Abstract
Verbal fluency tasks have been widely used to evaluate language and executive control processes in the human brain. FMRI studies of verbal fluency, however, have used either silent word generation (which provides no behavioral measure) or cued generation of single words in order to contend with speech-related motion artifacts. In this study, we use a recently developed paradigm design to investigate the neural correlates of verbal fluency during overt, free recall, word generation so that performance and brain activity could be evaluated under conditions that more closely mirror standard behavioral test demands. We investigated verbal fluency to both letter and category cues in order to evaluate differential involvement of specific frontal and temporal lobe sites as a function of retrieval cue type, as suggested by previous neuropsychological and neuroimaging investigations. In addition, we incorporated both a task switching manipulation and an automatic speech condition in order to modulate the demand placed on executive functions. We found greater activation in the left hemisphere during category and letter fluency tasks, and greater right hemisphere activation during automatic speech. We also found that letter and category fluency tasks were associated with differential involvement of specific regions of the frontal and temporal lobes. These findings provide converging evidence that letter and category fluency performance is dependent on partially distinct neural circuitry. They also provide strong evidence that verbal fluency can be successfully evaluated in the MR environment using overt, self-paced, responses.
Introduction
Neuropsychological investigations have shown that verbal fluency, as measured by the ability to generate lists of words aloud under time constraint, relies on the coordinated activity of a number of brain areas, particularly in the frontal and temporal lobes of the left hemisphere. Damage to the left frontal lobe, especially to left inferior frontal cortex (LIFC) has consistently been shown to impair verbal fluency, even in patients who are not overtly aphasic (e.g., Baldo & Shimamura, 1998; Milner, 1964; Thompson-Schill et al., 1998). In addition, there is evidence that the generation of word lists to letter cues (letter fluency, e.g., “tell me all the words you can think of that begin with the letter A”) relies on a partially different network of brain regions than the generation of word lists to semantic category cues (category fluency e.g., “tell me all the animals you can think of”). Studies have shown, for example, that frontal lobe damage results in disproportionate impairment to letter fluency (e.g. Hodges et al., 1999; Miller, 1984; Baldo et al. 2001; Moscovitch et al., 1994), while temporal lobe damage impairs semantic category fluency to a greater extent than letter fluency (e.g. Hodges et al. 1999; Newcombe, 1969, Butters et al.; 1987; Monsch, 1994; Chan et al; 1993; Baldo et al., 2006). Functional neuroimaging studies, using positron emission tomography (Mummery et al.,1996; Gourovitch et al., 2000) and fMRI (e.g., Perani et al., 2003) have generally supported these findings (for review, see Costafreda et al., 2006).
Motivated by studies of patients with focal brain lesions, verbal fluency tasks have been used increasingly to evaluate language-related and executive control processes in a variety of non-focal disorders including traumatic brain injury (Henry et al., 2004), depression (Wolfe et al., 1987), Alzheimer’s disease (Monsch et al., 1992, Monsch et al., 1994), Huntington’s disease (Monsch et al., 1994), schizophrenia (Saykin et al., 1991; Phillips et al., 2004), attention deficit/hyperactivity disorder (Geurts et al., 2004), and autism (Turner et al., 1999). It is therefore of considerable interest to elucidate the neural systems involved in performing these tasks using non-invasive methods.
The fluency paradigms used in nearly all fMRI studies, however, have differed markedly from the procedure used in neuropsychological investigations. Perhaps most important among these differences, the standard behavioral paradigm in neuropsychological studies requires free recall, with subjects producing words aloud as quickly as possible within a limited period of time. In contrast, in order to mitigate task-related motion artifacts, fMRI studies have typically required either covert word generation (e.g., Gurd et al., 2002; Hirshorn & Thompson-Schill, 2006; Perani et al., 2003), or overt, but experimenter-paced single word production (e.g., Phelps et al., 1997; Abrahams et al., 2003). Covert word generation lacks a behavioral correlate, and the results are therefore difficult to interpret and validate, particularly when studying patient groups. While providing a behavioral correlate, paced overt single word production tasks are also problematic. These procedures reduce cognitive demands relative to the behavioral task by allowing subjects more time to reflect upon their word choice, while increasing the need to inhibit responses (see Basho et al., 2007, and Abrahams et al., 2003 for discussions of these issues). Verbal fluency tasks are often used clinically because they provide measures of the efficiency of selecting and retrieving phonological/orthographic and semantic category information, and require efficient task initiation, planning, organization, and flexibility. These demands are likely to be markedly reduced or absent when responses are generated covertly or are artificially constrained by experimenter-determined pacing.
Fortunately, paradigms for allowing self-paced overt responses in fMRI while mitigating the artifacts from motion have recently been introduced. Basho et al. (2007), for example, investigated category fluency in an overt self-paced design. An overt speech baseline was included in an attempt to control for task-related motion artifacts (e.g., Barch et al., 1999). A potential difficulty with this design is that the main effect of speaking aloud cannot be investigated. This, in turn, may have contributed to the surprising finding that covert word generation lead to greater neural activity in a number of brain regions, relative to overt word generation. In contrast, no brain region showed more activity for the overt, relative to the covert task (Basho et al. , 2007). A different approach to reducing task-related motion artifacts is by using an event-related paradigm or a blocked design with relatively short (10s) task and rest periods (Birn et al., 2004; Soltysik & Hyde, 2006). These paradigms have been shown to produce reliable measures of activation using relatively simple speech tasks (e.g., cued single word reading). However, their ability to distinguish differences in activation expected to result from variations in fluency task demands has not been investigated.
