Abstract
Herein we demonstrate that highly sensitive conductometric gas nanosensors for H2S can be synthesized by electrodepositing gold nanoparticles on single-walled carbon nanotubes (SWNTs) networks. Adjusting the electrodeposition conditions allowed for tuning of the size and number of gold nanoparticles deposited. The best H2S sensing performance was obtained with discrete gold nanodeposits rather than continuous nanowires. The gas nanosensors could sense H2S in air at room temperature with a 3 ppb limit of detection. The sensors were reversible and increasing the bias voltage reduced the sensor recovery time, probably by local Joule heating. The sensing mechanism is believed to be based on the modulation of the conduction path across the nanotubes emanating from the modulation of electron exchange between the gold and carbon nanotubes defect sites when exposed to H2S.
Keywords: Electrodeposition, Single-Walled Carbon Nanotube, Hydrogen Sulfide, Sensor, Gold, Nanoparticles
Introduction
Sensitive detection and monitoring of gaseous reduced sulfur compounds such hydrogen sulfide (H2S) is important because of toxicity, corrosion and odor nuisance concerns. H2S is generated in large amounts in coal and natural gas processing, petroleum industries, biogas production and sewage treatment plants1. The odor threshold of H2S is around 3 ppb in air which is well below any known toxic effects, while the threshold limit value (TLV) was set to 10 ppm; at concentrations exceeding 150 ppm, H2S is extremely toxic and hazardous2. Hence, it is important to develop sensors that can monitor H2S in real-time, and can detect concentrations from as low as few of ppb to the several hundreds ppm in air. A number of H2S detection techniques have been developed in the past few decades, with electrochemical detection as the most widely utilized method in compact and portable H2S gas monitors3,4. These devices often rely on solid state sensors made of semiconducting metal oxides such as tungsten oxide and tin oxide, or metals such as gold. H2S detection is usually based on determining conductivity changes of thin films upon exposure to H2S gas4,5. While there are a number of successful devices available commercially, drawbacks of existing H2S monitors include high power consumption (e.g., in metal oxides sensors that require high operating temperatures), low sensitivity making them unsuitable for environmental monitoring, short life-time (usually less than one year), short linear dynamic range, interference from other gases (e.g., NH3, NOx, etc), and high cost.6
Recently, chemical sensors based on one-dimensional nanostructures such as bare or functionalized semiconducting single-walled carbon nanotubes (SWNTs)6,7, metal oxide and conducting polymer nanowires8 have attracted a great deal of attention because of their superior sensing performance. In particular, these sensors offer promising perspectives for real-time monitoring of gases or vapors with high sensitivity and low-power consumption, and potentially at low cost6. Their small size will also allow future integration with low-power microelectronics.
There has been only few gas nanosensors developed for H2S detection. Sun et al.9 reported a catalytic chemiluminescence sensor made of α-Fe2O3 nanotubes for rapid and selective detection of H2S down to 10 ppm H2S. However, it required heating to over 110 °C for catalytic oxidation of H2S to occur as well as complex electronics for chemiluminescence measurement. Geng et al.10 demonstrated that suppressed electron hoping occurred in gold nanoparticles after exposure to pure H2S. Although the sensors do not appear to be suitable for monitoring purposes because of low sensitivity, the study provided some mechanistic insight. Recently, we reported on the synthesis and H2S gas sensing performance of networks made of hybrid polyaniline (PANI) nanowires and gold nanoparticles11. Template-free electrochemical polymerization was used to synthesize 250–320 nm diameter PANI nanowires that were then decorated with gold nanoparticles by electrodeposition. The nanostructures exhibited superior H2S gas sensing, with a detection limit of 0.1 ppb H2S in air at room temperature and a linear response from 0.1 to 500 ppb. However, polymer-based sensors are often subject to drift over time and have a low operating temperature range.8
In this report, we demonstrate site-specific electrodeposition of gold nanoparticles on SWNT networks and use these nanostructures to study the mechanisms and demonstrate the effectiveness of H2S gas sensing at room temperature. SWNTs were selected as templates for growing nanoparticles because of their unique physical and chemical properties along with their ultra high surface area to volume ratio. Also SWNTs have little or no chemical interaction with H2S or other gases that may interfere.6 By varying the electrodeposition conditions, gold nanoparticles of different diameter and density were deposited. The sensor synthesis conditions were optimized to enhance H2S sensing performance. Sensing data suggested that the adsorption of H2S molecules on to the gold nanoparticles surface altered the work function of the gold which in turn altered the carbon nanotubes conductivity.
