Abstract
Multiple regulatory mechanisms control the activity of the protein serine/threonine phosphatase 2A catalytic subunit (PP2Ac), including post-translational modifications and its association with regulatory subunits and interacting proteins. Alpha4 is a PP2Ac-interacting protein that is hypothesized to play a role in PP2Ac ubiquitination via its interaction with the E3 ubiquitin ligase Mid1. In this report, we show that alpha4 serves as a necessary adaptor protein that provides a binding platform for both PP2Ac and Mid1. We also identify a novel ubiquitin-interacting motif (UIM) within alpha4 (amino acid residues 46-60) and analyze the interaction between alpha4 and ubiquitin using NMR. Consistent with other UIM-containing proteins, alpha4 is monoubiquitinated. Interestingly, deletion of the UIM within alpha4 enhances its association with polyubiquitinated proteins. Lastly, we demonstrate that addition of wild-type alpha4 but not an alpha4 UIM deletion mutant suppresses PP2Ac polyubiquitination. Thus, the polyubiquitination of PP2Ac is inhibited by the UIM within alpha4. These findings reveal direct regulation of PP2Ac polyubiquitination by a novel UIM within the adaptor protein alpha4.
Protein serine/threonine phosphatase 2A (PP2A) is an abundant cellular enzyme with numerous substrates that modulate a wide variety of cellular functions. Considering the multitude of cellular processes under the control of PP2A, it is not surprising that several different mechanisms exist to regulate phosphatase activity. These regulatory mechanisms include association with specific regulatory subunits and post-translational modifications of PP2Ac (i.e. phosphorylation, carboxymethylation, and ubiquitination) (1-3). Both biochemical and structural studies of PP2A have provided key mechanistic insights to explicate regulation of phosphatase holoenzyme composition and activity via phosphorylation and carboxymethylation (1, 2, 4, 5); however, little is known about PP2Ac ubiquitination beyond the initial report demonstrating the polyubiquitination and degradation of microtubule-associated PP2Ac (3). The E3 ubiquitin ligase responsible for targeting PP2Ac for proteasome degradation is Mid1, a protein linked to the congenital disorder Opitz Syndrome (OS). Human fibroblasts derived from a fetus with OS exhibit a loss of PP2Ac ubiquitination, increased levels of microtubule-associated PP2Ac, and a concomitant decrease in the phosphorylation of general microtubule-associated proteins as compared to age-matched control fibroblasts (3). Thus it appears that deregulation of PP2Ac ubiquitination culminates in the pathogenesis of OS.
Another key player in Mid1-dependent PP2Ac ubiquitination is alpha4, a mostly alpha-helical protein purported to serve as a scaffold for Mid1 and PP2Ac (3, 6-8). Alpha4 contains independent binding sites for PP2Ac and Mid1 on its N- and C-terminus, respectively (3, 8, 9); yet, its biochemical associations with Mid1 and PP2Ac have been primarily studied independently of one another. Nevertheless, colocalization of alpha4 and exogenous Mid1 at microtubule structures suggests that alpha4 plays an important role in PP2Ac ubiquitination (3, 8). Another report suggested that alpha4 facilitates dephosphorylation of Mid1 by PP2Ac, as increased alpha4 expression caused a reduction in the cellular levels of phosphorylated Mid1 protein (8). Indeed, it is reasonable to posit that alpha4 can support cross-regulation of both Mid1 and PP2Ac – PP2Ac regulates Mid1 activity via dephosphorylation, and conversely, Mid1 regulates PP2Ac activity via ubiquitination. However, the precise mechanism by which alpha4 modulates these processes remains unclear.
Here, we report experimental evidence to verify the role of alpha4 as an adaptor protein that facilitates formation of a Mid1•alpha4•PP2Ac ternary complex. Interestingly, we also demonstrate that alpha4 interacts with ubiquitin and possesses a ubiquitin-interacting motif (UIM). Finally, we show that wild-type alpha4, but not an alpha4 UIM deletion mutant, suppresses PP2Ac polyubiquitination. Together, these studies reveal that alpha4 serves as an adaptor protein to directly regulate PP2Ac ubiquitination via its UIM domain.
MATERIALS AND METHODS
Antibodies
The mouse monoclonal PP2Ac antibody was from BD Biosciences Pharmingen (San Diego, CA). Rabbit polyclonal Flag antibody was from Sigma (St. Louis, MO), and mouse monoclonal c-myc antibody (9B11) was from Cell Signaling Technology, Inc. (Danvers, MA). The rabbit polyclonal alpha4 antibody was from Bethyl Laboratories (Montgomery, TX). The rat monoclonal HA antibody (3F10) was from Roche Diagnostics Corporation (Indianapolis, IN).
