Abstract
Pre-existing immunity to adenovirus (Ad) reduces the efficacy of Ad-based vaccines. The goal of this study was to define the prevalence, magnitude, functionality and phenotype of Ad-specific human T cells directly ex vivo. To study the magnitude of T-cell responses to Ad, we developed a highly reproducible whole Ad vector stimulation assay for use with polychromatic flow cytometry. Ad-specific CD4+ and CD8+ T cell were detected in all 17 human subjects tested and were capable of proliferating upon restimulation. Ad-specific CD4+ T cells were primarily monofunctional CD4+ T cells that produced IL-2, IFN-γ or TNFα and expressed the memory markers CD27 and CD45RO. In contrast, Ad5-specific CD8+ T cells were more polyfunctional, expressing effector-like combinations of IFN-γ, MIP1α and perforin, and generally lacked CD27 and CD45RO expression. Ad-specific CD4+ and CD8+ T cell responses against chimpanzee-derived AdC6 and AdC7 were found in all subjects, indicating the commonality of cross-serotype reactivity of Ad-specific T cells. This cross-reactivity is due in part to extensive CD4+ and CD8+ T cell recognition of hexon regions conserved between multiple Ad serotypes. The prevalence, cross reactivity and effector like functions of Ad-specific T-cells in humans may affect the efficacy of Ad vector-based vaccines by eliminating vector infected cells even when rare serotype Ad vectors are employed.
Introduction
Adenoviruses (Ad) vectors are commonly used as vaccine carriers because of their ability to induce insert-specific CD8+ T cell responses. However, pre-existing Ad-specific immunity represents a major obstacle for Ad-based vaccines(Casimiro and others 2004; Casimiro and others 2003). In animal models and humans, vaccination is less effective in the presence of neutralizing antibodies (nAb)(Casimiro and others 2003; Priddy and others 2008; Yang and others 2003). It has also been shown that significant levels of nAb are generated after a single Ad5 injection, thereby reducing the efficacy of a homologous vaccine boost(McCoy and others 2007). The prevalence of nAb to the commonly used Ad5 varies worldwide, and was shown to be as high as 90% in Africa. Seroprevalence of the other ∼50 identified human Ads also fluctuate globally with the occurrence of natural infection. To avoid the potential limitations imposed by preexisting immunity, vectors based on alternative Ad serotypes are in development, including Ad26, 35, 48, and the chimpanzee-derived AdC6, C7, and C68. Neutralizing titers to these various rare Ad serotypes are typically low in humans, with seroprevalence to AdC6 and AdC7 less than 5% of adults in the United States and less than 10% seropositive in equatorial Africa, the natural habitat for chimpanzees(Xiang and others 2006).
Although the prevalence and effects of Ad-specific nAb on vaccine efficacy have been studied, little work has been done to characterize the naturally occurring T cell response to Ad, or the potential of Ad-specific T cells to influence Ad-based vaccine efficacy. Ad-specific CD8+ T cell responses can limit the effectiveness of Ad-vectored vaccines in animal models(McCoy and others 2007; Sumida and others 2005), presumably due to the direct elimination of vector-transduced antigen presenting cells. Such studies, however, have not been performed in the setting of natural Ad infections in the human. Ad-specific T cells have been detected ex vivo in humans, both before and after Ad vector vaccination, in peripheral blood and mucosal tissues(Calcedo and others 2009; Leen and others 2008; Leen and others 2005; Leen and others 2004; McElrath and others 2008). Several MHC class II-restricted CD4+ T cell epitopes have been identified in the Ad5 hexon, residing primarily in regions conserved between disparate Ad serotypes, such as the HLA-DP4 restricted CD4+ T cell epitope (hexon 910-924)(Leen and others 2008; Tang and others 2004; Veltrop-Duits and others 2006). MHC class I restricted CD8+T cell epitopes have also been identified in the Ad hexon, penton, and fiber(Leen and others 2008; Tang and others 2006). Responses to Ad appear to be almost ubiquitous in the human population(Calcedo and others 2009; McElrath and others 2008); however, beyond simple quantification, little is known regarding the functionality and phenotype of Ad-specific CD4+ and CD8+ T cells in humans. Moreover, while serotype cross reactivity has been noted for both Ad-specific CD4+ and CD8+ T cells, it is unclear whether Ad-specific T cells cross-reacting with a disparate Ad serotype will function in a similar manner.
To address these issues, we have developed a highly reproducible polyfunctional flow cytometry-based assay to quantify and characterize Ad-specific CD4+ and CD8+ T cells directly ex vivo from human peripheral blood lymphocytes. Herein, we describe the functional and phenotypic properties of human Ad-specific T cells against human Ad5 and cross-reactive responses against chimpanzee-derived AdC6 and AdC7.
Material and Methods
Subjects
Peripheral blood mononuclear cells (PBMCs) were obtained from apheresis of HIV and HCV negative adult healthy donors by the University of Pennsylvania Center for AIDS Research Immunology Core, under the institutional guidelines required for conduct of experiments on human samples.
