated with inexpensive behavioral adjustments (13). Insufficient saliva may also increase the risk for esophagitis, since the bicarbonate in saliva serves as a neutralizing mechanism that protects the esophagus from inflammation in the presence of acidic gastroesophageal reflux (14).
Sensory Changes
Sensory input for taste, temperature and tactile sensation changes in many older adults (14). For instance, sensory discrimination thresholds in the oral cavity and laryngopharynx have been shown to increase with age (12, 13). This disruption of sensory-cortical-motor feedback loops may interfere with proper bolus formation and the timely response of the swallowing motor sequence, as well as detract from the pleasure of eating. Thus, reduced sensation may explain the failure of some older adults, such as those with dementia or Parkinson’s disease, to spontaneously swallow when food, liquid or saliva is pooling in the pharynx. The predictive value of an absent gag reflex for aspiration is limited, as many healthy normal swallowers lack a strong gag. Nonetheless many clinicians still use this to screen for altered pharyngeal sensation as part of an assessment battery (15) while recognizing that its usefulness alone is questionable (16).
Research is demonstrating texture and flavor enhancement may be important to positively influence oral intake with aging. Single textures are simpler for an aging oral system to manipulate safely. Moderate sucrose, high salt, and high citric acid levels elicit higher lingual swallowing pressures (17). Flavor enhancement should be considered and potentially incorporated into standard diets for the elderly dysphagic patient.
Sarcopenia
Structurally, sarcopenia is associated with age-related reductions in muscle mass and cross sectional area, a reduction in the number or size of muscle fibers, and a transformation or selective loss of specific muscle fiber types (18). Sarcopenia is inherently associated with diminished strength. There are reports in the literature of sarcopenia-like changes in muscles of the upper aerodigestive tract (19–21) and the observed age-related changes in strength and function (5, 6) suggest pervasive changes in lingual muscle composition (22–24). Ongoing work is generating novel interventions effective for diminishing sarcopenia and increasing strength. Although most of the initial work in this area has been performed in the limb musculature, emerging work in cranial-innervated muscles is quite relevant to swallowing in older individuals and will be discussed later in more detail.
Medications
Older patients frequently report difficulty swallowing pills as the first sign of a swallowing problem. Polypharmacy, unfortunately, in old age is routine practice as the incidence of certain medical conditions increases with age. While difficulty swallowing pills can be an indicator of dysphagia, the drugs themselves can be part of the problem. More than 2000 drugs can cause xerostomia or influence lower esophageal sphincter relaxation (thereby exacerbating gastroesophageal reflux) via anticholinergic mechanisms. An equally large number affect cognition and mental status, or influence the tongue and bulbar musculature by delaying neuromuscular responses or inducing extrapyramidal effects, which can hinder safe and sufficient oral intake (Table 1). Angiotensin-Converting Enzyme (ACE) inhibitors have been considered for elderly dysphagic patients even when they do not have hypertension, secondary to studies showing lower rates of pneumonia in patients treated with ACE inhibitors (25); however, ACE inhibitors also are associated with symptoms such as chronic cough that may mimic, mask or exacerbate dysphagia symptoms and therefore should be prescribed judiciously in the older adult at risk for dysphagia.
Table 1.
Medications Affecting Swallowing
| Medications Producing Xerostomia | Anticholinergics (e.g., sedating antihistamines; medications used for Parkinson’s disease) |
| Antihypertensives (e.g., diuretics) | |
| Opiates | |
| Retinoids | |
| Antipsychotics | |
| Medications Altering Cognitive Function/Alertness | Antianxiety |
| Antihypertensives, especially centrally acting | |
| Antiepileptics | |
| Antiemetics | |
| Medications Associated with Esophagitis | Antibiotics |
| Nonsteroidal anti-inflammatory drugs (NSAIDS) | |
| Other (e.g., Warfarin, Diazepam, Phenobarbital) | |
Age Related Diseases Associated with Dysphagia
Neurologic and neuromuscular disorders are among the principal risks for dysphagia (Table 2). Neurologic diseases rise in prevalence in older cohorts of the population. Stroke, brain injury, Alzheimer’s disease and other dementia syndromes, and parkinsonism all place older adults at increased risk for dysphagia with its incipient consequences.
Table 2.
Neurologic Disorders Causing Dysphagia
| Stroke |
| Head Trauma |
| Parkinson’s disease and other movement and neurodegenerative disorders |
| Progressive supranuclear palsy |
| Olivopontocerebellar atrophy |
| Huntington’s disease |
| Wilson’s disease |
| Torticollis |
| Tardive dyskinesia |
| Alzheimer’s disease and other dementias |
| Motor neuron disease (amyotrophic lateral sclerosis) |
| Guillain-Barre syndrome and other polyneuropathies |
| Neoplasms and other structural disorders |
| Primary brain tumors |
| Intrinsic and extrinsic brainstem tumors |
| Base of skull tumors |
| Syringobulbia |
| Arnold-Chiari malformation |
| Neoplastic meningitis |
| Multiple sclerosis |
| Postpolio syndrome |
| Infectious disorders |
| Chronic infectious meningitis |
| Syphilis and Lyme disease |
| Diphtheria |
| Botulism |
| Viral encephalitis, including rabies |
| Myasthenia gravis |
| Myopathy |
| Polymyositis, dermatomyositis, inclusion body myositis, and sarcoidosis |
| Myotonic and oculopharyngeal muscular dystrophy |
| Hyper- and hypothyroidism |
| Cushing’s syndrome |
| Iatrogenic conditions |
| Medication side effects |
| Postsurgical neurogenic dysphagia |
| Neck surgery |
| Posterior fossa surgery |
| Irradiation of the head and neck |
Because cognitive function and/or communication may be impaired, it is important for the practitioner to note the warning signs associated with dysphagia and a risk of aspiration (Table 3). Between 50 and 75 percent of patients who have had a recent acute stroke develop eating and swallowing problems, with ensuing complications of aspiration developing in 50 percent, malnutrition in 45 percent, and pneumonia in 35 percent. Other delayed adverse consequences have also been reported, with up to 15 percent of patients who have suffered a cerebrovascular accident (CVA) developing pneumonia within 1 year of the acute insult. Brainstem or bilateral hemispheric strokes predictably produce dysphagia, but unilateral lesions also can contribute to dysphagia.
Table 3.
Warning Signs Associated with Dysphagia and Aspiration Risk
Decreased alertness or cognitive dysfunction
|
Changes in approach to food
|
Manifestations of impaired oropharyngeal functions
|
Patient complaints or observations of
|
A host of common problems involving the head and neck can directly damage the effector muscles of swallowing and increase the risk for dysphagia. Head and neck injury, carcinoma, complex infections, thyroid conditions, and diabetes are associated with age-related dysphagia. Although vertebral osteophytes are common, these bony growths alone rarely cause dysphagia. Dysphagia more commonly results from the presence of osteophytes in conjunction with neuromuscular weakness or discoordination. This can be caused by combinations of several underlying conditions or co-morbidities such as diabetes, chronic obstructive pulmonary disease, congestive heart failure, renal failure, an immunocompromised status, and/or cachexia for which an individual no longer can draw an adequate reserve to effectively compensate.
Sometimes dysphagia can have iatrogenic causes. Health care interventions can result in drug-induced delirium, protracted hospital stays, and ultimately malnutrition. Indwelling nasogastric tubes, airway intubation, and medication effects may all predispose a frail older adult with borderline airway protection to develop frank aspiration. Understanding the iatrogenic causes of dysphagia can alter medical practice and may reduce its incidence and complications. Several different treatments can either directly or indirectly damage swallowing effector organs as described previously. Head and neck cancer surgeries, some spinal cord surgeries, thyroid surgeries, and any intervention that can jeopardize the recurrent laryngeal nerve may result in dysphagia. A number of chemotherapy and radiotherapy regimes can cause oropharyngeal injury. The prospective outcome of dysphagia should be incorporated into the risk benefit discussions of these procedures.
Dysphagia, Care Transitions and Bounce-Backs
Relatively recently, attention has been directed toward health complications that arise during transitions of care (26, 27). Patients with challenging health conditions, many of which are accompanied by dysphagia (e.g., acute stroke), frequently undergo “complex transitions” or “bounce backs” defined as a transition from a less intensive to more intensive care setting (e.g., readmission to the hospital or ER visit after discharge home).