Plasmid constructs
The HA-ubiquitin plasmid was a gift from Dr. Hal Moses (Vanderbilt University), and the myc-Mid1/pCMVtag3A plasmid was a gift from Dr. Susann Schweiger (University of Dundee). The HA3-PP2Ac/pKHA3 plasmid was kindly provided by Dr. David Brautigan (University of Virginia). Construction of the human Flag-alpha4/pcDNA5TO expression vector was described previously (10). The 6xHis-alpha4(1-222)/pET28 plasmid was generated by PCR amplification of human alpha4 amino acid residues 1-222 using Flag-alpha4/pcDNA5TO as a template with the forward primer 5′ – GTA CGT ACG CAT ATG GCT GCT GAG GAC GAG TTA – 3′ and reverse primer 5′ – GTG GTG GGA TCC TTA GTC TCT TTC TCT CAG GAT CTT TAT TTC CTG – 3′. The PCR product was subcloned into the pET28 plasmid using NdeI and BamHI restriction sites. The Flag-alpha4 mutants were generated using Flag-alpha4/pcDNA5TO as a template and the QuikChange kit (Stratagene, La Jolla, CA) with the following primers: Δ51-53 forward 5′ – GGC TTG GAC CTC CTT GAG GAA ATG TTA TCG CAG CTC GAC – 3′; Δ51-53 reverse 5′ – GTC GAG CTG CGA TAA CAT TTC CTC AAG GAG GTC CAA GCC – 3′; Δ46-60 forward 5′ – GTC CAG GAG AAG GTG TTC AAG GGC TTC AGC CGA AAT GAA GAT TTG G – 3′; Δ46-60 reverse 5′ – CCA AAT CTT CAT TTC GGC TGA AGC CCT TGA ACA CCT TCT CCT GGA C – 3′; R155E/K158D forward 5′ – GGC ATC TCA AGA GCA GGC TGA TAT ACA GAG – 3′; R155E/K158D reverse 5′ – GTA TCT CTG TAT ATC AGC CTG CTC TTG AGA TG – 3′. Proper construction of all plasmids was verified by automated sequencing (Vanderbilt University DNA Core Facility).
Cell culture and transfection
HEK293 cells were grown at 37°C in a humidified atmosphere with 5% CO2 in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were transfected using Fugene 6 (Roche, Indianapolis, IN) according to manufacturer's directions.
Immunoprecipitations
Cells were lysed in IP buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Igepal, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 1 mM PMSF, and 1 μg/ml leupeptin) and centrifuged for 10 min at 12,000g. Clarified lysates were incubated at 4°C with 20 μl of a 50% slurry of anti-Flag M2 agarose (Sigma), 15 μl of a 50% slurry of anti-Myc (clone 4A6) agarose (Millipore, Billerica, MA), or 15 μl of a 50% slurry of anti-HA agarose (Roche). After three washes in IP buffer, bound proteins were eluted in SDS sample buffer and subjected to western analysis. For use in ubiquitination assays, Flag-alpha4 was eluted from the anti-Flag resin by incubation for 30 min at 4°C with 100 μg/ml Flag peptide in 50 μl of ubiquitination assay buffer (50 mM HEPES, pH 8.0, and 0.5 mM DTT).
Protein Purification
N-terminal His-tagged ubiquitin was overexpressed in a BL21 (DE3) E. coli strain. The bacteria were grown in minimal media with 15NH4Cl as the sole nitrogen source. The protein was purified by Ni-affinity chromatography (Qiagen) followed by gel filtration chromatography (Superdex-200, Amersham Pharmacia). Pure His-ubiquitin was concentrated and dialyzed against 50 mM sodium phosphate (pH 6.5). N-terminal His-tagged human alpha4 (amino acid residues 1-222) was overexpressed in a BL21 (DE3) E. coli strain and purified by metal affinity chromatography (Talon resin, Clontech) followed by gel filtration chromatography (Superdex-200). N-terminal His-tagged mouse alpha4 was purified in the same manner as human alpha4.
NMR titrations
The interaction between alpha4 and ubiquitin was observed by inspection of the signals in the 1H-15N HSQC spectrum of ubiquitin for a series of varying alpha4:ubiquitin ratios. Buffer-matched samples of 100 μM ubiquitin alone and 100 μM ubiquitin with 1.9 mM alpha4 were sequentially mixed to acquire spectra of samples at 0:1 4:1, 7:1, 12:1, 15:1, and 19:1 alpha4 to ubiquitin molar ratios. NMR experiments were conducted on a Bruker Avance 500 MHz spectrometer at 25°C. The samples contained 50 mM sodium phosphate, pH 6.5, 5% D2O, and 0.1% sodium azide. The data were analyzed with Sparky (11) using previously reported assignments for ubiquitin (12). For each titration point, a normalized peak height was determined by comparison to the peak height observed for ubiquitin alone. Resonances experiencing a signal loss with addition of the alpha4 protein greater than 1.5 times the standard deviation were identified as significantly perturbed.
