Abstract
Background:
The lung allocation score (LAS) was initiated in May 2005 to allocate lungs based on medical urgency and posttransplant survival. The purpose of this study was to determine if there is an association between an elevated LAS at the time of transplantation and increased postoperative morbidity and mortality.
Methods:
The United Network for Organ Sharing provided de-identified patient-level data. Analysis included lung transplant recipients aged ≥ 12 years who received transplants between April 5, 2006, and December 31, 2007 (n = 3,836). Recipients were stratified into three groups: LAS < 50 (n = 3,161, 83.87%), LAS 50 to 75 (n = 411, 10.9%), and LAS ≥ 75 (n = 197, 5.23%), referred to as low LAS (LLAS), intermediate LAS (ILAS), and high LAS (HLAS), respectively. The primary outcome was posttransplant graft survival at 1 year. Secondary outcomes included length of stay and in-hospital complications.
Results:
HLAS recipients had significantly worse actuarial survival at 90 days and 1 year compared with LLAS recipients. When transplant recipients were stratified by disease etiology, a trend of decreased survival with elevated LAS was observed across all major causes of lung transplant. HLAS recipients were more likely to require dialysis or to have infections compared with LLAS recipients (P < .001). In addition, length of stay was higher in the HLAS group when compared with the LLAS group (P < .001).
Conclusions:
HLAS is associated with decreased survival and increased complications during the transplant hospitalization. Whereas the LAS has improved organ allocation through decreased waiting list deaths and waiting list times, lower survival and higher morbidity among HLAS recipients suggests that continued review of LAS scoring is needed to ensure optimal long-term transplant survival.
The lung allocation score (LAS) was adapted by the Organ Procurement and Transplant Network as well as the United Network for Organ Sharing (UNOS) in May 2005 in an effort to improve organ allocation and transplant outcomes.1 Before the LAS, organs were allocated based on time accrued on the waiting list.2,3 An increase in the number of patients awaiting transplantation as well as a heightened concern for improved survival following transplantation led to the development of the LAS.4,5
The LAS, which ranges from 0 to 100, was developed as a multivariate model that is a weighted combination of predicted risk of waiting list death and the predicted likelihood of survival during the first year after transplantation.6 The LAS weighs expected survival on the waiting list more heavily than expected posttransplant survival. A concern during initiation of the LAS was whether a score that places greater emphasis on waiting list urgency would result in increased posttransplant morbidity and mortality. Previous studies demonstrated that implementation of the LAS system decreased waiting list mortality and waiting time, with no significant effect on survival.7-10 However, during the LAS era, higher rates of primary graft dysfunction and increased intensive care unit stays have also been observed.7
The objective of this study is to determine if posttransplant outcomes, including morbidity and mortality, are associated with LAS at the time of transplantation. We hypothesize that extremely high LAS at the time of transplantation is associated with poor posttransplant outcomes.
Materials and Methods
Data Collection
Use of data in this analysis is consistent with the regulations of our university’s institutional review board and the UNOS Data Use Agreement. The Standard Transplant Analysis and Research Dataset was provided by UNOS (data source No. 022009-3). The dataset contains information collected from the UNetsm database forms, including the Transplant Candidate Registration form, the Transplant Recipient Registration form, and the Transplant Recipient Follow-up form. These data are the basis for the UNOS Thoracic Registry.
Study Population
UNOS provided de-identified data for all lung transplant candidates and recipients in the United States aged 12 and older with reported LAS between April 5, 2006, and December 31, 2007 (n = 3,836). Follow-up data were provided through February 20, 2009. Patients were followed from the date of transplant until death, retransplantation, or the last known follow-up, which was the last day of follow-up data provided by UNOS. Recipients who underwent simultaneous transplantation of another organ (n = 10, 0.26%) and those with missing LAS data (n = 57, 1.4%) were excluded from the analysis.
Outcome Measures
The primary end point was posttransplant graft survival at 1 year. Secondary end points included: length of stay during the transplant hospitalization, in-hospital complications (primary graft failure [PGF] at 30 days, dialysis, any antibiotic-treated infection, airway dehiscence, and stroke), and bronchiolitis obliterans (BO)-free survival.
Data Analysis
The study population was divided into three groups by absolute LAS score to determine if a stepwise relationship existed between LAS and the outcomes of interest. Because no patient in the dataset was transplanted with LAS of less than 25 and the maximum LAS is 100, the three groups were divided by 25-point intervals. Recipients with LAS < 50, LAS of 50 to 75, and LAS ≥ 75 are referred to as low LAS (LLAS), intermediate LAS (ILAS), and high LAS (HLAS), respectively. Continuous variables were reported as means ± interquartile range. Baseline categorical variables were compared using a χ2 test, while continuous variables with parametric distributions were compared using an analysis of variance with Bonferroni correction for post hoc group comparison. The conventional P value of .05 or less was used to determine the level of statistical significance. All reported P values are two sided. All data were analyzed using the statistical software package Stata 9 (Stata Corp; College Station, TX).