Methods
Nanosensor fabrication
Sensor arrays were microfabricated as described previously12 on silicon substrate using standard lithographic patterning. Approximately one micron thick SiO2 film was first deposited on a lightly doped p-type (100) oriented silicon wafer using chemical vapor deposition (CVD) to insulate the substrate. The electrode area was defined by photolithography using positive photoresist, followed by e-beam evaporation of a 20 Å-thick Cr adhesion layer and a ~3000 Å-thick gold layer. Finally, electrodes (200 μm × 200 μm) separated by a gap of approximately 5 μm were defined using lift-off techniques. To fabricate SWNT interconnects across the electrodes; first, carboxylated-SWNTs (SWNT-COOH 80-90% purity) (Carbon Solution, Inc. Riverside, CA, USA) were dispersed (1 μg/mL) in dimethyl formamide (DMF, Sigma Aldrich, MO, USA) using ultrasonic force for 60 min. Then, a 50 nano-liter of the SWNT solution was manually dispensed on top of the electrodes gap using a micro syringe. After the evaporation of the solution, a network of randomly oriented SWNTs bridged each electrode gap. Atomic force microscopy observation of the networks (not shown) revealed that 80% of the SWNTs or SWNTs bundles were in the 3-5 nm diameter range, with lengths ranging from few microns to about 10-20 microns. The sensors were then annealed at 300 °C for 30 minutes under reducing atmosphere (5% H2 + 95% N2) to minimize the contact resistance between the CNT network and the gold pads and to remove any DMF residues. The number of SWNTs bridging the electrode gap was controlled by adjusting the concentration of the SWNTs in the DMF solution and the droplet size.
Electrochemical functionalization
Electrodeposition of gold nanoparticles on the SWNT networks was performed using a three electrode electrochemical cell configurations. The linear sweep voltammetry (LSV) and chronoamperometry (potentiostatic) measurements were carried out using a VMP2-potentiostat (Princeton Applied Research, TN, USA). For particle characterization studies, gold nanoparticles were electrodeposited on spray printed SWNT film on gold substrates. Commercially available ready-to-use gold electroplating solution from Technic Inc (Techni – gold 25 ES, CA) was used as electrolyte. Spray printed SWNT films on gold electrodes served as working electrode, Pt mesh as counter and standard glass Ag/AgCl (Satd. KCl) as reference electrode. The apparent surface area of electrode was fixed at 1cm2. For gas sensing studies, first, a 3 μL droplet of Au electrolyte solution was placed on top the SWNT network bridging the electrode gap. An alkaline cyanide-free gold electrolyte (pH = 7.5) was selected to prevent the dissolution of Cr adhesion layer which can be readily attacked in an acidic environment. Next, platinum and Ag/AgCl wires were positioned inside the droplets using a micropositioner. The annealed SWNT networks along with the gold electrodes served as the working electrode, while platinum and Ag/AgCl wires served as the counter and pseudo reference electrodes, respectively. All depositions were carried out in potentiostatic mode (constant potential) at 25 °C and ambient pressure, with deposition charge as the stopping criteria. After deposition the electrodes were rinsed with deionized water and acetone to remove any metal salt residues and impurities.
Characterization
The size and number of gold nanoparticles on SWNTs were determined using field emission - scanning electron microscopy (Leo model # 1550, MA, USA) and atomic force microscopy (PSIA, Inc. model # XE100, CA, USA). AFM images were obtained using a non-contact mode at a very low scan rate (0.1 Hz) for accurate representation of particle size and density. Five sets of functionalized SWNT (5 μm by 5 μm) were selected for particle size and density determinations. For measuring the particle size, particle height was measured rather than particle width to avoid AFM tip convolution effects. A good agreement was observed with the particle width measurement obtained from SEM images indicating that nanoparticles formed on the SWNTs were more or less spherical in shape. The temperature dependent electrical transport properties of Au-SWNTs were characterized using a Physical Property Measurement System from Quantum Design, Inc (CA, USA) and I-V characteristics were determined using a semiconductor parameter analyzer (HP model # 4155A, CA, USA).
Gas sensing studies
For gas sensing studies, the sensors were wire-bonded and each sensor was connected in series with a load resistor. The value of the load resistor was chosen to be as close as possible to the initial sensor resistance to optimize the resolution obtained from the measurements. The sensors typically had a baseline resistance in the range of 20 KΩ at room temperature. This baseline resistance value was selected based on optimization studies carried out earlier12. The circuit was subjected to a fixed voltage and the electrical resistance of the sensor was determined by continuously monitoring the differential voltage over the load resistor and applying Ohm’s law. The applied voltage across the sensor was varied from 1-5 V to study the effect of applied bias on sensing performance.
A 3.6 cm3 sealed glass chamber with gas inlet and outlet ports for gas flow-through was positioned over the sensor chip. All experiments were conducted with hydrogen sulfide (purity: 99.998 %) diluted in dry air (purity: 99.998%) at a gas flow of 200 std. cm3 min−1. The H2S and dry air gas flow rates were regulated by mass flow controllers (Alicat Scientific Incorporated, Tucson, AZ, USA). A custom Lab view computer program was developed to continuously control and monitor the voltage of the circuit using field point analog input and output modules (National Instruments, Austin, TX, USA). In all the experiments, sensors were first exposed to air to obtain the baseline, then to a desired concentration of H2S gas, and then back to air which completed one cycle.
