Abstract
Purpose
The bestrophin family of proteins has been demonstrated to generate or regulate Ca2+-activated Cl− conductances. Mutations in bestrophin-1 (Best1) cause several blinding eye diseases, but little is known about other bestrophin family members. This study involved disruption of the Best2 gene in mice.
Methods
The mouse Best2 gene was disrupted by replacing exons 1, 2, and part of exon 3 with a Lac Z. The expression profile of Bestrophin-2 (Best2) was examined using RT-PCR, X-gal staining, and immunohistochemistry. Intraocular pressure (IOP) was measured by anterior chamber cannulation.
Results
RT-PCR of mouse tissues revealed Best2 mRNA in eye, colon, nasal epithelia, trachea, brain, lung, and kidney. X-gal staining, confirmed expression in colon epithelia and in the eye, in the nonpigmented epithelia (NPE). Best2 was not expressed in RPE cells. Best2 protein was observed only in NPE and colon epithelia. The absence of Best2 had no obvious deleterious effect on the mice. However, the Best2−/− mice were found to have significantly (P < 0.02) diminished IOP with respect to the Best2+/+ and Best2+/− littermates. The Best2−/− and Best2+/− mice responded better to the carbonic anhydrase inhibitor brinzolamide than did their Best2+/+ littermates, although the β-blocker timolol brought IOP to the same level, regardless of genotype.
Conclusions
Best2 plays a role in the generation of IOP by regulating formation of aqueous humor, and inhibition of Best2 function represents an attractive new avenue for regulating IOP in individuals with glaucoma.
Bestrophins are a recently recognized family of proteins linked to Ca2+ sensitive Cl− transport.1,2 It is clear on the basis of multiple studies that these proteins play an important role in Ca2+-sensitive Cl− transport, although they may have other functions as well.1,2 There are four bestrophin genes in mammals, designated Best1 through Best4.3 The genes were originally designated VMD2 (Best1) and VMD2L1 through VMD2L3. Best4 (formerly designated VMD2L2) is a pseudo-gene in mice, and there is no evidence that the gene is functionally expressed in humans.4 Best1, is the prototypic member of the bestrophin family in mammals. Mutations in human BEST1 are causally associated with the blinding eye diseases Best vitelliform macular dystrophy (BVMD, OMIM 153700; Online Mendelian Inheritance in Man; http://www.ncbi.nlm.nih.gov/Omim/ provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD), Adult-onset vitelliform macular dystrophy (OMIM 608161), and autosomal dominant vitreoretinochoroidopathy (OMIM 193220).2 All three diseases exhibit dominant inheritance and with only a few exceptions, all the disease-causing mutations are either missense or single amino acid deletions.
The association of human Best1 with BVMD, a disease characterized by a diminished electrooculogram light peak,5,6 a response generated by activation of a Ca2+-sensitive Cl− conductance,7 led to the hypothesis that bestrophins are Cl− channels.8 Best1 is an integral membrane protein that forms dimers and potentially higher-order oligomers.9 All mammalian bestrophins are predicted to have at least four transmembrane domains, a structure that is supported with experimental data for Best1.10,11 Heterologous expression of bestrophins results in the de novo activation of Cl− currents with unique, bestrophin-specific, I/V relationships.8 Best2-associated Cl− currents are perhaps the best characterized.12–14 Extensive mutagenesis of the second transmembrane domain in Best2 has identified amino acid residues that appear to confer ion selectivity, suggesting that bestrophins can form channel pores.13,14 However, the disruption of mouse Best1 does not diminish the light peak, nor does it cause obvious changes in Ca2+-sensitive Cl− conductances measured in freshly isolated RPE cells.15 In addition, expression of human BEST1 and disease causing mutants in RPE-J cells results in changes in the kinetics of voltage-dependent Ca2+ channels.16 Studies in which VDCC (voltage-dependent Ca2+ channel) inhibitors have been used, and mice in which VDCC subunits are disrupted, have demonstrated that VDCCs are necessary for proper generation of the light peak response in mice and rats.15–17 Taken together, these data suggest that bestrophins may not generate Ca2+-sensitive Cl− conductances but may be involved in regulating Ca2+ signaling.
In an effort to gain a better understanding of the role of bestrophins, we characterized a mouse in which the Best2 gene was disrupted by targeted insertion of the LacZ gene. In addition to ablating expression of Best2, the LacZ gene is under control of the endogenous Best2 promoter, allowing for reporter analysis of Best2 gene expression. In this article, we report that Best2 is expressed predominantly in the nonpigmented ciliary epithelium (NPE) of the eye and in the epithelial cells of the colon. Gene expression data for these sites is confirmed by immunostaining using a well-characterized antibody against murine Best2. We found that disruption of the Best2 gene results in a significantly diminished IOP and altered sensitivity to drugs that affect the generation of aqueous humor. Based on these findings we conclude that Best2 represents a novel target for the development of new drugs to lower IOP by diminishing aqueous inflow.