PP2Ac ubiquitination assay
Ubiquitination assays were performed using a ubiquitin-protein conjugation kit (BostonBiochem, Cambridge, MA), which contains various purified conjugation enzymes from rabbit reticulocyte Fraction II (13). Fraction A contains E1 and E2 enzymes, and Fraction B contains E3 ligases and deubiquitinating enzymes. The kit also supplies ubiquitin protein and an ATP-containing energy solution. Ubiquitination assays were carried out using 16 μg (20 μl) of combined Fractions A and B, 34 μg of ubiquitin protein, 2.5 μl of energy solution, ~50 ng of purified bovine PP2Ac (a gift from Dr. Greg Moorhead, University of Calgary), and ~200 ng of immunopurified wild-type Flag-alpha4 or Flag-alpha4 Δ46-60 UIM mutant. Reaction mixtures were incubated at 37 °C for 4 h, quenched with 10 mM EDTA, and then subjected to Western analysis or Flag immunoprecipitations and Western analysis.
Western analysis
SDS-solubilized protein samples were subjected to SDS-PAGE (10% acrylamide gels), and transferred to 0.45 μm nylon-supported nitrocellulose membranes. Membranes were blocked in Odyssey buffer (LI-COR, Lincoln, Nebraska). All primary antibodies were used at a dilution of 1:1000. For detection with the Odyssey Infrared Imaging system, primary antibodies and the appropriate secondary fluorophore-conjugated antibodies (1:20,000 dilution; Molecular Probes, Eugene, OR or Rockland Immunochemicals, Gilbertsville, PA) were diluted in Odyssey blocking buffer. Bound antibodies were visualized via the Odyssey Infrared Imaging System and Odyssey software (LI-COR, Lincoln, Nebraska).
RESULTS AND DISCUSSION
Alpha4 serves as an adaptor protein linking Mid1 and PP2Ac
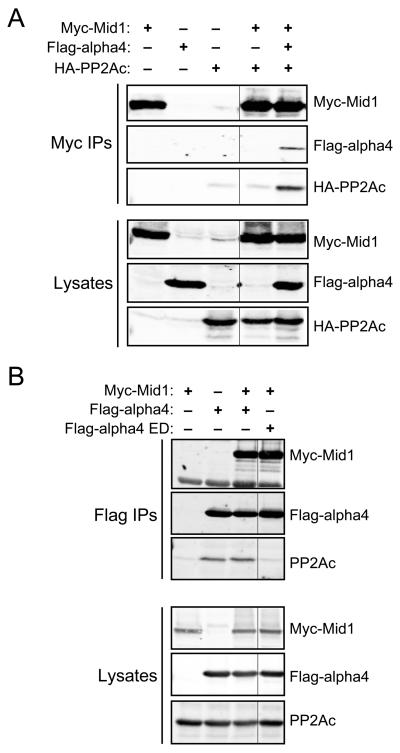
It has been reported that alpha4 facilitates the ubiquitination of PP2Ac via direct interactions with Mid1 and PP2Ac (3). Since the Mid1- and PP2Ac-binding domains within alpha4 are non-overlapping, it is possible that a Mid1•alpha4•PP2Ac ternary complex exists in cells (3, 8). Although prior immunoprecipitation experiments have revealed that alpha4 associates with PP2Ac and Mid1 (8), it is unclear whether this is representative of one complex (Mid1•alpha4•PP2Ac) or two distinct complexes (Mid1•alpha4 and alpha4•PP2Ac). To investigate whether alpha4 acts as a scaffold protein to promote PP2Ac ubiquitination by Mid1, we examined the protein composition of Mid1 immune complexes from lysates of HEK293 cells lacking or co-expressing Flag-alpha4 and HA-PP2Ac. As shown in Fig. 1A, we were able to detect an interaction of Mid1 with PP2Ac in myc-Mid1 immune complexes isolated from cells co-expressing Flag-alpha4 and HA-PP2Ac; however, we were unable to detect HA-PP2Ac in myc-Mid1 immune complexes from lysates of cells lacking Flag-alpha4. The finding that PP2Ac does not associate with Mid1 in the absence of co-expressed alpha4 supports the conclusion that alpha4 is an adaptor protein necessary for the formation of a Mid1•alpha4•PP2Ac complex. To determine if an association between alpha4 and PP2Ac is necessary for their interaction with Mid1, we exploited an alpha4 mutant protein lacking the PP2Ac binding determinants (Flag-alpha4 R155E/K158D), which was characterized in a previous report (7). Mid1, but not PP2Ac, co-immunoprecipitated with Flag-alpha4 R155E/K158D (Fig. 1B), indicating that alpha4 can interact with Mid1 independently of PP2Ac. Together, these data demonstrate that alpha4 serves as a scaffolding protein to promote formation of a Mid1•alpha4•PP2Ac complex.