Our primary goal was to investigate the neural correlates of verbal fluency during overt, free recall, word generation so that performance and brain activity could be evaluated under conditions that more closely mirror standard behavioral test demands. While the 10 seconds of word generation required is relatively short compared to typical behavioral word generation tasks, and will therefore be easier to complete, it does require self paced spontaneous generation of multiple words, a fundamental aspect of behavioral fluency tasks. Furthermore, the briefer word generation time will be important if this paradigm is to be used in the future with clinical groups that are impaired on behavioral word fluency tasks. It can be difficult to interpret functional imaging findings on tasks for which behavior is not equated. It is our clinical observation in both Alzheimers Disease (AM) and Autism Spectrum Disorders (LK) that the initial 10 seconds of response is similar to typical controls, whereas later in the course of one minute, word production drops off. Therefore, it is hoped that a briefer interval of self-paced word generation will tap fundamental processes associated with verbal fluency while allowing equivalent word output in typical and clinical groups. In contrast to previous studies of overt self-paced verbal fluency, our paradigm included both letter and category cues to evaluate differential involvement of specific frontal and temporal lobe sites as a function of retrieval cue type, as suggested by previous neuropsychological and neuroimaging investigations. We also incorporated a switching manipulation that required subjects to alternate retrieval according to two cues (two letters, two categories), in order to place greater emphasis on executive control processes typically linked to frontal lobe functioning (Baddeley et al., 2001). Switching fluency tasks have been commonly used to evaluate cognitive flexibility in a variety of clinical populations including schizophrenia (Gourvitch et al., 1996), Parkinson’s Disease (Gurd & Oliveira 1996), Alzheimer’s Diease (Houston et al., 2005), Obsessive Compulsive Disorders (Martin et al., 1993), and HIV-associated cognitive deficits (Iudicello et al., 2008). Finally, we included an automatic speech condition that required subjects to produce a highly over-learned sequence of words to provide a language production baseline and to control for language output effects.
The neural substrate for automatic speech is of interest in its own right. In the late 1800’s, Hughlings Jackson suggested that nonpropositional, automatic speech may be under right hemisphere control (Jackson, 1879). This idea has received some support from lesion studies showing that speech automatisms (e.g., over-learned phrases, curse words) often occur in patients with left hemisphere brain damage and aphasia, whereas, relative to patients with left-sided lesions, patients with right hemisphere damage are impaired in producing automatic speech (for review see Code, 1997).
We had a number of predictions based on previous neuropsychological and neuroimaging findings. First, we expected that, relative to automatic speech, the neural network associated with word generation during the fluency tasks (i.e., in response to specific letter and semantic category cues) would be strongly lateralized to the left-hemisphere, even though it would be expected that many more words would be produced under the automatic than the other word generation conditions. Conversely, we expected that automatic speech would show more extensive right hemisphere activity. We also expected that both the letter and semantic tasks would be associated with activity in a number of brain regions, prominently including LIFC (reflecting selection and retrieval demands) and left posterior temporal cortices (reflecting the site of stored information being retrieved). Moreover, we expected letter fluency to yield greater LIFC involvement than category fluency due to increased selection demands associated with retrieving words based on spelling rules (words beginning with a specific letter) relative to word retrieval based on a semantic category (words denoting objects belonging to a single, common category). In contrast, the category tasks would be expected to produce more posterior temporal lobe activity as a reflection of conceptually-driven word retrieval demands that define these, but not letter fluency tasks (e.g., Martin et al., 1994). Finally, relative to the single cue conditions, the two cue switching conditions should also produce increased LIFC (Sohn et al., 2000) and/or posterior parietal (Gurd et al., 2002, 2003) activity because of the greater demands these tasks place on controlled retrieval processes.
Methods
Subject and Imaging Parameters
Fourteen, right-handed, healthy volunteers participated in the study (7 female; mean age = 32.2 years, range 22 – 48). All subjects spoke English as their first language, had normal or corrected to-normal visual acuity, and no known history of neurological impairments or reading/vocabulary difficulties. Informed consent was obtained in writing under an approved National Institute of Mental Health protocol. All participants were financially compensated for their participation according to NIH guidelines.
Time series of T2*-weighted echo-planar MR images were acquired on a 3 T General Electric (GE) MRI scanner (Waukesha, WI, USA) using a quadrature birdcage RF coil. Whole brain coverage was achieved using 27–28 sagittal slices. (TR: 2000 ms, TE: 30 ms, FOV: 24 cm, slice thickness: 5 mm, matrix: 64×64, 115 image volumes per time series.) For anatomical reference, a higher resolution volume was acquired at the beginning of each scan session using a T1-weighted Magnetization Prepared Rapid Gradient Echo (MP-RAGE) pulse sequence (flip angle: 10°, resolution: 0.94 × 0.94 × 1.2mm3). The subject’s head was immobilized using a vacuum pillow (S&S Par Scientific, Houston, TX, USA).
During scanning, the subject’s spoken responses were recorded using an optical microphone with active noise cancellation (Phone-Or, Inc., Israel). This microphone and the associated processing software allowed the subject’s response to be separated from the scanner sounds.
Task Paradigm
The task was performed in a blocked design, with 10 s periods of task performance alternated with 10 s periods of rest (subjects instructed to stare at a central fixation cross). Using this design, blood oxygenation level dependent (BOLD) signal changes are delayed by a quarter cycle relative to the motion-induced signal changes, which occur in synchrony with the task. As a result, the correlation between BOLD and motion-induced signal changes is small, and the number of false positives resulting from speech-related motion artifacts (when performing a standard regression analysis) is minimized (for details see Birn et al., 2004).
In each 10 s block, subjects were presented with one of five possible task cues: a single letter, a single semantic category, two letters, two categories, or a control condition. Written cue letters were presented in the center of the screen and remained visible for the duration of the 10 s block. In the control condition, subjects were presented with an over-learned category – the word “months” appeared - and subjects named the months of the year in chronological order starting from January. When presented with a single letter or category, subjects were asked to generate as many words as they could think of starting with that letter, or that were members of the category, until the fixation cross appeared. When presented with two letters (or two categories), subjects were required to generate one word corresponding to one of the letters (or categories), then switch to the other letter (or category), and continue to alternate between the two cues (e.g. when presented with the cue “color/fruit” subjects would generate “blue, apple, red, banana, …”). Each condition was presented twice, in random order, in each of 8 runs for a total of 16 unique blocks for each of the letter and category conditions.
