Abstract
An increasing number of fMRI studies are using the correlation of low-frequency fluctuations between brain regions, believed to reflect synchronized variations in neuronal activity, to infer “functional connectivity”. In studies of autism spectrum disorder (ASD), decreases in this measure of connectivity have been found by focusing on the response to task modulation, by using only the rest periods, or by analyzing purely resting-state data. This difference in connectivity, however, could result from a number of different mechanisms – differences in noise, task-related fluctuations, task performance, or spontaneous neuronal activity. In this study, we investigate the difference in functional connectivity between adolescents with high-functioning ASD and typically developing control subjects by examining the residual fluctuations occurring on top of the fMRI response to an overt verbal fluency task. We find decreased correlations of these residuals (a decreased “connectivity”) in ASD subjects. Furthermore, we find that this decrease was not due to task-related effects, block-to-block variations in task performance, or increased noise, and the difference was greatest when primarily rest periods are considered. These findings suggest that the estimate of disrupted functional connectivity in ASD is likely driven by differences in task-unrelated neuronal fluctuations.
Introduction
An increasing number of neuroimaging studies are using functional MRI (fMRI) to investigate not only task-induced neuronal activation, but also the connections between different brain regions. This estimate of “connectivity” is typically derived by measuring the correlation of time series fluctuations between brain areas. Synchronized fluctuations in the fMRI signal intensity time series can, of course, be task-induced, but also have been shown to occur in the absence of an external stimulus or explicit task, particularly at low temporal frequencies (<0.1Hz). It is believed that these signal fluctuations reflect synchronized variations in the neuronal activity of a network of regions. These correlations are often referred to as “functional connectivity,” a phenomenon first studied in fMRI by Biswal et al., 1995 in the motor cortex. Since then a number of studies have identified a consistent set of resting state networks in motor cortex, auditory cortex, visual cortex, attentional areas and the “default mode network” areas (Damoiseaux et al., 2006; De Luca et al., 2006; Greicius et al., 2003; Raichle et al., 2001). The default mode network (DMN) consists of areas that consistently show deactivations (relative to rest) during a wide range of attention-demanding tasks (McKiernan et al., 2003; Raichle et al., 2001). This network, which includes the medial prefrontal cortex, posterior cingulate/precuneus, and angular gyrus, is of particular interest because it is believed to reflect areas that are more active during rest. Since this network is characterized by decreases in activity during many cognitively demanding tasks, it is also referred to as the Task-Negative Network (TNN). In contrast, the attention network consisting of the pre-supplementary motor area, intraparietal sulcus, and superior precentral sulcus is sometimes referred to ask the Task-Positive Network (TPN) (Fox et al., 2005; Kennedy et al., 2008; Raichle et al., 2001).
Changes in functional connectivity have been investigated in numerous psychiatric and neurological disorders, including Alzheimer’s disease (Li et al., 2002; Wang et al., 2006), multiple sclerosis (Cader et al., 2006; Lowe et al., 2002), epilepsy (Waites et al., 2006; Whalley et al., 2005), schizophrenia (Bluhm et al., 2007; Garrity et al., 2007; Lawrie et al., 2002; Liang et al., 2006; Zhou et al., 2007), attention deficit hyperactivity disorder (Tian et al., 2006), depression (Anand et al., 2005) and autism spectrum disorders (ASD) (Cherkassky et al., 2006; Just et al., 2004; Kennedy et al., 2008). For example, some studies have observed an increased functional connectivity in the default mode network in schizophrenia (Zhou et al., 2007), while others have observed a widespread (Liang et al., 2006) or frontotemporal (Lawrie et al., 2002) decrease in connectivity in this disorder. Likewise, a general theory of “underconnectivity” in autism has become prevalent in the literature (Brock et al., 2002; Just et al., 2007). It offers a potential explanation for many ASD characteristics and particularly afflicted individuals’ inability to integrate information. Though underconnectivity in autism is not ubiquitous, thalamo-cortical connectivity having been shown to be greater in ASD (Mizuno et al., 2006; Turner, 1999), it has been observed during sentence comprehension, verbal working memory, semantic judgments of sentences, executive processing on the tower of London task, visuomotor coordination, emotion perception and other executive function tasks (Just et al., 2007; Just et al., 2004; Kana et al., 2006; Kleinhans et al., 2005; Koshino et al., 2005; Villalobos et al., 2005; Welchew et al., 2005). Decreased connectivity in ASD has also been found using data from subjects during a resting state (Cherkassky et al., 2006; Kennedy et al., 2008).
The difficulty with interpreting the results of fMRI studies investigating functional connectivity is that the measures of connectivity are computed in a number of different ways and under a variety of conditions (also, see (Horwitz, 2003)). The term “functional connectivity” has most generally been defined as the “temporal correlation between remote neurophysiological events” (Friston et al., 1993). This definition, however, has been applied to both resting and task data. Functional connectivity has been computed from data acquired during task performance to determine the synchrony of brain networks while engaged in a task (Bokde et al., 2001; Buchel et al., 1997; Bullmore et al., 2000; Hampson et al., 2002). For example, in one of the early investigations of functional connectivity in ASD, Just et al., 2004 focused on the correlation of task effects by only using the task blocks in their analysis. Conversely, Cherkassky, et al., 2006 looked at functional connectivity in ASD using only the rest blocks of datasets from six different block-design experiments (including that from Just et al, 2004 aforementioned). More recently, Kennedy et al, 2008, investigated the functional connectivity in ASD using continuous resting-state data. The analysis of functional connectivity is particularly well suited to resting data, where an expected task response is not known (Biswal et al., 1995; Cordes et al., 2000; Lowe et al., 1998). In addition, resting-state designs are attractive for patient studies since they require no task compliance and hence minimal effort by the subject. Another alternative measure of connectivity that has been proposed is to compute the correlation between residual fluctuations in task-activation datasets after task effects have been regressed out. This was done by Villalobos et al. 2005, where a box-car nuisance regressor was applied to a dataset with mixed simple (index-only) and complex (pressing fingers in a six-digit sequence) finger tapping tasks. The resulting residual time series may reflect the variability between the two tasks, the trial-to-trial variability within each task, spontaneous neuronal fluctuations, and other sources of noise, making the measured functional connectivity more, but likely not entirely, driven by task-unrelated fluctuations. In another task regression technique, Fair et al., 2007, removed the task response from an event-related design using a deconvolution approach. This technique should model the task-response more effectively than a box-car regression, but trial-to-trial task variability would still be present in the data.
Because of the variety of techniques used for measuring functional connectivity, it is often difficult to draw direct comparisons between studies. Which fluctuations in the time series are driving the measure of connectivity? What is the source of the time series fluctuations that result in areas being functionally “connected?” Perhaps more importantly, when a change in functional connectivity is observed in a particular disorder, what exactly is it that changes? For example, a decreased connectivity (or, more precisely, a decrease in the temporal correlation between two or more regions) in a patient group could be due either to increased noise or to decreased “signal” (correlated fluctuations) in that population. Differences in connectivity observed during a task can also be influenced by variations in the task performance, a particular concern for studies involving patients that may have an impaired ability to perform the task. In this case, connectivity (correlation) differences may simply reflect task induced activation differences.