Fig. 1. Alpha4 is an adaptor protein linking Mid1 and PP2Ac.
A, HEK293 cells were transfected with myc-Mid1, HA-PP2Ac, Flag-alpha4, or the indicated combinations of these constructs. Myc immune complexes (Myc IPs) were isolated from cell lysates and analyzed by SDS-PAGE and immunoblotting using myc, Flag, and HA antibodies to detect the epitope-tagged forms of Mid1, alpha4, and PP2Ac, respectively. Aliquots of the cell lysates were analyzed in the same manner. B, HEK293 cells were co-transfected with HA-ubiquitin and the indicated construct(s). Flag immune complexes (Flag IPs) were isolated from cell lysates and analyzed by SDS-PAGE and immunoblotting using myc, Flag, and PP2Ac antibodies to detect the epitope-tagged forms of Mid1 and alpha4, as well as endogenous PP2Ac. The data are representative of at least three separate experiments.
Alpha4 contains a ubiquitin-interacting motif (UIM) and directly binds to ubiquitin
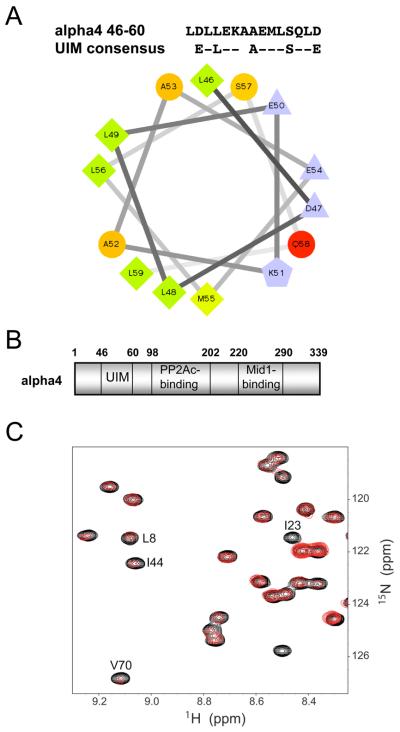
Proteins that function as adaptor molecules or scaffolds generally possess protein-protein interaction motifs to support dynamic and low-affinity interactions. Ubiquitin-binding domains have been uncovered in several proteins (e.g. p62, TAB2, TAB3, etc.; (14, 15)) that serve as adaptors to link the ubiquitination machinery with its substrates. Since alpha4 appears to function as an adaptor for ubiquitination machinery of PP2Ac, we analyzed its primary sequence for potential ubiquitin-binding domains (e.g. CUE, GAT, GLUE, NZF, PAZ, UBA, UEV, UIM, and VHS). A short stretch of amino acids corresponding to residues 46-60 of alpha4 (LDLLEKAAEMLSQLD) was found to conform to the consensus sequence of the ubiquitin-interacting motif (UIM) (E-L--A---S--E) (Fig. 2A). Consistent with the alpha-helical structures of other crystallized UIMs (16, 17), amino acid residues 46-60 of alpha4 lie within a predicted alpha-helix in the X-ray structure of the yeast homolog (7). Furthermore, like all UIM helices, this region of alpha4 is amphipathic in nature (Fig. 2A).
Fig. 2. Alpha4 contains a ubiquitin-interacting motif (UIM) and directly binds to ubiquitin.
A, Helical wheel representation of the UIM within alpha4 (amino acids 46-59). Circles denote hydrophilic residues, diamonds denote hydrophobic residues, triangles denote acidic residues, and pentagons denote basic residues. Hydrophobic residues are green; the level of green decreases in scale with hydrophobicity. Hydrophilic residues are red; the level of red decreases in scale with hydrophilicity. Charged residues are light blue. B, Schematic representation of the UIM (46-60), PP2Ac-binding (98-202), and Mid1-binding (220-290) domains of alpha4. C, Binding of alpha4 to ubiquitin perturbs specific residues in the ubiquitin I44/UIM interaction interface. A region of the 1H-15N HSQC spectra of Ub in the absence (black) and presence (red) of 12 molar equivalents of alpha4 demonstrate the selective line broadening and intensity loss observed upon formation of the Ub•alpha4 complex. The NMR data are representative of two independent experiments with five titration points each.