Analysis
All image analyses were performed using AFNI (Cox et al., 1996). Reconstructed images were first corrected for bulk head motion using a rigid-body volume registration routine. Time series were then corrected for slice-timing differences, spatially smoothed using a Gaussian blur with a root-mean-square (RMS) width of 4mm, and converted to percent signal changes. Activation amplitudes for each of the five conditions were determined in each subject using a multiple regression analysis. BOLD signal changes were modeled using the stimulus timing convolved with a gamma-variate (Cohen et al., 1997). Task-related motion artifacts were modeled using a boxcar waveform representing the task timing.
Group-level analyses were performed using a mixed effects ANOVA with subjects as a random factor and task as a fixed factor. In the first analysis, all five fluency tasks were included (one-way ANOVA with five levels: one letter, two letter, one category, two category, months) to allow us to define brain regions more active during strategic (letter and category cued) than automatic word production. We then evaluated how activity within these regions was modulated by fluency task via a second level analysis – a two-way ANOVA of Condition (letter, category) and Number of cues (i.e., switching: one, two).
Statistical maps were corrected for multiple comparisons by thresholding each contrast – category vs. letter, one cue vs. two cues, and fluency tasks vs. control condition – at a single-voxel p-value of p<0.001, and then rejecting activation differences below a cluster size of 770 mL. This cluster size was derived from a Monte Carlo simulation of false positive rates of different cluster sizes (using the program AlphaSim from the AFNI package), and results in a corrected p-value of p<0.05 for each contrast.
Averaged response time series were obtained by deconvolution. These time courses were averaged over different regions of interest, defined by different contrasts in the ANOVAz analysis – all fluency tasks vs. the control condition, category vs. letter fluency, and one cue vs. two cues.
Finally, an additional analysis was performed in order to assess and confirm the effectiveness of this paradigm design in isolating the speech related movement artifacts. The multiple regression analysis was repeated 21 times, each time with a different shift of the ideal hemodynamic response, from −10s to +10s, in 1s steps. In this analysis, the motion-induced signal change was not modeled with additional regressors. Our hypothesis was that motion induced signal changes would show a maximal correlation with the ideal response several seconds earlier than the true BOLD signal changes.
Results
Behavior
As expected, subjects generated more words during the automatic speech condition (“months”) (mean +/− std. error of the mean: 12.0 +/− 1.4 words) than during any of the other fluency conditions (all p’s <0.0001; 1 letter: 5.0 +/− 0.4 words, 1 category: 6.0 +/− 0.5 words, 2 letters: 5.2 +/− 0.4 words, 2 categories: 4.9 +/− 0.3 words). The number of words produced during the different fluency tasks, however, did not differ significantly (all p’s > .10).
Imaging
Relative to the 10 s rest periods, word generation during the fluency tasks produced robust and widespread bilateral activation of frontal, parietal, and occipitotemporal cortices, and the anterior cingulate. In comparison to the automatic speech condition, however, fluency-related activity was limited largely to the left hemisphere, whereas automatic speech yielded greater right hemisphere activity. Specifically, relative to the category and letter fluency tasks, repeated retrieval of the names of the months of the year in chronological order yielded increased activity in bilateral precentral gyrus (motor and premotor cortices), precuneus, left superior temporal gyrus, and a more extensive region of activation in the right hemisphere covering both right superior temporal gyrus and right supramarginal gyrus (Figure 1a; Table 1). In contrast, relative to automatic speech, the fluency conditions elicited robust activity in broad expanses of left ventral occipitotemporal cortex, including fusiform gyrus, (Fig 1b) left parietal and left frontal cortices (Fig 1c), as well as the supplemental motor area (SMA), and bilateral thalamus extending into the left caudate (Fig. 1c, Table 1). In each of these regions, the fluency tasks yielded more activity than automatic speech even though, on average, only half as many words were generated.
Figure 1.
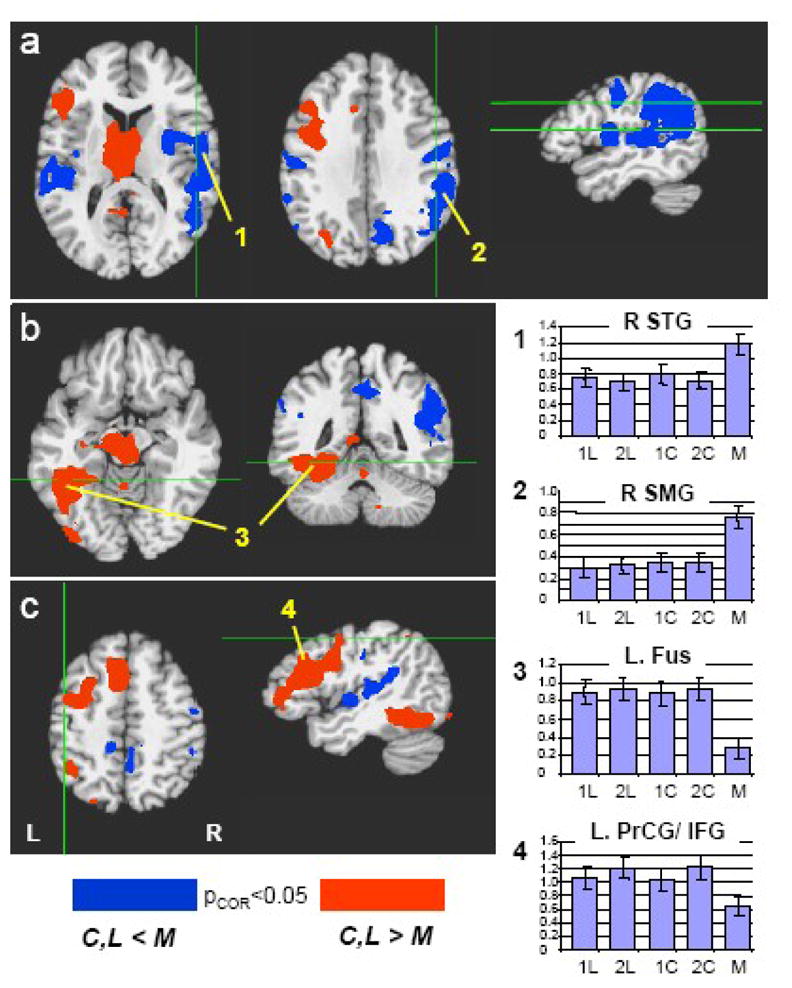
Category and letter fluency tasks (C,L) vs. “Months” automatic speech control condition (M). Blue areas indicate regions with a greater activation during the automatic speech; Red areas indicate regions with a greater activation during the fluency tasks. Activations are thresholded at p<0.05 (corrected for multiple comparisons). Bar graphs on right indicate the average signal intensity during each of the 5 tasks, averaged over all subjects and over the region of interest indicated by the number (1–4). (R.STG = Right Superior Temporal Gyrus, R.SMG = Right Supramarginal Gyrus, L.Fus = Left Fusiform Gyrus, L.PrCG/IFG = Left Precentral Gyrus/Inferior Frontal Gyrus.) Note that activation associated with automatic speech is greater in the right hemisphere, whereas activation associated with the fluency tasks is left hemisphere lateralized.
