Abstract
Toll-like receptors (TLRs) are germline-encoded receptors that recognize various pathogen-associated molecular patterns (PAMPs). They are key components of the innate immunity which are activated in response to pathogens as well as non-pathogenic components of damaged tissues. TLR agonists have been developed to treat allergies, cancers, and chronic infections by upregulating the innate immune system. TLR antagonists may be used to treat a number of inflammatory conditions, such as rheumatoid arthritis and systemic lupus erythematosus. Recent research also has shown that TLRs are involved in the pathogenesis of atherosclerosis, thrombosis, myocardial remodeling, ischemic/reperfusion injury, and valvular disease. This article reviews the current experimental and clinical evidence for the role of TLRs in the cardiovascular system, and examines the mechanisms by which TLR antagonists could potentially be used in targeted therapy.
Keywords: Atherosclerosis, Cardiovascular disease, Myocardial Ischemic/reperfusion injury, Thrombosis, TLR antagonist, TLRs, Valvular disease
Introduction
The innate immune system has long been regarded as the first line of defense against foreign pathogens. However, more recently it is recognized that the involvement of nonpathogenic components of necrotic or damaged tissues can activate innate immunity. Thus, rather than simply responding to “foreign” material, the innate immune system responds to “danger” signals that can be either microbial or endogenous in origin [1]. Toll-like receptors (TLRs) are germline-encoded receptors that recognize an array of pathogen-associated molecular patterns. TLRs induce a rapid innate immune response and serve as a bridge to long-term adaptive immune responses. There are 10 human and 13 murine TLRs characterized to date. Each recognizes different ligands that produce distinctive responses depending on the context, such as the nature of the “danger” signal and the specific cell type activated. The last decade has seen major advances in the field of TLR research. As further insight is gained, various drugs have been developed to target individual receptors for the treatment of specific diseases. Most of the TLR drugs marketed today or that are in development are agonists. The rationale behind TLR agonist development centers on the activation of effector cells of the innate immune system in order to trigger the adaptive immune response against specific targets, such as viruses and cancer cells. Numerous clinical trials have been launched to study the efficacy and safety profile of TLR agonists directed at treating allergies, cancer, and chronic infections such as HCV and HIV.
Investigations of TLR agonists have also drawn interest into the prospective use of TLR antagonists to decrease inflammatory responses mediated by the innate immune system. For example, TLR4 antagonists Eritoran and Tak242 were developed for the treatment of severe sepsis. In addition to infectious agents, TLRs have also been shown to respond to a variety of endogenous ligands derived from substances released as a result of cell death or tissue injury [2]. Thus, other research efforts are under way to study the role of TLR signaling in the pathogenesis of noninfectious conditions such as lupus, rheumatoid arthritis, inflammatory bowel disease, and interestingly, cardiovascular diseases.
In addition to the classic immune cells such as macrophages and monocytes, other non-bone marrow-derived cells were also shown to actively participate in the inflammatory cascade through TLR signaling. TLRs are expressed in almost all cells of the heart—TLR2, 3, 4, and 6 are found in cardiomyocytes, and TLR1 through 6 are found in the smooth muscle and endothelial cells of the vasculature [3]. Therefore, it is not surprising that TLRs are involved in the development of atherosclerosis, thrombosis, myocardial remodeling, ischemic/reperfusion injury, and even valvular disease. Here we review the current experimental and clinical evidence for the role of TLRs in the cardiovascular system and examine the mechanisms by which TLR antagonists could potentially be used in targeted therapy.
Atherosclerosis and Coronary Artery Disease
Atherosclerosis is a chronic inflammatory disease of the arterial vasculature, largely due to the interplay between modified lipoproteins and cytokines, which eventually results in plaque initiation and progression (Figure 1). There is evidence that TLRs, notably TLR2 and TLR4, are involved in the development of atherosclerotic disease.
Figure 1.