Vector
Ad5, C6 and C7 vector was prepared using previously described methods16. For cell stimulation we used an E1-deleted Ad5 vector that expressed the rabies virus glycoprotein(Crawford-Miksza and Schnurr 1996; De Jong and others 1999). The Ad5 vector was grown on HEK293 cells in DMEM supplemented with 10% fetal calf serum, antibiotics and glutamine(Olive and others 2002). Vectors were purified by CsCl gradients and quality controlled (infection unit to vp ratios, lack of replication competent Ad5 virus, genome integrity, lack of LPS contamination, sterility)
Antibodies
Directly conjugated antibodies were obtained from the following: BD Biosciences (San Jose, CA): TNFα (Pe-Cy7), IFN-γ (Alexa700); Caltag (Carlsbad, CA): CD14 (APC-Alexa750), CD19 (APC-Alexa750), and CD4 (Pe-Cy5.5); Beckman Coulter (Fullerton, CA): CD8 (ECD), CD27 (Pe-Cy5) and R&D systems (Minneapolis, MN): MIP1α (FITC), and IL-2 (APC). The following antibodies were conjugated in our laboratory: CD3 (QD585), CD57 (QD565), CD45RO (QD705), and Perforin (PacBlue). The unconjugated CD45 and CD57 were obtained from AbD Serotec (Raleigh, NC), perforin from Diaclone (Besançon, France) and CD3 OKT3 from American Type Culture Collection (Manassas, VA). Pacific Blue and Quantum Dots were obtained from Invitrogen (Carlsbad, CA).
Cell Stimulation and Staining
T cell responses were measured to whole replication defective AdH5, AdC6 and AdC7 vectors expressing an antigen to which only very few humans are immune (rabies glycoprotein). To measure responses, 2×106 PBMCs were incubated overnight with 1×1011 Ad vp and costimulatory antibodies (αCD28 and 49d, 1 μg/ml; BD Biosciences) at 37°C and 5% CO2 in 1 ml complete RPMI media (RMPI 1640 with 10% heat inactivated FBS, 100 U/mL Penicillin, 100 μg/mL streptomycin sulfate and 1.7 mM sodium glutamate). A positive control was stimulated with Staphylococcus enterotoxin B (SEB, 1 μg/ml; Sigma-Aldrich) and a negative control received only costimulatory antibodies. The following morning each of the secretion inhibitors Monensin (Golgi Stop, 0.7 μg/ml; BD Biosciences) and Brefeldin A (1 μg/ml; Sigma-Aldrich) were added to each sample and the cells were incubated for six hours at 37°C and 5% CO2. Samples were then washed (PBS with 1% BSA and 0,1% sodium azide) and stained for viability (Aqua live/dead amine reactive dye; Invitrogen) followed by treatment with surface antibodies. The cells were then washed, permeabilized and fixed using the Cytofix/Cytoperm kit (BD Biosciences) then stained with intracellular fluorochome-labeled antibodies. Following staining cells were washed, fixed (2% paraformaldehyde in PBS) and stored at 4°C in the dark until analysis.
Flow Cytometry
Cells were analyzed on a modified LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA) with 200,000 to 1,000,000 events collected per sample. Data analysis was performed using FlowJo 8.7.1 (TreeStar, San Carlos, CA). Cells were initially gated on using forward scatter area versus forward scatter height to remove doublets followed by a lymphocytes gate an forward scatter area versus side scatter area. Dead cells were then removed by gating CD3 versus Aqua blue removing dead cells that are Aqua blue bright. Contaminating CD14+/CD19+ cells were then removed before gating sequentially on CD3+, CD8+/CD4+ and CD4-/CD8- versus IFNγ to account for receptor down-regulation. A gate was then made for each respective function and the Boolean gating platform used to create the array of 32 possible functional combinations. Data are reported after background subtraction of the no stimulation condition.
Statistics: variability in the assays was tested using Levene's robust variance test with Brown and Forsythe's 10% trimmed mean alternative. This method is robust to non-normality in the data. Analyses were conducted using Stata MP 10.0. The T-cell response to different vectors and peptide pools were compared using a Friedman Statistic. This method is similar to an ANOVA for non-parametric matched data. When two groups were compared a wilcoxons sum rank test, a non-parametric t-test, was used.