Table 1.
| Contrast | Talairach Coords (Cntr. of Mass) | Talairach Coords (Peak voxel) | Name | Mean t-stat | Peak t-stat | Volume (mm3) |
|---|---|---|---|---|---|---|
| M > (C,L) | ||||||
| (44,−10,7) | (29,−9,3) | Right Superior Temporal Gyrus | 5.1123 | 8.6319 | 11799 | |
| (−48,−25,11) | (−45,−39,22) | Left Superior Temporal Gyrus | 5.0104 | 7.6841 | 6645 | |
| (50,−45,25) | (56,−38,33) | Right Supramarginal Gyrus | 5.1499 | 8.5728 | 15434 | |
| (7,−47,38) | (11,−69,34) | Cingulate/Precuneus | 5.0178 | 8.6982 | 6810 | |
| (−51,−15,32) | (−49,−17,32) | Left Post-Central Gyrus | 4.7842 | 6.2972 | 1342 | |
| (51,−10,36) | (53,−11,42) | Right Post-Central Gyrus | 4.8598 | 6.5613 | 2297 | |
| (C,L) > M | ||||||
| (−38,12,30) | (−39,22,24) | Left Inferior Frontal Gyrus | 5.6177 | 11.357 | 17049 | |
| (−36,−51,−14) | (−45,−51,−11) | Left Fusiform Gyrus | 5.6046 | 13.254 | 11366 | |
| (−5,−14,6) | (−7,−3,13) | Left Caudate/Thalamus | 5.5381 | 12.437 | 19285 | |
| (−4,16,44) | (−3,16,45) | SMA | 5.7732 | 9.6589 | 5927 | |
| (−29,2,50) | (−34,−4,47) | Left Middle Frontal Gyrus | 5.1673 | 8.928 | 4334 | |
| (−27,−70,36) | (−31,−66,31) | Left Superior Parietal Lobule | 4.9452 | 7.9471 | 1964 | |
| (−4,−25,−8) | (−5,−26,−4) | Brain stem/Substantia Nigra | 5.3757 | 10.112 | 5252 | |
| L > C | ||||||
| (−45,0,27) | (−46,−3,31) | Left Inferior Frontal Gyrus. | 5.119 | 7.3631 | 2820 | |
| (−36,−46,44) | (−34,−45,45) | Left Superior Parietal Lobule | 5.0381 | 8.4761 | 2769 | |
| (0,−28,25) | (−5,−33,25) | Precuneus | 5.2196 | 7.2782 | 2186 | |
| (42,−57,−9) | (40,−59,−9) | Right Fusiform Gyrus | 4.9342 | 6.5318 | 2072 | |
| (−40,−62,−8) | (−38,−66,−8) | Left Fusiform Gyrus | 5.0883 | 7.455 | 1890 | |
| (16,−66,36) | (15,−67,32) | Right Superior Parietal Lobule | 4.8283 | 6.4391 | 791 | |
| C > L | ||||||
| (−2,−65,5) | (−9,−62,7) | Visual Cortex | 5.4843 | 12.933 | 17353 | |
| (−19,23,49) | (−21,11,45) | Left Middle Frontal Gyrus. | 6.3948 | 10.348 | 6799 | |
| (−42,−69,27) | (−48,−66,24) | Left MTG | 5.0957 | 8.6699 | 2990 | |
| (−23,−45,−7) | (−15,−50,−1) | Left Fusiform Gyrus | 5.039 | 7.7621 | 1138 | |
| (−42,−24,10) | (−36,−27,12) | Left Superior Temporal Gyrus | 4.9257 | 7.1258 | 934 | |
| 2 > 1 | ||||||
| (−27,−9,45) | (−30,−8,50) | Left Pre-Central Gyrus | 5.1067 | 8.3642 | 4555 | |
| (26,−7,50) | (22,−11,51) | Right Pre-Central Gyrus | 4.8202 | 6.5381 | 2230 | |
| (−22,−80,11) | (−28,−69,23) | Left Superior Parietal Lobule | 5.2697 | 10.079 | 8975 | |
| (26,−79,10) | (24,−67,31) | Right Superior Parietal Lobule | 5.3566 | 11.186 | 8877 | |
| (−30,−68,−12) | (−35,−57,−6) | Left Fusiform Gyrus | 4.9488 | 7.1721 | 8915 | |
| (32,−62,−11) | (34,−73,−11) | Right Fusiform Gyrus | 5.276 | 7.948 | 6634 | |
| (−9,−63,44) | (−7,−70,40) | Precuneus | 5.0516 | 9.7959 | 6467 | |
Differences between the fluency task conditions were also observed. Relative to category fluency, letter fluency yielded greater activity in the left inferior frontal gyrus, bilateral superior parietal cortex, and in the bilateral ventral occipitotemporal cortex centered on the occipital temporal sulcus. In contrast, relative to letter fluency, category fluency yielded greater activity in occipital (visual) cortex, the left fusiform gyrus – anterior and medial to the activity associated with letter fluency - and the left middle frontal gyrus, anterior to the region more active for letter than the category fluency tasks (indicated by arrows in Fig. 2c) (see Figure 2, Table 1).