In the present study, we investigate the difference in functional connectivity between adolescents with high-functioning ASD and typically developing (TD) control subjects during an overt verbal fluency task designed to investigate deficits in language and executive function. Such deficits are pervasive characteristics of autism (Howlin, 2003; Kenworthy et al., 2005; Lord et al., 1997; Muller et al., 1998; Tager-Flusberg, 2003; Tager-Flusberg, 2004). The differences in activation observed between ASD and TD subjects performing this task, and the resulting neuroscientific and clinical interpretation, are the focus of a separate study. The focus of this study is to delve more deeply into measures of functional connectivity, particularly from a methodological point of view. We compare functional connectivity measures obtained during task modulation, and those obtained from residual fluctuations occurring on top of the task. The primary goals of this study are: 1) to determine whether differences in functional connectivity in ASD subjects compared to typical controls can be seen in residual fluctuations on top of task modulations; and 2) to determine the sources of these changes in functional connectivity. We investigate the connectivity both between areas active in the fluency task, as well as between regions within the task negative (TNN) and task positive (TPN) networks, which have been implicated in previous studies of functional connectivity in autism (Cherkassky et al., 2006; Kennedy et al., 2008). Based on previous studies, our hypotheses are that there are task-unrelated fluctuations, occurring on top of the task-induced signal changes, which are correlated between functionally related areas – such as the areas activated during the verbal fluency task and the areas of the task-negative (“default mode”) network. Furthermore, based on prior studies of ASD during a resting state and the deficits in the performance of executive function tasks typically observed in ASD (Hill, 2004; Kenworthy et al., 2008; Pennington et al., 1996; Sergeant et al., 2002; Turner, 1999), we predict that the correlation of these fluctuations (i.e., the “functional connectivity”) is lower in ASD subjects, and that this difference in connectivity is driven in large part by differences in neuronal activity unrelated to the verbal fluency task performed by the subjects. Although this analysis is applied to a particular disorder, ASD, our techniques to identify the sources of connectivity differences and the insights gained from this investigation should extend more broadly to other studies of functional connectivity.
Methods
General Scanning Information
We scanned a total of 23 adolescent high-functioning ASD male subjects and 20 typically developing (TD) male subjects on a 3T GE Signa MRI scanner (Waukesha, WI). All ASD subjects met DSM-IV criteria for ASD in the judgment of a clinician orteam of clinicians experienced with the assessment and diagnosis of individuals with ASD (American Psychiatric Association, 1994). In addition, all ASD participants also met criteria for an ASD on the Autism Diagnostic Interview - Revised (ADI-R, (Lord et al., 1994)) and/or the Autism Diagnostic Observation Schedule (ADOS, (Lord et al., 2000)) according to criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (CPEA; see Lainhart et al., 2006). Because the ADI and ADOS do not have an algorithm for Asperger syndrome, Lainhart and colleagues developed criteria that include an individual in the broad autism spectrum if they: meet the ADI cut off for autism in the social domain and at least one other domain or meet the ADOS cutoff for the combined social and communication score. These criteria are relatively inclusive, but appropriate for capturing the full autism spectrum as long as they are used in conjunction with clinical assessment. TD participants were recruited from the community, and parents of all TD participants underwent telephone screenings. TD participants were excluded from participation if they had been given a psychiatric diagnosis, ever received mental health treatment for anxiety, depression, or any other psychiatric condition, taken psychiatric medications, required special services in school, or had trauma/injury that could potentially affect cognitive functioning and/or brain development. All participants in both groups included for analysis had Full Scale IQs (FSIQ) ≥ 85, as measured by the Wechsler Abbreviated Scale of Intelligence (ASD: n=12, TD: n=18), Wechsler Adult Intelligence Scale-III (ASD: n=2), Wechsler Intelligence Scale for Children-III (ASD: n=1), or Wechsler Intelligence Scale for Children-IV (ASD: n=1). Participants were group-matched on FSIQ. Seven ASD subjects were excluded from the analysis: one because of a scanner malfunction, another because of an uncorrectable susceptibility artifact (braces), another because they made no behavioral responses in the majority of the runs, another because they had an IQ below 80, and three because of excessive head motion (motion exclusion criterion detailed below). No TD subjects were excluded. The data for included subjects were: 17 ASD, age: 16.1 ±2.6 yrs, IQ: 117.5 ±16.4; and 20 TD, age: 17.1 ±2.1 yrs, IQ: 114.0 ±9.0. There was no significant difference between the groups in age or IQ (age: p=0.22; IQ: p=0.42).
Subjects were scanned using a quadrature birdcage RF head coil (GE Medical, Waukesha, WI), with TR/TE=2000ms/30ms, resolution: 3.8×3.8×5mm3, 115 time points per run, and eight runs per imaging session. The subject’s head was immobilized using a vacuum pillow (S&S Par Scientific, Houston, TX, USA). During the imaging runs, subjects performed a self-paced overt verbal fluency task. The task was performed in a blocked design, with 10s periods of task performance alternated with 10s periods of rest (subjects instructed to stare at a central fixation cross). Using this design, blood oxygenation level dependent (BOLD) signal changes are delayed by a quarter cycle relative to the motion-induced signal changes, which occur in synchrony with the task. As a result, the correlation between BOLD and motion-induced signal changes is small, and the number of false positives resulting from speech-related motion artifacts (when performing a standard regression analysis) is minimized (Birn et al., 2004). In each 10s block, subjects were presented with one of five possible task cues: a single letter (1L), a single semantic category (1C), two letters (2L), two categories (2C), or a control condition (M). Written cue letters were presented in the center of the screen and remained visible for the duration of the 10 s block. In the control condition, subjects were presented with an over-learned category – the word “months” appeared - and subjects named the months of the year in chronological order starting from January. When presented with a single letter or category, subjects were asked to generate as many words as they could think of starting with that letter, or that were members of the category, until the fixation cross appeared. When presented with two letters, or two categories, subjects were required to generate one word corresponding to one of the letters or categories, then switch to the other letter or category, and continue to alternate between the two cues. Each condition was presented twice, in random order, in each of 8 runs for a total of 16 unique blocks for each of the letter and category conditions per run. The subject’s spoken responses were recorded using an optical microphone with active noise cancellation (Phone-Or, Inc., Israel). This microphone and the associated processing software allowed the subject’s response to be separated from the scanner sounds.