Results and Discussion
Effect of deposition parameters on metal deposition
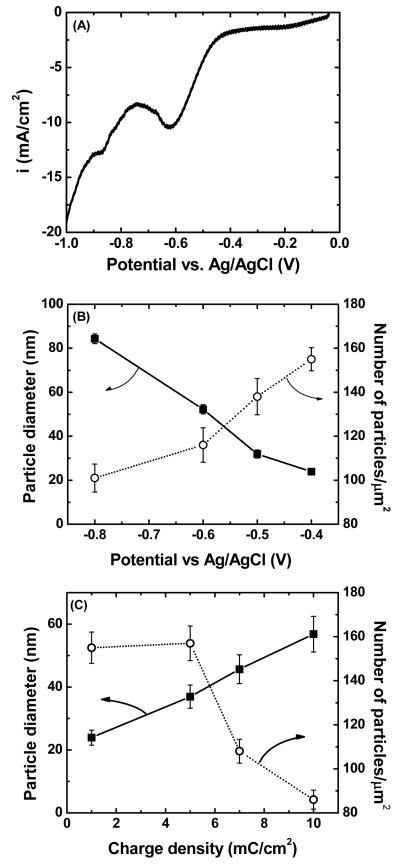
For topographic studies, gold nanoparticle electrodeposition was conducted at constant potential mode. The deposition potential and duration were varied and gold nanoparticle size and density was determined. Figure 1A shows a typical LSV obtained for gold deposition on spray printed SWNTs. For all these determinations, high density SWNT networks on gold substrate were used instead of actual sensors, for easier optimization purposes. And also for spray printed SWNTs, the electrolyte is mainly in contact with the surface of SWNTs therefore, any changes observed during electrodeposition studies is attributed to changes occurring on surface of SWNTs instead of the gold electrodes. The LSV indicated that electrodeposition of gold on SWNTs started when the applied deposition potential became more negative than −0.4 V (vs. Ag/AgCl) where the cathodic current sharply increased. The effect of deposition potential on particle size and density for a constant charge passed (1 mC/cm2) is shown in Figure 1B. Low overpotentials (−0.4 to −0.5 V vs. Ag/AgCl) resulted in low deposition rate and lead to small and sparsely dispersed gold nanoparticles suggesting that nucleation was mostly taking place at the SWNT defect sites13. This is consistent with the observation of an energetic barrier for nucleation on defect free SWNT surfaces14. At more negative overpotentials, both the size of the deposits and coverage by gold nanoparticles increased due to an increased deposition rate. As the size of the deposits increased, significant metal particle coalescence was observed. This implied that metal clusters were being deposited on SWNTs rather than deposition as a monolayer, which is not a typical of deposition onto surfaces having lower interfacial energies15.
Figure 1.
Effect of electrodeposition parameters on nanoparticles size and density: (A) linear sweep voltammogram of gold nanoparticles deposition on spray-printed SWNTs (1 cm2). The scan rate was fixed at 10 mV/s. The reference was a standard Ag/AgCl electrode (sat. KCl). Effect of deposition potential (B) and charge density (C) on gold nanoparticles size and density on spray printed SWNTs. The charge density was fixed at 1 mC/cm2 for B and deposition potential was fixed at −0.4 V for C.
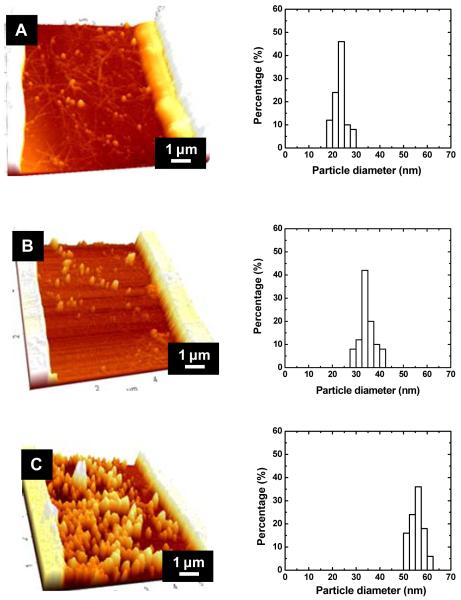
The effect of deposition time on particle size and density was determined at a fixed potential (Figure 1C). For this experiment, a low cathodic deposition potential was selected (−0.4 V vs. Ag/AgCl) as it resulted in the smallest deposits. The particle density remained constant at lowest charge densities (1-5 mC/cm2) consistent with the hypothesis that gold initially deposits at the SWNT defect sites. A sharp decrease in particle density was observed as the charge density was increased, most probably because coalescence of the deposits as they grew in size. The particle diameter increased with charge density similar to earlier experiments in which palladium were deposited on SWNTs12, except that here a quasi-linear increase was observed. The observations are consistent with AFM images and gold deposit size histograms sensors shown in Figure 2. AFM images at low charge densities (Figures 2AB) show that gold deposits are clearly located on the SWNTs. As the charge density increased, larger gold clusters and greater coverage of the SWNTs with gold deposits were observed. At 10 mC/cm2, extensive coverage of the sensing area with gold deposits is accomplished. Examination of the deposit size histograms reveals that the size distribution remained relatively constant with the charge density. This is probably because the number of nucleation sites is not affected by the charge density.
Figure 2.