Survival Analysis
Survival analysis was performed using the Kaplan-Meier actuarial method. To assess the impact of the LAS on early and late mortality, the incidence rate of death (IRD) was calculated at multiple time intervals (0-1 years, 1-2 years, and 2-3 years). The Kaplan-Meier analysis with a log-rank test was used to assess BO-free survival. For this analysis, recipients were censored at the time of death, retransplantation, or last known follow-up.
Results
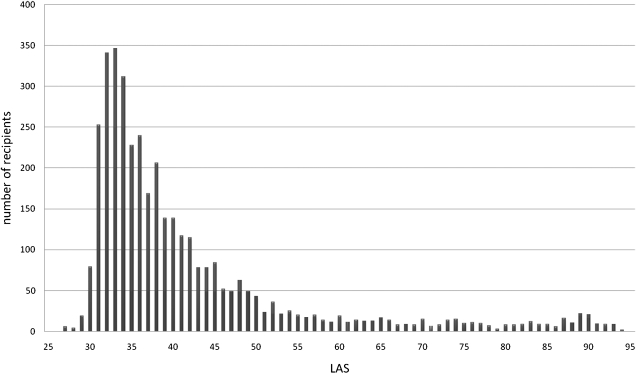
A total of 3,769 lung transplant recipients were included in the analysis. LAS scores were distributed as follows: LLAS (n = 3,161, 83.87%), ILAS (n = 411, 10.9%), and HLAS (n = 197, 5.23%). Mean follow-up time was 1.54 ± 0.90 (0-3.49) years. The distribution of LAS was right skewed (Fig 1), with a mean LAS of 42.1 ± 13.4 (range = 27.7-94.2). Baseline donor and recipient characteristics are shown in Table 1. There was no significant difference in baseline donor age, recipient age, peripheral vascular disease in the recipient, pulmonary infection in the donor, and diabetes in the donor. Recipients in the HLAS group had a slightly higher BMI compared with the LLAS group. The proportion of diabetic recipients, recipients on glucocorticoids, recipients with active infection, and recipients hospitalized and intubated at the time of transplant was higher in the HLAS and ILAS groups relative to the LLAS group. There were a higher proportion of double lung transplants in the ILAS and HLAS groups, and the ischemic time was slightly higher in the HLAS vs LLAS group. Receiver operating characteristic curves generated by plotting sensitivity on the ordinate and one-specificity on the abscissa with LAS as a continuous variable and mortality (at 1 year) as a binary outcome gave an area under the curve of 0.589 (0.566-0.611).
Figure 1.
Distribution of LAS in lung transplant recipients. LAS =lung allocation score.
Table 1.
—Baseline Donor and Recipient Characteristics and Recipient Pulmonary Disease Causes
| Characteristics | LLAS | ILAS | P Value | HLAS | P Value |
| No.a | 3161 | 411 | 197 | ||
| Age, recipient (IQR) | 51.81 (17) | 52.18 (16) | .6143 | 51.64 (20) | .877 |
| BMI, recipient (IQR) | 24.46 (7.52) | 24.59 (8.09) | .6063 | 25.17 (7.07) | .0483 |
| Diabetes mellitus, recipient (%) | 503 (15.91) | 103 (25.06) | < .001 | 50 (25.38) | < .001 |
| eGRF, recipient (IQR) | 58.59 (6.33) | 58.26 (7.29) | .3301 | 55.96 (12.33) | < .001 |
| Glucocorticoids, recipient (%) | 1479 (46.79) | 230 (55.96) | < .001 | 123 (62.44) | < .001 |
| Infection, recipient (%) | 286 (9.04) | 72 (17.52) | < .001 | 55 (27.92) | < .001 |
| Peripheral vascular disease, recipient (%) | 20 (0.63) | 4 (0.97) | .427 | 2 (1.02) | .519 |
| Double lung transplants (%) | 1,923 (60.84) | 271 (65.94) | .046 | 140 (71.07) | .004 |
| One previous lung transplant (%) | 116 (3.67) | 33 (8.03) | < .001 | 27 (13.71) | < .001 |
| Two previous lung transplants (%) | 3 (0.09) | 1 (0.24) | .387 | 2 (1.02) | .0304 |
| Hospitalized at time of transplant (%) | 329 (10.41) | 124 (30.17) | < .001 | 137 (69.54) | < .001 |
| Hospitalized in ICU at time of transplant (%) | 140 (4.43) | 70 (17.03) | < .001 | 102 (51.78) | < .001 |
| Intubated at time of transplant (%) | 62 (1.96) | 40 (9.73) | < .001 | 71 (36.04) | < .001 |
| On ECMO at time of transplant (%) | 11 (0.35) | 3 (0.73) | .244 | 10 (5.08) | < .001 |
| Cause of death | |||||
| Cystic fibrosis (%) | 463 (14.65) | 47 (11.44) | .080 | 19 (9.64) | .052 |
| COPD (%) | 1,090 (34.48) | 23 (5.60) | < .001 | 13 (6.60) | < .001 |
| Pulmonary fibrosis (%) | 899 (28.44) | 234 (56.93) | < .001 | 112 (56.85) | < .001 |
| Sarcoidosis (%) | 114 (3.61) | 17 (4.14) | .591 | 7 (3.55) | .969 |
| Reoperative lung transplant (%) | 98 (3.10) | 28 (6.81) | < .001 | 25 (12.69) | < .001 |
| Other (%) | 57 (1.80) | 15 (3.65) | .0122 | 3 (1.52) | .773 |
| Age, donor (IQR) | 33.15 (26) | 32.11 (24) | .1773 | 34.97 (25) | .0908 |
| Ischemic time (IQR) | 4.98 (2.08) | 5.13 (2.03) | .0807 | 5.24 (2.08) | .040 |
| Pulmonary infection, donor (%) | 852 (26.95) | 106 (25.79) | .617 | 55 (27.92) | .767 |
| Insulin-dependent diabetes mellitus, donor (%) | 64 (2.03) | 4 (0.98) | .142 | 7 (3.55) | .148 |
P values were calculated using patients with an LLAS as the reference group. ECMO = extracorporeal membrane oxygenation; eGFR = estimated glomerular filtration rate; HLAS = high lung allocation score; ILAS = intermediate lung allocation score; IQR = interquartile range; LLAS = low lung allocation score.