Functional Connectivity ROI Definition
Seed ROIs for the connectivity analysis were taken from activation maps generated by a multiple linear regression analysis of the fluency task. All functional image analysis was done using the software package AFNI (Cox, 1996). Every run was motion corrected (3dvolreg) – all images being registered to the first volume. Every slice was time shift corrected (3dTshift) to temporally align all slices with the first slice in the acquisition. The images were spatially smoothed (3dmerge) by a Gaussian kernel with FWHM = 5mm. Every voxel was converted to percent signal change (3dcalc) by normalization to its mean over the run. The first five data-points from every run were excluded for transient T1 effects and all eight runs were concatenated for every subject to ease further analysis. BOLD response amplitudes for each of the five conditions were obtained by general linear model on the concatenated subject datasets. BOLD signal changes were modeled using the stimulus timing convolved with a gamma-variate (Cohen, 1997). These beta weights were then converted to Talairach space and submitted to a group ANOVA (pooled across both groups, ASD and TD). We pooled the two groups (ASD and TD) in the ANOVA to eliminate biases toward one subject group in the ROI definitions. Regions with a significant difference in the response to category (C) vs. letter (L) fluency, and regions with a significant difference between the more demanding fluency tasks (1C, 2C, 1L, 2L) compared to the control task (M), were used for the functional connectivity analysis (see Fig 1, Table 2). Seventeen anatomically distinct ROIs were drawn from these contrasts and are tabulated in Table 2. In addition, six spherical ROIs of diameter 12 mm in the Task Negative Network (TNN) and Task Positive Network (TPN) were included in the analysis for comparison to previous studies of default mode network activity in ASD (Kennedy et al., 2008). Three were in the TNN and three were in the TPN, and they were centered on the same locations used previously by (Kennedy et al., 2008) and (Fox et al., 2005) (Table 2). All 23 ROIs were then converted back to every individual subject’s native space, creating 23 distinct ROIs for every subject.
Figure 1.

Activation maps pooled across all subjects (ASD and TD) used to generate ROIs for the connectivity analysis. The upper contrast is for Letter (L) vs. Category (C) at a threshold of p < 1*10-4. The lower contrast is for Control (M) vs. Other Tasks (C,L) at a threshold of p < 1*10-8. Left is Left.
Table 2.
These ROIs were used for the connectivity analysis.
| ROI | CODE | CONTRAST | THRESH | COORDS (x,y,z) |
|---|---|---|---|---|
| Left Fusiform | LFUS1 | C>L | p < 10−8 | (−22,−40,−13) |
| Left Fusiform | LFUS2 | L > C | p < 10−8 | (−44,−65,−5) |
| Right Fusiform | RFUS | L > C | p < 10−8 | (43,56, −10) |
| Left Inferior Frontal Gyrus | LIFG1 | L > C | p < 10−8 | (−45,24,13) |
| Left Inferior Frontal Gyrus | LIFG2 | L > C | p < 10−8 | (−45,3,23) |
| Left Inferior Frontal Gyrus | LIFG3 | L>C, ASD>TD | p < 10−2 | (−40,20,14) |
| Right Inferior Frontal Gyrus | RIFG1 | L > C | p < 10−4 | (45,−24,12) |
| Right Inferior Frontal Gyrus | RIFG2 | L > C | p < 10−4 | (43,3,26) |
| Left Posterior Cingulate | RPC | C > L | p < 10−8 | (−7,−57,11) |
| Right Posterior Cingulate | RPC | C > L | p < 10−8 | (7,57,13) |
| Right Lingual Gyrus | RLG | C > L | p < 10−8 | (14, −81,−7) |
| Left Inferior Parietal Lobule | LIPL | L > C | p < 10−8 | (−37,−48,−44) |
| Right Inferior Parietal Lobule | RIPL | L > C | p < 10−8 | (34,−50,38) |
| Left Precentral Gyrus | LPCG | M > (C,L) | p < 10−8 | (−48,−16,34) |
| Right Precentral Gyrus | RPCG | M > (C,L) | p < 10−8 | (49,−12,7) |
| Left Superior Temporal Gyrus | LSTG | M > (C,L) | p < 10−8 | (−50,−12,33) |
| Right Superior Temporal Gyrus | RSTG | M > (C,L) | p < 10−8 | (53, −18,4) |
| TNN Left Angular Gyrus | TNN_LAG | N/A | N/A | (−45, −67,36) |
| TNN Medial Prefontal Cortex | TNN_MPFC | N/A | N/A | (−1,47, −4) |
| TNN Posterior Cing/Precuneus | TNN_PPC | N/A | N/A | (−5,−49,40) |
| TPN Left Intraparietal Sulcus | TPN_LIPS | N/A | N/A | (−25,−57,46) |
| TPN Left Medial Temporal Region | TPN_LMTR | N/A | N/A | (−45, −69, −2) |
| TPN Right Sup. Precentral Sulc. | TPN_RSPS | N/A | N/A | (25,−13,50) |
Center of mass coordinates are in Tailarach space. C = Category, L = Letter, M = Months.
Functional Connectivity Analyses
Pre-processing steps for the functional connectivity analysis were similar to that used for the task activation mapping. The primary differences were that in the functional connectivity analysis, images were not smoothed, and time series were low pass filtered with a cutoff of 0.1Hz. Time points with excessive head motion were censored from further analysis. The method implemented was very similar to that proposed by Kennedy et al., 2008. The six motion parameter time courses created by 3dvolreg were first individually concatenated for all eight runs. The square root of the sum of squares of the derivatives (SSD, eq. 1) of these six time courses was calculated for every subject:
| (1) |
Where x, y, and z are the translations (in mm), and roll, pitch, and yaw are the rotations (in degrees). Any point with an SSD greater than 1 (and its two immediately neighboring points) was ignored. In the case of pure translation (i.e. no rotation), an SSD threshold of 1 could be thought of as a translation of 1mm in any one translational direction, or a combination of translations of 0.577mm in all three translational directions, in the time of one TR. This is a conservative illustration because any rotational components would decrease the maximum contribution of any other one component and because neighboring data points were also ignored. In addition, a rotation of 1 degree, measured by the registration program around the center of the image, would cause a voxel shift of 1.5 mm at the edge of the brain (about 84mm from the center of the image), but less shift of voxels inside the brain closer to the center. Any subject with greater than 25% of its data points being ignored was excluded from the analysis.
Functional connectivity was computed using time courses (either with or without regressing out the task effects, as detailed below) averaged over the ROIs. Two different methods of computing functional connectivity were implemented. The first consisted of correlating all 17 task-defined ROIs with each other, producing a correlation matrix with dimensions 17×17 for every subject. These correlation matrices were converted to Fisher-Z scores and averaged across the two groups (ASD and TD). A t-test was then performed on each element of the correlation matrix, showing differences in functional connections between the groups. These computations were performed in Microsoft Excel (Redmond, WA), and plotted using Mathcad (Parametric Technology Corp., Needham, MA). The second type of connectivity involved correlating the average time courses from the 17 task-defined ROIs and 6 TNN and TPN ROIs with the entire brain, creating connectivity maps. These maps were then converted to Fisher-Z scores and averaged within groups. A t-test was performed on each voxel in these maps to depict areas where the groups showed differences in connectivity, unbiased by multiple ROI definitions.