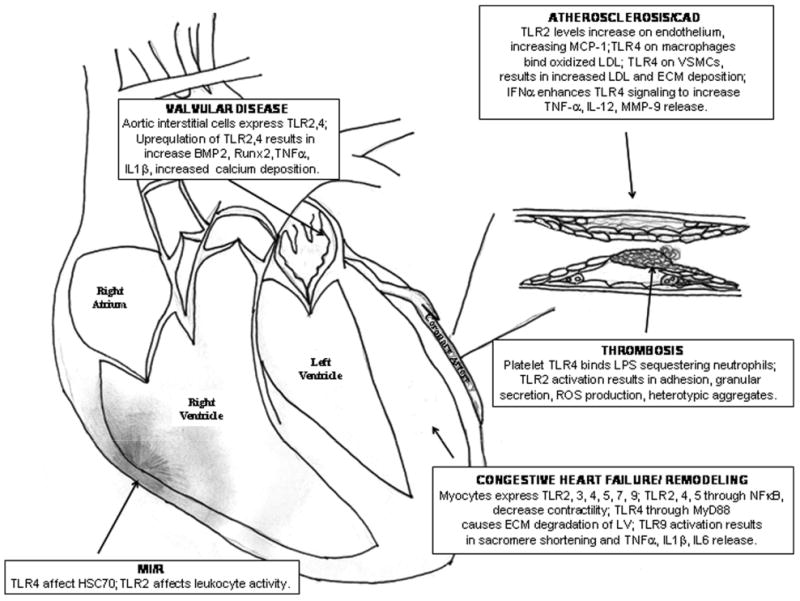
Schematic summarizing the role of TLRs in cardiovascular disease.
TLR2 expression was recently shown to be increased in endothelial cells in the regions with disturbed blood flow in atherosclerosis-prone LDLr−/− mice. Furthermore, diet-induced hyperlipidemia increased TLR2 expression at these sites [4]. TLR2 has also been shown to be critical in the progression of atherosclerosis in mouse models. Inactivation of TLR2 leads to a reduction in lipid accumulation in ApoE−/− mice as well as a reduction in chemokine production, specifically MCP-1 levels. These findings suggest that TLR2 is important in atherosclerosis independent of dietary cholesterols [5].
TLR4 also plays an important role in atherosclerotic development and progression. TLR4 is expressed by macrophages in both human atherosclerotic lesions and at the site of plaque rupture in patients with acute myocardial infarction [6]. It has been established that oxidized LDL and its components serve as endogenous TLR4 ligands [7]. Levels of endogenous TLR ligands, EDA and heat shock protein 60 (hsp60), and the mRNA encoding TLR2 and TLR4 are increased in ApoE−/− mice with advanced atherosclerosis [8]. TLR4 protein expression was also shown to be upregulated in vascular smooth muscle cells (VSMCs) of atherosclerotic arteries [9]. Signaling through TLR4 in VSMCs resulted in a proinflammatory phenotype and increased LDL and extracellular matrix deposition, which contributes to the acceleration of atherosclerotic lesions [10].
Studies have also shown that exogenous ligands, such as lipopolysaccharide (LPS) in sepsis or components of Porphyromonas gingivalis in periodontal infections, accelerate atherosclerosis by engaging TLRs. In addition, IFN-α, produced by plasmacytoid dendritic cells in atherosclerotic plaques, was found to enhance TLR4 signaling by sensitizing these cells to the TLR4 ligand, LPS. IFN-α upregulation of TLR4 expression increases the production of TNF-α, IL-12, and MMP-9, some of the key players in plaque destabilization [11]. These data suggest that TLRs may be the link between inflammation and atherosclerotic disease, in the presence or absence of infection [12].
It is unclear whether TLR2 and TLR4 independently or cooperatively contribute to atherosclerosis. There is additional evidence to suggest that there may be synergy between the two receptors in the progression of atherosclerosis. Transfection of cDNA encoding human TLR2 and TLR4 in the carotid arterial walls of rabbits accelerates atherosclerosis, while a less significant effect is seen with the transfection of either receptor individually [13].
The evidence accumulated by basic research suggests that TLRs play a critical role in atherosclerosis and coronary artery disease, which has led many groups to examine their clinical significance. One group has found that TLR2 and TLR4 expressions correlated with the extent and severity of coronary artery disease in patients with stable angina [14].