Results
Optimization of Ad particle stimulation for Ad-specific T cell responses by intracellular cytokine staining
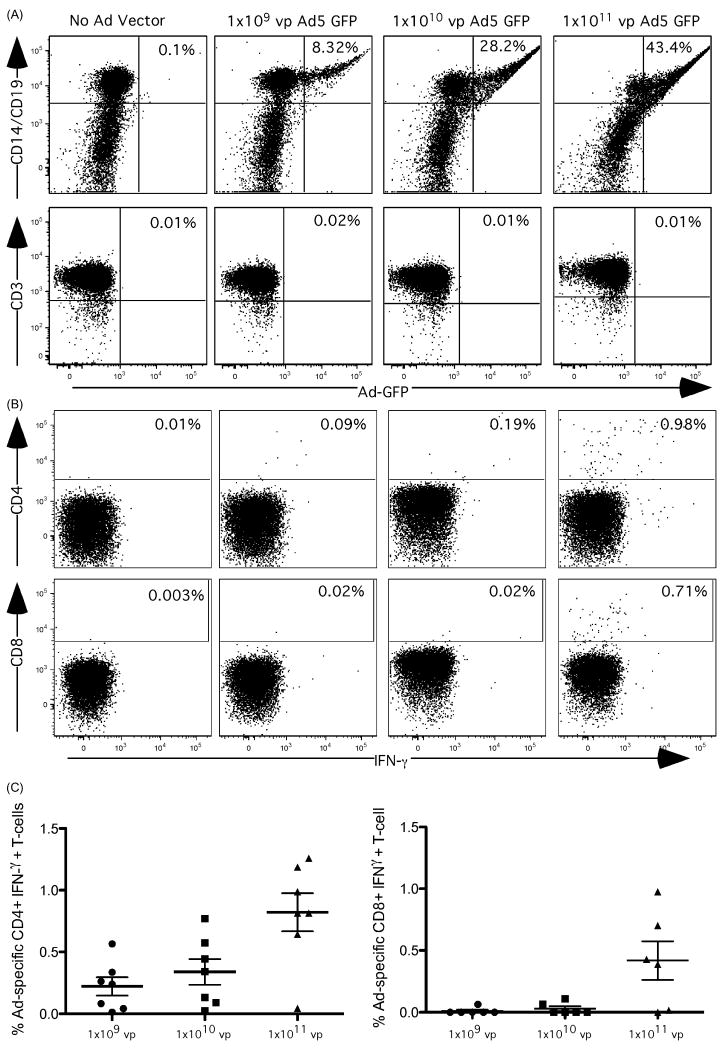
Previous studies of Ad-specific T cell responses have focused primarily on Ad hexon protein-specific T cell responses using ELISpot assays. To more completely quantify and characterize human Ad-specific T cell responses, we developed a stimulation procedure using intact Ad particles, followed by a standard intracellular cytokine staining (ICS) assay. Initially, we determined the optimal concentration of intact Ad particles to maximize expression within peripheral blood mononuclear cells (PBMC) for presentation to T cells. We incubated an Ad5 vector expressing green fluorescent protein (GFP) at various concentrations ranging from 1×109 vp to 1×1011 with PBMC for 18 hrs and assessed GFP expression in B cells (CD19+), monocytes (CD14+), T cells (CD3+/CD4+/CD8+), or the remaining PBMC negative for these markers. After an overnight incubation, GFP was detected in CD14+ monocytes and CD19+ B cells in a dose dependent manner (Figure 1A). No GFP was expressed in other cell types. Along with increasing vector concentration we also detected an Ad-specific CD4+ and CD8+ T cell response as measured by IFN-γ production (Figure 1B) that appeared to increase in frequency with the Ad dose applied to the cells. To confirm the optimal concentration of Ad5 vector for stimulation, we titrated the vector from 1×109 vp to 1×1011 vp Ad5 using PBMC from 6 normal donors. Optimal IFN-γ expression was observed at the maximal dose of 1 × 1011 Ad particles/million PBMC (Figure 1C).
Figure 1.
Measuring Adenovirus Specific T-cell responses following whole vector stimulation. PBMCs from healthy adults were cultured overnight at 37°C in 5% CO2 with Ad5 vector and costimulatory antibodies αCD28 and αCD49d. The following morning golgi secretion inhibitor brefeldin A and monensin were added for 6 hours before standard intracellular cytokine staining. A) PBMCs were incubated with 1×109-1×1011 vp Ad5 expressing green fluorescent protein (GFP). The upper graphs show GFP expression in CD14+ monocytes and CD19+ B-cells, the lower graph shows expression in CD3+ T-cells. B) PBMCs were stimulated with 1×109-1×1011 vp Ad5 vector and CD4+ and CD8+ T-cell responses were measured by IFN-γ production. C) Ad-specific IFN- γ + CD4 and CD8 T-cell responses were measured in seven donors following stimulation with 1×109-1×1011 vp Ad5.