Figure 2.
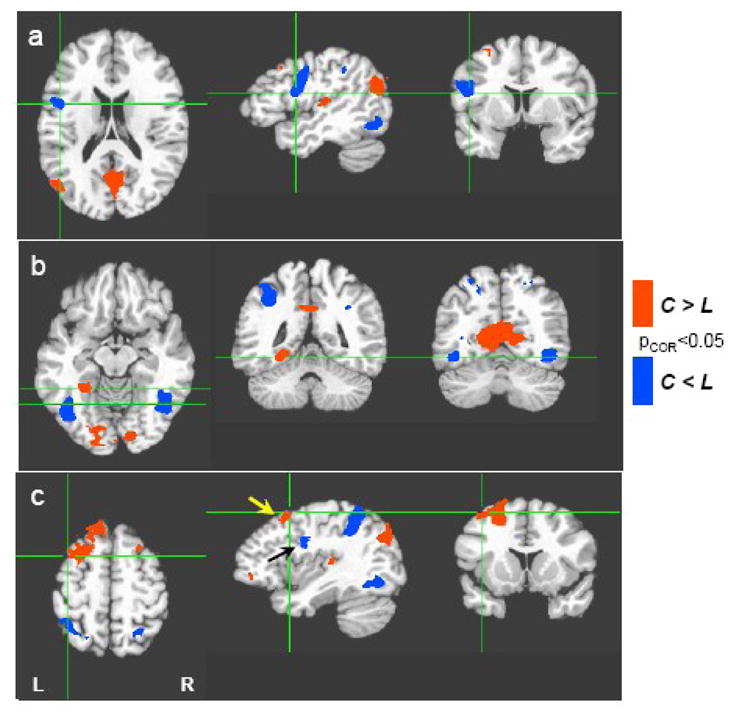
Category vs. Letter Fluency. Regions with greater activation during the category fluency tasks (C) are shown in red, while regions with greater activation during the letter fluency tasks (L) are shown in blue. Activations are thresholded at p<0.05 (corrected for multiple comparisons). Compared to category fluency, letter fluency resulted in greater activation in left precentral/inferior frontal gyrus (a), bilateral ventral occipito-temporal cortex (b), and bilateral superior parietal cortex (c). Category fluency resulted in greater activation in occipital (visual) cortex, fusiform (b), and left middle frontal gyrus (c). Frontal activation with a greater response to category cues (left middle frontal gyrus, yellow arrow) is located superior and anterior to the frontal activation with a greater response to letter cues (left precentral/inferior frontal gyrus, black arrow).
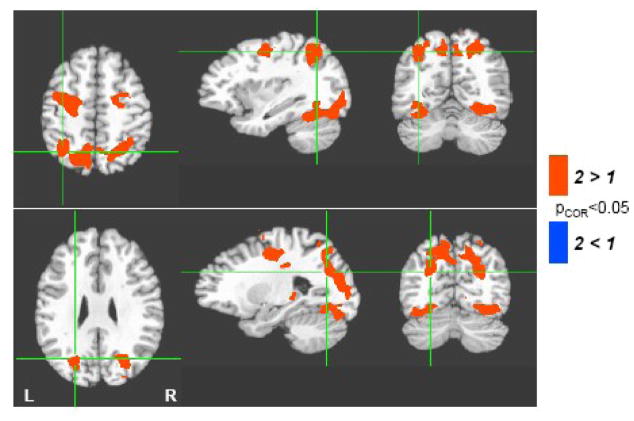
The additional task demand of switching between two categories or two letters resulted in greater activation in a number of these regions including the left middle frontal gyrus, left superior parietal cortex, left fusiform, and the precuneus (Figure 3 and Table 1). The task switching also resulted in greater activation in the right middle frontal gyrus, right fusiform, and right superior parietal cortex, but these regions showed no significant difference between the fluency tasks and the automatic speech condition. No regions showed a weaker response to the switching than to the single cue conditions, and no significant interactions between fluency task type (category, letter) and switching (one cue, two cues) were found.
Figure 3.
Main effect of task-switching. Regions with greater activation during verbal fluency in response to two categories or letters (2) compared to single category or letter cues (1) are indicated in red. This includes bilateral premotor areas, superior parietal cortex, ventral occipito-temporal cortex, and posterior cingulate. No regions show a greater activation to single category or letter cues (blue).
Analysis of motion artifacts
As expected, the averaged signal intensity time courses from the above described activated regions show a delayed response, characteristic of the typical hemodynamic BOLD signal. In contrast, signal intensity time courses in regions that are particularly vulnerable to task-related motion artifacts –– orbital frontal cortex, anterior inferior temporal cortex, and the edges of the brain –– showed more rapid signal intensity changes that were synchronous with the task timing and not significantly correlated with the ideal delayed BOLD response (Figure 4). These rapid signal intensity changes preceded the expected BOLD response by approximately 3–4 seconds, and therefore likely reflect task-related motion. In some brain regions, such as the orbitofrontal cortex, the signal intensity time course not only showed features consistent with task-related motion artifact, but also showed a slower return to baseline during the rest period, indicating that this region could contain a combination of motion-induced signal changes and task-related neuronal activity. The presence of the motion artifact, however, makes a precise estimation of the activation amplitude more difficult, and as a result this region was not included in the above described results.
Figure 4.