AFM images and histograms of gold nanoparticles decorated SWNTs with different charge density: (A) 1 mC/cm2, (B) 5 mC/cm2, and (C) 10 mC/cm2. The deposition potential was fixed at −0.4 V versus pseudo reference electrode (chlorinated Ag/AgCl wire). The initial resistance of SWNTs before decoration was in the range of 10 to 40 KΩ.
Overall, the results of Figures 1 and 2 show that electrodeposition allows to precisely control the size and density of the metal nanoparticle deposits by varying the charge density and the deposition potential.
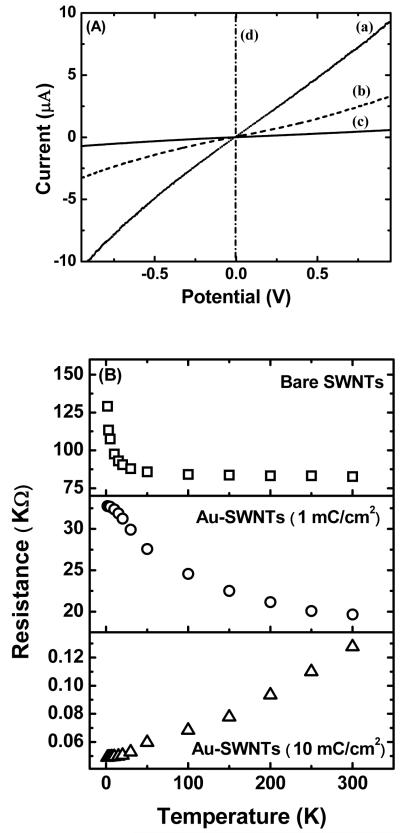
The room temperature I-V characteristics of randomly oriented carboxylated SWNTs and gold nanoparticle decorated SWNTs are shown in Figure 3A. For carboxylated SWNTs and those with a low density of gold deposits, the I-V characteristics are S-shaped, indicating Schottky contacts which are usually observed for SWNTs on gold pads. However, as was shown in Figurer 2C, at the higher density of gold deposits, the nanotubes are almost continuously covered with nanoparticles forming a gold nanowire or a film-like structure and consequently, a linear Ohmic behavior is observed. The temperature dependent resistance studies provided further insight on the conduction path of electrons across the source and drain electrodes. For sparse gold nanoparticle deposits, the resistance of the device increased with decrease in temperature indicating that the current conduction path is through the semiconducting SWNTs (Figure 3B), while at a high deposit density, the resistance decreased with decrease in temperature which is a characteristic feature of metals. This indicated that the underlying SWNTs no longer played a role in the conduction path and that conduction was through a continuous gold wire. The thermal activation energy of the devices for the low density deposit case, calculated by plotting ln R vs. 1/T (see Figure S1 in Supporting Information), was found to be about 3.5 meV, which is comparable to the thermal activation energy value of 5 meV for Pd decorated SWNTs12 and 6 meV for carboxylated SWNT networks16. The activation energy values further confirm that the dominant conduction pathways is across the semi-conducting nanotubes and/or due to the tunneling barriers between the nanotube networks.
Figure 3.
I-V characteristics (A) and temperature dependent electrical resistance (B) of Au-SWNTs as a function of deposition charge density. (a) Carboxylated SWNTs, Au-SWNTs with a charge density of (b) 1 mC/cm2, (c) 5 mC/cm2, and (d) 10 mC/cm2. All the devices were made at a constant deposition potential of −0.4 V versus chlorinated Ag/AgCl wire.
H2S gas sensing
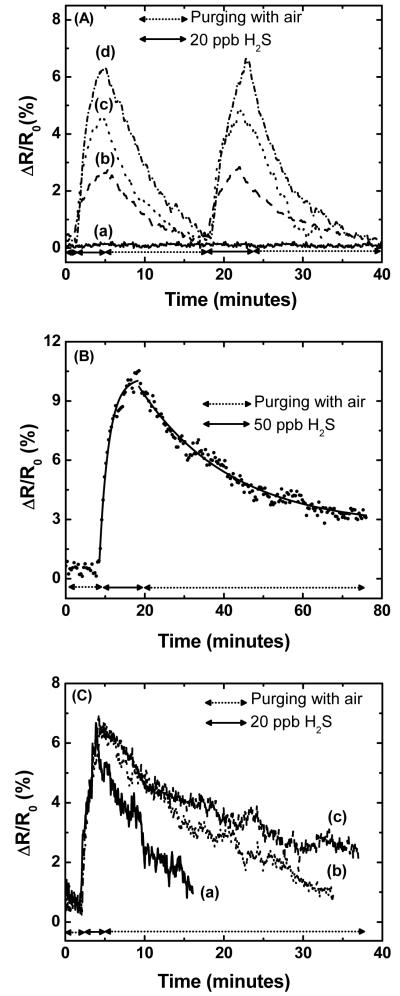
For H2S sensing studies, three topographically different sensors were selected: (i) sensors with the smallest gold particle diameter (−0.4 V, 1 mC/cm2), (ii) sensors with the same particle density but about two fold larger gold particle (−0.4 V, 5 mC/cm2), and (iii) sensors with large and dense gold deposits and overall metallic behavior (−0.4 V, 7 mC/cm2). Figure 4A shows the dynamic responses of these sensors compared to the response of carboxylated SWNTs for two repeated exposures to 20 ppb H2S at room temperature. The carboxylated SWNTs has no chemical interaction with H2S, hence no change in the resistance of the device was observed (Figure 4A (a)). This also indicates that the gold pads did not exhibit any response to H2S exposure at such low concentrations. The resistance of SWNT decorated with gold nanoparticles sharply increased upon exposure to H2S; the effect was most pronounced for the sample with the smallest size and largest number of nanoparticles (Figure 4A (d)). The increase in resistance observed here is consistent with the measured increase in resistance in gold films5 and nanoparticles10 attributed to reduced electron hopping, while a recent nanosensor made with polyaniline (PANI) nanowires decorated with gold nanoparticles exhibited a decrease in resistance when exposed to H2S11.