A direct interaction of human alpha4 and ubiquitin was suggested by the putative UIM found in alpha4 (Fig. 2B). Because UIM-Ub interactions usually exhibit weak binding (~1 mM affinity), the interaction between alpha4 and ubiquitin was probed by NMR. This approach is extremely sensitive to effects of binding and provides direct and unambiguous evidence of interactions between two titrated molecules. In fact, several studies of UIM-containing proteins have employed this methodology to demonstrate binding to Ub (16, 18, 19). Titration of human alpha4 into 15N-ubiquitin resulted in substantial effects on the 1H −15N HSQC NMR spectrum, including small chemical shift perturbations for some signals and progressive loss of signal intensity as the titration proceeded (Fig. 2C). The loss of signal intensity is due to line broadening arising from formation of the Ub•alpha4 complex. Changes in the spectrum throughout the entire titration indicate that the binding is very weak, with a Kd value substantially higher than 100 μM.
Six residues in ubiquitin were found to have significantly greater signal loss than the average of all residues over the entire titration series, thus indicating that these residues are specifically affected by alpha4 binding. Three of these residues (L8, I44, and V70) are located on the well-characterized I44 ubiquitin interface, which has been shown to be essential for function (20). The other three significantly perturbed residues (I23, L56, and E64) are located adjacent to the known interface. In addition to these backbone amides, resonances arising from the side chain amides of Q31, Q40 and Q49, which span the I44 interface, experience a much larger decrease in intensity than other side chain amides. Small significant chemical shift perturbations were observed for three residues (T9, K48, and L71) established as part of the I44 ubiquitin interface. We also noted a similar pattern of shifts and intensity loss when the titration was repeated with murine alpha4, confirming a direct interaction with alpha4 and the I44 face of ubiquitin (data not shown). Thus, ubiquitin and alpha4 appear to have weak binding similar to that observed for other UIMs binding to the ubiquitin I44 interaction surface.
Alpha4 is targeted for monoubiquitination
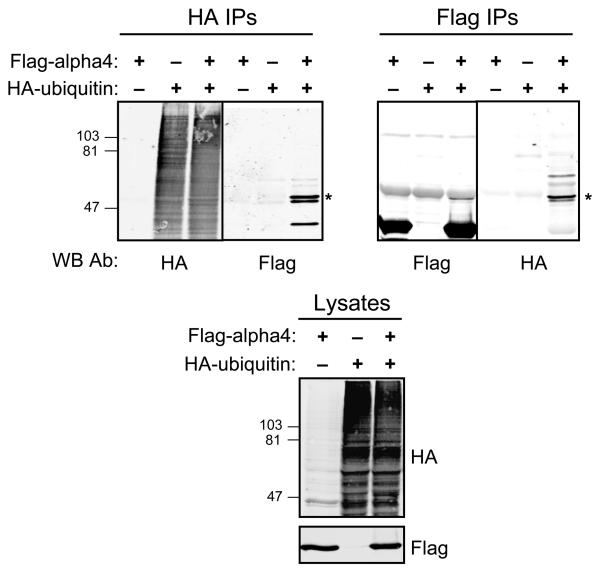
Several UIM-containing proteins are themselves ubiquitinated, although the functional relevance of this phenomenon is unclear (21-23). To determine whether alpha4 is ubiquitinated, Flag-alpha4 and HA-ubiquitin were co-transfected into HEK293 cells, and reciprocal immunoprecipitations were performed using anti-Flag- and anti-HA-agarose beads. A ~50 kDa protein immunoreactive with Flag and HA antibodies was detected in HA and Flag immune complexes, respectively, from lysates of cells co-expressing Flag-alpha4 and HA-ubiquitin (Fig. 3); this protein is the approximate size of monoubiquitinated Flag-alpha4. A 50 kDa protein immunoreactive with Flag antibody was also observed in Flag immune complexes from lysates of cells expressing Flag-alpha4 (Fig. 3). Alpha4 did not appear to be polyubiquitinated under these experimental conditions as no high molecular weight proteins were detected on the immunoblot. Moreover, treatment of cells with a proteasome inhibitor (MG132) did not alter the levels of ubiquitinated Flag-alpha4 (data not shown). These data indicate that alpha4 is itself monoubiquitinated, consistent with observations reported for other UIM-containing proteins (23, 24).
Fig. 3. Alpha4 is targeted for monoubiquitination.
HEK293 cells were transfected with HA-ubiquitin, Flag-alpha4, or both HA-ubiquitin and Flag-alpha4. Immunoprecipitations were performed from the cell lysates using Flag M2 agarose (Flag IPs) or HA-agarose beads (HA IPs). Bound proteins were analyzed by SDS-PAGE and immunoblotting with HA and Flag antibodies. The asterisk denotes the migration of monoubiquitinated alpha4. Lysates were analyzed in the same manner (bottom panel). The migration of SDS-PAGE standards is shown on the left. The data are representative of at least three separate experiments.