Materials and Methods
Targeted Disruption of the Mouse Best2 Gene and Southern Blot Analysis
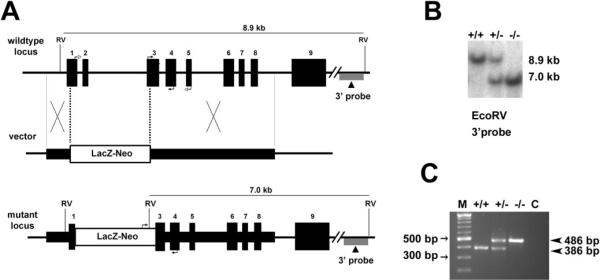
The targeting vector was constructed using 0.8-kb (5′) and 2.9-kb (3′) mouse Best2 genomic DNA fragments as homology arms. The two arms flanked a promoterless lacZ and a neomycin-resistant gene cassette (lacZ-neo). Homologous recombination in mouse embryonic stem cells resulted in the insertion of the lacZ-neo cassette, replacing a region spanning exon 1 through a part of exon 3 of the mouse Best2 locus. Germ-line–transmitting chimeric mice generated from the targeted embryonic stem cells were bred with C57BL/6 mice to produce Best2+/− mice (Deltagen, San Mateo, CA). Intercrossing of heterozygous mice generated Best2−/− mice. Southern blot analysis was performed for identification of homologous recombinants on genomic DNA digested with EcoRV, separated on an 0.8% agarose gel (SeaKem Gold; Cambrex, East Rutherford, NJ) and transferred to a nylon membrane (Hybond-N+; GE Healthcare, Piscataway, NJ) by capillary blotting. The membrane was hybridized with a 3′ external probe located outside of the homologous arm regions (Fig. 1A). The probes were labeled with 32P via random priming with a DNA labeling system (Megaprime DNA Labeling System; GE Healthcare) and purified (Probe-Quant G-50 Micro Columns; GE Healthcare). Hybridization was performed with a commercial solution (MiracleHyb; Stratagene, La Jolla, CA), according to the manufacturer's instructions. PCR was performed for subsequent genotyping. All studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Figure 1.
Targeted disruption of the mouse Best2 gene. (A) The wild-type locus, targeting vector, and mutant locus. Thick lines: fragments used for constructing the targeting vector 5′ and 3′ arms. Thin lines: genomic DNA or vector backbone sequence. Numbered solid boxes: Best2 exons. Labeled boxes: the LacZ and neor expression cassette. The external 3′ probe used for Southern blot analysis in (B) is indicated under the wild-type and mutant loci. Filled arrows at exons 3 and 4 of the wild-type locus and LacZ-neor and exon 4 of mutant locus indicate the PCR primers used for genotyping in (C). Open arrows at exons 1 and 5 of wild-type locus indicate the primers used for RT-PCR in Figure 2. RV, EcoRV. (B) Southern Blot analysis was performed to identify homologous recombinants. Mouse genomic DNA was digested with EcoRV. Wild-type (+/+) fragment, 8.9 kb; mutant (−/−) fragment, 7.0 kb. The heterozygous mice (+/−) had both fragments. (C) Genotyping was confirmed using a multiplex PCR with two forward primers in exon 3 and the LacZ and neor expression cassette, respectively, and one reverse primer in exon 4. The 386-bp product represents the wild-type allele and is absent from the Best2−/− mice. The 486-bp product representing the disrupted allele is present only in the Best2+/− and Best2−/− mice. M, molecular weight marker.
Reverse Transcription–Polymerase Chain Reaction
Reverse transcription–PCR (RT-PCR) was performed to confirm the absence of Best2 expression in the homozygous mice. Total RNA was isolated (TRIzol; Invitrogen, Carlsbad, CA) and reverse transcribed (Superscript III; Invitrogen). A 3′ primer set corresponding to the Best2 cDNA sequence was used in the PCR. This primer set (5′-ATGGCACTAAGCGCCGCCTATC-3′ and 5′-CTGGGTGTAGACGAGGGGTA-3′) generates a 585-bp fragment. A set of mouse fibulin-3 primers, generating a 372-bp fragment, were used as a positive control.
RT-PCR was also performed to identify expression of human BEST1 and BEST2 in isolated human ciliary body and RPE. Donor eyes were obtained from the Cleveland Eye Bank. The following primers sets were used: BEST1 (5′-atgtactggaataagcccgag-3′ and 5′-ttaggaatgtgcttcatccctg-3′), 752-bp and BEST2 (5′-ATTGTGACCAGTATGCCAGCCTCA-3′ and 5′-TTTCAAACTTCTTGCGCTCCTCGC-3′), 315 bp. β-Actin primers resulting in a 234-bp product were used as a control (5′-GGACTTCGAGCAAGAGATGG-3′ and 3′-AGCACTGTGTTGGCGTACAG-3′).
X-gal Staining
Staining for expression of the LacZ reporter was performed on 10-μm frozen sections of tissue fixed in 4% paraformaldehyde in PBS. Sections were postfixed for 1 minute in 2% formaldehyde, 0.2% glutaraldehyde in PBS, containing 0.1% Tween 20, rinsed twice in 100 mM Tris-HCl (pH 7.4), 100 mM NaCl, 50 mM MgCl2, and 0.1% Tween 20, and incubated for 3 hours or overnight in a 1-mg/mL solution of X-gal (Tissue Stain Solution; Chemicon, Temecula, CA). After three washes in PBS containing 0.1% Tween 20, the samples were counterstained with nuclear fast red. The sections were examined with a microscope (model E-600) and photographed with a color CCD camera using ACTII software (Nikon, Tokyo, Japan).
Transfection and Western Blot Analysis
HEK293 cells were transfected with the plasmid pAdlox-mBest2 by means of a lipophilic transfection reagent (Lipofectamine; Invitrogen), as described elsewhere.18 Forty-eight hours after transfection, the cells were lysed in SDS-PAGE sample buffer and resolved on a 10% SDS-PAGE gel. The gel was transferred to a PVDF membrane and blotted with a 1:1000 dilution of affinity-purified rabbit anti-Best2 antibody B4947A, as described.18
Immunohistochemistry
Immunohistochemistry was performed as before19 on unfixed, frozen sections (ABC kit with VIP as substrate; Vector Laboratories, Burlingame CA) with affinity-purified rabbit anti-Best2 antibody B4947A at a 1:500 dilution. Immunofluorescent staining of unfixed, frozen sections was performed as before12 using a 1:50 dilution of the affinity-purified rabbit anti-Best2 antibody B4947A12 and a CY3-conjugated goat anti-rabbit IgG antibody.