Figure 4.
Sensor response (A) to 20 ppb H2S at room temperature for (a) carboxylated SWNTs, and gold decorated SWNT with the following charge deposited: (b) 7 mC/cm2, (c) 5 mC/cm2, and (d) 1 mC/cm2. (B) Resistance change of Au-SWNT sensors to 50 ppb H2S. Dotted line are experimental data while red line is a fit using the kinetic model developed by Lee et al.21 (C) Sensor response (−0.4 V, 1 mC/cm2) to 200 ppb H2S for different applied bias potential (a) 4 V (b) 2.5 V (c) 1.0 V.
The sensing mechanism here is due to change in the electronic structure at the gold-SWNT interface resulting from the modification of the electronic properties of the gold nanoparticles upon exposure to H2S gas. H2S is known to adsorb strongly onto gold because of the high chemical affinity between gold and sulfur10. Leavitt and Beebe found that H2S adsorb (85 K) and desorb molecularly (165 K) at low temperatures from Au (111) surfaces18. At such low temperatures H2S binds weakly to the gold surface hence it easily undergoes adsorption and desorption process molecularly. However, at higher temperatures (165<T<520 K), Leavitt and Beebe found that adsorbed H2S decomposes to form SH which is chemisorbed onto the gold surface while H2 is released. The adsorbed SH remained adsorbed on the gold surface until the onset of even greater temperatures (>520 K) at which SH undergoes disproportionation to form H2S and adsorbed sulfur still bound to the gold surface. The latter can be removed as elemental sulfur at temperatures above 900 K. With respect to electronic properties, it has been reported that gold-sulfur bonds in alkylthiol self-assembled monolayers can lower the surface work-function of gold by as much as 1 eV19. Thus, we propose the following sensing mechanism. During electrodeposition, gold nanoparticles are preferentially deposited on the defect sites of the SWNTs15 because of the higher surface energy of defect sites compared to clean sp2 bonded lattice allowing easier electronic interaction between gold and carbon nanotubes. Upon exposure to H2S, hydrogen sulfide is chemisorbed on the gold nanoparticles, and undergoes decomposition to form either Au-SH or Au-S species on the surface of the particles. This lowers the surface work function of the gold particles. Such changes in the electronic state of the gold nanoparticles cause a change in the extent of electron exchange between the gold and the defect sites of carbon nanotubes. As the work function of the gold is reduced, electrons are donated from the gold to the SWNT. Since the latter is a p-type20 semiconductor at ambient conditions, this results in an increase in its electrical resistance. We hypothesize that the absence of change in resistance for SWNTs (Figure 4A (a)) is because of the fact that the carboxylated SWNTs bind to the gold pads by Vander Waals forces and no charge transfer or Schottky barrier modulation takes place between the gold pads and the SWNTs when exposed to low concentrations of H2S unlike what is happening when gold is deposited on the defect sites of SWNTs. A detailed study on understanding the role of gold nanoparticles and gold contacts in H2S sensing is currently under investigation; transfer characteristics of Au-SWNT devices operated in field effect transistor (FET) mode are consistent with the proposed mechanism. In addition, no change in resistance was observed when gold nanoparticles formed a continuous wire on the SWNTs (Figure S2, Supporting Information). This was because the main conduction path was through the gold nanoparticle wire and any modulation of the SWNT resistance by adsorbed H2S became irrelevant. The decreasing sensor response with increasing deposition charge density (Figure 4A (b through d)) is attributed to the greater specific interfacial area per unit gold volume of the sensors with the smaller gold nanoparticles, and to the larger effect of short range electronic interactions discussed above.
We also investigated the molecular sensing mechanism for H2S on gold decorated SWNTs using previously reported semi-theoretical models7b, 21. Rate constants for H2S adsorption can be extracted from a highly simplified model developed by Lee and Strano21 based on reversible adsorption kinetics of the analyte. The model equations for adsorption and desorption kinetics were fitted to the experimental data (Figure 4B) to extract the rate constants. From the fit, the adsorption (kads) and desorption (kdes) rate constants were estimated to be 4.37 × 103 s−1 and 0.72 × 10−3 s−1, respectively. The above kinetic constants reflect that H2S desorption from the gold surface is much slower than its adsorption, consistent with the fact that disproportionation of SH from the gold surface is only favored at high temperatures. The calculated adsorption and desorption rate constants can be indirectly probed to determine the binding energy. The equilibrium rate constant K (kads/kdes) determined is exponentially related to the enthalpy and free energy of the reaction by van-Hoff’s relation22 given in equation 1.