Thrombosis
Platelets are key components of thrombosis, a process in which vessel damage results in clot formation, eventually leading to ischemia of the damaged area (Figure 1). Recent studies have shown that human platelets express TLRs [15,16]. The functionality of TLRs in platelets was demonstrated in several studies focused on the stimulation of TLR4 by LPS, thereby inducing neutrophil sequestration and thrombocytopenia. In addition, TLR4 stimulation causes the priming of platelet aggregation activated by epinephrine, ADP, and arachidonic acid [17,18]. Besides TLR4, TLR2 is also a functional receptor in platelets [19]. Treatment with PAM3CSK4, a synthetic ligand of the TLR2/TLR1 heterodimer, directly resulted in platelet aggregation, adhesion, granular secretion, interaction with leukocytes, and reactive oxygen species (ROS) production. These effects were mediated by PI3-K/Akt signaling [19].
Based on these findings, it is likely that the activation of the innate immune system, either by pathogens or by tissue injury, can lead to thrombosis and subsequent coronary events. These mechanisms could explain the relationship between inflammation and thrombosis, as described in several studies [20,21]. Further studies are needed to investigate the clinical significance of TLR upregulation in thrombosis and myocardial ischemia.
Congestive Heart Failure and Myocardial Remodeling
Congestive heart failure occurs in many pathological conditions including coronary artery disease, valvular disease, and hypertension. Particularly following myocardial infarction, a compensatory but maladaptive remodeling occurs in order to maintain cardiac output (Figure 1). However, this remodeling process leads to diastolic impairment and further decline of cardiac function.
There is evidence that TLRs are involved in the pathogenesis of heart failure and myocardial remodeling. Studies have shown that TLR2, 3, 4, 5, 7, and 9 are expressed on cardiac myocytes. TLR2, 4, and 5 signaling results in a robust inflammatory response via NFκB, as well as decreased contractility both in human myocardium and in murine cardiomyocyte cell lines [22,23].
Among the TLR family, TLR4 is the most extensively studied in the pathogenesis of cardiomyopathy. TLR4 activation not only triggers an inflammatory response but also results in extracellular matrix degradation, an important step in left ventricular (LV) remodeling. On the other hand, TLR4 deficiency leads to a reduction in LV remodeling and a preservation of systolic function post-myocardial infarction (MI) in mice [24]. In addition, studies using TLR4−/− mice demonstrated a concomitant decrease in protein levels of TLR4 adaptor MyD88 and an increase in antihypertrophic JNK signaling, resulting in a significantly improved survival post-MI [25]. Another study investigating the prognostic value of activated monocytic TLR4 after acute myocardial infarction found that activated TLR4 was an independent predictor of 30-day major adverse clinical outcomes, including advanced Killip score, overt congestive heart failure, New York Heart Association (NYHA) class ≥2, or death [26].
Interestingly, one recent study also implicates TLR9 in the reduction of myocardial contractility by showing the inhibitory effects of CpG-ODN, a synthetic exogenous TLR9 ligand, on sarcomeric shortening. In addition, TLR9 activation in cardiomyocytes leads to a strong inflammatory response marked by the release of TNF-α, IL-1β, IL-6, and activation of NFκB and iNOS [27].
Myocardial Ischemic/Reperfusion Injury
Myocardial ischemic/reperfusion (MI/R) injury is caused by the restoration of blood flow to the heart following an ischemic event. Ironically, this reperfusion process leads to an inflammatory response that causes further damage to viable tissue around the infarct, likely through accelerated apoptosis (Figure 1). TLRs are implicated in MI/R injury, suggested by the fact that the use of a TLR4 antagonist Eritoran in mice protects against this injurious process [28]. This protective mechanism may be attributed to attenuated inflammation, such as decreased myeloperoxidase activity, leading to smaller infarcts compared to controls [29]. Further studies have suggested that extracellular heat shock cognate protein 70 (HSC70), released by the myocardium during MI/R injury, plays a key role in the postischemic inflammatory response via TLR-4 signaling [30]. TLR2 also plays a role in MI/R injury, likely related to its modulation of leukocyte activity, which mediates coronary endothelial dysfunction [31].