In previous investigations of ASD, the functional connectivity between brain regions has been computed during the resting state (Kennedy et al., 2008), during rest blocks (Cherkassky et al., 2006) or during task blocks (Just et al., 2004). In order to investigate the effect of task-related responses on the measure of connectivity in our study, three different methods of task regression were done prior to averaging the time courses over the ROIs, and prior to computing the correlation between these ROI-average time series. The first measure of “connectivity” used the raw preprocessed time courses with task responses included (Method 1. Abbr. M1). This method is similar to a multiple regression analysis of the task activation because the signal contains the response of each task vs. the fixation baseline. The second measure of connectivity used the residual fluctuations after removing the task response, but where all 5 task conditions were considered to have the same amplitude and shape (Method 2. Abbr. M2). This was done by performing a deconvolution analysis that models the average response to a task block for each voxel. The resulting residual fluctuations, after the average task block response is regressed out, have had the task vs. fixation differences removed, but the variability between the different task types preserved. Computing the functional connectivity with this pre-processing method is similar to computing the functional connectivity from a design that includes continuous task switching without rest intervals, or a design that preserves the task activation differences to different task types, such as that implemented by Just et al., 2004. The third measure of connectivity used the residual fluctuations after removing the task response deconvolved over every individual task condition (Method 3. Abbr. M3). Again, this task regression does not assume a fixed shape of the hemodynamic response, but instead removes the mean response averaged over all similar task blocks. The resulting residual time course is similar to resting-state data, but will contain the trial-to-trial variability in the task-related response. The task-related responses in M2 and M3 were removed together with other nuisance regressors (e.g. the subject motion and its derivatives). This regression was performed prior to computing the correlation between ROI time series. This is mathematically identical to including the nuisance regression together with the seed-ROI time series in a single regression step, since the nuisance regressors are removed from all ROI time series, including the seed (the regressor of interest in the regression analysis).
Analysis of Potential Sources for Connectivity Differences
Several additional analyses were performed to investigate other aspects of the signal, the contributions to the measure functional connectivity, and the causes for differences in functional connectivity between groups. One possible contribution to functional connectivity is a block-to-block variability in the subject behavior. To test whether behavioral variability contributed to the residual fluctuations, particularly in M3 (which excluded explicit task modulations), we created behavioral regressors to correlate with the ROI time courses. These behavioral regressors were created by convolving the typical gamma-variate hemodynamic response function (HRF) with the number of words produced in each task block. To make regressors analogous to M3, we removed the average number of words produced in each task from those task blocks prior to convolving with the gamma-variate. We then correlated these regressors with the ROI time courses to determine which correlations might be driven by behavioral output. We also performed a t-test between groups to investigate if differences between ASD and TD could be explained by this factor.
We also wanted to investigate whether a difference in functional connectivity between subject groups was simply a function of differences in noise characteristics between ASD and TD. It is conceivable that reduced functional connectivity in ASD is simply due to increased noise. To test this, we took the standard deviations of the individual ROI time courses and submitted them to t-tests between groups.
Significant differences in functional connectivity between ASD and control subjects have been found using data taken exclusively from task blocks (Just et al., 2004), or exclusively from rest blocks (Cherkassky et al., 2006). To evaluate the relative contributions of rest and task periods to functional connectivity, we recomputed the connectivity measure after ignoring, or censoring, several blocks of the time course data. A censor file was first created that removed all of the task blocks from the functional connectivity calculation (i.e. computing the correlation using only the rest blocks). This case is similar to that used by Cherkassky et al., 2006. We then repeated the functional connectivity calculation multiple times, each time after shifting the censor file by a TR increment, until the rest blocks were censored (i.e. computing the correlation using only the time points during the task blocks). The latter case is similar to that performed by Just et al., 2004. By performing this recursive censoring, we can determine how the correlations vary as a function of which parts of the signal we censor.
Several previous studies of functional connectivity regress out global signal fluctuations (i.e. the average signal over the entire brain at each point in time) prior to correlation analysis in order to reduce the influence of global fluctuations in blood flow and oxygenation. Such a preprocessing technique, however, can be problematic since it changes the distribution of correlation values across the brain and could induce false anti-correlations between brain regions (Murphy et al., 2008). We therefore did not include this as a standard preprocessing step in our analyses described above. Instead, in order to evaluate the effect of global signal regression on both the correlation values and the difference in correlation observed between subject groups, we performed an additional functional connectivity analysis, similar to method 3, but adding global signal regression as an additional preprocessing step.
Results
Activation Results and ROI Definitions
The general activation results used to define the ROIs can be seen in Figure 1. The selectivity (C, L or M) of the active areas are tabulated in Table 2 and can be visualized in Figure 1. The ROIs for the connectivity analysis were drawn according to the spatial extent of activation seen in Figure 1.
Connectivity Results
Figure 2 shows the effect of regressing out the task from M1 to M2 and M3. The full BOLD responses to the tasks can be seen in M1, while the residual task-to-task variability in the BOLD responses can be seen in M2 (Fig. 2). As expected, there is no average BOLD response to any task remaining in M3 (Fig. 2). Note that a large amount of variance remains in signal both after regressing out the response common to all tasks (M2) in addition to the response specific to every task (M3) (Fig. 2).
Figure 2.

Time series (right) for the three task regression methods and associated average time series (left). Time series taken from the LIFG ROI seed for TD subject number 18.
The correlation between the fluctuations in the various regions interest (i.e. the “connectivity”) was highly significant for both TD and ASD groups for most of the ROI comparisons in M1, M2, and M3. The connectivity was generally reduced in ASD compared to TD subjects for all three methods of task regression (Figs. 3, 4, and 5). Twenty-two of the 136 ROI comparisons showed a lower connectivity in ASD compared to TD for M1 (p < 0.05, uncorrected) (Fig. 3). Of these 22, seven were still significant at p<0.05 (uncorrected) after regression of the common task response in M2 (i.e. after removing signal modulations of the task vs. fixation baseline) (Fig. 4). Similarly, the difference between correlations in ASDs and TDs remained significant for seven ROI comparisons in M3 (after regressing out all task effects), five of which were the same as M2 (Figs. 4 and 5).
Figure 3.
ROI connectivity matrix with raw preprocessed subject time courses (M1). Correlation coefficients were converted to fisher-z scores, averaged across groups (ASD and TD), and then reconverted back to correlation coefficients. Within each cell, TD values are on top, ASD on bottom. Highlighted cells represent significant differences between groups (all ASD < TD, two-tailed t-test, p < 0.05 uncorrected).