| (Eq. 1) |
The entropy change can be assumed constant for the binding event23 and hence at constant temperature, equilibrium constant is exponentially related to enthalpy. Because the barrier to the desorption event is much higher compared to the adsorption event, the amount of energy required to desorb a molecule from a surface can be correlated to enthalpy of binding7b. Hence equation 1 can be rewritten as,
| (Eq. 2) |
Where EB, is the binding energy of the molecule and was determined to be 0.19 eV. The binding energy determined experimentally (0.19 eV) is close to the computed values (0.3 eV ~0.4 eV) for binding energy of H2S on gold surfaces18.
Significant research efforts have been directed at means to improve the regeneration rate of gas nanosensors, the most common being increasing the operating temperature or UV irradiation7b. Here we tried a different approach. The applied bias potential was increased during sensing in an attempt to improve the regeneration rate. The dynamic responses of a sensor subjected to different applied bias potentials while sensing H2S are plotted in Figure 4C. The sensor responses to H2S were almost identical for the three different bias potentials applied. However recovery to the baseline when switching off H2S was accelerated with increasing bias potential. Desorption rates improved (0.72 × 10−3 s−1(1V), 0.3 × 10−1 s−1 (2.5 V), 0.6 × 101 s−1 (4V)) with increasing applied bias. The most probable explanation for the faster recovery at higher bias potentials is the greater local Joule heating at the defect sites with increasing applied bias potential. SWNTs are excellent thermal conducting materials, but surface defects are known to result in localized phonon scattering and increased Joule heating. Thus temperature at defect sites should increase with increasing bias potential and the associated Joule heating.
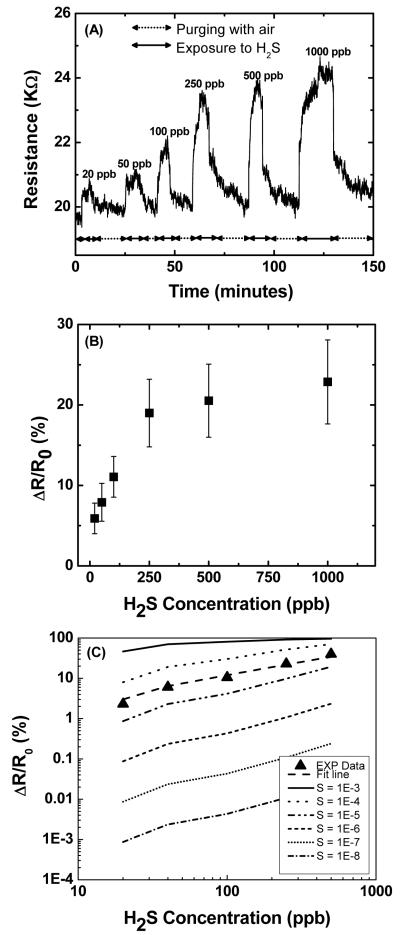
Finally, selected sensors were subjected to different concentration of H2S ranging from 20 to 1000 ppb (Figure 5A). In each case, the sensor promptly reached a steady-state after exposure to the analyte, and returned to its baseline when the analyte gas feed was discontinued. The sensor exhibited a steep and linear (r=0.986) response for concentrations ranging from 20 to 250 ppb (Figure 5B) while saturation was observed above 250 ppb. The sensitivity (defined as sensor response/analyte concentration) was found to be 0.07% per ppb in the linear range. The lower detection limit (defined as the concentration providing a signal-to-noise ratio of at least 3) was 3 ppb which is about the same as odor threshold level for humans. This is higher than our previous report on gold functionalized polyaniline nanowires11, however, polymer-based sensors are have a low upper temperature range (usually about 60-80 °C), and tend to have a poor stability over time,8 Further, electrodeposition on conducting polymer can only be made in a narrow range of cathodic potentials, as the polymer can be reduced at excessive potentials, which would affect its conductivity. These limitations do not exist in metal functionalized SWNT-based sensors. The response time was typically in the range of 6-8 minutes for all selected concentrations, while the recovery time was around 10 minutes for lower concentrations (20 -100 ppb) and almost twice that value for higher (200-1000 ppb) H2S concentrations. This is in the upper middle range of response time for nanomaterials-based gas sensors6. The sensors exhibited some cross sensitivity to NH3. Examination of the responses (see Figure S3 in Supporting Information) obtained by bare carboxylated SWNTs and Au-SWNTs when exposed to NH3 in air revealed only minor differences. This indicates the Au nanodeposits played no role in the NH3 sensing response. In contrast, major modulation of sensor resistance was obtained for H2S exposures. This property could be exploited in sensor arrays in order to discriminate H2S from interfering gases. The sensors were stable over time, with less than 25% loss in H2S sensitivity after being stored for six months and no major change of the response or recovery times (see Figure S4 in Supporting Information).
Figure 5.