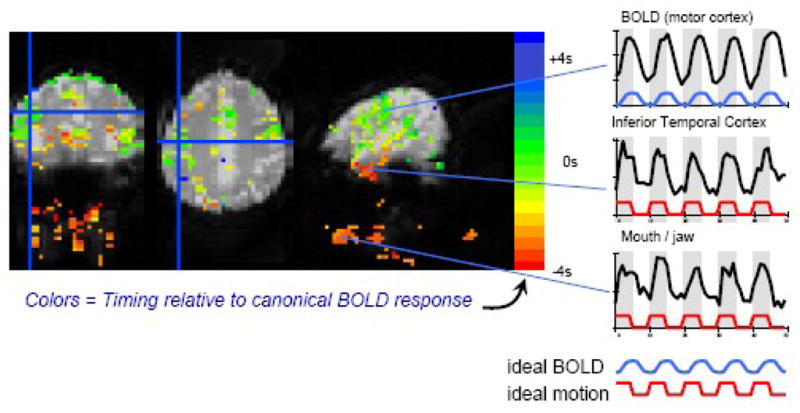
Latency analysis - Main effect of task vs. fixation: Colored regions show the main effect of the task vs. fixation. The color scale indicates the timing of the signal changes relative to the typical BOLD response. Red = earlier than typical BOLD response (i.e. synchronous with task timing, and indicative of motion); Green = timing similar to typical BOLD response. Signal changes occurring in synchrony with the task (indicated by gray regions in the time series) are visible near the mouth and in the inferior temporal lobe. These motion-induced signal changes occur several seconds before BOLD signal changes in motor and language regions. Time courses from 3 regions are shown on the right. The blue and red curves represent the ideal BOLD and motion induced signal changes, respectively. Signals from the mouth/jaw area and the inferior temporal cortex are more rapid and occur primarily during the task, indicative of motion artifact.
Discussion
This study provides strong evidence that different types of verbal fluency tasks can be successfully performed and evaluated in the MR environment using overt responses that are self-paced. Although the feasibility of the paradigm used in this study as been demonstrated before (Birn et al., 2004), the prior study used a task that was highly constrained – reading aloud single words. The current study is the first to show that this paradigm can be successfully used to evaluate overt free recall. In addition, although there have been a few previous reports on overt fluency; the current study is the first to investigate overt, unpaced verbal fluency under multiple retrieval conditions. In the following sections, we will first discuss the specific findings on the neural circuitry supporting single word retrieval and then turn to some final comments on our paradigm.
Fluency tasks vs. automatic speech
As expected, subjects produced significantly more words when repeatedly reciting the months of the year in chronological order than when retrieving words to specific letter and category cues. Consistent with this increased output, a number of brain regions - including the superior temporal and precentral gyri, bilaterally - showed enhanced activity during this automatic speech condition relative to the controlled retrieval conditions. Activation of the superior temporal gyri included primary and secondary auditory processing zones, and thus likely reflected, at least in part, auditory processing of the subject’s own output since many more words were produced during the automatic than controlled fluency conditions. Bilateral activation of the precentral gyrus was also observed, likely reflecting overt speech production (articulation). An interesting aspect of the activations in both of these sites is that they were considerably more extensive in the right than left hemisphere (see Table 1). Moreover, other activated regions, including a large region of posterior temporal-parietal cortex centered on the supramarginal gyrus, were lateralized to the right hemisphere. Thus, repeated retrieval of the same highly over-learned sequence of words led to enhanced right hemisphere activity relative to considerably more effortful tasks requiring the generation of a unique list of words on every trial. This finding is particularly noteworthy given the extensive literature showing that neural activity typically decreases when stimuli and tasks are repeated (i.e., repetition suppression; Grill-Spector et al., 2006; Schacter et al., 2007).
The interpretation of this finding is not, however, straightforward. One possibility is that this finding suggests a right hemisphere superiority for producing automatic speech, consistent with the clinical literature noted previously (see Code 1997 for review). Previous neuroimaging studies have not been particularly informative on this issue. Automatic speech tasks have been used in several studies (e.g., generating months of the year, days of the week, number counting: Gourovitch et al., Hutchinson et al., Schlosser et al) but enhanced activations associated with these baseline conditions were typically not assessed or reported. Larsen and colleagues, however, reported a 10 percent rCBF increase in right, but not left, hemisphere blood-flow during an automatic speech task (Larsen et al., 1978). Thus, our finding is in agreement with some prior neuroimaging and clinical reports. Nevertheless, it should be noted that the hemispheric difference we observed could in principle reflect any of the ways that the automatic speech and fluency conditions differed. Thus, our results provide no more than a tantalizing clue that the right hemisphere may be more involved than the left in the production of highly over-learned verbal sequences.
Regardless of the interpretation of the automatic speech findings, this condition proved to be highly successful for isolating fluency-related activations nearly exclusively to the left hemisphere. Both the semantic and phonemic fluency tasks produced greater activation in the left frontal, posterior ventral temporal, and superior parietal cortices, compared to the over-learned category control condition. In fact, in some of these regions, automatic speech resulted in almost no detectable BOLD response compared to rest, whereas the fluency tasks produced robust responses, consistent with claims that these left hemisphere sites are involved in strategic lexical and semantic search and retrieval processes (see Fig 1).
Letter vs. Category Fluency
While letter fluency and category fluency require many of the same cognitive processes, including sustaining attention, devising a search strategy, selecting appropriate words, inhibiting competitors, engaging working memory, and articulating the output, there are important differences. Letter fluency requires selecting and retrieving information based on spelling (orthography). Category fluency, on the other hand, places a greater demand on conceptual knowledge stores. Our fMRI data show activations consistent with these different requirements. In the left hemisphere, letter fluency was associated with enhanced responses in the left premotor/inferior frontal gyrus, relative to the category fluency tasks. In contrast, category fluency showed enhanced activity in the left fusiform and left middle frontal gyrus. These findings are consistent with previous studies associating letter fluency with the left frontal lobe, especially the more posterior regions of the left inferior frontal gyrus (for review, see Costafreda et al., 2006), and semantic fluency with increased activation of the more anterior regions of the frontal lobes and with posterior regions of temporal cortex (Gourovitch et al., 2000; Mummery et al., 1996; Perani et al., 2003).