Overall Cost of Bounce-Backs
Movements from less intense to more intense health care settings within 30 days of hospital discharge (i.e. “bounce-backs” or complicated transitions) represent a significant strain on the U.S. health system (27, 28). Approximately 20% of all hospitalized Medicare patients experience bounce-backs within the first 30 days of discharge (27, 28). The overwhelming majority (>90%) of these bounce-backs are emergency room visits or rehospitalizations (27), accounting for over $15 billion in payments annually (29). Sub-acute care patients, who are primarily older adults with complicated medical conditions such as stroke, disproportionately share this burden (29). There is evidence that dysphagia, an addressable yet under-diagnosed condition, may play a major role in bounce-backs especially within the stroke population.
Bounce-Backs in Stroke
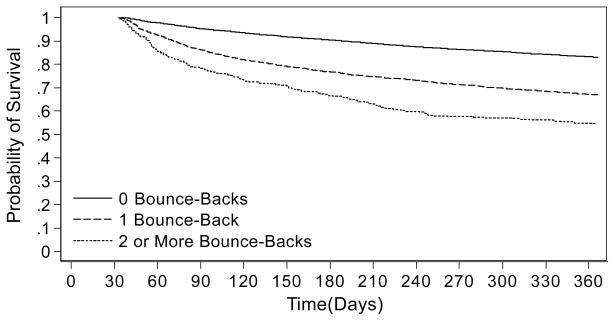
Acute ischemic stroke patients bounce-back at high rates. Twenty percent of recently discharged stroke patients experience at least one bounce-back and 16% of those experience more than one (27). Stroke patients with gastrostomy tubes or who are initially discharged to skilled nursing facilities are the most likely to experience bounce-backs, with these factors increasing the odds of bouncing-back by 52% and 36% respectively (27). Bounce-backs may have important future repercussions. Acute stroke patients who bounce-back within the first 30 days have markedly poorer survival and higher health care payments over the subsequent year than their counterparts with no bounce-backs (Figure 4) (30).
Figure 4.
Kaplan-Meier curve depicting 1-year survival of acute ischemic stroke patients with zero, one, and two or more bounce-backs within the first 30 days (N511,729). Time zero is discharge from index stroke hospitalization. To be included in the sample, patients had to survive at least 30 days from discharge. Reprinted from Kind AJ, Smith MA, Liou JI, Pandhi N, Frytak JR, Finch MD. The price of bouncing back: one-year mortality and payments for acute stroke patients with 30-day bounce-backs. Journal of the American Geriatrics Society. 2008; 56(6):999–1005 with permission from Wiley-Blackwell.
There is evidence that dysphagia may contribute to a large number of stroke bounce-backs through its frequent complications of aspiration and pneumonia (31). Firstly, infections and aspiration pneumonitis are the most frequent reasons for rehospitalization within 30 days of an acute stroke and contribute to 51% of all stroke deaths within the first 30 days (32–35). These diagnoses account for 25–43% of all rehospitalizations in stroke patients discharged directly to skilled nursing facilities and 38% of all rehospitalizations in stroke patients who die within the first 30 days (34). Secondly, aspiration pneumonia is a known complication of gastrostomy tube use (36). A high rate of gastrostomy tube use in stroke patients, especially within patients who are rehospitalized and die within the first 30 days (29%)(34), likely contributes to many of the rehospitalizations for infection/aspiration pneumonitis. Lastly, the risk of rehospitalization for infection or aspiration pneumonia can be decreased through specialized stroke unit care (33, 37). The early mobilization and universal swallowing consultations provided within these stroke units have been cited as the keys to their success (33, 37). Targeted dysphagia programs can also be helpful in decreasing pneumonia rates (38).
Overall, the aspirations and infections which result from dysphagia have a significant impact on the U.S. health system and directly contribute to a large number of the costly bounce-backs experienced within the stroke population. The prevention of these dysphagia complications is critical for the greater health system as well as for individual patients. Fortunately, a number of interventions have directly targeted this critical bounce-back problem by enhancing the quality of patient care transitions from the hospital to the post-hospital setting (39–45).
Interventions to Prevent Bounce-Backs
In the current system of specialized health care, patients with complex chronic health conditions often require care across multiple settings with numerous care transitions (27, 28). Poor care transitions can result in heightened patient vulnerability, medication errors, decreased patient satisfaction, care fragmentation and unnecessary resource utilization (21–25). The risk of a poor transition is especially relevant for patients unable to advocate for themselves or without a caretaker to advocate for them, such as the elderly and the disenfranchised. However, all inpatients experience a transition at some point in their discharge process. Despite the commonality of this experience, patients and their caregivers are often unprepared for the realities of the post-hospital care setting and unable to successfully manage their medical conditions (i.e. dysphagia) once they leave the hospital (26–28). Taken together, these factors conspire to increase bounce-backs and health care costs (13, 23, 29).
Existing bounce-back prevention efforts work by improving post-hospital communication. These studies employ nurses to meet personally with high risk patients both before and after discharge to educate patients about their medication and disease condition management, perform medication reconciliation, ensure medical follow-up is in place and to provide a contact if problems arise. Such interventions reduce hospital readmissions by up to 45% (39–41). Less comprehensive interventions which utilize enhanced discharge counseling and/or telephone follow-up have also proven to be effective, particularly in congestive heart failure populations (42–45). Thus far these interventions have not specifically focused on patients with dysphagia or on reinforcing therapies particular to dysphagia treatment. Given that dysphagia likely plays a major role in precipitating bounce-backs, particularly in stroke, this is an area ripe for further study.
Dysphagia, Nutrition and Hydration
Dysphagia has a profound effect on nutritional status often resulting in malnutrition and dehydration and may compromise nutrient status as a result of diminished capacity to eat or drink, anorexia or fear of eating. When dysphagia occurs in the elderly population in tandem with sarcopenia, or loss of skeletal muscle mass and strength (46), the risk for malnutrition especially protein-energy malnutrition is increased (47). Consequences of the dysphagia-malnutrition relationship include: weight loss, dehydration, muscle breakdown, fatigue, aspiration pneumonia, and a general decline in functional status. Moreover, a recent study identified swallowing problems and sarcopenia as predictive of nosocomial infections in hospitalized elderly patients (48). Increased morbidity and mortality are documented outcomes of undiagnosed or untreated dysphagia that have progressed to protein-energy malnutrition (49, 50).
Nutrient Requirements of Older Adults
Emerging research suggests that the nutrient requirements of older adults are unique. The Institute of Medicine, Dietary Reference Intakes (51) subdivides the “greater than age 50 years” category into 51–70 years and greater than 70 years of age. The greater than 70 years age group is especially vulnerable to compromised nutritional status (52). With advancing age, energy needs decrease due to loss of muscle mass and decreased energy expended in physical activity whereas the need for vitamins and minerals either remains the same or increases due to changes in the efficiency of absorption and utilization of nutrients (51). Previous efforts to identify older adults at nutrition risk targeted individuals who are vulnerable to compromised nutrient status based on inadequate energy intakes (52). However, current data suggest that healthy adults approaching 70 years of age will more likely be faced with problems of excessive energy intake leading to overweight and obesity (53), as well as deficiencies in vitamins and minerals due to consumption of low nutrient density foods. Energy needs of older adults can be estimated initially as 20 kcal per kg body weight for those who are overweight and 25–30 kcal per kg body weight for those within normal body weight. Continued unintentional weight loss in the elderly is associated with increased mortality.
Protein
There is controversy regarding whether the requirement for dietary protein increases with advancing age. The current recommended dietary allowance (RDA) for protein intake for all men and women aged 19 years and older is 0.8 g protein/kg body weight/day or 46 and 56 g protein per day for reference women and men, respectively (51). These recommendations are based primarily on data from short-term nitrogen balance studies performed on young men and extrapolated to older adults (54, 55). Recent studies suggest that a moderately higher protein intake of 1.0–1.3 g protein/kg body weight/day or 20–35% of energy from protein may be required to maintain nitrogen balance and offset a potentially lower energy intake, decreased protein synthetic efficiency, and impaired insulin action in older adults (56–59). Between 15–41% of adults in the United States consume less than the RDA of 0.8 g protein/kg body weight/day (60). But for adults 70 years of age and older the risk of inadequate protein intake is greater with ~40% consuming less than 100% of the RDA and ~16% consuming less than 75% of the RDA, which may be only the minimal protein requirement for elderly populations (61). Protein intake in the United States has been relatively stable at 14–15% of energy from protein since 1971 (62). Caution is needed for the use of high-protein diets (>45% energy from protein or >2 g protein/kg body weight/day) in individuals with impaired renal function, which may occur in the elderly. Lastly, the beneficial effects of resistance exercise to help counteract sarcopenia and maintain muscle mass and function is clearly established in aging populations (63). In contrast, the beneficial effects of protein supplementation or interaction between protein supplementation and resistance exercise to improve muscle mass and function are less clear and require further research (46, 56). However, recent data from the Health, Aging and Body Composition study indicate that higher energy-adjusted protein intake was associated with a significant reduction in loss of lean body mass over 3 years in 70–79 year old, community dwelling adults (61).