IOP Measurements
IOP was measured in the mice by cannulation of the anterior chamber as described by others,20–22 with Avertin anesthesia (300 mg/kg injected intraperitoneally). In brief, the anterior chamber was cannulated with a borosilicate glass microneedle (1-mm outer diameter with a 50-μm inner diameter tip, beveled to 45°; Humagen, Charlottesville, VA) filled with Hanks' balance salt solution (HBSS) and connected to a pressure transducer (BLPR; World Precision Instruments, Sarasota, FL). The signal was amplified (Bridge8 amplifier; World Precision Instruments), converted from analog to digital (Iworx model 108 converter; CB Sciences, Dover, NH), and recorded (LabScribe software ver.1.6; CB Sciences, Dover, NH). IOP was recorded for a period of >90 seconds, and the IOP value determined from the average of each recording. Recordings were discarded if the variance during the recording period exceeded 1 mm Hg or did not immediately return to zero after withdrawal of the needle. All measurements were performed between 2 and 6 PM to avoid diurnal pressure variation. The apparatus was calibrated using a fluid reservoir the height of which could be adjusted to generate a series of known pressures. For experiments using timolol (0.5% solution; Bausch & Lomb, Tampa, FL) or brinzolamide (Azopt 1% solution; Alcon, Fort Worth, TX), 10 μL of solution was administrated topically to each eye 2 hours before cannulation.
Measurements of EVP (episcleral venous pressure) were performed as described by Aihara et al.22 IOP was first measured as described earlier, after which the height of the saline-filled reservoir was adjusted to equal the IOP, and the stopcock between the transducer and reservoir was opened. The height of the reservoir was decreased 0.5 mm Hg (0.68 mm H2O) per minute. EVP was determined as the pressure at which reflux of blood from collector channels into Schlemm's canal could be observed through a dissecting microscope. The reservoir was then returned to its original height, and the measurement repeated in the same eye. Differences in IOP and EVP between experimental groups were compared by t-test.
Results
Generation of Mice with a Disrupted Best2 Gene
The murine Best2 gene was disrupted by replacing exons 1 and 2 and part of exon 3 with a promoterless LacZ and a neomycin-resistance gene cassette through homologous recombination (Fig. 1A). The LacZ gene contained a sequence encoding a nuclear localization signal. The disruption of the gene was confirmed by Southern blot analysis (Fig. 1B) and PCR (Fig. 1C). The Best2−/− mice were viable, grew normally, were fertile, and demonstrated no obvious physical or behavioral abnormalities.
Expression Profiling ofBest2
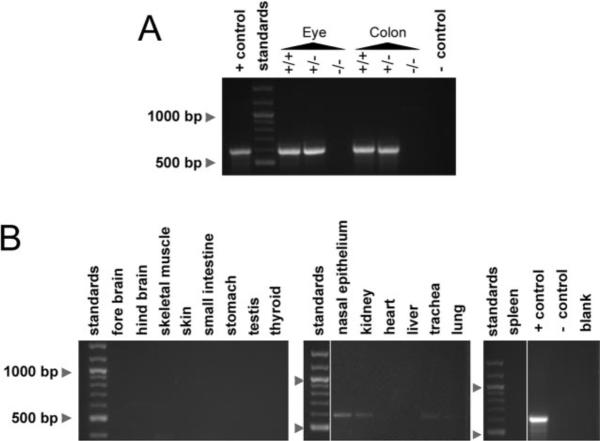
The Best2 gene is reportedly expressed in multiple mouse tissues and organs, including RPE, colon, testis, and nasal epithelia.3,23 To determine whether we had generated a true null mutant, we performed RT-PCR on total RNA from colon and eye. As shown in Figure 2A, the 585-bp reaction product obtained from the Best2+/+ and Best2+/− mice was absent from these organs in mRNA isolated from the Best2−/− mice. We used RT-PCR to scan additional mouse organs for Best2 mRNA. As shown in Figure 2 and Table 1, positive results were obtained from multiple organs with bands for eye, colon, and nasal epithelium being strongest and most reproducible.
Figure 2.
The expression of Best2 mRNA was probed using RT-PCR in multiple tissues. Initially, eye and colon were examined to validate that a true null mutant had been generated (A). A PCR product of the correct size (585 bp), representing Best2 mRNA was present in eye and colon in the Best2+/+ and Best2+/− mice, but not in the Best2−/− mice (A). Next other organs were probed for Best2 mRNA expression (B). Weak bands for Best2 were identified in hind brain, nasal epithelium, kidney, trachea, and lung. +control, mBest2 cDNA used as a template; −control, template omitted.
Table 1.
Expression of Best2 in Mouse Organs/Tissues
| Organ/Tissue | Lac Z Gene Expression* | RT-PCR | Protein Expression† |
|---|---|---|---|
| Eye (whole eye) | NA | +++ | NA |
| Eye (ciliary body) | +++ | ND | +++ |
| Eye (RPE/choroid) | — | ND | — |
| Eye (other) | — | ND | — |
| Nasal epithelium | +/− | ++ | — |
| Fore brain | — | — | ND |
| Hind brain | — | + | ND |
| Liver | — | — | ND |
| Kidney | — | + | — |
| Heart | — | — | ND |
| Trachea | — | + | ND |
| Lung | — | + | ND |
| Skeletal muscle | — | — | ND |
| Spleen | — | — | ND |
| Skin | +/− | — | — |
| Colon | +++ | +++ | +++ |
| Small intestine | — | — | ND |
| Stomach | — | — | ND |
| Testis | +/− | — | ND |
ND, not done; NA, not applicable.