Deletion of the UIM within alpha4 results in enhanced binding of ubiquitinated proteins
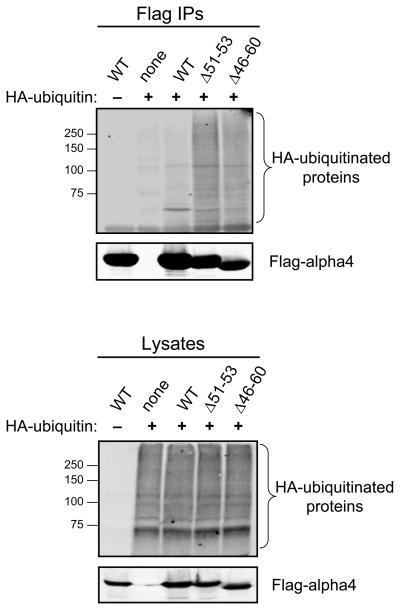
To test whether alpha4 can bind ubiquitinated species in cells, HEK293 cells were co-transfected with HA-ubiquitin and wild-type Flag-alpha4 or Flag-alpha4 with a mutated UIM. Flag immune complexes were isolated from the cell lysates and probed for polyubiquitinated proteins by immunoblotting with an anti-HA antibody. As shown in Fig. 4, a high molecular weight protein smear indicative of polyubiquitinated proteins was detected in the immune complexes of both alpha4 UIM mutants (Δ51-53 and Δ46-60), but not in the wild-type alpha4 immune complex. The high molecular weight proteins do not appear to be representative of polyubiquitinated forms of the alpha4 mutants, as these proteins failed to be recognized with the Flag antibody (data not shown). These findings indicate that partial and full deletions of the UIM increase the association of polyubiquitinated proteins with alpha4.
Fig. 4. Deletion of the UIM within alpha4 results in enhanced binding to polyubiquitinated proteins.
HEK293 cells were transfected with HA-ubiquitin, wild-type Flag-alpha4 (WT), or the combination of the indicated Flag-alpha4 protein (WT,Δ51-53, and Δ46-60) and HA-ubiquitin. Immunoprecipitations were performed from the cell lysates using Flag M2 agarose beads (Flag IPs). Bound proteins were analyzed by SDS-PAGE and immunoblotting using HA and Flag antibodies. Aliquots of the cell lysates were analyzed in the same manner. The migration of SDS-PAGE standards is shown on the left. The data are representative of at least three separate experiments.
The UIM within alpha4 regulates PP2Ac ubiquitination
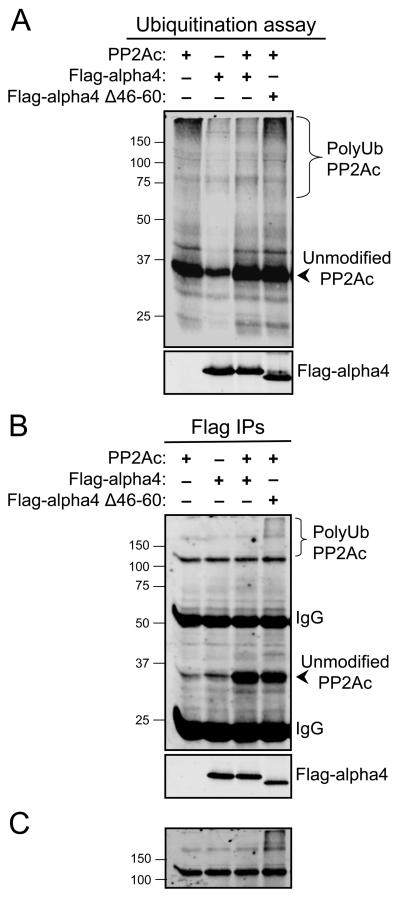
The UIM is located near the N-terminus of alpha4 at a site distinct from the PP2Ac- and Mid1-binding regions (Fig 2B). Therefore, it is conceivable that alpha4 can interact with all three proteins at one time, forming a quaternary complex with ubiquitin, Mid1, and PP2Ac. Although the exact function of this UIM remains unclear, it is possible that this motif: i) helps to orient the ubiquitin molecule for attachment to PP2Ac, ii) forms an intramolecular contact with the ubiquitin moiety attached to alpha4, and/or iii) forms an intermolecular contact with the ubiquitin moiety attached to PP2Ac. If the latter is the case, then the UIM of alpha4 may function to “cap” the ubiquitin chain formed on PP2Ac and prevent further covalent additions of ubiquitin molecules. In the absence of the UIM, PP2Ac polyubiquitination would occur. To test this possibility, PP2Ac ubiquitination assays were performed in the presence or absence of purified wild-type alpha4 or the alpha4 UIM-deficient mutant lacking amino acid residues 46-60. The inclusion of wild-type alpha4 in the in vitro reaction mixture suppressed PP2Ac ubiquitination, whereas the addition of equal amounts of the alpha4 UIM deletion mutant did not affect PP2Ac ubiquitination (Fig. 5A). No appreciable PP2Ac ubiquitination was observed in control reaction mixtures lacking either the E1 and E2 enzymes (Fraction A), the E3 ligases (Fraction B), or the ATP-containing energy solution (Fig. S1). We next analyzed whether alpha4 could physically interact with ubiquitinated PP2Ac species by immunopurifying alpha4 from the ubiquitination assay mixture. As expected, both the wild-type and alpha4 UIM deletion mutant associated with unmodified PP2Ac (Fig. 5B). Interestingly, the alpha4 UIM deletion mutant, but not wild-type alpha4, coimmunoprecipitated polyubiquitinated PP2Ac species (Figs. 5B and 5C). The increase in polyubiquitinated PP2Ac species associated with the alpha4 UIM deletion mutant, as compared with wild-type alpha4, supports the hypothesis that the UIM “caps” the ubiquitin chain formed on PP2Ac and prevents further covalent additions of ubiquitin molecules. In the absence of the UIM, PP2Ac polyubiquitination occurs.