Figure 4.
ROI connectivity matrix after removal of the common task response (M2). Correlation coefficients were converted to fisher-z scores, averaged across groups (ASD and TD), and then reconverted back to correlation coefficients. Within each cell, TD values are on top, ASD on bottom. Highlighted cells represent significant differences between groups (all ASD < TD, two-tailed t-test, p < 0.05 uncorrected).
Figure 5.
ROI connectivity matrix after removal of the individual task responses (M3). Correlation coefficients were converted to fisher-z scores, averaged across groups (ASD and TD), and then reconverted back to correlation coefficients. Within each cell, TD values are on top, ASD on bottom. Highlighted cells represent significant differences between groups (all ASD < TD, two-tailed t-test, p < 0.05 uncorrected).
The TNN is clearly visible in both the TD and ASD groups (Fig. 7). In the TD group, the highest correlations exist in typical default mode areas: dorsal and ventral medial prefrontal cortex, the posterior cingulate/precuneus and left and right angular gyrus. Though to a lesser extent, the same areas are shown in the ASD group (Fig. 7). These connectivity maps show striking similarity to the TNN maps generated by Kennedy et al., 2008 in resting-state TD and ASD data as well as those generated by Fox et al., 2005, from which the ROIs were defined. Significant differences in the connectivity to the left angular gyrus (i.e. where the left angular gyrus (TNN_LAG) is the seed ROI) were observed in the medial prefrontal cortex, right angular gyrus, and right cerebellum (p<0.01, corrected for multiple comparisons; indicated by green arrows in Fig. 7). In addition, a significant difference in the connectivity between the posterior cingulate and the medial prefrontal gyrus was observed, using a seed time series in the posterior cingulate/precuneus. However, these differences were not significant after global signal regression (using the same correlation coefficient threshold).
Figure 7.

Images of the Task Negative Network (TNN) derived from the correlation with a time series from the left angular gyrus (TNN_LAG). The three left images are without global signal regression (GR), whereas the right three images are with GR. The average ASD maps are on top, TD in the middle and difference on the bottom. Unthresholded images are included to provide a representation of the functional connectivity measure unbiased by the choice of a particular threshold. Significant differences in the correlation with the left angular gyrus seed time series (indicated by the green arrows) were observed in the right angular gyrus and the right cerebellum, at a p<0.05 (corrected for multiple comparisons). These differences, however, did not pass the same significance (t-statistic) threshold after global signal regression.
Additional Analysis
The correlations between the behavioral regressors and the residuals from M3 were quite low, < 0.12 (Fig. 8). However, the highest correlations were observed in the pre central gyrus (RPCG and LPCG) and superior temporal gyrus (RSTG and LSTG), bilaterally (Fig. 8), areas known to be involved in motor control (motor cortex) and speech production (Wernicke’s Area), respectively. These areas also showed a greater response to the “months” control condition, during which subjects produced almost twice as many words as compared to the other more effortful category and letter fluency conditions (Fig. 1).
Figure 8.
Correlations between the subject time courses and the behavioral regressors averaged over ASD and TD groups. The behavioral regressors were created by convolving the gamma variate with the number of words produced in each block. Error bars represent standard deviation. No significant differences between ASD and TD were found (two-tailed t-test, p < 0.05).
The average standard deviations of the ROI time courses from Method 3 are depicted in Figure 9 and show that TD subjects tend to have a slightly higher variance than ASD subjects. However, of the 23 ROIs, only two showed a significant difference (p < 0.05), TD showing greater variance than ASD in the right posterior cingulate (RPC) and the medial prefrontal cortex of the TNN (TNN_MPFC) (Fig. 9). A measure of standard deviation does make the assumption that the noise is normally distributed, which is not strictly true in fMRI data. The difference in variance of the time series was therefore additionally assessed using a Levene’s test (Levene, 1960; Neter et al., 1996), computed by concatenating the ROI time series for each of the subject groups, and comparing the fluctuations in the two concatenated datasets. Similarly, only two ROIs showed significant differences in this measure, the LIPL and the TPN_LIPS.
Figure 9.
Temporal Standard deviations of the subject time courses (after regressing out average task responses, M3) averaged over the ASD and TD groups. The behavioral regressors were created by convolving the gamma variate with the number of words produced in each block. Error bars represent standard deviation (of the temporal standard deviation). Significant differences were found in the RPC and TNN_MPFC, TD greater than ASD (two-tailed t-test, p < 0.05).
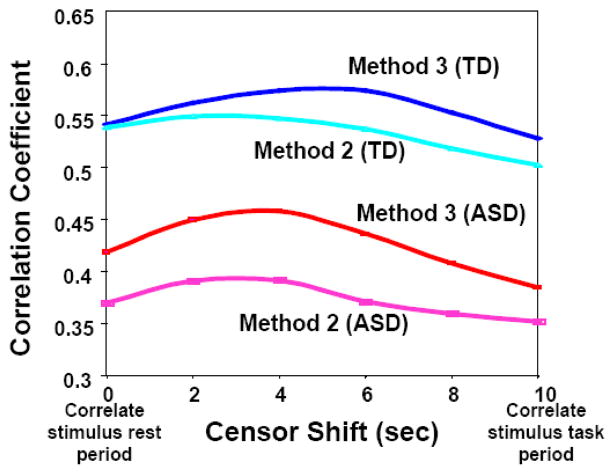
Figure 10 indicates that the correlation reached a maximum at a censor lag of approximately three to five seconds. Due to the hemodynamic lag of the BOLD contrast, this indicates that the peak correlation occurs during the hemodynamic “rest period” between task blocks. This is consistent across ROIs, and we only present the LFUS1-LIFG1 comparison as an example.
Figure 10.
Correlations between the LIFG1 and LFUS1 averaged across the ASD and TD groups after censoring the subject time courses at various lags. Correlations were first made only on the rest periods and then recalculated after TR shifts of the censor file until only the task periods were included in the correlation.
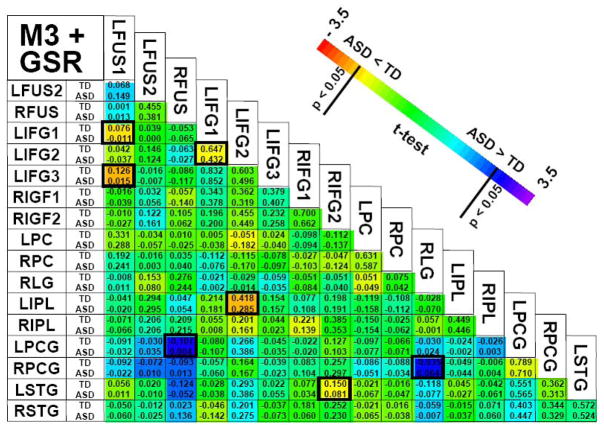
Global signal regression substantially changes both the connectivity maps as well as the statistical difference maps (Fig. 7). The same areas retain the highest correlations both before and after global signal regression, however, outside these areas, the correlation coefficients have been dramatically reduced (Fig. 7). Furthermore, noting the blue areas in Figure 7, weak negative correlations have actually been introduced in some areas, a phenomenon of particular concern (Murphy et al., 2008). Additionally, only the LIFG1-LFUS1, LIFG1-LFUS3 and LIFG1-LIPL correlations show a similar difference in functional connectivity as obtained from M3, with a significantly lower (p < 0.05, uncorrected) connectivity in ASD (Fig. 6). Two other ROI correlations showed significantly lower connectivity in ASD that were not evident in M3 (LIFG1-LIFG2 and RIFG2-LSTG) while two more ROI correlations actually became greater in ASD (RFUS-LPCG and RLG-RPCG) (Fig. 6). In other words, the group effect on connectivity is dramatically changed when global signal regression is used as a physiological correction.