Polyfunctional analysis of Ad-specific T cell responses
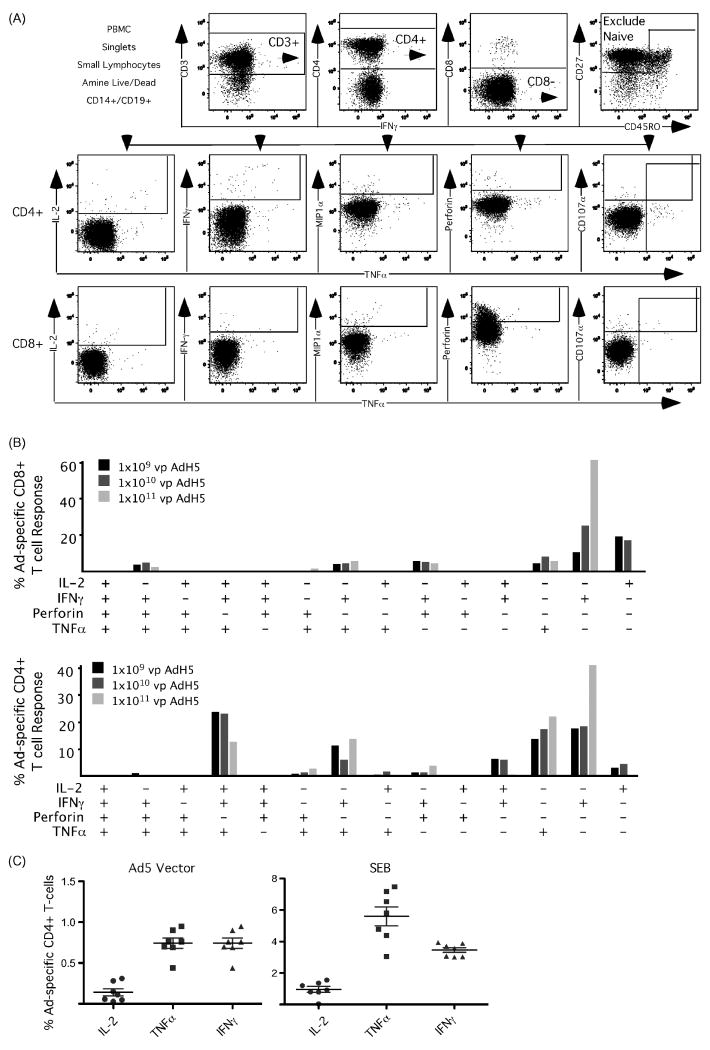
Having established the optimal conditions to detect IFN-γ producing Ad-specific T cells using whole Ad particles, we next adapted the procedure to a polychromatic flow cytometry panel that simultaneously detects T cell memory phenotype and 5 unique effector functions, i.e. IL-2, IFN-γ, TNFα, MIP1α, and perforin, along with standard T cell lineage markers, and exclusion markers. To ensure that we were detecting only Ad-specific T cells and minimizing background, we followed a strict gating strategy that removes dead cells as well as CD14+ monocytes and CD19+ B cells. We were able to detect Ad-specific CD4+ and CD8+ T cells capable of eliciting multiple functions after stimulation (Figure 2A). We next re-evaluated our Ad vector titration to determine if there were differential functional responses based on vector dose. The Ad-specific T cell response was similar at all vector concentrations for CD8+ T cells, which produced a combination of IFN-γ, TNFα and perforin and small amounts of IL-2. CD4+ T cells predominantly produced IFN-γ, IL-2, and TNFα (Figure 2B, average of six subjects). At 1×1011 vp Ad5 the percentage of CD4+ and CD8+ T cell producing only IFN-γ was appreciably higher than the other functional combinations. Finally, we examined the reproducibility of the assay system in the setting of simultaneous IL-2, IFN-γ, and TNFα production (Figure 2C). Using cryopreserved samples, we measured the Ad-specific T cell response at the 1×1011 Ad particle dose with the same batch of PBMC obtained from a single donor on seven separate days. The variability in Ad-specific CD4+ and CD8+ T cell frequency producing IL-2, TNFα, and IFN-γ was low, and significantly lower than the range of variability observed for the superantigen positive control (SEB, p<0.05).
Figure 2.
Adenovirus Particle Stimulation for Measuring Polyfunctional Ad-specific T-cell Responses. A) Gating strategy for determining Ad-specific CD4+ and CD8+ T-cells producing IL-2 IFN-γ MIP1α, Perforin and TNF α. B) Polyfunctional CD4+ and CD8+ T-cell responses following stimulation with 1×109-1×1011 vp Ad5. C) PBMCs from a single donor were stimulated with 1×1011 vp Ad5 on 7 different days to test the reproducibility of the whole vector stimulation assay. The percentage of CD4+ T-cells producing IL-2, IFN-γ and TNF α was compared following Ad vector and positive control streptococcus enterotoxin B (SEB) stimulation. The degree of variability was significantly less (p<0.05) following Ad stimulation compared with SEB.
Ad5-specific CD4+ and CD8+ T cells are common in humans
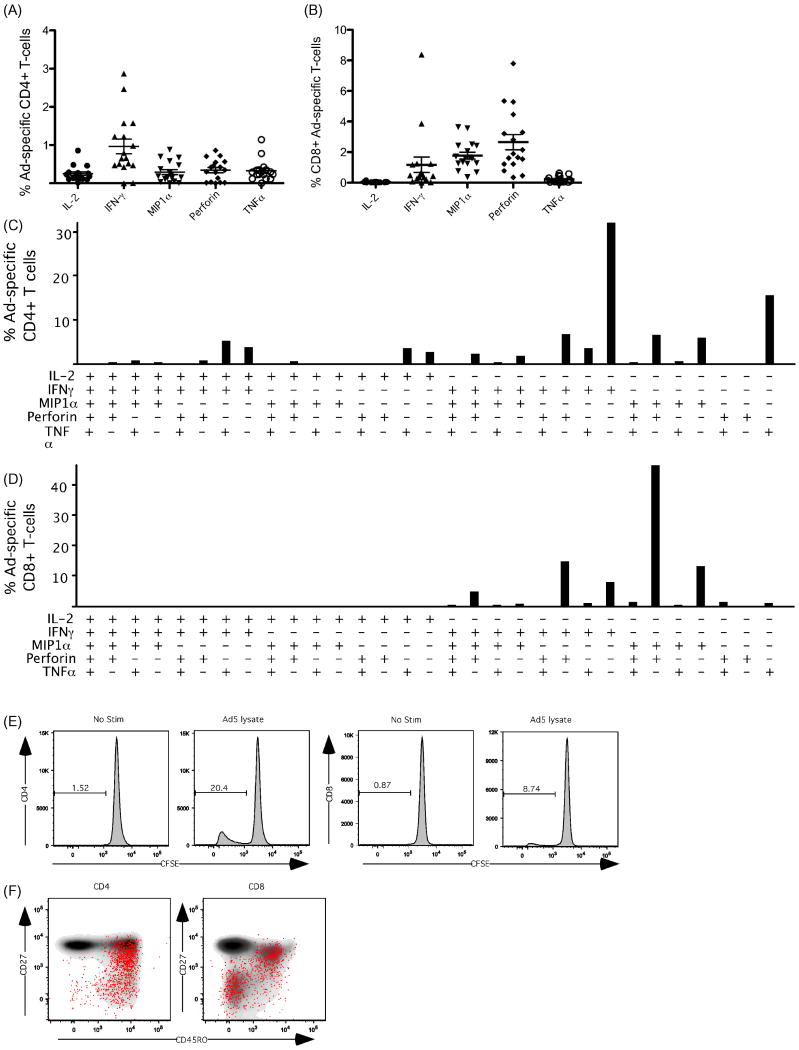
We next assessed the functionality and phenotype of Ad5 reactive T cells in 17 healthy adults. CD4+ and CD8+ T cell responses to Ad5 were detected in all subjects. Most subjects had primarily monofunctional CD4+ T cells that produced IL-2, IFN-γ or TNFα (Figures 3A, C). In contrast, Ad5-specific CD8+ T cells were more polyfunctional, expressing effector-like combinations of IFN-γ, MIP1α and perforin (Figures 3B, D). With the exception of monofunctional responses, nearly all Ad5-specific CD8+ T cells produced perforin together with at least one other function (Figure 3D). Ad5-specific CD4+ and CD8+ T cells were also capable of proliferating upon stimulation (Figure 3E). Ad-specific CD4+ T cells generally exhibited a central memory-like phenotype (CD27+CD45RO+) with a small contribution of effector memory (CD27-CD45RO+) cells (Figure 3F, left panel). In marked contrast, the majority of Ad-specific CD8+ T cells displayed an effector-like phenotype (CD27-CD45RO-), with a smaller contribution of central memory-like cells (Figure 3F, right panel).