(A) Response of a 20 K Au-SWNT sensor (Vdep: −0.4 V, CDep: 1 mC/cm2) to different H2S concentrations for an applied bias of 4 V. (B) Resistance change (ΔR/R0) for different H2S concentration and (C) calculated (ΔR/R0) for Au-SWNT sensor plotted versus H2S concentration for different sticking coefficient (S). The solid triangles are the experimental data.
From the above sensing results, experimental sticking coefficient (ratio of adsorbate molecules that adsorb to the surface to the total number of molecules that reaches the surface for a fixed period of time at fixed temperature) for H2S was also determined using a Langmuir isotherm model as suggested by Qi et al.7b Assuming that the relative change in resistance (ΔR/R0) is proportional to the adsorption of H2S molecules on gold nanoparticles, ΔR/R0 was plotted as a function of H2S gas concentrations for different sticking coefficients (Figure 5C) from 10−3 to 10−8. Examination of the figures reveals that a sticking coefficient for H2S on Au-SWNT network of 3×10−5 provides an adequate representation of the experimental data. Less than 4% change observed for the conductivity of carboxylated SWNTs when exposed to H2S concentrations as high as 20 ppm (see Figure S3 in in Supporting Information). This indicates that H2S molecules do not “stick” or adsorb to significant extents on carboxylated SWNTs and that gold functionalization significantly increases the binding affinity of H2S towards these hybrid nanostructures.
Conclusions
Novel gas nanosensors consisting of gold nanoparticles electrodeposited on SWNTs were synthesized. The chemiresitive sensors allowed rapid and quantitative detection of trace H2S concentrations at room temperature. The sensing mechanism is believed to be based on the modulation of the conduction path across the nanotubes, by changing the extent of electron exchange between the gold and the defect sites of nanotubes when exposed to various concentrations of H2S. Electrochemical functionalization of gold nanoparticles on SWNTs has many advantages including simple processing and site-specific functionalization of nanostructured materials. The sensor characteristics could be tuned by changing the sensor synthesis conditions such as deposition potential, deposition charge and initial resistance of the sensor. The SWNTs were functionalized with gold ranging from about 20 nm nanoparticles up to continuous deposits forming a nanowire depending on the deposited charge. The sensors exhibited superior sensitivity towards H2S (0.07%/ppb at room temperature with a detection limit of 3 ppb) over many conventional gas sensors. Increasing the bias voltage during sensing reduced the sensor recovery time, probably by local Joule heating. Sensors such as the ones presented herein offer tremendous potential for the development of powerful sensor arrays and their integration in low cost monitoring devices.
Supplementary Material
Acknowledgement
Funding for the project was provided by Bourns, Inc., University of California Discovery Grant (UC Discovery), the Defense Microelectronic Activity (DMEA) under agreement number H94003-05-2-0505 and the NIH Genes, Environment and Health Initiative through award U01 ES 016026.
Footnotes
Supporting Information Available
Plot of ln R vs. 1/T for low density Au deposit case; response of a high density deposit case sensor to H2S. Sensing of NH3 and H2S by carboxylated and Au-SWNT sensors. Comparison of as-prepared sensor response (25 ppb H2S in air) and sensor response after six months storage.
References
- (1).a) Watts SF. Atmos. Environ. 2000;34:761–779. [Google Scholar]; b) Gabriel D, Deshusses MA. Proc. Natl. Acad. Sci. USA. 2003;100:6308–6312. doi: 10.1073/pnas.0731894100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).a) Beauchamp RO, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. CRC Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]; b) Glass DC. Ann. Occup. Hyg. 1990;34:323–327. doi: 10.1093/annhyg/34.3.323. [DOI] [PubMed] [Google Scholar]
- (3).a) Sedlak JM, Blurton KF. Talanta. 1976;23:445–448. doi: 10.1016/0039-9140(76)80125-7. [DOI] [PubMed] [Google Scholar]; b) Bergman I. J. Electroanal. Chem. 1983;157:59–73. [Google Scholar]; c) Opekar F, Bruckenstein S. Anal. Chem. 1984;56:1206–1209. [Google Scholar]; d) Redondo R, Cunha Machado V, Baeza MM, Lafuente J, Gabriel D. Anal. Bioanal. Chem. 2008;391:789–798. doi: 10.1007/s00216-008-1891-5. [DOI] [PubMed] [Google Scholar]