Figure 6.
ROI connectivity matrix after removal of the individual task responses (M3) and after global signal regression (GSR). Correlation coefficients were converted to fisher-z scores, averaged across groups (ASD and TD), and then reconverted back to correlation coefficients. Within each cell, TD values are on top, ASD on bottom. Highlighted cells represent significant differences between groups (two-tailed t-test, p < 0.05 uncorrected).
The correlation between ROIs generally increased after regressing out task differences (M3 compared to M2) (Figs. 4, 5 and 10). Of the 136 ROI comparisons, 74 correlations were significantly greater for M3 compared to M2 in TD subjects, and 35 were significantly greater for M3 compared to M2 in ASD subjects (p < 0.05, uncorrected). Conversely, in both ASD and TD subjects, six ROI pairs had a significantly greater correlation when task difference effects were present (M2) compared to when they were regressed out (M3) (p < 0.05, uncorrected). This occurred between the right and left precentral gyrii (RPCG and LPCG) and the right and left superior temporal gyrii (RSTG and LSTG), the same areas that were preferentially active for months and that showed the greatest effect of behavioral variability (Fig. 1, Fig. 8 and Table 2). The majority of all other ROI comparisons showed a trend of a greater correlation in M3 compared to M2, but did not reach significance.
Discussion
In this study, functional connectivity was computed in a number of different ways. Computing the functional connectivity (correlation) from the signal intensity time course without removing any task-related effects (M1) shows areas with a similar response to the induced task compared to baseline. Differences in correlation of ROI time courses for TD compared to ASD subjects, in this case, could simply reflect the differences in the task-related response. This is similar to a more conventional regression analysis, and whether this should really be called a measure of “connectivity” is debatable. In Method 2, the relatively large signal changes of the task relative to fixation have been removed, but the difference in response between different task types remains. The correlation of time series from this method are perhaps more interesting than those from Method 1, in that they reflect the similarity of more subtle task modulation effects (e.g. Category vs Letter fluency) as well as other residual fluctuations, instead of primarily the similarity of the response to task vs fixation. However, it is unclear using only Method 2 whether the correlations are driven by the task modulations, or other residual fluctuations. In Method 3, the average task-related response for each task type has been removed. Regions that are significantly correlated in this method therefore reflect regions with similar residual fluctuations occurring on top of the task, and not including any similar average responses to the task or task modulation. What is particularly interesting is that the correlation of the fluctuations between most of the ROIs increases when task modulations are removed (going from M2 to M3). This suggests that the correlation of time courses in M2 is driven by fluctuations occurring on top of the average task-related responses, rather than the task-induced modulation. Such fluctuations may be similar to what is observed during the resting state, where no explicit task, or task-modulation, is performed.
Highly significant correlations of the residual fluctuations occurring on top of task-related responses were found between a number of functionally related areas. These correlated networks were similar to many of the “functional connectivity” networks observed during rest (e.g. the motor network, or the default mode network as shown in Figure 7). In general, these networks were highly similar in adolescents with high functioning autism compared to typically developing control subjects. However, a difference in the correlation of residual fluctuations (M3), occurring on top of task-induced responses, was observed between specific brain regions, consistent with other reports of decreased connectivity in ASD (Belmonte et al., 2004; Brock et al., 2002; Cherkassky et al., 2006; Just et al., 2007; Kennedy et al., 2008). While many of these differences were only significant at an uncorrected p<0.05, and should therefore be regarded as preliminary, it is important to note that the average correlation for ASD subjects was always lower than the average correlation for TD subjects, for each one of these ROI pairs. If the difference in connectivity (correlation) for ASD compared to TD subjects were due purely to chance, then some of these differences would likely be positive, while others would be negative. In particular, we found lower correlations (a decreased “connectivity”) in ASD subjects, primarily between frontal and posterior cortices. This included a reduced connectivity between the left inferior frontal gyrus and left fusiform gyrus, the right inferior frontal gyrus and right fusiform, the left inferior frontal gyrus and the left superior parietal lobule, and the right inferior frontal gyrus and right inferior parietal lobule (Fig. 5). A decreased connectivity in ASD subjects was also observed between the left and right fusiform gyrii, as well as the left and right precentral gyrii. A difference in connectivity of the task negative network (TNN) was observed, similar to the results by (Kennedy et al., 2008), with decreased connectivity in ASD subjects particularly between the medial prefrontal cortex and the left angular gyrus and posterior cingulate (p<0.05, corrected for multiple comparisons).
It is important to note that these residual fluctuations reflect the deviation from the average response to each task. Thus, even though the ROIs were defined by the task, the correlation between these residuals reflects novel information not captured in the activation mapping linear regression analysis. That is, the correlation between two regions cannot simply be the result of a similarity in the average task-related response. Furthermore, the decreased correlations of residual fluctuations in ASD are likely not due to block-to-block variations in the behavior (the number of words produced in each block), the correlation values between the behavioral regressors and the residuals being quite low (Fig. 8). Likewise, the differences in correlation between ASD and TD are likely not due to differences in noise, since the standard deviations over time in the ROIs showing a difference in connectivity are similar between the groups (Fig. 9). In fact, there appears to be a trend towards TDs having a higher temporal standard deviation than ASDs. The difference in connectivity is therefore more likely due to decreased signal in the ASD patients rather than an increased level of noise. Also, the correlation between specific brain areas was higher after removal of all task related effects; that is, the correlations go up from M2 to M3 (Figs. 4 and 5). This again suggests that task-related effects are likely not driving the correlation, or the difference in correlation, between specific brain regions such as the fusiform and the inferior frontal gyrus. Consistent with this observation, the correlations are highest when the BOLD responses to the task blocks are ignored, the censor file being shifted by the hemodynamic delay (Fig. 10). These observations support the hypothesis that correlated fluctuations, and the differences in these fluctuations between ASD and TD subjects, are driven by task-unrelated neuronal fluctuations.