Figure 3.
Adenovirus 5 specific T-cell responses are common in humans. We assessed the functionality and phenotype of Ad5-specific T-cells in 17 healthy adults by stimulating PBMCs with whole Ad5 vector and performing intracellular cytokine staining. A) The percentage of Ad5-specific CD4+ T-cells producing IL-2, IFN-γ MIP1α, Perforin and TNFα. B) The percentage of Ad5-specific CD4+ T-cells producing IL-2, IFN-γ, MIP1α, perforin and TNFα. C) The average polyfunctional Ad5-reactive CD4+ T-cell response from 17 donors. D) The average polyfunctional Ad5-reactive CD8+ T-cell response from 17 donors. E) Ad5-reactive CD4+ and CD8+ T-cells proliferate in response to Ad5 stimulation. The percentage of CFSE low dividing cells is shown following no stimulation or stimulation with Ad5 vector. F) Memory phenotype of Ad5-specific CD4+ and CD8+ T-cells. Memory phenotype of all CD4+ and CD8+ T-cells is shown in grey. Red dots represent Ad-specific cells positive for IL-2, IFN-γ, MIP1α, Perforin and or TNFα. Ad5-specific CD4+ cells are primarily central memory like (CD27+CD45RO+) and effector memory (CD27-CD45RO+) phenotypes. Ad5-specific CD8+ T-cells are primarily effector (CD27-CD45RO-) and central memory like (CD27+CD45RO+) phenotypes.
Ad5 hexon-specific responses are commonly observed in humans and are directed against both conserved and variable regions
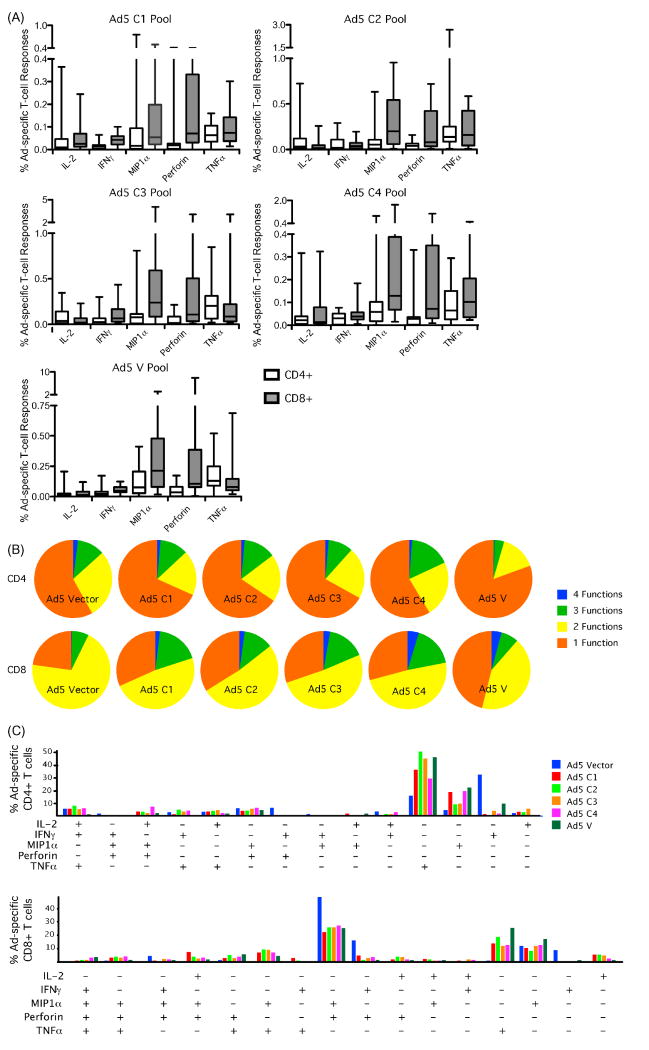
Next, we determined whether Ad5 hexon-specific responses were directed against conserved or variable hexon regions. We also assessed the functionality of responses to the different regions of hexon. The latter analysis was conducted to elucidate differences in responses to the constant regions that had presumably been recalled repeatedly due to infections with different Ad serotypes and those to the variable loops that presumably have a different stimulation history. Starting with overlapping 15-mer peptides spanning the hexon amino acid sequence, we divided the conserved regions into 4 linear pools of ∼ 44 peptides each (C1-C4), and made a single pool consisting of peptides from variable (V) regions. We could readily detect CD4+ T cell responses to all 5 pools (Figure 4A, open bars), indicating that CD4+ T cells can recognize epitopes spread throughout the hexon, with the largest responses in the C2, C3, and V pools. There was no significant difference in the summation of the total response between the C2 and C3 pools compared with the V pool, while responses to the C1 (p<0.001) and C4 (p<0.02) pools were significantly lower. Perforin production tended to be low in the Ad-specific CD4+ T cells, while IL-2, IFN-γ, MIP1α, and TNFα dominated the responses. While there were multifunctional responses (two or more functions simultaneously), the bulk of Ad-specific CD4+ T cells were monofunctional (Figure 4B, top), producing either