Fig. 5. The UIM within alpha4 regulates PP2Ac ubiquitination.
A, In vitro ubiquitination reactions were carried out using purified PP2Ac as a substrate in the absence (−) or presence (+) of Flag-alpha4 or Flag-alpha4 UIM deletion mutant Δ46-60. Reaction mixtures were analyzed by SDS-PAGE and immunoblotting with antibodies recognizing PP2Ac (top panel) and alpha4 (bottom panel). The ~35 kDa protein detected in reaction mixtures lacking purified PP2Ac (lane 2) represents PP2Ac present in the ubiquitin-protein conjugation kit; the weak PP2Ac polyubiquitination signal detected in this lane is defined as background PP2Ac ubiquitination. B, Immunoprecipitations were performed from ubiquitination reactions in A using Flag M2 agarose (Flag IPs), and analyzed by SDS-PAGE and immunoblotting with PP2Ac and alpha4 antibodies. The migration of SDS-PAGE standards is shown on the left. C, Higher intensity scan of top portion of the blot shown in B. The data are representative of at least three separate experiments.
In summary, the data presented in this report demonstrate that alpha4 is a UIM-containing scaffold protein that modulates PP2Ac polyubiquitination. During preparation of this manuscript, Kong and colleagues reported that alpha4 prevents PP2Ac ubiquitination and protects the phosphatase from proteasomal-mediated degradation (25). Importantly, our results provide a mechanistic explanation for the regulation of PP2Ac ubiquitination by alpha4 – the UIM in alpha4 “caps” associated monoubiquitinated forms of PP2Ac and prevents polyubiquitination of PP2Ac. While previous studies have suggested that UIMs may serve to “cap” the ubiquitination of an associated protein (24), our results provide direct evidence to support this hypothesis. Although additional studies are warranted to explore whether the UIM of alpha4 may form an intramolecular contact with the ubiquitin moiety on alpha4 and/or influence the ubiquitin ligase activity of Mid1, our present studies provide novel insights into the role of alpha4 in the control of PP2Ac ubiquitination.
Supplementary Material
Abbreviations
- PP2A
Protein serine/threonine phosphatase 2A
- PP2Ac
PP2A catalytic subunit
- Mid1
midline-1
- OS
Opitz syndrome
- UIM
ubiquitin-interacting motif
- Ub
ubiquitin
- HEK
human embryonic kidney
- NMR
nuclear magnetic resonance
- HSQC
heteronuclear single quantum coherence
Footnotes
This work was supported by grants from the National Institutes of Health (DK070787 and GM051366 to BEW; GM075156 to WJC; Training Grant T32 CA09582 to SES); the Vanderbilt-Ingram Cancer Center (CA68485); the Center for Molecular Neuroscience (MH19732); the Vanderbilt Diabetes Research and Training Center (DK20593); and an American Cancer Society Grant (58-009-49 to BWS).