Valvular Heart Disease
Few studies have investigated the role of the innate immunity on valvular disease, which often involves calcification (Figure 1). Recent research has shown that human aortic valve interstitial cells express TLR2 and TLR4. Furthermore, TLR2 and TLR4 upregulation results in an increase in gene expression of osteogenic factors, such as BMP-2 and Runx2, likely contributing to the pathogenesis of aortic stenosis [32,33]. A small-scale study showed that plasma and tissue TNF-α and IL-6 levels correlated with the amount of calcium deposits in human aortic valve stenosis, which may be a result of upregulated TLR2 and TLR4 [34]. More studies are needed to examine the role of the innate immunity and TLRs in valvular heart disease.
Potential Uses of TLR Antagonists
As more data emerge supporting the role of TLRs in various cardiovascular diseases, there is a growing interest in therapeutics targeting TLRs and components of the downstream proinflammatory signaling cascade.
Since TLRs contribute significantly to the pathogenesis of atherosclerosis and other cardiovascular diseases, researchers have been prompted to study the effects of available anti-inflammatory cardiovascular drugs on TLR activity. For instance, statins have been shown to inhibit the TLR4-mediated inflammatory response in certain individuals with a specific TLR4 genotype, explaining the added benefit of statins on the cardiovascular risk of a specific subset of the population [35]. One study showed that fluvastatin negatively regulates monocyte TLR4 signaling in patients with congestive heart failure, suggesting a possible beneficial effect of statins on cardiac remodeling [36]. In addition, endothelial lipase was shown to be upregulated by LPS through TLR4, which leads to the uptake of LDL by macrophages. This increase was shown to be blocked by simvastatin [37]. Thus, statins could provide an additional level of cardioprotection by modulating TLR activity, secondary to its well-established effects on hyperlipidemia.
Angiotensin receptor blockers (ARBs) have been shown to have TLR antagonist activity, a study based on the rationale that angiotensin II is involved in the vascular inflammatory response [38]. Stimulation with TNF-α and angiotensin II increased TLR4 mRNA levels in cultured human VSMCs [9]. Candesartan inhibits PAM3CSK4 and LPS-induced TLR2 and TLR4 mRNA and protein expression in human monocytes in vitro [39]. Thus, ARBs, in addition to their antihypertensive and cardiac remodeling effects, have potential added benefits in treating other types of cardiovascular diseases by modulating TLR-mediated inflammatory response.
Although some currently marketed drugs have shown to have TLR antagonist activity, targeted TLR2 and TLR4 antagonists may prove to be more effective. Drugs can be developed to target several different steps in TLR2 and TLR4 signaling: (1) interaction between the ligand and receptor; (2) interaction between the receptor and adaptors of the signaling pathway; and (3) enzymatic activity of downstream factors. Blocking of the ligand–receptor interaction can be done either by using a neutralizing antibody, soluble decoy receptors, or a mimetic ligand. For example, synthetic derivatives of LPS lipid A from P. gingivalis were found to be potent antagonists of human TLR4, as shown by Zhang et al. [40]. Soluble forms of human TLR2 (sTLR2) have been shown to be released by monocytes, and the depletion of sTLR2 resulted in an exaggerated inflammatory response [41]. Patients with post-MI heart failure have been shown to have markedly decreased sTLR2 compared to controls [42]. Anti-TLR4 neutralizing antibodies were also found in many studies to suppress NFκB activity, making it another potential for drug development [43]. Thus, development of synthetic, soluble TLRs may be an effective way to block TLR signaling.
Downstream targets of TLR signaling are also candidates for drug design. Adaptors such as MyD88 and Mal, as well as kinases like IRAK, p38, and JNK, could be antagonized to attenuate TLR-mediated inflammation.