IFN-γ, IL-2, TNFα, or MIP1α only (Figure 4C, top). The CD4+ T cell responses against the hexon peptide pools were similar in functionality compared to the whole Ad vector, with the exception that whole Ad vector responses were dominated by IFN-γ production, while responses elicited by hexon peptides tended to skew to TNFα production (Figure 4C, top). This difference may be attributed to the different assay conditions used for testing whole vector (overnight) vs. hexon peptides (6 hrs), or could reflect different levels of antigenic stimulation.
Figure 4.
Ad5-specific T-cell recognize variable and conserved regions of the hexon. We assessed the functionality and phenotype of Ad5-specific T-cells in 17 healthy adults by stimulating PBMCs with overlapping 15mer Ad5 hexon peptides. Approximately 35 peptides were combined into 5 pools: 1 for all sequences in the variable regions of the hexon, and 4 containing linear sequences within the conserved regions of the hexon. A) The percentage of CD4+ and CD8+ T-cells responding to the conserved 1 (C1), conserved 2 (C2), conserved 3 (C3), conserved 4 (C4), and variable (V) hexon pools. Cells staining positive for IL-2, IFN-γ, MIP1α, Perforin and or TNFα were summed to compute the total percentage of responding cells. CD4+ responses are depicted in white bars and CD8+ responses in grey. Center line represent the mean with whiskers depicting the standard error. B) Percentage of Ad-specific cells with a polyfunctional response. Pies represent all responding Ad-specific cells making IL-2, IFN-γ, MIP1α, Perforin and or TNFα following stimulation with Ad5 vector or Ad5 hexon pools C1, C2, C3, C4 or V. Each slice represents the proportion of the cells producing four of five cytokines (blue), three of five (green), two of five (yellow) and one of five (orange). C) Percentage of Ad-specific cells with a polyfunctional response. Bars represent the percentage of CD4+ (top) and CD8+ (bottom) T-cells making each combination of IL-2, IFN-γ, MIP1α, Perforin and or TNFα following stimulation with Ad5 vector (blue) or Ad5 hexon pools C1 (red), C2 (neon green), C3 (orange), C4 (pink) or V (dark green). Plus signs represent cells staining positive (+) for each cytokine.
CD8+ T cells also responded potently to Ad hexon (Figure 4A, grey bars). The magnitude of responding CD8+ T cells was higher than that of CD4+ T cells with the largest responses against the C3 and V pool. In contrast to hexon-specific CD4+ T cells, perforin, TNFα, and MIP1α dominated the hexon-specific CD8+ T cell responses, and IL-2 and IFN-γ tended to be lower. The overall level of functionality in the hexon-specific CD8+ T cell response tended to be higher than the CD4+ hexon-specific response, with the majority of CD8+ T cells responding by at least two functions (Figure 4B, bottom). Similar to whole Ad vector responses, hexon-specific CD8+ T cells were highly skewed towards effector like activity, with perforin clearly dominating the entire response. With the exception of monofunctional responses, perforin was present in combination with another function in nearly every hexon-specific CD8+ T cell (Figure 4C, bottom). There was no apparent difference in functionality between hexon-specific CD8+ T cell responses directed against conserved or variable regions.