Some areas, notably bilateral precentral gyrus and superior temporal gyrus, did show a slight correlation with block-to-block variability in behavior (Fig 8). These areas are hypothesized to be active in word production. It therefore makes sense that their activity would be modulated by behavioral output to a higher degree than other areas. The fact that the correlation with the behavioral regressor is not very high (correlation coefficient ~ 0.1), however, suggests that this behavioral variability only accounts for a small portion of the residual variability. It is also interesting to note that in almost all of the regions of interest, the correlation between the residual signal time course and the behavior is slightly higher in ASDs. While this is not a significant difference, the trend is in the opposite direction to what would be expected if residual behavioral differences are driving the connectivity and the differences in connectivity. A higher correlation of residual task-related effects should result in a greater connectivity, whereas a lower connectivity was in fact observed in ASD subjects.
A Previous study by Villalobos et al., 2005 regressed out the task during a boxcar paradigm by shifting a smoothed boxcar regressor. In our study, task-effects were removed by deconvolution. Rather than assuming a fixed shape for the task response, deconvolution removes any response that is time locked with the task performance and consistent across performances of the same task. This procedure is less prone to errors resulting from an inaccurate ideal model of the BOLD response.
Some task-related effects, such as nonlinearities in the BOLD response or block-to-block variability in behavior not captured by the number of words produced, may still remain in the signal even after deconvolution of the task. For this to affect the difference in connectivity observed between ASD and TD subjects would require a different nonlinearity in the BOLD responses between patient and control groups, or a systematic difference in the timing or pattern of responses between ASD and TD subjects.
Physiological noise is, of course, another potential confound in functional connectivity studies (Bhattacharyya et al., 2004; Birn et al., 2006; Cordes et al., 2001; Lowe et al., 1998; Lund, 2001; Wise et al., 2004). The data used in this study were acquired prior to our previously published studies on the effects of physiological noise in functional connectivity analysis (Birn et al., 2006) and before physiological recording equipment was in place on our scanners. As a result, traditional physiological corrections (e.g. RETROICOR, (Glover et al., 2000); RVT-COR, (Birn et al., 2006)), were not able to be performed. The spatial pattern of the connectivity maps in individual subjects, however, did not closely resemble that expected for respiration. Signal changes correlated with breath-to-breath changes in the respiration depth and rate occur throughout gray matter, but are particularly large signal changes in the Circle of Willis, medial visual areas, posterior cingulate, and precuneus (Birn et al., 2006). While the connectivity maps prior to global signal regression do show a certain amount of correlation with the seed throughout gray matter, the highest correlations to each seed ROI (i.e. the “hot spots” on the correlation map) are not in locations that typically show the largest respiration-induced signal changes (e.g. see Fig 7).
We also investigated the effect of global signal regression, a common surrogate for physiological correction. The motivation for this correction step, traditionally, is that correlated fluctuations are expected to occur in smaller localized regions, and that any global variations in signal intensity are uninteresting. The difficulty with this pre-processing step is that it relies on the assumption that global signal fluctuations are non-neuronal (or otherwise uninteresting). In addition, global signal regression can induce anti-correlations between a seed region and other voxels (Fox et al., 2009; Murphy et al., 2009). Erroneous changes to the correlation coefficients are particularly large when the fluctuations of interest are a significant contribution to the global signal. Global signal regression may therefore not be advisable when the functionally correlated network being investigated spans across several brain regions or covers large areas of the cortex. In our analysis, we found that global signal regression does make the observed signal correlations more focal in space, particularly on group maps. Without this preprocessing step, relatively high correlation with each seed ROI was observed throughout gray matter, but with the highest signal changes in more focal regions. These more focal regions largely overlapped with the other ROIs (i.e. areas activated during the fluency task), and likely reflect brain areas that are functionally related, or “connected.” After global signal regression, significant correlations with the seed ROI were more focal. However, it is unclear to what degree the correlation with true neuronal fluctuations are also altered by this preprocessing step. Some of the “hot spots” present prior to global signal regression (i.e. regions with the highest correlation to the seed ROI time course) were no longer significant after global signal regression. In other words, the two connectivity maps (before vs. after global signal regression) could be made similar, but not identical, by changing the correlation coefficient thresholds. It is unclear which of these maps is more correct – whether global signal regression removed an artifactual correlation, a true neuronal correlation, or both. In addition, we found that global signal regression not only changes the correlation values substantially, but also changes the significant differences in the correlation values between ASD and TD subjects (Figs. 5, 6 and 7). Our study cannot definitively show whether true differences between ASD and TD subjects are revealed by removing a global confound, or whether false differences are introduced by this preprocessing step, and therefore both results are presented. Given that the verbal fluency task involves the coordinated activity of a relatively large network of brain areas, the BOLD response from synchronized spontaneous neuronal fluctuations within this network could contribute a significant portion to the global signal changes. Consequently great care should be taken in interpreting results when global signal regression is used.
Functional connectivity in ASD, as well as other disorders, has been measured in many different ways – by looking at either the correlation of signal fluctuations in response to a task (Just et al., 2004); signal fluctuations in the absence of an external stimulus or explicit task, either from a continuous resting run (Kennedy et al., 2008) or by considering only the rest blocks from a block design experiment (Cherkassky et al., 2006); or signal fluctuations on top of task-related responses (Villalobos et al., 2005). What is the source of the fluctuations that are driving the correlations in each of these connectivity measures? To what extent is the measure of functional connectivity influenced by preceding tasks, or by the performance of a concurrent task?
Measures of connectivity obtained from an explicit task modulation are almost certainly influenced by the performance of the task. Previous studies have shown that such task-related functional connectivity measures can be significantly different from measures of connectivity obtained during rest (Calhoun et al., 2008; Harrison et al., 2008). Differences in the connectivity measures for ASD vs TD subjects obtained from Method 1 and Method 2 in this study could therefore be at least partially reflective of differences in the task-induced activation between the two subject groups. However, such observed differences between connectivity measures obtained from task-modulation and those obtained from resting data should not be surprising and do not invalidate either technique. Rather they may point to the different functional roles of parts of a network.
Measures of connectivity can also be affected by prior tasks. Waites et al., 2005, for example, found that resting state connectivity maps following a language task were significantly different from maps obtained from resting runs before these tasks. In contrast, Fair et al., 2007 found only small differences between connectivity measures obtained from resting blocks extracted from a blocked design object recognition task compared to a continuous resting scan. It is possible that the discrepancy between these studies reflects the sensitivity of functional connectivity measures to subtle differences in the cognitive state – which may be altered by some preceding tasks, but not others.