Other differences between category and letter fluency were also observed that were not directly predicted by these earlier studies. Specifically, letter fluency was found to show greater activity than category fluency in relatively discrete bilateral regions of the occipitotemporal cortex centered on the occipital temporal sulcus. These activations likely do not reflect early low-level visual processing, since the activity was greater during viewing of a single letter, compared to viewing an entire word (the category and control conditions). In fact, viewing whole words activated primary visual cortex, bilaterally, to a greater extent relative to viewing single letters (see Fig 2). Interestingly, the region of posterior temporal cortex more active for the letter than category fluency conditions overlaps with an area implicated by previous studies as being involved in the visual processing of word forms (i.e., the visual word form areas, VWFA; McCandliss et al., 2003), as well as with other word processing sites that appears to have a multimodal function; i.e., for combining orthographic and phonological information (Cohen et al., 2004). Based on these studies, one could speculate that this area may be more heavily involved in letter than category fluency because letter fluency requires an initial mapping of the letter cue to phonologic and/or orthographic information, and checking that the orthography of the retrieved word matches the initial letter cue. In other words, this activity may reflect top-down activation of VWFA required by the demands of the letter fluency task that are not necessary when retrieving words to category cues.
The effect of externally cued task-switching demands
Task switching requires increased attention, working memory, and other executive control processes. In the current study, switching between two letters or two categories resulted in greater activation in bilateral premotor regions and posterior temporal cortices, likely reflecting increased demand on word selection, retrieval, and storage sites. In addition, increased activation was observed in bilateral posterior parietal regions, a finding consistent with previous reports using covert verbal fluency tasks (Gurd et al., 2002; Gurd et al., 2003). Surprising, but again consistent with previous studies (Gurd et al., 2002; Gurd et al., 2003), no enhanced activity was found in the more anterior frontal region (the middle frontal gyrus) found to be more active for category compared to letter fluency. The apparent conflict between this finding and previous clinical research emphasizing the role of prefrontal cortex during switching may reflect differences in task demands. For example, in a behavioral investigation of patients with dementia, Troyer et al (1998) found that spontaneous switching to facilitate word generation during semantic verbal fluency tasks was related to frontal lobe functioning. In contrast, the task used in the Gurd studies, like ours, provides specific cues to switch. Baldo et al. (2001) differentiate endogenous switching (a spontaneous internally generated strategy) from exogenous, or externally cued, switching during a fluency task, and report that while patients with frontal lobe lesions are more impaired on verbal fluency tasks than the control participants, both groups are comparably affected by an explicit requirement to switch between cues. The lack of prefrontal findings in this study may also relate to the Baddeley et al (2001) finding that switching costs are small when the need to remember the switches is removed by providing visual cues.
The influence of task-related motion
This study shows that neuronal activation during an overt self-paced verbal fluency task can be successfully assessed and separated from task-related motion effects by using a block design with task (speech) and rest (non-speech) block durations of 10s. This paradigm design exploits the latency difference between the delayed hemodynamic BOLD response and the motion artifact, minimizing the likelihood that motion artifacts appear as false positives (Birn et al., 2004). It should be noted, however, that there is a possibility of either missing activation or incorrectly estimating the response amplitude when the BOLD response and task-related motion-induced signal changes occur in the same voxel. Previous studies have shown that these task-induced motion artifacts occur primarily in the anterior inferior temporal lobes, orbito-frontal cortex, and at the edges of the brain (Birn et al., 1998, 2004). A closer look at the time courses within these regions indeed show significant and rapid signal changes, but these were not correlated with the ideal BOLD response and were therefore not classified as “active” regions in our analysis. Recovering the BOLD response within these regions would require either a more accurate modeling of motion-induced signal changes, or ignoring the time points during the speech epochs (Birn et al., 2004). Ignoring the corrupted time points could, perhaps, be done selectively in those regions severely affected by motion in order to preserve the degrees of freedom in unaffected regions.
Conclusions
In this study we were able to measure brain activity when subjects generated aloud lists of words to specific retrieval cues, thereby more closely mirroring standard behavioral test demands for unpaced word generation than in previous fMRI investigations. Consistent with previous findings, we found that letter and category fluency tasks were associated with differential involvement of frontal and temporal lobes – with a greater activation in left pre-central and inferior frontal gyrus for letter fluency, and greater activation more anterior in the left middle frontal gyrus as well as in the left fusiform gyrus for category fluency. Contrary to expectations, we also found greater activation of left occipitotemporal sulcus/posterior fusiform gyrus during word retrieval to letter than category cues. We speculated that this activity may be related to word-form processing demands that are greater when retrieval is guided by letter rather than by category cues. The additional demand of task-switching increased activation in parietal areas as well as pre-motor regions, partially overlapping with areas more active during the letter fluency task, but not the category fluency task, suggesting a functional role for the middle frontal gyrus in verbal fluency beyond lexical search and retrieval. While bilateral activations were observed in many of these tasks, right hemisphere activations were typically greater during automatic speech produced in response to an over-learned category, while left hemisphere activations were greater during the category and letter fluency tasks that placed greater demands on executive function processes. Overall, our findings show that both the location and amount of cortical activity can be modulated by varying verbal fluency task demands. They also demonstrate that these differences can be identified and evaluated when subjects speak aloud in a self-paced manner in the magnet. This, in turn, suggests that our paradigm should be useful for evaluating the integrity of neural systems in clinical populations using verbal fluency tasks and other procedures that require overt speech.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, Andrew CM, Leigh PN. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum Brain Mapp. 2003;20(1):29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Chincotta D, Adlam A. Working memory and the control of action: evidence from task switching. J Exp Psychol Gen. 2001;130(4):641–57. [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12(2):259–67. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc. 2001;7(5):586–96. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10(6):642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Basho S, Palmer ED, Rubio MA, Wulfeck B, Muller RA. Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia. 2007;45(8):1697–706. doi: 10.1016/j.neuropsychologia.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magnetic Resonance in Medicine. 1998;40(1):55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–58. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Butters N, Wolfe J, Granholm E, Martone M. An assessment of verbal recall, recognition and fluency abilities in patients with Huntington’s disease. Cortex. 1986;22(1):11–32. doi: 10.1016/s0010-9452(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Chan AS, Butters N, Paulsen JS, Salmon DP, Swenson MR, Maloney LT. An Assessment of the Semantic Network in Patients with Alzheimer’s Disease. Journal of Cognitive Neuroscience. 1993;5(2):254–261. doi: 10.1162/jocn.1993.5.2.254. [DOI] [PubMed] [Google Scholar]