The therapeutic effect of two TLR4 antagonists, including E5564 (Eritoran) by Eisai, Inc., and TAK-242 by Takeda Pharmaceutical Company, are currently undergoing phase III clinical trials, mainly for the treatment of severe sepsis. Eisai announced in 2005 that phase II trials for Eritoran showed a 12% reduction in the mortality rate in septic patients in the high-dose treatment group compared to placebo [44]. The drug was largely well-tolerated, although self-limited phlebitis was noted in 6.7% of the patients. For cardiovascular diseases, Eritoran also seemed to have some preclinical benefits. As previously mentioned, it was shown to attenuate myocardial I/R injury by inhibiting TLR4 [28]. Thus, more experiments are warranted to study the therapeutic and side effects of Eritoran and TAK-242 on other TLR4-mediated cardiovascular diseases. Currently, there are no additionally published clinical trial data looking at TLR antagonists as a therapeutic for cardiovascular diseases.
Conclusions
There is growing evidence implicating TLR2 and TLR4 in the pathogenesis of atherosclerosis, thrombosis, congestive heart failure/myocardial remodeling, ischemic/reperfusion injury, and valvular disease (Figure 1). Antagonism of these TLRs may modulate the inflammatory response and potentially lessen the deleterious processes that lead to the pathogenesis of certain cardiovascular diseases. More studies are necessary to examine the use of these drugs in specific cardiovascular diseases, along with long-term evaluation of the effects of TLR antagonists.
Acknowledgments
We would like to thank Hannah Iafrati for her assistance with the illustration.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
References
- 1.Matzinger P. The danger model: A renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 2.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: Toll-like receptor 4—sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 4.Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC., III Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa Y, Satoh M, Itoh T, Minami Y, Takahashi Y, Akamura M. Local expression of toll-like receptor 4 at the site of ruptured plaques in patients with acute myocardial infarction. Clin Sci. 2008;115:133–140. doi: 10.1042/CS20070379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 8.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atheroscerotic lesion development and Toll-like receptor 2 and 4. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M. Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels. 2007;22:416–422. doi: 10.1007/s00380-007-1001-1. [DOI] [PubMed] [Google Scholar]
- 10.Loppnow H, Werdan K, Buerke M. Vascular cells contribute to atherosclerosis by cytokine and innate-immunity-related inflammatory mechanisms. Innate Immun. 2008;14:63–87. doi: 10.1177/1753425908091246. [DOI] [PubMed] [Google Scholar]
- 11.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 12.Gibson FC, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci. 2008;13:2041–2059. doi: 10.2741/2822. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara M, Hirata K, Yamashita T, Takaya T, Sasaki N, Shiraki R. Local expression of toll-like receptors at the vessel wall induces atherosclerotic lesion formation: Synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol. 2007;27:2384–2391. doi: 10.1161/ATVBAHA.106.139253. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi E, Orihara K, Hamasaki S, Ishida S, Kataoka T, Ogawa M. Association between toll-like receptors and the extent and severity of coronary artery disease in patients with stable angina. Coron Artery Dis. 2007;17:31–38. doi: 10.1097/MCA.0b013e328010a474. [DOI] [PubMed] [Google Scholar]
- 15.Shiraki R, Inoue N, Kawasaki S, Takei A, Kadotani M, Ohnishi Y. Expression of toll-like receptors on human platelets. Thromb Res. 2004;113:379–385. doi: 10.1016/j.thromres.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, Garraud O. Evidence of toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 17.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 18.Montrucchio G, Bosco O, Del Sorbo L, Fascio Pecetto P, Lupia E, Goffi A. Mechanisms of the priming effect of low doses of lipopolysaccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb Haemost. 2003;90:872–881. doi: 10.1160/TH03-02-0085. [DOI] [PubMed] [Google Scholar]
- 19.Blair PS, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S. Stimulation of toll-like receptor 2 in human platelets induces a thrombo-inflammatory response through activation of phosphoinositide 3-kinase. Circ Res. 2009;104:346–354. doi: 10.1161/CIRCRESAHA.108.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 21.Jayachandran M, Brunn GJ, Karnicki K, Miller RS, Owen WG, Miller VM. In vivo effects of lipopolysaccharide and TLR4 on platelet production and activity: Implications for thrombotic risk. J Appl Physiol. 2007;102:429–433. doi: 10.1152/japplphysiol.01576.2005. [DOI] [PubMed] [Google Scholar]