Particularly relevant to the measure of connectivity obtained from Method 3 in this study is the potential difference in the correlation of fluctuations occurring on top of an explicit task compared with fluctuations occurring during rest (in the absence of any task demand). The study by Fair et al., 2007, for example, showed that using the residuals on top of task-related activation can lead to slightly different connectivity maps compared to using either the resting blocks from a blocked design study or a continuous resting-state scan. The study concluded that connectivity measurements obtained from residuals occurring on top of tasks should therefore be interpreted with caution. However, an alternative source of the difference in the connectivity maps obtained during rest vs. from residuals on top of tasks is that during the “resting” state, the subject may be engaged in a series of cognitive “tasks,” such as mind wandering, monitoring of the environment or body state (Mason et al., 2007), or reflecting on prior tasks (Waites et al., 2005). Some of these activities may be reduced during the performance of an explicit task. The correlation of fluctuations on top of task-induced responses may therefore more accurately reflect the spontaneous neuronal fluctuations within the brain’s multiple networks. The investigation of connectivity based on residual fluctuations occurring on top of a task should therefore not be ruled out. Rather, it provides additional and potentially clinically relevant information beyond what is obtained from a more conventional regression analysis of task-related differences. While the present study rules out some potential mechanisms, further investigation is needed to conclusively determine the source of the correlated fluctuations occurring on top of a task.
Conclusions
In this study, we found strong correlations in the residual fluctuations occurring on top of a task in both TD and ASD subjects. The connectivity maps derived from these residuals are highly similar to maps seen in other resting state studies. The high correlation of the residuals is likely driven by task-unrelated fluctuations, since the correlation increases when task effects are regressed out and when task blocks are ignored. Furthermore, we find differences in these correlations between two subject groups – adolescents with ASD compared to typical controls.
Many studies have shown significant differences in functional connectivity between patient and control groups, particularly for autism. For all functional connectivity studies, it is important to remember that the correlation of fluctuations between two or more regions is used as a measure of connectivity. All studies demonstrating changes in connectivity should therefore investigate what aspects of the signal are changing to cause this difference in correlation. In this study we find differences in fluctuations occurring on top of task-induced responses between ASD and TD subjects performing a verbal fluency task. The differences in the correlation of these fluctuations are not due to a difference in overall noise level, are not related to block-to-block variations in one aspect of behavior (the number words produced per block), and are higher both when task effects are regressed out and when task periods are ignored. These findings suggest that the functional connectivity of residuals on top of task responses, and differences in functional connectivity observed in autism, are driven by task-unrelated fluctuations, possibly spontaneous neuronal fluctuations.
Table 1.
Age and Full Scale IQ (FSIQ) for subjects with autism spectrum disorder (ASD) and typically developing (TD) control subject.
| ASD (N=17) | TD (N=20) | |
|---|---|---|
| Age | 16.02 (2.45) | 17.05 (2.10) |
| FSIQ | 117.50 (15.84)^ | 114.00 (9.04)* |
| ADI Social Interaction | 20.94 (4.99)^ (range=8–28) | – |
| ADI verbal Communication | 15.43 (5.00)^ (range=6–26) | – |
| ADI Repetitive Behaviors | 6.69 (2.95)^ (range=3–12) | – |
| ADOS Social + Communication | 11.71 (4.29) (range=5–17) | – |
ASD and TD subjects were matched for age and FSIQ. Scores from Autism Diagnostic Interview (ADI) and Autism Diagnostic Observation Schedule (ADOS) for autistic subjects.
n=16
n=l8
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30 (7):1334–44. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24 (42):9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya PK, Lowe MJ. Cardiac-induced physiologic noise in tissue is a direct observation of cardiac-induced fluctuations. Magn Reson Imaging. 2004;22(1):9–13. doi: 10.1016/j.mri.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–48. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33 (4):1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30(2):609–17. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14 (2):209–24. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7 (8):768–78. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? Neuroimage. 2000;11 (4):289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129(Pt 2):527–37. doi: 10.1093/brain/awh670. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29(7):828–38. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17 (16):1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22 (7):1326–33. [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol. 2000;21 (9):1636–44. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research. 1996;29 (3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29 (4):1359–67. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J Neurophysiol. 2009 doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13 (1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164 (3):450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–62. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yucel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci U S A. 2008;105(28):9781–6. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8 (1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19 (2 Pt 1):466–70. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. J Autism Dev Disord. 2003;33 (1):3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17 (4):951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129 (Pt 9):2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39 (4):1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychol Rev. 2008;18 (4):320–38. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy LE, Black DO, Wallace GL, Ahluvalia T, Wagner AE, Sirian LM. Disorganization: the forgotten executive dysfunction in high-functioning autism (HFA) spectrum disorders. Dev Neuropsychol. 2005;28 (3):809–27. doi: 10.1207/s15326942dn2803_4. [DOI] [PubMed] [Google Scholar]
- Kleinhans N, Akshoomoff N, Delis DC. Executive functions in autism and Asperger’s disorder: flexibility, fluency, and inhibition. Dev Neuropsychol. 2005;27 (3):379–401. doi: 10.1207/s15326942dn2703_5. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24 (3):810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51 (12):1008–11. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Levene H. Robust Tests for Equality of Variances. In: Olkin I, editor. Contributions to Probability and Statistics. Stanford University Press; Palo Alto, California: 1960. pp. 278–292. [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225 (1):253–9. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17 (2):209–13. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Lord C, Paul R. Language and communication in autism. In: Cohen DJ, Volkman FR, editors. Handbook of autism and pervasive developmental disorders. John Wiley; New York: 1997. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30 (3):205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24 (5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7 (2):119–32. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224 (1):184–92. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lund TE. fcMRI--mapping functional connectivity or correlating cardiac-induced noise? Magn Reson Med. 2001;46(3):628–9. doi: 10.1002/mrm.1238. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15 (3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104 (1):160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Muller RA, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, Chugani HT. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neurosci Lett. 1998;245 (1):1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The Impact of Global Signal Regression on Resting State Correlations: Are Anti-Correlated Networks Introduced? NeuroImage. 2008 doi: 10.1016/j.neuroimage.2008.09.036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44 (3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Vol. 4. WCB McGraw-Hill; Boston, MA: 1996. [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37 (1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130 (1–2):3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Language impairments in children with complex neurodevelopmental disorders: The case of autism. In: Levy Y, Schaever JC, editors. Language competence across populations: Toward a definition of specific language impairment. Lawrence Erlbaum Associates; Mahway, NJ: 2003. pp. 297–321. [Google Scholar]
- Tager-Flusberg H. Language and communicative deficits and their effects on learning and behavior. In: Prior M, editor. Asperger syndrome:Behavioral and educational aspects. Guilford Press; New York: 2004. pp. 85–103. [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400 (1–2):39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Turner MA. Generating novel ideas: fluency performance in high-functioning and learning disabled individuals with autism. J Child Psychol Psychiatry. 1999;40 (2):189–201. [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25 (3):916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59 (2):335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24(1):59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31 (2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biol Psychiatry. 2005;57 (9):991–8. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128(Pt 9):2097–108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21 (4):1652–64. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97 (1–3):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]