Based on X-gal staining.
Based on immunohistochemical staining.
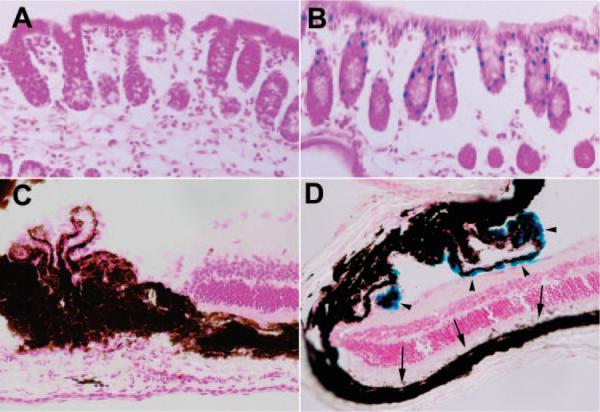
In the Best2-knockout mouse, the Lac Z gene was inserted into the Best2 gene placing it under control of the endogenous Best2 promoter. This method provided a second means of analysis of Best2 expression in the Best2−/− and Best2+/− mice by using X-gal staining. As summarized in Table 1, of the tissues surveyed, only the colon and eye (Fig. 3) were consistently and strongly positive for X-gal staining. Nasal epithelia were positive with prolonged incubation times; however, in every instance, the control (wild-type) nasal tissues were positive as well (data not shown). Results were ambiguous for testis, skin, and kidney. Lung, trachea, hind brain, and skeletal muscle, all positive by RT-PCR, were negative for X-gal staining. In colon, X-gal-positive cells appeared limited to a subset of epithelia and was absent from the base of the crypts. In the eye, we were surprised to find that X-gal staining was confined to NPE cells rather than the RPE as anticipated. No X-gal staining was observed in other ocular tissues.
Figure 3.
X-gal staining was used to identify tissues in which the Best2 promoter functions. Of multiple tissues surveyed (Table 1), only colon (A, B) and eye (C, D) were consistently positive, as represented by the accumulation of a blue reaction product in the nuclei of a subset of colon epithelia (B) and in NPE cells (D, arrowheads). No reaction product was observed in the RPE (D, arrows). LacZ was present only in the Best2+/− and Best2−/− mice (B, D); we placed sections from the Best2+/+ mice (A, C) on the same slides as the control sections. No reaction product was observed in eye and colon in the Best2+/+ mice (A, C).
Since we expected that Best2 would be expressed in the RPE, we further examined its expression using RT-PCR of total RNA isolated from human RPE and ciliary body. Human tissues were used, because the larger eyes facilitate dissection of RPE and ciliary body without cross-contamination. As shown in Figure 4, RPE was positive for Best1 but not Best2, while ciliary body was positive for Best2 and not Best1, confirming in the human eye, the expression pattern observed in mouse.
Figure 4.
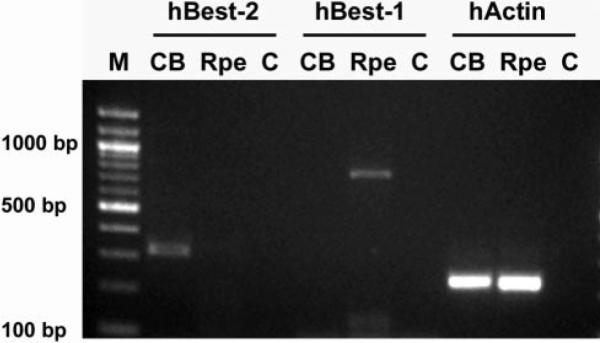
The expression of Best2 in ciliary body (CB) and RPE was compared by RT-PCR. A band of 314 bp representing BEST2 was observed only in CB and not RPE. As a control for tissue specificity and purity, BEST1 expression was analyzed in these tissues as well. A band of 752 bp representing BEST1, which is uniquely expressed in the RPE, was detected only in RPE and not in CB. PCR performed with human actin (hActin) primers demonstrates that similar amounts of cDNA were used in each reaction. No reaction products were observed in lanes in which the template was omitted (C).
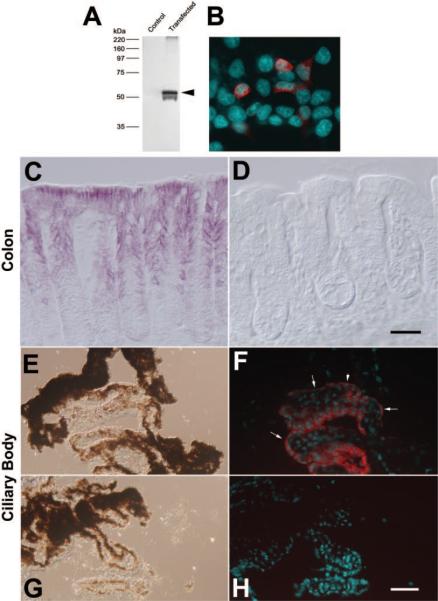
Localization of Best2 Protein
To examine the expression of Best2, we used an antibody raised against the C terminus of the murine protein.12 The antibody recognizes recombinant mBest2 expressed in HEK 293 cells by Western blot as well as immunofluorescence (Figs. 5A, 5B). Similar to our findings with Best1,15 we could not reliably detect Best2 in mouse tissues by Western blot. In colon, signal amplification with avidin-biotin was necessary to detect Best2 by immunohistochemistry (Figs. 5C, 5D). Immunohistochemical staining for Best2 using horseradish peroxidase labeled streptavidin and the substrate VIP to amplify the signal revealed a dark-purple reaction product associated with the basolateral membranes of epithelial cells in the colon (Fig. 5C). No antibody staining for Best2 was observed in sections of colon from the Best2−/− mice stained simultaneously on the same slide (Fig. 5D). Of note, as observed with X-gal staining (Fig. 3B), antibody staining was absent from cells at the base of the crypts. We could not on the basis of immunohistochemical staining determine whether Best2 is expressed solely in goblet cells, as suggested by X-gal staining, since the goblet cells are intermingled with transporting epithelia, and staining of lateral membranes was most prominent.