- 22.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT. TLR4 expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyocytes decreases contractility and initiates an NF-κB dependent inflammatory response. Cardiovasc Res. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–264. doi: 10.1161/CIRCRESAHA.107.158220. [DOI] [PubMed] [Google Scholar]
- 25.Riad A, Jäger S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol. 2008;180:6954–6961. doi: 10.4049/jimmunol.180.10.6954. [DOI] [PubMed] [Google Scholar]
- 26.Sheu JJ, Chang LT, Chiang CH, Youssef AA, Wu CJ, Lee FY. Prognostic value of activated toll-like receptor-4 in monocytes following acute myocardial infarction. Int Heart J. 2008;49:1–11. doi: 10.1536/ihj.49.1. [DOI] [PubMed] [Google Scholar]
- 27.Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rüdiger M. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: Role of toll-like receptor 9. Cardiovasc Res. 2008;78:26–35. doi: 10.1093/cvr/cvn011. [DOI] [PubMed] [Google Scholar]
- 28.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ. Inhibition of toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:270–274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 29.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 30.Zou N, Ao L, Cleveland JC, Jr, Yang X, Su X, Cai GY. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:2805–2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF. Toll-like receptors 2-deficient mice are protected against post-ischemic coronary dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 32.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M. Expression of functional toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:29–35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 33.Babu AN, Meng X, Zou N, Yang X, Wang M, Song Y. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86:71–76. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Toli K, Paraskevas KI, Poulakou MV, Agrogiannis G, Kavantzas N, Xanthopoulos V. Association between plasma levels and immunolocalization of cytokines in heart valve lesions: A possible target for treatment? Expert Opin Ther Targets. 2008;12:1209–1215. doi: 10.1517/14728222.12.10.1209. [DOI] [PubMed] [Google Scholar]
- 35.Hodgkinson CP, Ye S. Statins inhibit toll-like receptor-mediated lipopolysaccharide signaling and cytokine expression. Pharmacogenet Genomics. 2008;18:803–813. doi: 10.1097/FPC.0b013e3283050aff. [DOI] [PubMed] [Google Scholar]
- 36.Földes G, von Haehling S, Okonko DO, Jankowska EA, Poole-Wilson PA, Anker SD. Fluvastatin reduces increased blood monocyte toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int J Cardiol. 2008;124:80–85. doi: 10.1016/j.ijcard.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda T, Hirata K, Ishida T, Kojima Y, Tanaka H, Okada T. Endothelial lipase is increased by inflammation and promotes LDL uptake in macrophages. J Atheroscler Thromb. 2007;14:192–201. doi: 10.5551/jat.e502. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Lemus E, Murakami Y, Larrayoz-Roldan IM, Moughamian AJ, Pavel J. Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology. 2008;149:5177–5188. doi: 10.1210/en.2008-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasu MR, Riosvelasco AC, Jialal I. Candesartan inhibits toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis. 2009;202:76–83. doi: 10.1016/j.atherosclerosis.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Gaekwad J, Wolfert MA, Boons GJ. Synthetic tetra-acylated derivatives of lipid A from Porphyromonas gingivalis are antagonists of human TLR4. Org Biomol Chem. 2008;6:3371–3381. doi: 10.1039/b809090d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M. Soluble forms of toll-like receptor 2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol. 2003;171:6680–6689. doi: 10.4049/jimmunol.171.12.6680. [DOI] [PubMed] [Google Scholar]
- 42.Ueland T, Espevik T, Kjekshus J, Gullestad L, Omland T, Squire IB. Mannose binding lectin fand soluble toll-like receptor 2 in heart failure following acute myocardial infarction. J Card Fail. 2006;12:659–663. doi: 10.1016/j.cardfail.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Blanco AM, Vallés SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 44.Wickelgren I. Targeting the tolls. Science. 2006;312:184–187. doi: 10.1126/science.312.5771.184. [DOI] [PubMed] [Google Scholar]