Vitamins and Minerals
Current data for mean nutrient intakes suggest that as a group, older adults are at risk for not meeting the RDA or Adequate Intake (AI) values for calcium, vitamins D, E, and K, and potassium and fiber as shown in Table 4 (52). Individuals over the age of 70 years consume a mean of 33–50% of the RDA or AI values for these shortfall nutrients. Dairy or milk products are excellent sources of high-quality protein and calcium although lactose intolerance may restrict consumption of dairy products in older adults. Interestingly, the prevalence of lactose intolerance increases with age, although intolerance symptoms among lactose maldigesters tend to decrease with age which suggests that as people get older they may have more flexibility in their choice of calcium-rich dairy foods (64). Older adults are at increased risk for inadequate intake of vitamin D because of low intake from foods as well as decreasing ability of the skin to synthesize vitamin D after exposure to sunlight with age. Strikingly, no more than 2% of individuals greater than 70 years of age meet the AI level for vitamin D from food sources with a large proportion of vitamin D coming from supplement usage (65). Food sources of vitamin D include fortified cereal, milk but not cheese, eggs, liver, salmon, tuna, catfish and herring. Current regulations permit vitamin D fortification of calcium-fortified juices and juice drinks, nutrient supplemented meal replacements and a wide variety of dairy products in addition to fluid milk (52). Greater consumption of fruits and vegetables should be encouraged in older adults as this will generally improve intakes of vitamins E and K, potassium and fiber. Additional fluid is needed when increasing fiber intake from whole-grain cereals and breads, legumes, fruits and vegetables. Slowly adding both fiber and fluid allows the gastrointestinal tract time to adapt to the additional bacterial substrates.
Table 4.
Shortfall nutrients for individuals 70 years and older in NHANES 2003–20041
| Nutrient | Men | Women | Men | Women |
|---|---|---|---|---|
| RDA or AI | Mean Intake (% below RDA or AI) | |||
| Vitamin D, μg/d | 15 | 15 | 8.1(8.8)3 | 8.0(8.8)3 |
| Calcium,2 mg/d | 12002 | 12002 | 743(62)2 | 668(56)2 |
| Vitamin E, mg/d | 15 | 15 | 6(40) | 5(33) |
| Vitamin K,2 μg/d | 120 | 90 | 92(77) | 56(62) |
| Potassium, mg/d | 4700 | 4700 | 2441(52) | 2332(50) |
| Fiber,2 g/d | 30 | 21 | 15(50) | 14(67) |
Adapted from A Lichtenstein, J Nutr 138: 5–11, 2008
AI, otherwise RDA
Food plus supplements
Older adults often consume nutritional supplements and fortified foods which can be helpful, particularly to augment shortfall intakes of calcium, vitamin D and vitamin B-12 from foods. However, high rates of nutrient supplement use among older adults increases the risk of overconsumption of nutrients especially folate which can mask or precipitate vitamin B-12 deficiency (51). Risk for vitamin B-12 deficiency is increased in the elderly due to a high prevalence of atrophic gastritis which limits the absorption of protein-bound vitamin B-12 from foods and affects 10–30% of individuals greater than 60 years of age (66). Vitamin B-12 deficiency leads to irreversible neurological damage, walking and balance disturbances, and cognitive impairment including confusion and mood changes. Supplements of free vitamin B-12 are well absorbed, as stomach acid is not needed to free the vitamin B-12 from food proteins before binding to the intrinsic factor that is needed to absorb vitamin B-12 in the ileum.
Fluid Intake
Achieving adequate fluid intake to avoid dehydration is often a challenge in older adults with dysphagia because of the risk of choking and aspiration. Fluid needs in older adults are variable and greatly influenced by fever, level of physical activity, ambient temperature, renal function and use of medications. Moreover, homeostatic mechanisms such as loss of the thirst sensation are often compromised and can result in dehydration (67, 68). Fluid needs can be estimated as one mL of fluid per calorie eaten with a minimum daily fluid intake of 1500 mL in patients weighing 50–80 kg to cover losses from urine, feces, lung vapor and sweat (69). Unfortunately, studies investigating the fluid intakes of institutionalized elderly patients show low intakes ranging from 500–1200 mL per day (70). Fluid needs may be met in a variety of ways including drinking water or other beverages, water in foods (which ranges from 37% in bread to 99% by weight in fruits and vegetables,) and thickened-fluids used in dysphagia diets. A study in dysphagic stroke patients indicates that those who received thickened-fluid dysphagia diets failed to meet their fluid requirements whereas patients on enteral feeding and intravenous fluid regimens received ample fluid (70). Given the likelihood of inadequate fluid intake in dysphagic patients it is important to monitor clinical symptoms of dehydration such as dry membranes, low urine output, elevated urine specific gravity and loss of body weight, as well as biochemical tests, including hematocrit and elevated concentrations of sodium, blood urea nitrogen and albumin.
Nutritional Assessment
Given the strong relationships between dysphagia, malnutrition and poor clinical outcome (50), it is important that everyone with dysphagia receive nutritional assessment to identify individuals who have or are at risk for protein-energy malnutrition or specific nutrient deficiencies. The results of the nutritional assessment will determine nutrient needs and serve as the basis for the development of an individualized nutrition care plan (49). A number of tools are available for nutritional screening and assessment in the elderly (see comprehensive review in Nutr In Clin Prac Aug–Sept 2008 issue). Herein we briefly review features of two validated nutritional assessment methods which correlate well with each other and require follow-up with biochemical measurements - the Subjective Global Assessment (SGA, see Figure 5 in reference 71) and the Mini Nutritional Assessment® (MNA®, see Figure 3 in reference 71). The SGA examines 5 features of patient history: change in body weight, change in dietary intake, gastrointestinal symptoms, functional capacity, and disease and its relation to nutrition requirements. Fluid status and the ability to tolerate various types of fluids (as discussed under Interventions) are not included in the SGA and must be assessed separately. The combination of SGA and a serum albumin concentration is suggested to be one of the best clinical tools to identify patients with or suspected of having malnutrition (72). MNA® was developed to assess malnutrition in elderly populations. It consists of anthropomorphic measurements, 6 global assessment questions, 8 dietary questions including fluid intake, and subjective perception of health questions; assessment of functional capacity is not included. Validation studies indicate that approximately 75% of elderly patients can be correctly classified as well nourished, at risk of malnutrition or malnourished using the MNA® without biochemical parameters (73). Regardless of which tool is used, assessment of change in body weight over time is the most simple and reliable index of nutritional status. The following guidelines are useful in classifying the amount of weight loss in an individual as significant or severe (74). Significant weight loss is defined as a 5% loss of body weight in 1 month, 7.5% loss in 3 months or 10% loss in 6 months and severe weight loss is defined as any loss of body weight that exceeds the values for significant weight loss. A recent review emphasizes the need to distinguish between starvation, sarcopenia and cachexia in malnourished patients to develop an individualized nutrition care plan and understand the expected clinical response (46). In contrast to starvation, both sarcopenia and cachexia show a poor response to refeeding. The primary goal for nutrition therapy of the dysphagic patient is adequate and safe intake of nutrients. Regular diets are not sufficient for patients with dysphagia and specialized nutritional supplements and individualized dietary modifications are often needed.