Figure 5.
Expression of Best2 protein was probed with the antibody B4749A. Specificity was tested in transfected HEK293 cells by Western blot (A) and immunofluorescence (B). Best2 (red) was expressed in a subpopulation of cells. The expression of Best2 was probed by immunohistochemistry with antibody B4947A in colon from the Best2+/+ (C) and Best2−/− (D) mice. (C) Purple VIP reaction product was deposited along the lateral borders of epithelia in the mid-to-luminal regions, but not at the base of the crypts in the Best2−/− mice. Reaction was absent from the Best+/+ sections processed on the same slide. The localization of Best2 to the NPE of the ciliary body was examined by using immunofluorescence on unfixed cryosections of eyes (F, H). (E, G) DIC images of the sections shown in (F) and (H), respectively. Red staining for Best2 expression was observed only in the NPE of the Best2+/+ mice (F) and not the NPE of the Best2−/− mice (H). In regions in which the NPE were in cross section, Best2 staining appeared to be associated only with the basal surface of the cells (F, arrows). Nuclei (cyan) in (B), (F), and (H) were stained with DAPI (4′,6′-diamino-2-phenylindole). Scale bars, 60 μm.
In the eye, signal amplification was not necessary, and so we used immunofluorescence to localize Best2. Best2 was observed only in NPE cells (Fig. 5F). The protein appeared to be polarized along the basal membranes of the cells. No staining was observed in the RPE, even with signal amplification (not shown), and no staining was observed in sections from the Best2−/− mice stained simultaneously on the same slide (Fig. 5H). These results agree with our observations obtained with X-gal staining.
We also examined nasal tissues for expression, by using immunohistochemistry with avidin-biotin signal amplification, but could not detect any Best2 reactivity (data not shown).
Phenotype Analysis of the Best2−/− Mice
The Best2−/− mice exhibited no obvious phenotypic abnormalities. Because Best2 was reported by other investigators to be expressed in nasal epithelia,23 we assayed the mice for impaired olfactory responses. Mice were starved for 24 hours and then challenged to find hidden food. In this assay, mice with olfactory defects due to a disruption of the Bbs2 gene were incapable of finding the food within a 10-minute period, whereas wild-type mice found it within 240 seconds.24 The Best2−/− mice found the food in an average of 266 ± 144 seconds (n = 10). Their performance was similar to that of their Best2+/+ littermates, which found the food in 231 ± 141 seconds (n = 10). We conclude from this that Best2−/− mice have no significant olfactory deficit. We observed no obvious gastrointestinal distress in the mice. Despite the absence of expression of Best2 in colon, the mice did not exhibit diarrhea or any other obvious symptom of colon dysfunction.
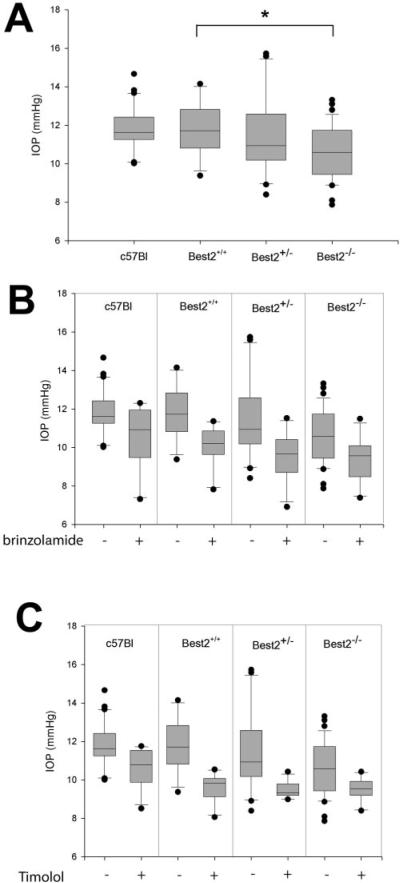
The expression of Best2 in the eye has been predicted.3,4 While we anticipated on the basis of these prior studies that Best2 would be expressed in RPE cells, we found that it was instead expressed in the NPE cells of the ciliary body. The ciliary epithelial cells play a critical role in the generation of aqueous humor. Since IOP is determined by the balance between aqueous humor secretion and outflow facility, we hypothesized that the disruption of Best2 might affect IOP. We measured IOP in mice according to the methods of John et al.,21 and Aihara et al.22 in which a glass micropipette connected to a pressure transducer is inserted through the cornea and into the anterior chamber of the eye. Our wild-type C57BL/6 mice exhibited IOPs of 11.78 ± 0.22 (average ± SE, n = 28; Table 2) consistent with prior reports22 of the use of this technique. The Best2+/+ and Best2+/− littermates exhibited comparable IOPs that did not differ significantly (P = 0.979 and P = 0.989, respectively) from the C57BL/6 control group (Fig. 6A, Table 2), although there was more variability in the Best2+/− mice. In contrast, the Best2−/− mice exhibited significantly (P < 0.02) lower IOPs than did C57BL/6 mice or their Best2+/+ and Best2+/− littermates (Fig. 6A, Table 2). Measurements of the EVP of the mice identified no differences between genotypes, with all mice exhibiting an EVP of 6.3 ± 0.4 mm Hg (average ± SD, n = 12). Because of the reduced IOP in the Best2−/− mice and its expression in NPE cells, we conclude that Best2 probably contributes to the formation of aqueous humor.