- Code C. Can the right hemisphere speak? Brain Lang. 1997;57(1):38–59. doi: 10.1006/brln.1997.1833. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22(1):466–76. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry. 2004;45(4):836–54. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Goldberg TE, Weinberger DR. Verbal fluency deficits in patients with schizophrenia: Semantic fluency is differentially impaired as compared with phonologic fluency. Neuropsychology. 1996;10(4):573–577. [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14(3):353–60. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR. Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain. 2002;125(Pt 5):1024–38. doi: 10.1093/brain/awf093. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Oliveira RM. Competitive inhibition models of lexical-semantic processing: experimental evidence. Brain Lang. 1996;54(3):414–33. doi: 10.1006/brln.1996.0083. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Weiss PH, Amunts K, Fink GR. Within-task switching in the verbal domain. Neuroimage 20 Suppl. 2003;1:S50–7. doi: 10.1016/j.neuroimage.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A Meta-Analytic Review of Verbal Fluency Performance in Patients With Traumatic Brain Injury. Neuropsychology. 2004;18(4):621–628. doi: 10.1037/0894-4105.18.4.621. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–57. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Ward R, Garrard P, Bak T, Perry R, Gregory C. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology. 1999;13(1):31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- Houston WS, Delis DC, Lansing A, Jacobson MW, Cobell KR, Salmon DP, Bondi MW. Executive function asymmetry in older adults genetically at-risk for Alzheimer’s disease: verbal versus design fluency. J Int Neuropsychol Soc. 2005;11(7):863–70. doi: 10.1017/s1355617705051015. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Schiffer W, Joseffer S, Liu A, Schlosser R, Dikshit S, Goldberg E, Brodie JD. Task-specific deactivation patterns in functional magnetic resonance imaging. Magn Reson Imaging. 1999;17(10):1427–36. doi: 10.1016/s0730-725x(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL, Grant I, Group TH. Cognitive mechanisms of switching in HIV-associated category fluency deficits. J Clin Exp Neuropsychol. 2008:1–8. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH. On affections of speech from disease of the brain. In: Taylor J, editor. Selected writings of John Hughlings Jackson. Vol. 2. Staples Press; London: 1879. 1958. [Google Scholar]
- Larsen B, Skinhoj E, Lassen NA. Variations in regional cortical blood flow in the right and left hemispheres during automatic speech. Brain. 1978;101(2):193–209. doi: 10.1093/brain/101.2.193. [DOI] [PubMed] [Google Scholar]
- Martin A, Pigott TA, Lalonde FM, Dalton I, Dubbert B, Murphy DL. Lack of evidence for Huntington’s disease-like cognitive dysfunction in obsessive-compulsive disorder. Biol Psychiatry. 1993;33(5):345–53. doi: 10.1016/0006-3223(93)90323-6. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Lalonde F, Mack C. Word retrieval to letter and semantic cues: a double dissociation in normal subjects using interference tasks. Neuropsychologia. 1994;32(12):1487–94. doi: 10.1016/0028-3932(94)90120-1. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Miller E. Verbal fluency as a function of a measure of verbal intelligence and in relation to different types of cerebral pathology. Br J Clin Psychol. 1984;23 (Pt 1):53–7. doi: 10.1111/j.2044-8260.1984.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Milner B. Some effects of frontal lobectomy in man. In: Warren J, Akert K, editors. The frontal granular cortex and behavior. McGraw-Hill; New York: 1964. pp. 313–331. [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Paulsen JS, Salmon DP, Bruyer D, Swenson M. A comparison of category and letter fluency in Alzheimer’s and Huntington’s disease. Neuropsychology. 1994;8:25–30. [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–8. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive Resources and Dual-Task Interference Effects at Retrieval in Normal People: The Role of the Frontal Lobes and Medial Temporal Cortex. Neuropsychology. 1994;8(4):524–534. [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263(1373):989–95. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Newcombe F, Russel WR. Dissociated visual perceptual and spatial deficits in focal lesions of the right hemisphere. J Neurol Neurosurg Psychiat. 1969;32:73–81. [Google Scholar]
- Perani D, Abutalebi J, Paulesu E, Brambati S, Scifo P, Cappa SF, Fazio F. The role of age of acquisition and language usage in early, high-proficient bilinguals: an fMRI study during verbal fluency. Hum Brain Mapp. 2003;19(3):170–82. doi: 10.1002/hbm.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Basso A, Fazio F. A fMRI study of word retrieval in aphasia. Brain Lang. 2003;85(3):357–68. doi: 10.1016/s0093-934x(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8(2):561–5. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, James AC, Crow TJ, Collinson SL. Semantic fluency is impaired but phonemic and design fluency are preserved in early-onset schizophrenia. Schizophr Res. 2004;70(2–3):215–22. doi: 10.1016/j.schres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, Bhati MT, Valdez JN, Kohler CG, Siegel SJ, Gur RC, Gur RE. Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophrenia Research. 2008;99:312–323. doi: 10.1016/j.schres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–24. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr Opin Neurobiol. 2007;17(2):171–6. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64(4):492–8. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97(24):13448–53. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysik DA, Hyde JS. Strategies for block-design fMRI experiments during task-related motion of structures of the oral cavity. NeuroImage. 2006;29 (4):1260–1271. doi: 10.1016/j.neuroimage.2005.08.063. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95(26):15855–60. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G, Leach L, Freedman M. Clustering and switching on verbal fluency tests in Alzheimer’s and Parkinson’s disease. Journal of the International Neuropsychological Society. 1998;4:137–143. doi: 10.1017/s1355617798001374. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999;40(2):189–201. [PubMed] [Google Scholar]
- Wolfe J, Granholm E, Butters N, Saunders E, Janowsky D. Verbal memory deficits associated with major affective disorders: a comparison of unipolar and bipolar patients. J Affect Disord. 1987;13(1):83–92. doi: 10.1016/0165-0327(87)90077-2. [DOI] [PubMed] [Google Scholar]