Screening and Evaluation of Dysphagia: The Team
Present day dysphagia evaluation varies dependent upon the clinical setting. The comprehensive diagnostic approaches available for the hospitalized dysphagic senior (e.g., videofluoroscopic oropharyngeal swallowing study) may not logistically always be feasible for institutionalized elders (75). Likewise interdisciplinary dysphagia teams are frequently available only in academic or larger hospital systems (76). This places the responsibility for screening for swallowing problems on nursing staff who interact with patients on a daily basis. Dietitians are also key members of the dysphagia team, conducting mandatory nutrition screening and assessment and meal observations. The Registered Dietitian is particularly skilled at discerning why patients are selecting or avoiding particular food items and advocating for a more comprehensive swallowing evaluation. Once alerted via a physician’s referral, speech language pathologists (SLPs), who focus on facilitating bolus transfer safely and efficiently through the upper aerodigestive tract, are well trained to conduct bedside (also referred to as “non-instrumental”) examinations that include history-taking, oropharyngeal sensiorimotor assessment, and evaluation of trial swallows. While the screening process increases the suspicion of dysphagia, instrumental assessment by a SLP is often necessary to rule out aspiration with an acceptable level of confidence. Effective dysphagia intervention relies on accurate diagnosis of the specific pathophysiology. That is, the underlying movement or structural disorder that results in disordered bolus flow in terms of its direction, duration, and clearance, must be defined and remediated in order to eliminate or minimize the dysphagia. Most frequently instrumental methods are necessary to clarify the underpinnings that must be modified to affect safe and efficient bolus flow.
Recognizing complaints associated with dysphagia and differentiating them from symptoms of common age-related diseases can be challenging, especially since many individuals with swallowing problems present with concomitant language or cognitive difficulties that hinder their ability to clearly express their symptoms. For example, frail individuals may manifest depression solely by experiencing weight loss and diminished eating. On the other hand, in individuals with cognitive impairment or dementia, the only sign of dysphagia may be refusal of foods or retention of food in the mouth after extended chewing. Because swallowing is not something a patient traditionally considers, it may be necessary to ask related questions until a particular word or phrase triggers association in the patient’s experience (e.g., moving food to the throat, cough, throat clearing) or rely on family members and friends to provide information about the patient’s mealtime behavior. Certain symptoms may help the dysphagia team identify swallowing difficulties during their day-to-day interaction with patients, only some of which are provided in Table 5.
Table 5.
Condensed List of Signs and Symptoms of Dysphagia
| Oropharyngeal dysphagia | food spillage from the mouth |
| excessive drooling | |
| sense of difficulty initiating the swallow | |
| residual food in the oral cavity | |
| choking, coughing | |
| alteration of voice quality during or after eating, drinking or taking medications | |
| recurrent pneumonia or exacerbation of asthma or COPD | |
| Esophageal dysphagia | food “sticking” in the throat or chest |
| neck pain, chest pain or heartburn | |
| regurgitation of food or pills | |
| coughing when lying down or after meals | |
| recurrent pneumonia or exacerbation of asthma or COPD | |
Are Feeding Tubes A Solution?
Oropharyngeal dysphagia is potentially life threatening. In the older population, crucial decisions often must be made that influence the patient’s safety, health and QOL. Among these perplexing issues is the question of continuing oral intake or providing nonoral enteral nutrition.
Percutaneous Endoscopic Gastrostomy
Percutaneous endoscopic gastrostomy (PEG) tubes were first introduced into practice in 1980 with the purpose of providing enteral nutrition in children and young adults (77). Now they are the preferred method of delivering long-term enteral nutrition when oral intake is deemed inadequate. PEG tube placement rates have risen steadily over the last twenty years from 15,000 in 1989 to 121,000 in 1995 and more than 216,000 in the year 2000 (78, 79). PEG tubes are placed for a variety of reasons and clinical situations. The American Gastroenterological Association supports the placement of PEG tubes when the patient cannot or will not eat, when the gut is functional, and when the patient can safely tolerate the procedure (80). However, the decision involves a complex process taking into account the patient’s wishes as well as the religious and moral convictions of the surrogate decision makers. The use of PEG tube feeding has been validated specifically in patients with acute stroke with dysphagia and oropharyngeal malignancy where improvements in both morbidity and mortality have been shown (81, 82). However, they are more frequently being placed in older patients with chronic and degenerative diseases where the outcomes are not well established. These indications include, but are not limited to prolonged illness, neurological and psychiatric disorders, anorexia, attempt to prevent aspiration pneumonia, treating malnutrition, and with the intent to provide comfort and improve functional status (78, 83).
Complications and risks
While PEG tube placement is a relatively easy procedure with low procedure-related mortality (rates of approximately 1% to 2%) (84, 85), it is not without risks. Review of the literature provides a list of adverse events related to this procedure that can include local complications (wound infection, bleeding at the insertion site, leakage around the site, tube occlusion, erosion of the bumper into the abdominal wall, abdominal wall abscess, necrotizing fasciitis, colocutaneous fistula), gastrointestinal complications (ileus, diarrhea, nausea, vomiting, increased reflux), and other complications such as aspiration pneumonia, metabolic or electrolyte disturbances with refeeding, need for restraint use, and loss of the social interaction with feeding (78, 83, 84, 86).
In addition to these adverse events, 30-day mortality should also be considered. Sanders et al studied 361 consecutive patients requiring PEG tubes over a five year period from two hospitals. The 30-day mortality rate in this cohort was 28% and up to 52% at six months (81). Another study including 74 patients referred for PEG placement over one year found a mortality rate of 19% at one month and 42% at six months (84). In both of these studies, indications for PEG placement covered a variety of conditions such as cerebrovascular disease with dysphagia, chronic neurological conditions, trauma, head and neck cancers, and dementia. In most cases, the overall morbidity and mortality rates can be attributed to the underlying disease processes and comorbidities that served as the indication for the procedure in the first place (87). This illustrates that careful consideration of the risks and benefits should be undertaken prior to placing a PEG tube. More specifically, the factors that should be addressed include any known outcome data for that subgroup of patients, the natural course of the underlying illness, comorbidities, quality of life, and the patient’s overall life expectancy (78).
Outcomes of PEG in elderly patients with dementia
Among elderly patients requiring tube feeding, the most well studied subgroup are patients with dementia. Approximately 30% of all PEG tubes are placed in patients with dementia and as many as 10% of institutionalized elderly patients are being tube fed (88). The use of PEG tubes in patients suffering from dementia is controversial. Difficulty eating is common in advanced dementia and is usually a marker for the terminal phase of the illness (86, 88, 89). Individuals with advanced disease may not be interested in food, may have problems managing the food bolus once it is in the mouth, or may aspirate when swallowing. In 1999, Finucane et al (83) published a thorough review of the literature to date addressing enteral nutrition in the elderly. The questions they analyzed were whether tube feeding in advanced dementia can prevent aspiration pneumonia, prolong survival, reduce the risk of pressure sores or infections, improve function, or provide palliation. They found no data to suggest that tube feeding improves any of these outcomes.
The risk of aspiration pneumonia is one of the most frequently used reasons by physicians for requesting a feeding tube in patients with dementia despite the lack of evidence or efficacy (79). Tube feeding does not reduce the risk of regurgitation of gastric contents. In fact, animal studies and studies in children show that gastrostomy tube placement may reduce the lower esophageal sphincter pressure and increase reflux (83, 88). Tube feeding cannot prevent aspiration of oral secretions. The idea that jejunal placement versus gastric placement of the feeding tube will lower rates of aspiration pneumonia is controversial. Heyland et al (90) published a meta-analysis combining seven studies that showed shifting the level of infusion from the stomach to the small bowel was associated with a 24% reduction in the incidence of aspiration pneumonia, while an earlier meta-analysis comparing gastrostomy with jejunostomy tubes found no significant difference in aspiration pneumonia frequency (79).
PEG tubes have also been placed in elderly patients and patients with dementia with the intent of treating malnutrition, preventing and healing pressure sores, and improving functional status. Several studies show no significant improvement in nutritional markers such as hemoglobin, albumin, and cholesterol levels after placement of a feeding tube (88). In the geriatric population, there is weak evidence that nutritional status correlates with pressure sores and it is therefore not surprising that tube feeding also was not associated with healing of pre-existing pressure sores in at least two longitudinal studies involving 800 patients (86). On the contrary, tube feeding may actually be linked with worsening pressure sores due to the need for physical restraints in some patients with dementia who have PEG tubes (91). The data regarding the impact of feeding tubes on functional status is limited, but in their review Finucane et al (83) found no studies in which nutritional intervention facilitated recovery of functional status.