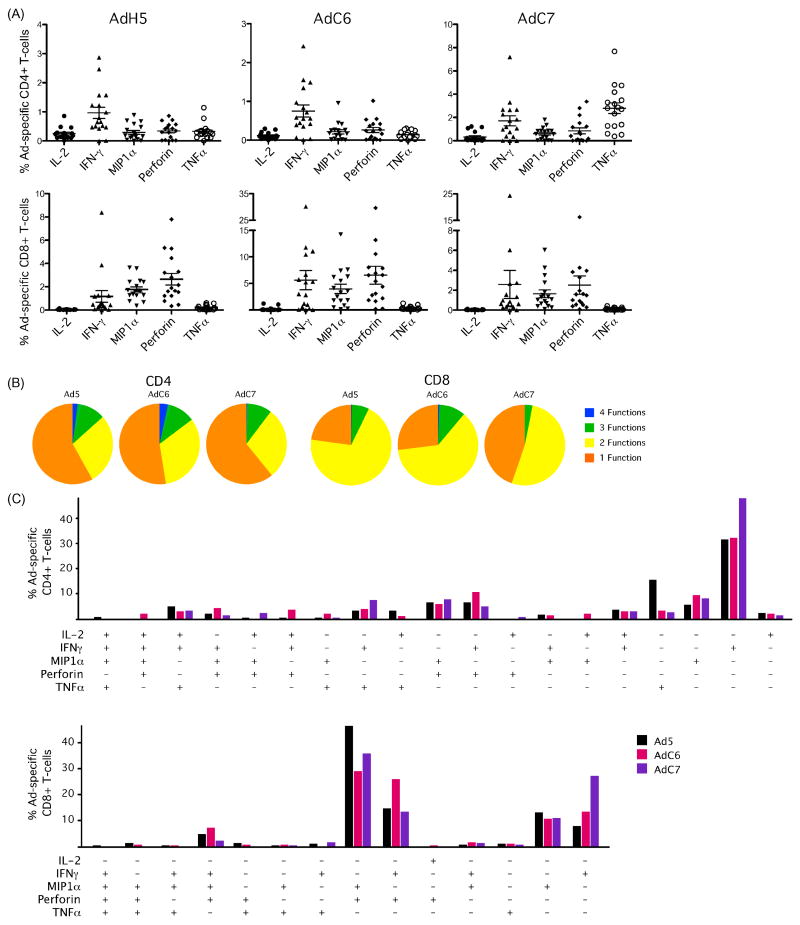
Cross-serotype reactivity of Ad-specific T cells in humans
To test the ability of Ad-specific T cells to cross-react with disparate Ad serotypes, we examined T cell responses against the chimpanzee-derived adenoviruses AdC6 and AdC7, which are only rarely found in humans. In all subjects examined CD4+ T cells responded to Ad5, AdC6 and AdC7, demonstrating a high level of cross-serotype reactivity. The predominant functional response differed between the vectors, with Ad5 and AdC7 inducing mainly IFN-γ, while AdC6 induced primarily TNFα (Figure 4A). Despite these differences, the overall functionality of the Ad-specific CD4+ T cell response was quite similar between all three vectors (Figure 4B, C). The CD4+ T cell response to AdC6 was significantly larger than to Ad5 (p<0.01). Cross-serotype reactive Ad-specific CD8+ T cells were also present in every donor, with no differences in magnitude and a similar degree of functionality (Figure 4B, C). There was no significant difference in the total magnitude of the CD8+ T cell response to Ad5, AdC6 and AdC7, and the functional profiles of cross-reactive Ad-specific CD8+ T cells were also similar.
Discussion
Ad vectors are commonly used to deliver transgenes in gene transfer and vaccination. It is well known that Ad-specific neutralizing antibodies can limit the effectiveness of Ad-based vectors; however the potential role of Ad-specific T cells to further curtail Ad vector efficacy is unclear. Here we provide a minimal estimate of the level of Ad-specific T cell responses in humans. We find that Ad-specific T cell responses are universal, as every subject we tested had a detectable CD4+ and CD8+ T cell response against Ad5, despite a seroprevalence of only 40% in the United States.
Our findings indicate that Ad-specific T cells are readily detectable using replication defective Ad particles or Ad hexon peptides. Although the response magnitude against each particular stimulant may vary between subjects, both CD4+ and CD8+ T cells responded in a similar fashion. Interestingly, Ad-specific T cells appear to be maintained continually in an effector like state, particularly Ad-specific CD8+ T cells. Unlike our observations for common human viral pathogens, including cytomegalovirus, Epstein-Barr virus, and Influenza, Ad-specific CD8+ T cells are highly prone to effector function upon stimulation (Makedonas, et al., manuscript in preparation). This is further manifested in the effector-like memory phenotype maintained by a substantial proportion of the Ad-specific CD8+ T cell response. Due to extensive sequence homology between various human Adenovirus serotypes, Ad-specific T cells in part cross-react and are likely repeatedly stimulated by periodic infections with different Ad serotypes(De Jong and others 1999; Leen and others 2004; Tang and others 2004). As a result, human Ad-specific CD8+ T cells are unlikely to be restricted specifically to a single Ad serotype, and able to recognize not only targets infected with virus homologous to the vector used for expansion but also heterologous virus from diverse serotypes. This high level of cross-reactivity very likely leads to the continual maintenance of Ad-specific CD8+ T cells in an effector-like state, as humans are expected to be repeatedly infected with different serotypes of Ad. Furthermore, Ad viruses persist in lymphatic tissues and if they remain transcriptionally active this would further maintain T cells at an activated state(Tatsis and others 2007).