Table 2.
Mean IOPs in Control and Drug Treated Best2-Knockout Mice
| C57BL/6 | Best2+/+ | Best2+/− | Best2−/− | |
|---|---|---|---|---|
| Untreated | 11.78 ± 0.22 | 11.73 ± 0.37 | 11.52 ± 0.44 | 10.56 ± 0.21 |
| n = 28 | n = 12 | n = 20 | n = 39 | |
| Brinzolamide | 10.51 ± 0.51 | 10.08 ± 0.33 | 9.56 ± 0.37 | 9.34 ± 0.34 |
| n = 11 | n = 10 | n = 12 | n = 11 | |
| Timolol | 10.53 ± 0.29 | 9.57 ± 0.22 | 9.48 ± 0.12 | 9.49 ± 0.18 |
| n = 10 | n = 11 | n = 11 | n = 11 |
Data are mean ± SE (in mm Hg).
Figure 6.
(A) The IOP of the C57BL/6 mice and Best2−/− mice along with their Best+/− and Best+/+ littermates was determined and compared. A significantly diminished IOP (*) was observed in the Best2−/− mice (n = 39) versus the Best2+/+ littermates (n = 11), Best2+/− littermates (n = 20), and C57BL/6 mice (n = 31). Each graph contains box plots in which the line within the box marks the median IOP, and the boundary of the box farthest from 0 indicates the 75th percentile. Error bars above and below the boxes indicate the 90th and 10th percentiles, respectively. (B) The effect of the carbonic anhydrase inhibitor brinzolamide was determined and compared to the general population of untreated mice of each strain/genotype. Brinzolamide was significantly more effective in lowering IOP in the Best2+/− mice (n = 12, P < 0.01) than other genotypes and reduced IOP in the Best2−/− mice (n = 11, P < 0.01) to a lower level than in C57BL/6 (n = 10, P < 0.02) or the Best2+/+ littermates (n = 10, P < 0.01). (C) Timolol caused a significant (P < 0.005) reduction in all groups (n = 11 in each timolol-treated group), with the median being similar in the Best2+/+, Best2+/−, and Best2−/−, mice. IOP was less efficiently lowered by timolol in the C7BL/6 mice.
To elucidate further the role of Best2 in generating aqueous humor, we treated mice with either the carbonic anhydrase inhibitor brinzolamide or the β-blocker timolol. Timolol has been shown to be effective in lowering IOP in mice,25 as have carbonic anhydrase inhibitors,25 and both drugs are currently used to lower IOP in humans with glaucoma. Brinzolamide was effective in lowering IOP in all mice tested (Fig. 6B, Table 2). Treatment with brinzolamide resulted in reduction to a lower IOP in the Best2+/− and Best2−/− mice than in the C57BL/6 or Best2+/+ mice. Of interest, despite the mean IOP being similar in the Best2+/+ and Best2+/− mice, brinzolamide was more effective in reducing IOP in the Best2+/− mice, reducing the overall pressure to the same level as observed in the Best2−/− mice treated with the drug (Fig. 6B, Table 2); approximately 1 mm Hg lower than was observed in the C57BL/6 or Best2+/+ mice. In contrast, timolol, which lowered IOP significantly (P < 0.005) in the C57BL/6 and Best2+/+ mice, brought the IOP to the same level in the Best2+/− and Best2−/− mice (Fig. 6C, Table 2), making it less effective in lowering IOP in the Best2−/− mice, and suggesting that Best2 plays a role downstream of the β2 adrenergic receptor that is the presumptive target of timolol.
Discussion
Data on the physiological role of any bestrophin in any tissue are sparse. Most of the available data are derived from Best1, for which we have previously disrupted the gene in mice15 and which when mutated can cause several blinding diseases. None of these disorders is associated with nonocular abnormalities. In the present study, we sought to further our knowledge on the role of bestrophins by disrupting the Best2 gene in mice. Similar to our observations in Best1−/− mice, we found that the Best2−/− mice exhibited no obvious defects. Using a combination of methods, we found that the gene and protein are clearly expressed only in the NPE cells of the ciliary body and in epithelial cells of the colon.
The restricted expression of Best2 observed in this study is similar to the restricted expression that we have reported for Best1, which indicates that the protein is expressed only in RPE cells.9,26 These data reinforce the idea that bestrophins are expressed in very specific cell types and are not widely distributed in tissues throughout the body. The limited expression of Best2 led us to identify an important ophthalmic phenotype: a significant reduction in the IOP of the Best2−/− mice and differences among genotypes with regard to sensitivity to drugs that affect the aqueous inflow pathway. These results suggest that Best2 plays a role in the generation of intraocular pressure, most likely by participating in the formation of aqueous humor. The data also suggest that Best2 is a potential therapeutic target for the treatment of glaucoma. Of note, the human BEST2 gene is located on the short arm of chromosome 19 near a strong glaucoma locus identified by sib pair analysis.27 Based on our findings, BEST2 represents a potential candidate for the glaucoma gene at this locus. Our data suggest that loss of function or diminished expression of BEST2 are protective against glaucoma and that BEST2 gain of function mutations predispose to glaucoma.