PEG tubes are often placed with the hope that they will prolong survival. Nonrandomized, retrospective observations of nursing home residents have found no survival advantage with tube feeding (83). In 2003, Murphy and Lipman (91) looked at survival between 23 patients with dementia who received a PEG tube and 18 patients with dementia in whom PEG tube placement was refused either by their surrogate or because the procedure violated their advance directives. Median survival was 59 days and 60 days respectively. They concluded that there is no survival benefit in patients with dementia who receive artificial feeding by PEG tube. Mortality among tube fed patients is not insignificant and among the subgroups of patients requiring tube feeding, patients with dementia have the worst prognosis. Sanders et al (81) published an elegant study comparing the mortality of patients with dementia who were tube fed to that of other subgroups of patients requiring PEG tubes. Mortality for the overall cohort at one month and one year was 28% and 63% respectively. Among the patients with dementia, 30-day mortality was 54% with a mortality of 90% at one year. This difference remained statistically significant even after adjusting for age at the time of PEG tube insertion. The finding that patients with dementia have a considerably worse prognosis than other subgroups of patients undergoing PEG tube placement was later confirmed by the same group in a prospective analysis (92).
Best practices with PEG tubes
If the decision has been made to place a PEG tube for delivery of enteral nutrition, there are best practices that can be followed to decrease the risk of complications. Roche (78) details a number of these recommendations including evaluating tubes regularly for redness and irritation from gastric contents, flushing the tube before and after feeds and medications to prevent tube occlusion, adjusting the skin bolster according to any change in the patient’s weight if necessary, ensuring that the patient is sitting up or laying down with the head raised 45 degrees during feeds and for one hour afterwards, and instituting plans for long-term monitoring of the patient’s nutritional status and electrolytes. To answer the question posed at the start of this section: Are feeding tubes a solution? – one must tailor the decision to each individual patient. A discussion should be facilitated by the healthcare provider with the patient and their surrogates addressing the current evidence that exists on outcome measures, the patient’s known comorbidities, the natural progression of the patient’s underlying illness, and the expectations of the patient and their family regarding PEG tube placement.
Optimal Intervention Strategies for Elders with Dysphagia
Treatment for senescent dysphagia is usually compensatory, rehabilitative, or a combination of the two approaches. Compensatory interventions avoid or reduce the effects of impaired structures or neuropathology and resultant disordered physiology and biomechanics on bolus flow. Rehabilitative interventions have the capacity to directly improve dysphagia at the biological level. That is, aspects of anatomic structures (e.g., muscle) or neural circuitry are the targets of therapy that may have a direct influence on physiology, biomechanics, and bolus flow.
Compensatory Dysphagia Interventions
Traditionally, interventions for dysphagia in elderly patients are compensatory in nature and are directed at modifying bolus flow by targeting neuromuscularly induced pathobiomechanics or by adapting the environment. Compensatory strategies are believed by clinicians to be less demanding on the patient in terms of effort because many of the strategies can be imposed on a relatively passive patient. A nonexclusive sampling of compensatory strategies includes postural adjustment, slowing the rate of eating, limiting bolus size, adaptive equipment, and the most commonly used environment adaptation, diet modification.
Postural Adjustments
Postural adjustments are relatively simple to teach to a patient, require little effort to employ, and can eliminate misdirection of bolus flow through biomechanical adjustment. A general postural rule for facilitating safe swallowing is to eat in an upright posture (90° seated) so that the vertical phases (pharyngeal) of the oropharyngeal swallow as well as esophageal motility capitalize on gravitational forces. Upright posture also can assist in precluding early spillage of food or liquid from the horizontal oral phase into the pharynx and a potentially open airway as well as diminishing the probability of nasal regurgitation. A less obvious postural adjustment is useful for patients with hemiparesis. For this group of patients, a common strategy is a head turn toward the hemiparetic side, effectively closing off that side to bolus entry and facilitating bolus transit through the nonparetic pharyngeal channel. If the pathophysiologic condition is the uncoupling of the oral from the pharyngeal phase of the swallow indicated by a delay in onset of airway protection (mechanisms including hyolaryngeal excursion and vocal fold closure), a simple chin tuck (45°) reduces the speed of bolus passage, thereby giving the neural system the time it needs to initiate the pharyngeal and airway protection events prior to bolus entry. Other postural adjustments facilitate safe swallowing and are designed to specifically compensate for pathophysiologic conditions analyzed and treated by a swallowing clinician on referral for a swallowing assessment and treatment.
Food and Liquid Rate and Amounts
Although we live in a “fast food” society, older individuals and especially those with dysphagia take longer to eat. Eating an adequate amount of food becomes a challenge not only because of the increased time required to do so but also because fatigue frequently becomes an issue. To promote a safe, efficient swallow in most individuals with swallowing and chewing difficulties, the following recommendations are useful.
Eat slowly with intent to implement control of bolus flow and allow enough time for a meal.
Do not eat or drink when rushed or tired.
Take small amounts of food or liquid into the mouth—use a teaspoon rather than a tablespoon.
Concentrate on swallowing only - eliminate distractions like television.
Avoid mixing food and liquid in the same mouthful – single textures are easier to swallow than multiple textures.
Place the food on the stronger side of the mouth if there is unilateral weakness.
Alternate liquids and solids to “wash down” residue
Use sauces, condiments and gravies to facilitate cohesive bolus formation and prevent pocketing or small food particles from entering the airway
Adaptive Equipment
Eating and drinking aids can assist in placing, directing, and controlling the bolus of food or liquid and in maintaining proper head posture while eating. For example, modified cups with cutout rims (placed over the bridge of the nose) or the use of straws prevent a backward head tilt when drinking to the bottom of a cup. A backward head tilt, which results in neck extension, should be avoided in most cases because food and liquid are more likely to be misdirected into the airway. Spoons with narrow, shallow bowls or glossectomy feeding spoons (spoons developed for moving food to the back of the tongue) are useful to individuals who require assistance in placing food in certain locations in the mouth. More importantly, these utensils and devices promote independence in eating and drinking. A speech pathologist can make suggestions regarding appropriate aids for optimizing swallowing safety and satisfaction. Occupational therapists are experts in the area of adaptive equipment and can be helpful in obtaining products that are often available commercially.
Diet Modification
The most common compensatory intervention is diet modification, a totally passive environmental adaptation. Withholding thin liquids such as water, tea, or coffee, which are most easily aspirated by older adults, and restricting liquid intake to thickened liquids is almost routine in nursing homes in an attempt to minimize or eliminate thin-liquid aspiration, presumably the precedent to the long-term related outcome, which is pneumonia. Increasing the viscosity of liquids using thickener additives decreases the rate of flow allowing patients more time to initiate airway protection and prevent or decreases aspiration. Despite the huge impact these seemingly unappealing practices may have on patient QOL, they have been commonly implemented in the absence of efficacy data.
The largest NIH-funded multisite, randomized clinical trial (RCT) in the field of dysphagia was recently completed. It was designed as two sequential RCTs to compare the efficacy of two of the most commonly prescribed dysphagia interventions: chin-down posture and thickened liquids of either nectar-thick (300cps) and honey-thick (3000cps) viscosity1 for patients with Parkinson’s disease (PD) and/or dementia in the short term and over a longer period of time. Seven hundred eleven patients were enrolled in a short-term arm of the study (Part I) where each intervention was evaluated using videofluoroscopy to assess its effectiveness in preventing the primary outcome: immediate aspiration. In Part II of the study, the long-term follow-up, 515 patients were randomly assigned to use one of these common interventions for 3 months and were monitored for incidence of the primary outcome: pneumonia. The primary outcome of Part I showed that aspiration was significantly reduced with honey-thick liquids as compared with both nectar-thick and chin-down posture interventions. The primary outcome of Part II showed equal value in chin-down posture and thickened liquids in pneumonia prevention. Several secondary outcomes also are of great importance. Patients with dementia (with or without PD), regardless of intervention, had significantly greater incidence of pneumonia than patients with PD only. Patients who aspirated more frequently with all of the interventions during the videofluoroscopic evaluation of swallowing also were more likely to incur pneumonia during Part II. Of the individuals who did get pneumonia, those randomized to drink honey-thick liquids (defined as 3000 centipoise which is very thick) for 3 months were hospitalized an average of 13 days longer than patients drinking nectar-thick liquids (4 days) or those performing the chin-down posture (6 days) while drinking.
With the appearance of the SWAL-QOL, a dysphagia-specific quality of life questionnaire, on the clinical scene, some of these ongoing practices can be evaluated from the patient’s perspective as well as by the clinicians who, with good intent, recommend them. In the NIH-funded RCT, significantly more patients who were cognitively intact reported preferring chindown posture with thin liquids over consumption of nectar-thick or honey-thick fluids to attempt eliminating aspiration. Between the thickened fluids, they found nectar-thick more satisfactory, or pleasant to drink, than honey-thick.