- (4).a) Tao WH, Tsai CH. Sens. Actuators B. 2002;81:237–247. [Google Scholar]; b) Lin HM, Hsu CM, Tang HY, Lee PY, Yang CC. Sens. Actuators B. 1994;22:63–68. [Google Scholar]; c) Kersen U, Holappa L. Anal. Chim. Acta. 2006;562:110–114. [Google Scholar]; d) Sberveglieri G, Groppelli S, Nelli P, Perego C. Sens. Actuators B. 1993;15/16:86–89. [Google Scholar]
- (5).Yoo KS, Sorensen IW, Glaunsinger WS. J. Vac. Sci. Technol. A. 1994;12:192–198. [Google Scholar]
- (6).Zhang T, Mubeen S, Myung NV, Deshusses MA. Nanotechnology. 2008;19:332001. doi: 10.1088/0957-4484/19/33/332001. [DOI] [PubMed] [Google Scholar]
- (7).a) Kong J, Chapline MG, Dai H. Adv. Mater. 2001;13:1384–1386. [Google Scholar]; b) Qi P, Vermesh O, Grecu M, Javey A, Wang Q, Dai H. Nano. Lett. 2003;3:347–351. doi: 10.1021/nl034010k. [DOI] [PubMed] [Google Scholar]; c) Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H. Science. 2000;287:622–625. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]; d) Zhang T, Nix MB, Yoo BY, Deshusses MA, Myung NV. Electroanalysis. 2006;18:1153–1158. [Google Scholar]
- (8).a) Kolmakov A, Zhang Y, Cheng G, Moskovits M. Adv. Mater. 2003;15:997–1000. [Google Scholar]; b) Xue XY, Chen YJ, Liu YG, Shi SL, Wang YG, Wang TH. Appl. Phys. Lett. 2006;88:201907. [Google Scholar]; c) Wang J, Bunimovich YL, Sui G, Savvas S, Wang J, Guo Y, Heath JR, Tseng HR. Chem. Commun. 2006:3075–3077. doi: 10.1039/b604426c. [DOI] [PubMed] [Google Scholar]; d) Liu H, Kameoka J, Czaplewski DA, Craighead HG. Nano. Lett. 2004;4:671–675. [Google Scholar]; e) Zhang D, Li C, Liu X, Han S, Tang T, Zhou C. Appl. Phys. Lett. 2003;83:1845–1847. [Google Scholar]
- (9).Sun Z, Yuan H, Liu Z, Han B, Zhang X. Adv. Mater. 2005;17:2993–2997. [Google Scholar]
- (10).Geng J, Thomas MDR, Shephard DS, Johnson BFG. Chem. Commun. 2005:1895–1897. doi: 10.1039/b418559e. [DOI] [PubMed] [Google Scholar]
- (11).Shirsat MD, Bangar MA, Deshusses MA, Myung NV, Mulchandani A. Appl. Phys. Lett. 2009;94:083502. [Google Scholar]
- (12).Mubeen S, Zhang T, Yoo BY, Deshusses MA, Myung NV. J. Phys. Chem. C. 2007;111:6321–6327. [Google Scholar]
- (13).Fan Y, Goldsmith BR, Collins PJ. Nature Mater. 2005;4:906–911. doi: 10.1038/nmat1516. [DOI] [PubMed] [Google Scholar]
- (14).Walter EC, Favier F, Penner RM. Anal. Chem. 2002;74:1546–1553. doi: 10.1021/ac0110449. [DOI] [PubMed] [Google Scholar]
- (15).a) Penner RM. J. Phys. Chem. B. 2002;106:3339–3353. [Google Scholar]; b) Zoval JV, Stiger RM, Biernacki PR, Penner RM. J. Phys. Chem. 1996;100:837–844. [Google Scholar]
- (16).Zhou C, Kong J, Dai H. Phy. Rev. Lett. 2000;84:5604–5607. doi: 10.1103/PhysRevLett.84.5604. [DOI] [PubMed] [Google Scholar]
- (17).Shirsat MD, Bangar MA, Deshusses MA, Myung NV, Mulchandani A. Appl. Phys. Lett. 2009;94:083502. [Google Scholar]
- (18).Leavitt AJ, Beebe TP., Jr. Surface Science. 1994;314:23–33. [Google Scholar]
- (19).a) Cui X, Freitag M, Martel R, Brus L, Avouris P. Nano. Lett. 2003;6:783–787. [Google Scholar]; b) Schoenenberger C, Jorritsma JC, Sondag-Huethorst JAM, Fokkink LGJ. J. Phys. Chem. 1995;99:3259. [Google Scholar]; c) Alloway DM, Hofmann M, Smith DL, Gruhn NE, Graham AL, Colorado R, Jr., Wysocki VH, Lee TR, Lee PA, Armstrong NR. J. Phys. Chem.B. 2003;107:11690. [Google Scholar]; d) Ah Qune LFN, Akiyama H, Nagahiro T, Tamada K, Wee ATS. App. Phy. Lett. 2008;93:083109. [Google Scholar]; e) Sushko ML, Shluger AL. Adv. Mater. 2009;21:1111. [Google Scholar]
- (20).a) Kong J, Franklin N, Zhou C, Chapline M, Peng S, Cho K, Dai H. Science. 2000;287:622–625. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]; b) Collins P, Bradley K, Ishigami M, Zettl A. Science. 2000;287:1801–1804. doi: 10.1126/science.287.5459.1801. [DOI] [PubMed] [Google Scholar]
- (21).Lee CY, Strano SM. Langmuir. 2005;21:5192–5196. doi: 10.1021/la046867i. [DOI] [PubMed] [Google Scholar]
- (22).Espenson JH. Chemical Kinetics and Reaction Mechanisms. McGraw-Hill, Inc.; New York: 1981. [Google Scholar]
- (23).Bohrer FI, Colesniuc CN, Park J, Ruidiaz ME, Schuller IK, Kummel AC, Trogler WC. J. Am. Chem. Soc. 2009;131:478–485. doi: 10.1021/ja803531r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.