How might Best2 affect IOP? IOP is determined from the sum of the EVP plus the ratio of aqueous humor formation and its drainage through the outflow facility. Since EVP was similar in all genotypes studied, Best2 must play a role in either inflow or outflow. We favor scenarios in which Best2 affects aqueous formation because of its localization on the basal surface of the NPE, its ability to generate or regulate Cl− conductances, and the differential effects across Best2 genotypes of drugs altering aqueous inflow (Figs. 6B, 6C). It has long been recognized that water transport across the ciliary epithelium is coupled to the transepithelial transport of Cl− by the NPE.28–30 NPE cells secrete Cl− via channels in their basal plasma membrane. Our data indicate that Best2 is present at the NPE basal plasma membrane, and so the simplest explanation is that Best2 is one of at least three species of Cl− channels that have been described in the NPE.28 The absence of one species of Cl− channel may result in a diminished IOP similar our observations. Although there is no evidence that NPE Cl− transport is Ca2+ sensitive, Best2 Cl− transport is Ca2+ regulated.12 However, the apparent affinity for Ca2+ is high so that a considerable fraction of Best2 channels are active at “resting” intracellular Ca levels.12 Furthermore, Best2 Cl− channel activity is also volume sensitive.27 Some Cl− channels in the basal NPE membrane are volume sensitive,31 and some models for fluid secretion by ciliary epithelia invoke volume-regulated anion channels. Cl− transport in the NPE is coupled to HCO3− transport, which is affected by carbonic anhydrase inhibitors. The altered sensitivity of the Best2+/− and Best2−/− mice to brinzolamide (Fig. 6B) is consistent with this idea. The β-blocker timolol brought IOP to the same level in all mice tested (Fig. 6C). Timolol affects adrenergic signaling via β2 adrenergic receptors in NPE cells.32,33 This pathway is thought to govern inflow by regulating the number of Cl− channels present in the basal membrane of the NPE,28 and so it is possible that Best2 represents a population of channels that can be mobilized in response to adrenergic signaling.
Another possible function for Best2 in the inflow pathway is as an antagonist to Ca2+ signaling, as we have proposed for Best1. According to Mitchell et al.,34 ATP stimulates P2Y2 receptors in ciliary pigmented epithelial (PE) cells and triggers Ca2+ release. PE cells release ATP due to cell swelling, which results in activation of a hypothesized Ca2+-dependent, negative-feedback loop sending Cl− into the stroma.28,34 But why then is Best2 on the NPE and not the PE? Perhaps it is the governor of the negative feedback loop or maybe it senses Cl− flux across the NPE basal membrane and alters Ca2+ signaling sensitivity to prevent this negative feedback loop from overtaking the positive flow of aqueous humor. In its absence, the failure to regulate Ca2+ properly could result in a faster move toward recycling of Cl− and diminished aqueous production. According to this scenario, the apparent amplification of the brinzolamide effect would be via changes in pH, perhaps via the Na+/H+ exchanger,35,36 with downstream effects via intracellular pH on overall Ca2+ signaling.
Although further studies are needed to clarify the role of Best2 in the generation of IOP, it is clear that Best2 represents a new potential target for the development of drugs to lower IOP. Because Best2-deficient mice continue to respond to drugs that have been traditionally used to lower IOP in patients with glaucoma, a drug that blocks Best2 activity could function synergistically with currently available drugs expanding the repertoire of therapies available to treat this blinding disease.
Acknowledgments
The authors thank Dennis Rice and Andy Whitlock for sharing their expertise with IOP measurements in mice and John Fingert and Dan Stamer for critical reading of the manuscript.
Supported by National Eye Institute Grants EY13160 and EY13847; the Macular Vision Research Foundation; Synfrämjandet and the Swedish Research Council (BB); a Career Development Award, and an unrestricted grant to the Department of Ophthalmology and Vision Science at the University of Arizona from Research to Prevent Blindness.
Footnotes
Disclosure: B. Bakall, None; P. McLaughlin, None; J.B. Stanton, None; Y. Zhang, None; H.C. Hartzell, None; L.Y. Marmorstein, None; A.D. Marmorstein, None
References
- 1.Hartzell C, Qu Z, Putzier I, Artinian L, Chien LT, Cui Y. Looking chloride channels straight in the eye: bestrophins, lipofuscinosis, and retinal degeneration. Physiology. 2005;20:292–302. doi: 10.1152/physiol.00021.2005. [DOI] [PubMed] [Google Scholar]
- 2.Marmorstein AD, Kinnick TR. Focus on molecules: bestrophin (Best-1) Exp Eye Res. 2006;85:423–424. doi: 10.1016/j.exer.2006.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer F, Stohr H, Weber BH. Cloning and characterization of the murine Vmd2 RFP-TM gene family. Cytogenet Genome Res. 2004;105:107–114. doi: 10.1159/000078016. [DOI] [PubMed] [Google Scholar]
- 4.Stohr H, Marquardt A, Nanda I, Schmid M, Weber BH. Three novel human VMD2-like genes are members of the evolutionary highly conserved RFP-TM family. Eur J Hum Genet. 2002;10:281–284. doi: 10.1038/sj.ejhg.5200796. [DOI] [PubMed] [Google Scholar]
- 5.Cross HE, Bard L. Electro-oculography in Best's muscular dystrophy. Am J Ophthalmol. 1974;77:46–50. doi: 10.1016/0002-9394(74)90603-5. [DOI] [PubMed] [Google Scholar]
- 6.Deutman AF. Electro-oculography in families with vitelliform dystrophy of the fovea. Br J Ophthalmol. 1969;81:305–316. doi: 10.1001/archopht.1969.00990010307001. [DOI] [PubMed] [Google Scholar]