An attempt by the American Dietetics Association (ADA) was made to standardize dysphagia diets by modifications in the texture of solid foods and/or increased viscosity of liquids (93). Four levels of their National Dysphagia Diet are presented in Table 6. Level 1 or the “pureed diet” includes cohesive foods that do not require bolus formation, controlled manipulation or chewing. The pureed diet is for individuals who have moderate to severe dysphagia and reduced ability to protect their airway. Level 2 or the “mechanically altered diet” includes foods that are moist, soft-textured, and easily formed into a bolus. This diet is a transition from the pureed textures to more solid textures and chewing ability is required. Level 3 or “dysphagia advanced” includes moist foods of nearly regular textures with the exception of hard and sticky foods. The use of sauces and gravies to minimize the formation of dry particles that may easily be misdirected into the airway is a common practice. This diet is a transition to a regular diet and is appropriate for individuals with mild oral and/or pharyngeal phase dysphagia (34). Small frequent meals are often used for individuals with dysphagia, although, recent data suggests five daily feedings does not improve energy intake when compared with three feedings (94). The dietitian should work closely with the team to ensure that the safest diet is provided and that it is effective in maintaining adequate nutrition and hydration, while also acceptable to patients in order to insure compliance.
Table 6.
National Dysphagia Diet Progression
| SOLIDS: | |||
|---|---|---|---|
| National Dysphagia Diet: | Description | Examples of Preferred Foods | Foods to Avoid |
| Diet 1- Pureed | Pureed, cohesive, pudding-like foods that are homogenous (no lumps) | Pureed meats/fruits/vegetables, Mashed potatoes with gravy Pregelled, slurried breads Puddings, souffles Hummus |
Gelatin Foods with lumps (fruited yogurt, cottage cheese, soups, cereals) Peanut butter Scrambled/fried/hard-cooked eggs |
| Diet 2-Mechanically Altered | Moist, cohesive, and soft-textured foods | Pancakes with syrup Scrambled/poached/soft-cooked egg Meats 1/4″ cubed or smaller, with gravy Mashable vegetables 1/2″ cubed or smaller Most canned/cooked fruits, fresh bananas Moistened cereals with little texture |
Crackers, bread, dry cake, rice Pineapple, fresh/frozen fruits Dry, tough meats Raw vegetables Fruits with skins or seeds/dry fruit Coconut, nuts Corn or peas |
| Diet 3-Advanced | Soft solid diet that includes most foods and requires more chewing ability than Diet 2 | Moist cake/bread Whole meats that are tender/moist Shredded lettuce Fruits with soft peel All tender, cooked vegetables Well-moistened cereal |
Dry bread/toast/crackers/cake Corn Nuts/seeds Sticky/very hard/crunchy foods Hard fruits/vegetables Coarse, dry cereal (shredded wheat) |
| Diet 4-Normal | Includes all foods | All | None |
| LIQUIDS: | |||
Liquid consistencies will be provided in combination with any of the above solid diets:
| |||
Knowing the Heimlich Maneuver
Educating care providers and family members about the signs of choking and the standard first-aid technique for clearing the airway, namely, the Heimlich maneuver, is essential. While the Heimlich maneuver can be self-administered, it is recommended that individuals with dysphagia eat in the company of someone who knows this first-aid technique. Family members should be trained in emergency techniques for clearing the airway.
Rehabilitative Dysphagia Interventions
Active Exercise
Rehabilitative exercises are, by nature, more active and rigorous than alternative interventions for dysphagia. Traditionally a rehabilitative approach to dysphagia intervention has been withheld from elderly patients because such a demanding activity has been assumed to deplete any limited remaining swallowing reserve, thus potentially exacerbating dysphagia symptoms. The treatment approaches mentioned thus far are compensatory in nature and do not directly change the function of the swallowing mechanism, thus requiring employment of these strategies for every single swallow. Sufficient treatment efficacy data are becoming available, and while assumption-based patterns of practice prevail, practice patterns are beginning to undergo change. A body of literature has emerged during the past decade that suggests that loss of muscle strength with age is, to a great extent, reversible through rehabilitation exercise.
Recent research on the benefits of lingual resistance exercise suggests that strength-building exercises for the tongue increase lingual muscle strength and mass and improve the timing of the swallowing components in healthy older adults, with implications for greater gains and carryover into swallowing-related outcomes in elderly dysphagic patients (5, 6). A description of two different exercise regimens that are supported with efficacy data for improving swallowing function and related outcome in the elderly follow.
One is a simple isotonic/isometric neck exercise performed over a 6-week period in which the patient simply lies flat on his back and lifts his head (keeping shoulders flat) for a specified number of repetitions. The improved physiologic outcome of upper esophageal sphincter (UES) opening that affects swallowing is speculated to result from strengthening the mylohyoid/geniohyoid muscle groups and possibly the anterior segment of the digastric muscle (95, 96).
Another exercise program demonstrating effectiveness in older dysphagic patients comprises an 8-week isometric resistance exercise for the tongue and related oropharyngeal musculature, and was shown in acute and chronic stroke patients to improve swallowing safety by reducing airway invasion, increasing lingual pressure generation both isometrically and during swallowing and increasing lingual structure, specifically volume, as measured via magnetic resonance imaging (97). Such findings suggest that older, dysphagic individuals are able to benefit from rehabilitative exercises focused on bulbar-innervated head and neck musculature. The methods hold promise for not only influencing safe, efficient bolus flow with significant functional gains but also appears to be restoring health and improving QOL as well.
Surgical Procedures
Although compensatory and rehabilitative strategies can facilitate more normal swallowing, some patients present with structural or functional impairments that are best managed surgically. For instance, patients with esophageal strictures, a cricopharyngeal prominence or a large Zenker’s diverticulum may immediately resume normal swallowing function once the structure is repaired. In specific circumstances, both swallowing techniques and surgery are combined to provide the best outcomes. For instance, a patient with limited UES opening may undergo dilation to decrease resistance at the distal end of the pharynx and also perform lingual exercises to increase bolus propulsion at the proximal end. All team members should carefully consider the risks and benefits of surgical intervention for elderly patients as these individuals may experience greater complications given their increased age and often are not good surgical candidates.
Oral Hygiene
Poor oral hygiene is a risk factor for pneumonia, and aspiration of saliva, whether or not it is combined with food or fluid, can increase the likelihood of infection. Therefore, patients should be encouraged to perform oral hygiene several times a day and undergo periodic dental examinations. Furthermore, products to relieve oral dryness, as well as alcohol-free mouth care products, can be recommended.
Summary
In summary, while oropharyngeal dysphagia may be life-threatening, so are some of the alternatives, particularly for frail elderly patients. Therefore, contributions by all team members are valuable in this challenging decision-making process, with the patient’s family or care provider’s point of view perhaps being the most critical. The state of the evidence calls for more research, including randomized clinical trials in this area. Until (and perhaps after) these data are collected and have been analyzed, the many behavioral, dietary, and environmental modifications described in this manuscript are compassionate and, in many cases, preferred alternatives to the always present option of tube feeding.
Acknowledgments
Supported by VA Merit Grant #C4796R, VA RR&D grant #RRP 07-314. This is GRECC Manuscript # 2008-29. The authors wish to thank Jacqueline Hind, MS, CCC-SLP, and Amanda Ganser, BS, for their contributions, and Abby Duane, BS, for manuscript preparation.
Footnotes
As a reference, water is 1cps and Karo syrup is 5,000cps.