SUPPORTING INFORMATION AVAILABLE PP2Ac ubiquitination in control in vitro ubiquitination reactions lacking either the E1 and E2 enzymes (Fraction A), the E3 ligases (Fraction B), or the ATP-containing energy solution (Fig. S1). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Balpha subunit. Biochem J. 1999;339(Pt 2):241–246. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 3.Trockenbacher A, Suckow V, Foerster J, Winter J, Krauss S, Ropers HH, Schneider R, Schweiger S. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29:287–294. doi: 10.1038/ng762. [DOI] [PubMed] [Google Scholar]
- 4.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Smetana JH, Oliveira CL, Jablonka W, Aguiar Pertinhez T, Carneiro FR, Montero-Lomeli M, Torriani I, Zanchin NI. Low resolution structure of the human alpha4 protein (IgBP1) and studies on the stability of alpha4 and of its yeast ortholog Tap42. Biochim Biophys Acta. 2006;1764:724–734. doi: 10.1016/j.bbapap.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Roe SM, Prickett TD, Brautigan DL, Barford D. The structure of Tap42/alpha4 reveals a tetratricopeptide repeat-like fold and provides insights into PP2A regulation. Biochemistry. 2007;46:8807–8815. doi: 10.1021/bi7007118. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Prickett TD, Elliott E, Meroni G, Brautigan DL. Phosphorylation and microtubule association of the Opitz syndrome protein mid-1 is regulated by protein phosphatase 2A via binding to the regulatory subunit alpha 4. Proc Natl Acad Sci U S A. 2001;98:6650–6655. doi: 10.1073/pnas.111154698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inui S, Sanjo H, Maeda K, Yamamoto H, Miyamoto E, Sakaguchi N. Ig receptor binding protein 1 (alpha4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood. 1998;92:539–546. [PubMed] [Google Scholar]
- 10.McConnell JL, Gomez RJ, McCorvey LR, Law BK, Wadzinski BE. Identification of a PP2A-interacting protein that functions as a negative regulator of phosphatase activity in the ATM/ATR signaling pathway. Oncogene. 2007;26:6021–6030. doi: 10.1038/sj.onc.1210406. [DOI] [PubMed] [Google Scholar]
- 11.Goddard TD, Kneller DG. Sparky3. 2007 [Google Scholar]
- 12.Wang AC, Grzesiek S, Tschudin R, Lodi PJ, Bax A. Sequential backbone assignment of isotopically enriched proteins in D2O by deuterium-decoupled HA(CA)N and HA(CACO)N. J Biomol NMR. 1995;5:376–382. doi: 10.1007/BF00182281. [DOI] [PubMed] [Google Scholar]
- 13.Hershko A, Heller H, Elias S, Ciechanover A. Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. J Biol Chem. 1983;258:8206–8214. [PubMed] [Google Scholar]
- 14.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Wooten MW, Geetha T, Seibenhener ML, Babu JR, Diaz-Meco MT, Moscat J. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J Biol Chem. 2005;280:35625–35629. doi: 10.1074/jbc.C500237200. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RD, Wang B, Alam SL, Higginson DS, Robinson H, Sundquist WI, Hill CP. Structure and ubiquitin binding of the ubiquitin-interacting motif. J Biol Chem. 2003;278:28976–28984. doi: 10.1074/jbc.M302596200. [DOI] [PubMed] [Google Scholar]
- 17.Swanson KA, Kang RS, Stamenova SD, Hicke L, Radhakrishnan I. Solution structure of Vps27 UIM-ubiquitin complex important for endosomal sorting and receptor downregulation. Embo J. 2003;22:4597–4606. doi: 10.1093/emboj/cdg471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haririnia A, D'Onofrio M, Fushman D. Mapping the interactions between Lys48 and Lys63-linked di-ubiquitins and a ubiquitin-interacting motif of S5a. J Mol Biol. 2007;368:753–766. doi: 10.1016/j.jmb.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao Y, Senic-Matuglia F, Di Fiore PP, Polo S, Hodsdon ME, De Camilli P. Deubiquitinating function of ataxin-3: insights from the solution structure of the Josephin domain. Proc Natl Acad Sci U S A. 2005;102:12700–12705. doi: 10.1073/pnas.0506344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beal R, Deveraux Q, Xia G, Rechsteiner M, Pickart C. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc Natl Acad Sci U S A. 1996;93:861–866. doi: 10.1073/pnas.93.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldham CE, Mohney RP, Miller SL, Hanes RN, O'Bryan JP. The ubiquitin-interacting motifs target the endocytic adaptor protein epsin for ubiquitination. Curr Biol. 2002;12:1112–1116. doi: 10.1016/s0960-9822(02)00900-4. [DOI] [PubMed] [Google Scholar]
- 22.Miller SL, Malotky E, O'Bryan JP. Analysis of the role of ubiquitin-interacting motifs in ubiquitin binding and ubiquitylation. J Biol Chem. 2004;279:33528–33537. doi: 10.1074/jbc.M313097200. [DOI] [PubMed] [Google Scholar]
- 23.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 24.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 25.Kong M, Ditsworth D, Lindsten T, Thompson CB. Alpha4 is an essential regulator of PP2A phosphatase activity. Mol Cell. 2009;36:51–60. doi: 10.1016/j.molcel.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.