One reason for the divergence between Ad serostatus and Ad responsive T cells is the presence of common T cell epitopes in conserved regions of the Ad hexon protein, which accounts for ∼80% of the entire hexon sequence. Similar to previous findings obtained with CD4+ and CD8+ T cell lines9, we find that both CD4+ and CD8+ T cell responses can be detected against the conserved and variable hexon peptide pools. Conservation of T cell epitopes in hexon leads to cross-reactivity even among divergent serotypes from chimpanzees, AdC6 and AdC7. Because of this high degree of cross-reactivity, it is impossible to know whether, for example, Ad5-reactive T cells are truly Ad5-specific and generated from natural Ad5 infection, or simply cross-reacting with Ad5 following infection with another Ad serotype. Finally, due to this extensive cross-reactivity, it is likely that transgene product-specific immune responses induced by Ad vectors derived from rare human serotypes, which are currently under development, may still be affected by Ad-specific cytotoxic T cells capable of recognizing Ad vector transduced cells.
Figure 5.
Ad-specific T-cells are cross reactive. Cross reactivity of Ad-specific T-cells was measured in seventeen healthy donors by stimulating PBMCs overnight with human Adenovirus 5 (Ad5), and chimpanzee 6 (AdC6) and chimpanzee 7 (AdC7) followed by intracellular cytokine staining. A) The total percentage of Ad5, AdC6 and AdC7 CD4+ (top row) and CD8+ (bottom row) T-cells. Ad-specific cells stained positive for IL-2, IFN-γ, MIP1α, Perforin and or TNFα. B) Percentage of Ad-specific cells with a polyfunctional response. Pies represent all responding Ad-specific cells making IL-2, IFN-γ, MIP1α, Perforin and or TNFα following stimulation with Ad5 AdC6 or AdC7 vector. Each slice represents the proportion of the cells producing four of five cytokines (blue), three of five (green), two of five (yellow) and one of five (orange). C) Percentage of Ad-specific cells with a polyfunctional response. Bars represent the percentage of CD4+ (top) and CD8+ (bottom) T-cells making each combination of IL-2, IFN-γ, MIP1α, Perforin and or TNFα following stimulation with Ad5 (black) AdC6 (pink) and AdC7 (purple) vector. Plus signs represent cells staining positive (+) for each cytokine.
Acknowledgments
This work was supported by NIH IPCAVD U19AI074078 to M.B. and H.E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Calcedo R, Vandenberghe LH, Roy S, Somanathan S, Wang L, Wilson JM. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol. 2009;83:2623–31. doi: 10.1128/JVI.02160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA, Chen M, Long R, Mckelvey T, Chastain M, et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78:11434–8. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford-Miksza L, Schnurr DP. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70:1836–44. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong JC, Wermenbol AG, Verweij-Uijterwaal MW, Slaterus KW, Wertheim-Van Dillen P, Van Doornum GJ, Khoo SH, Hierholzer JC. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbiol. 1999;37:3940–5. doi: 10.1128/jcm.37.12.3940-3945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–54. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Myers GD, Bollard CM, Huls MH, Sili U, Gee AP, Heslop HE, Rooney CM. T-cell immunotherapy for adenoviral infections of stem-cell transplant recipients. Ann N Y Acad Sci. 2005;1062:104–15. doi: 10.1196/annals.1358.013. [DOI] [PubMed] [Google Scholar]
- Leen AM, Sili U, Vanin EF, Jewell AM, Xie W, Vignali D, Piedra PA, Brenner MK, Rooney CM. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–40. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, et al. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J Virol. 2007;81:6594–604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–78. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–85. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- Tang J, Olive M, Champagne K, Flomenberg N, Eisenlohr L, Hsu S, Flomenberg P. Adenovirus hexon T-cell epitope is recognized by most adults and is restricted by HLA DP4, the most common class II allele. Gene Ther. 2004;11:1408–15. doi: 10.1038/sj.gt.3302316. [DOI] [PubMed] [Google Scholar]
- Tang J, Olive M, Pulmanausahakul R, Schnell M, Flomenberg N, Eisenlohr L, Flomenberg P. Human CD8+ cytotoxic T cell responses to adenovirus capsid proteins. Virology. 2006;350:312–22. doi: 10.1016/j.virol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-Mccoy KC, Hensley SE, Zhou D, Lin SW, Bian A, Xiang ZQ, Iparraguirre A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–23. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltrop-Duits LA, Heemskerk B, Sombroek CC, Van Vreeswijk T, Gubbels S, Toes RE, Melief CJ, Franken KL, Havenga M, Van Tol MJ, et al. Human CD4+ T cells stimulated by conserved adenovirus 5 hexon peptides recognize cells infected with different species of human adenovirus. Eur J Immunol. 2006;36:2410–23. doi: 10.1002/eji.200535786. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, Kalish ML, Ertl HC. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12:1596–9. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]