References
- 1.McHorney C, Bricker D, Kramer A, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults:I. Conceptual foundation and item development. Dysphagia. 2000;15 (3):115–121. doi: 10.1007/s004550010012. [DOI] [PubMed] [Google Scholar]
- 2.McHorney CA, Bricker DE, Robbins JA, Kramer AE, Rosenbek JC, Chignell KA. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: II. Item reduction and preliminary scaling. Dysphagia. 2000;15 (3):122–133. doi: 10.1007/s004550010013. [DOI] [PubMed] [Google Scholar]
- 3.McHorney CA, Robbins JA, Lomax K, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults:III. Extensive evidence of reliability and validity. Dysphagia. 2002;17:97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 4.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–9. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 5.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):M257–62. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 6.Nicosia MA, Hind JA, Roecker EB, Carnes M, Robbins JA. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol Med Sci. 2000;55A (11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 7.Tracy F, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugla C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4:90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 8.Shaw D, Cook IJ, Dent J, et al. Age influences oropharyngeal and upper esophageal sphincter function during swallowing. Gastroenterology. 1990;98:A390. [Google Scholar]
- 9.Shaw D, Cook IJ, Gabb M, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol Gastrointest Liver Physiol. 1995;268:G389–396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 10.Robbins J, Coyle J, Roecker E, Rosenbek J, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14 (4):228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 11.Pendergast DR, Fisher NM, Calkins E. Cardiovascular, neuromuscular, and metabolic alterations with age leading to frailty. J Gerontol. 1993;48(Spec No):61–7. doi: 10.1093/geronj/48.special_issue.61. [DOI] [PubMed] [Google Scholar]
- 12.Brandt N. Medications and dysphagia: How do they impact each other? Nutr Clin Prac. 1999;14:S27–S30. [Google Scholar]
- 13.Waite B, Palmer R, Nicosia M, et al. Intraesophageal reflux and intraesophageal stasis are distinct from gastroesophageal reflux as seen radiographically. Gastroenterology. 2002;122:283. [Google Scholar]
- 14.Ghezzi EM, Ship JA. Aging and secretory reserve capacity of major salivary glands. J Dent Res. 2003;82(10):844–8. doi: 10.1177/154405910308201016. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman SS. Perception of taste and smell in elderly persons. Crit Rev Food Sci Nutr. 1993;33(1):17–26. doi: 10.1080/10408399309527608. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder MF, Daniels SK, McClain M, Corey DM, Foundas AL. Clinical and cognitive predictors of swallowing recovery in stroke. J Rehabil Res Dev. 2006;43(3):301–10. doi: 10.1682/jrrd.2004.12.0154. [DOI] [PubMed] [Google Scholar]
- 17.Kenshalo DR. Somesthetic sensitivity in young and elderly humans. J Gerontol. 1986;41(6):732–742. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 19.Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging (Milano) 1996;8(2):99–105. doi: 10.1007/BF03339562. [DOI] [PubMed] [Google Scholar]
- 20.Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):137–41. doi: 10.1093/gerona/50a.special_issue.137. [DOI] [PubMed] [Google Scholar]
- 21.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):124–9. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 22.Price PA, Darvell BS. Force and mobility in the aging human tongue. Med J Aust. 1982;1:75–78. doi: 10.5694/j.1326-5377.1981.tb135325.x. [DOI] [PubMed] [Google Scholar]
- 23.Rastatter MP, McGuire RA, Bushong L, Loposky M. Speech-motor equivalence in aging subjects’. Percept Mot Skills. 1987;64(2):635–8. doi: 10.2466/pms.1987.64.2.635. [DOI] [PubMed] [Google Scholar]
- 24.Newton JP, Abel EW, Robertson EM, Yemm R. Changes in human masseter and medial pterygoid muscles with age: a study by computed tomography. Gerodontics. 1987;3(4):151–4. [PubMed] [Google Scholar]
- 25.Arai T, Sekizawa K, Ohrui T, et al. ACE inhibitors and protection against pneumonia in elderly patients with stroke. Neurology. 2005;64(3):573–4. doi: 10.1212/01.WNL.0000150897.14961.0F. [DOI] [PubMed] [Google Scholar]
- 26.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–6. doi: 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kind AJ, Smith MA, Frytak JR, Finch MD. Bouncing back: patterns and predictors of complicated transitions 30 days after hospitalization for acute ischemic stroke. J Am Geriatr Soc. 2007;55(3):365–73. doi: 10.1111/j.1532-5415.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–65. doi: 10.1111/j.1475-6773.2004.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medicare Payment Advisory Commission. (MedPAC) Report to the Congress: Medicare payment policy. 2007. [Google Scholar]
- 30.Kind AJ, Smith MA, Liou JI, Pandhi N, Frytak JR, Finch MD. The price of bouncing back: one-year mortality and payments for acute stroke patients with 30-day bounce-backs. Journal of the American Geriatrics Society. 2008;56(6):999–1005. doi: 10.1111/j.1532-5415.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71. doi: 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 32.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke. 2004;35(4):1005–9. doi: 10.1161/01.STR.0000120727.40792.40. [DOI] [PubMed] [Google Scholar]
- 33.Stroke Unit Trialists’ Collaboration (SUTC) How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke. 1997;28(11):2139–44. doi: 10.1161/01.str.28.11.2139. [DOI] [PubMed] [Google Scholar]
- 34.Kind AJH, Smith M, Pandhi N, Frytak JR, Finch M. Bouncing-Back: Rehospitalization in Patients with Complicated Transitions in the First Thirty Days After Hospital Discharge for Acute Stroke. Home Health Services Quarterly. 2007;26(4):37–55. doi: 10.1300/J027v26n04_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslanyan S, Weir CJ, Diener H-C, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11(1):49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 36.James A, Kapur K, Hawthorne AB. Long-term outcome of percutaneous endoscopic gastrostomy feeding in patients with dysphagic stroke. Age Ageing. 1998;27(6):671–6. doi: 10.1093/ageing/27.6.671. [DOI] [PubMed] [Google Scholar]
- 37.Smith MA, Liou JI, Frytak JR, Finch MD. 30-day survival and rehospitalization for stroke patients according to physician specialty. Cerebrovasc Dis. 2006;22(1):21–6. doi: 10.1159/000092333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doggett DL, Tappe KA, Mitchell MD, Chapell R, Coates V, Turkelson CM. Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: an evidence-based comprehensive analysis of the literature. Dysphagia. 2001;16(4):279–95. doi: 10.1007/s00455-001-0087-3. [DOI] [PubMed] [Google Scholar]
- 39.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–8. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 40.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA. 1999;281(7):613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 41.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–84. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 42.Institute for Healthcare Improvement. Reducing readmissions for heart failure patients. Vol. 2008. Hackensack University Medical Center; Cambridge, MA: 2004. [Google Scholar]
- 43.Institute for Healthcare Improvement. The MedProvider inpatient care unit -Congestive heart failure project. Vol. 2008. Cambridge, MA: 2004. [Google Scholar]
- 44.Berenson RA California Healthcare Foundation. Challenging the status quo in chronic disease care: Seven case studies. 2006. Sep, [Google Scholar]
- 45.VanSuch M, Naessens JM, Stroebel RJ, Huddleston JM, Williams AR. Effect of discharge instructions on readmission of hospitalised patients with heart failure: do all of the Joint Commission on Accreditation of Healthcare Organizations heart failure core measures reflect better care? Quality and Safety in Health Care. 2006;15(6):414–7. doi: 10.1136/qshc.2005.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26(4):389–99. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Akner G, Cederholm T. Treatment of protein-energy malnutrition in chronic nonmalignant disorders. Am J Clin Nutr. 2001;74(1):6–24. doi: 10.1093/ajcn/74.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Cosqueric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr. 2006;96(5):895–901. doi: 10.1017/bjn20061943. [DOI] [PubMed] [Google Scholar]
- 49.Brody R. Nutrition Issues in Dysphagia: Identification, Management, and the Role of the Dietitian. Nutrition in Clinical Practice. 1999;14(5):S47–S51. [Google Scholar]
- 50.Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. 2008;65(1):39–43. doi: 10.1001/archneurol.2007.12. [DOI] [PubMed] [Google Scholar]
- 51.IOM. The essential guide to nutrient requirements. National Academy of Sciences; Washington, DC: 2006. Dietary Reference Intakes. [Google Scholar]
- 52.Lichtenstein AH, Rasmussen H, Yu WW, Epstein SR, Russell RM. Modified MyPyramid for Older Adults. J Nutr. 2008;138(1):5–11. doi: 10.1093/jn/138.1.5. [DOI] [PubMed] [Google Scholar]
- 53.Statistics NCfH. [Accessed September 29, 2008];Prevalence of Overweight and Obesity Among Adults in the United States. 2007 http://www.cdc.gov/nchs/products/pubs/pubd/hestats/3and4/overweight.htm.