- 7.Gallemore RP, Hughes BA, Miller SS. Light-induced responses of the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford University Press; New York: 1998. pp. 175–198. [Google Scholar]
- 8.Sun H, Tsunenari T, Yau KW, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci USA. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanton JB, Goldberg AF, Hoppe G, Marmorstein LY, Marmorstein AD. Hydrodynamic properties of porcine bestrophin-1 in Triton X-100. Biochim Biophys Acta. 2006;1758:241–247. doi: 10.1016/j.bbamem.2006.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milenkovic VM, Rivera A, Horling F, Weber BH. Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J Biol Chem. 2007;282:1313–1321. doi: 10.1074/jbc.M607383200. [DOI] [PubMed] [Google Scholar]
- 11.Tsunenari T, Sun H, Williams J, et al. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu Z, Fischmeister R, Hartzell C. Mouse bestrophin-2 is a bona fide Cl(−) channel: identification of a residue important in anion binding and conduction. J Gen Physiol. 2004;123:327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu Z, Hartzell C. Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J Gen Physiol. 2004;124:371–382. doi: 10.1085/jgp.200409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu Z, Chien LT, Cui Y, Hartzell HC. The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J Neurosci. 2006;26:5411–5419. doi: 10.1523/JNEUROSCI.5500-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmorstein LY, Wu J, McLaughlin P, et al. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal R, Bakall B, Kinnick T, et al. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006;20:178–180. doi: 10.1096/fj.05-4495fje. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Marmorstein AD, Striessnig J, Peachey NS. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J Neurophysiol. 2007;97:3731–3735. doi: 10.1152/jn.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmorstein LY, Ouchi T, Aaronson SA. The BRCA2 gene product functionally interacts with p53 and RAD51. Proc Natl Acad Sci USA. 1998;95:13869–13874. doi: 10.1073/pnas.95.23.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakall B, Marmorstein LY, Hoppe G, Peachey NS, Wadelius C, Marmorstein AD. Expression and localization of bestrophin during normal mouse development. Invest Ophthalmol Vis Sci. 2003;44:3622–3628. doi: 10.1167/iovs.03-0030. [DOI] [PubMed] [Google Scholar]
- 20.Husain S, Whitlock NA, Rice DS, Crosson CE. Effects of latanoprost on rodent intraocular pressure. Exp Eye Res. 2006;83:1453–1458. doi: 10.1016/j.exer.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 21.John SW, Hagaman JR, MacTaggart TE, Peng L, Smithes O. Intraocular pressure in inbred mouse strains. Invest Ophthalmol Vis Sci. 1997;38:249–253. [PubMed] [Google Scholar]
- 22.Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44:5168–5173. doi: 10.1167/iovs.03-0504. [DOI] [PubMed] [Google Scholar]
- 23.Pifferi S, Pascarella G, Boccaccio A, et al. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci USA. 2006;103:12929–12934. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura DY, Fath M, Mullins RF, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA. 2004;101:16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avila MY, Carre DA, Stone RA, Civan MM. Reliable measurement of mouse intraocular pressure by a servo-null micropipette system. Invest Ophthalmol Vis Sci. 2001;42:1841–1846. [PubMed] [Google Scholar]
- 26.Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duggal P, Klein AP, Lee KE, Klein R, Klein BE, Bailey-Wilson JE. Identification of novel genetic loci for intraocular pressure: a genomewide scan of the Beaver Dam Eye Study. Arch Ophthalmol. 2007;125:74–79. doi: 10.1001/archopht.125.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do CW, Civan MM. Basis of chloride transport in ciliary epithelium. The J Membr Biol. 2004;200:1–13. doi: 10.1007/s00232-004-0688-5. [DOI] [PubMed] [Google Scholar]
- 29.Shahidullah M, Wilson WS, Yap M, To CH. Effects of ion transport and channel-blocking drugs on aqueous humor formation in isolated bovine eye. Invest Ophthalmol Vis Sci. 2003;44:1185–1191. doi: 10.1167/iovs.02-0397. [DOI] [PubMed] [Google Scholar]
- 30.Kong CW, Li KK, To CH. Chloride secretion by porcine ciliary epithelium: new insight into species similarities and differences in aqueous humor formation. Invest Ophthalmol Vis Sci. 2006;47:5428–5436. doi: 10.1167/iovs.06-0180. [DOI] [PubMed] [Google Scholar]
- 31.Do CW, Peterson-Yantorno K, Civan MM. Swelling-activated Cl− channels support Cl− secretion by bovine ciliary epithelium. Invest Ophthalmol Vis Sci. 2006;47:2576–2582. doi: 10.1167/iovs.05-0851. [DOI] [PubMed] [Google Scholar]
- 32.Avila MY, Stone RA, Civan MM. Knockout of A3 adenosine receptors reduces mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:3021–3026. [PubMed] [Google Scholar]
- 33.McLaughlin CW, Peart D, Purves RD, et al. Timolol may inhibit aqueous humor secretion by cAMP-independent action on ciliary epithelial cells. Am J Physiol. 2001;281:C865–C875. doi: 10.1152/ajpcell.2001.281.3.C865. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avila MY, Seidler RW, Stone RA, Civan MM. Inhibitors of NHE-1 Na+/H+ exchange reduce mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2002;43:1897–1902. [PubMed] [Google Scholar]
- 36.Counillon L, Touret N, Bidet M, et al. Na+/H+ and CI−/HCO3− antiporters of bovine pigmented ciliary epithelial cells. Pflugers Arch. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]