- 54.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 55.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77(1):109–27. doi: 10.1093/ajcn/77.1.109. [DOI] [PubMed] [Google Scholar]
- 56.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 57.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 58.Campbell WW, Trappe TA, Jozsi AC, Kruskall LJ, Wolfe RR, Evans WJ. Dietary protein adequacy and lower body versus whole body resistive training in older humans. J Physiol. 2002;542(Pt 2):631–42. doi: 10.1113/jphysiol.2002.020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56(6):M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 60.Campbell W, Carnell N, Thalacker A. Protein metabolism and requirements. In: Chernoff R, editor. Geriatric Nutrition the Health Professional’s Handbook. 3. Sudbury, MA: Jones and Bartlett Publishers; 2006. [Google Scholar]
- 61.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 62.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–31. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 63.Sheffield-Moore M, Paddon-Jones D, Sanford AP, et al. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288(5):E922–9. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 64.Di Stefano M, Veneto G, Malservisi S, Strocchi A, Corazza GR. Lactose malabsorption and intolerance in the elderly. Scand J Gastroenterol. 2001;36(12):1274–8. doi: 10.1080/003655201317097119. [DOI] [PubMed] [Google Scholar]
- 65.Moore C, Murphy MM, Keast DR, Holick MF. Vitamin D intake in the United States. J Am Diet Assoc. 2004;104(6):980–3. doi: 10.1016/j.jada.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 66.Hurwitz A, Brady DA, Schaal SE, Samloff IM, Dedon J, Ruhl CE. Gastric acidity in older adults. JAMA. 1997;278(8):659–62. [PubMed] [Google Scholar]
- 67.Phillips PA, Rolls BJ, Ledingham JG, et al. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311(12):753–9. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 68.Farrell MJ, Zamarripa F, Shade R, et al. Effect of aging on regional cerebral blood flow responses associated with osmotic thirst and its satiation by water drinking: a PET study. Proc Natl Acad Sci U S A. 2008;105(1):382–7. doi: 10.1073/pnas.0710572105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberg AD, Minaker KL. Dehydration. Evaluation and management in older adults. Council on Scientific Affairs, American Medical Association. JAMA. 1995;274(19):1552–6. doi: 10.1001/jama.274.19.1552. [DOI] [PubMed] [Google Scholar]
- 70.Finestone HM, Greene-Finestone LS, Wilson ES, Teasell RW. Malnutrition in stroke patients on the rehabilitation service and at follow-up: prevalence and predictors. Arch Phys Med Rehabil. 1995;76(4):310–6. doi: 10.1016/s0003-9993(95)80655-5. [DOI] [PubMed] [Google Scholar]
- 71.Anthony PS. Nutrition screening tools for hospitalized patients. Nutr Clin Pract. 2008;23(4):373–82. doi: 10.1177/0884533608321130. [DOI] [PubMed] [Google Scholar]
- 72.Detsky AS, Smalley PS, Chang J. The rational clinical examination. Is this patient malnourished? JAMA. 1994;271(1):54–8. doi: 10.1001/jama.271.1.54. [DOI] [PubMed] [Google Scholar]
- 73.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us? J Nutr Health Aging. 2006;10(6):466–85. discussion 485–7. [PubMed] [Google Scholar]
- 74.Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr. 1977;1(1):11–22. doi: 10.1177/014860717700100101. [DOI] [PubMed] [Google Scholar]
- 75.Barczi SR, Sullivan P, Robbins J. How should dysphagia care of the older adult differ? Establishing optimal practice patterns. Semin Speech Lang. 2000;21:347–361. doi: 10.1055/s-2000-8387. [DOI] [PubMed] [Google Scholar]
- 76.Gunter-Hunt G, Carnes M, Priefer B, Robbins J. Team evaluation of older patients with dysphagia. Federal Practitioner. 1997 October;:30–34. [Google Scholar]
- 77.Gauderer MW, Ponsky JL, Izant RJ., Jr Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872–5. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 78.Roche V. Percutaneous endoscopic gastrostomy. Clinical care of PEG tubes in older adults. Geriatrics. 2003;58(11):22–6. 28–9. [PubMed] [Google Scholar]
- 79.Garrow D, Pride P, Moran W, Zapka J, Amella E, Delegge M. Feeding alternatives in patients with dementia: examining the evidence. Clin Gastroenterol Hepatol. 2007;5(12):1372–8. doi: 10.1016/j.cgh.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 80.American Gastroenterological Association Medical Position Statement: guidelines for the use of enteral nutrition. Gastroenterology. 1995;108(4):1280–1. doi: 10.1016/0016-5085(95)90230-9. [DOI] [PubMed] [Google Scholar]
- 81.Sanders DS, Carter MJ, D’Silva J, James G, Bolton RP, Bardhan KD. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472–5. doi: 10.1111/j.1572-0241.2000.02079.x. [DOI] [PubMed] [Google Scholar]
- 82.Abuksis G, Mor M, Plaut S, Fraser G, Niv Y. Outcome of percutaneous endoscopic gastrostomy (PEG): comparison of two policies in a 4-year experience. Clin Nutr. 2004;23(3):341–6. doi: 10.1016/j.clnu.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Finucane TE, Christmas C, Travis K. Tube feeding in patients with advanced dementia: a review of the evidence. JAMA. 1999;282(14):1365–70. doi: 10.1001/jama.282.14.1365. [DOI] [PubMed] [Google Scholar]
- 84.Skelly RH, Kupfer RM, Metcalfe ME, et al. Percutaneous endoscopic gastrostomy (PEG): change in practice since 1988. Clin Nutr. 2002;21(5):389–94. doi: 10.1054/clnu.2002.0563. [DOI] [PubMed] [Google Scholar]
- 85.Johnston SD, Tham TC, Mason M. Death after PEG: results of the National Confidential Enquiry into Patient Outcome and Death. Gastrointest Endosc. 2008;68(2):223–7. doi: 10.1016/j.gie.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 86.DeLegge MH, McClave SA, DiSario JA, et al. Ethical and medicolegal aspects of PEG-tube placement and provision of artificial nutritional therapy. Gastrointest Endosc. 2005;62(6):952–9. doi: 10.1016/j.gie.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 87.McClave SA, Delegge MH. Predicting life expectancy before percutaneous endoscopic gastrostomy placement: a lesson in futility or an exercise of injustice? Gastrointest Endosc. 2008;68(2):228–30. doi: 10.1016/j.gie.2008.01.007. quiz 333, 335. [DOI] [PubMed] [Google Scholar]
- 88.Cervo FA, Bryan L, Farber S. To PEG or not to PEG: a review of evidence for placing feeding tubes in advanced dementia and the decision-making process. Geriatrics. 2006;61(6):30–5. [PubMed] [Google Scholar]
- 89.Vitale CA, Hiner T, Ury WA, Berkman CS, Ahronheim JC. Tube feeding in advanced dementia: an exploratory survey of physician knowledge. Care Manag J. 2006;7(2):79–85. doi: 10.1891/cmaj.7.2.79. [DOI] [PubMed] [Google Scholar]
- 90.Heyland DK, Drover JW, Dhaliwal R, Greenwood J. Optimizing the benefits and minimizing the risks of enteral nutrition in the critically ill: role of small bowel feeding. JPEN J Parenter Enteral Nutr. 2002;26(6 Suppl):S51–5. doi: 10.1177/014860710202600608. discussion S56–7. [DOI] [PubMed] [Google Scholar]
- 91.Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351–3. doi: 10.1001/archinte.163.11.1351. [DOI] [PubMed] [Google Scholar]
- 92.Sanders DS, Carter MJ, D’Silva J, et al. Percutaneous endoscopic gastrostomy: a prospective audit of the impact of guidelines in two district general hospitals in the United Kingdom. Am J Gastroenterol. 2002;97(9):2239–45. doi: 10.1111/j.1572-0241.2002.05778.x. [DOI] [PubMed] [Google Scholar]
- 93.National Dysphagia Diet Task F. The National Dysphagia Diet: Standardization for Optimal Care. American Dietetic Association; Chicago, IL: 2002. [Google Scholar]
- 94.Taylor KA, Barr SI. Provision of small, frequent meals does not improve energy intake of elderly residents with dysphagia who live in an extended-care facility. J Am Diet Assoc. 2006;106(7):1115–8. doi: 10.1016/j.jada.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 95.Shaker R, Kern M, Bardan E, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. American Journal of Physiology. 1997;272:G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- 96.Shaker R, Cook IJS, Dodds WJ, Hogan WJ. Pressure flow dynamics of the oral phase of swallowing. Dysphagia. 1988;3:79–84. doi: 10.1007/BF02412424. [DOI] [PubMed] [Google Scholar]
- 97.Robbins J, Kays SA, Gangnon R, Hewitt A, Hind J. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]