Total number of participants, both donors and recipients, was 3,769.
Mortality by LAS Group
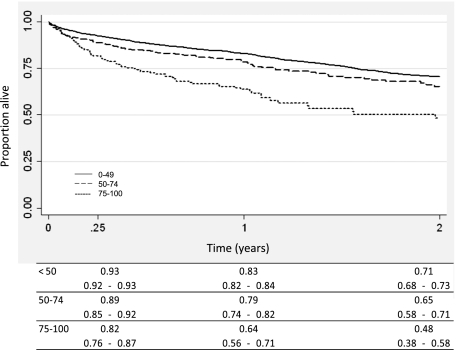
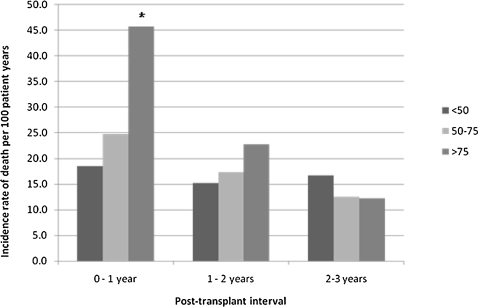
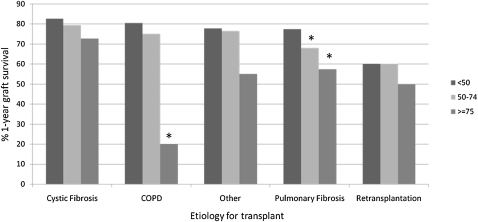
Compared with LLAS recipients, HLAS recipients had significantly worse survival at 90 days and 1 year (Fig 2). However, whereas the IRD was significantly higher among the HLAS group in the first year posttransplantation, there was no difference across groups in IRD during years 1 to 2 or 2 to 3 (Fig 3). When transplant recipients were stratified by disease etiology, a trend of decreased survival with elevated LAS was observed across all diseases (Fig 4).
Figure 2.
Kaplan-Meier survival curve of patients transplanted by LAS. See Figure 1 legend for expansion of the abbreviation.
Figure 3.
Incidence rate of death by LAS and posttransplant time interval. * = P < .05, compared with group that has LAS < 50. See Figure 1 legend for expansion of the abbreviation.
Figure 4.
One-year survival by cause of disease. * = P < .05, compared with group that has LAS < 50. See Figure 1 for expansion of the abbreviation.
Posttransplant Morbidity: In-Hospital Morbidity, Length of Stay, and BO
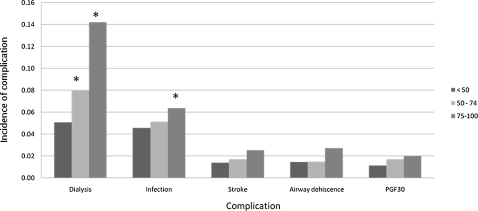
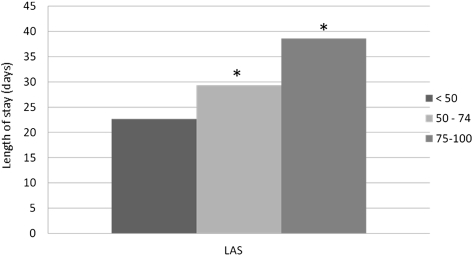
Compared with the LLAS recipients, both ILAS (P = .012) and HLAS recipients (P < .001) were significantly more likely to require dialysis. Likewise, compared with the LLAS recipients, HLAS recipients were significantly more likely to develop infection (P < .001). However, neither ILAS nor HLAS recipients experienced higher rates of PGF, airway dehiscence, or stroke when compared with LLAS recipients (Fig 5). Length of stay was longer in both HLAS (P < .001) and ILAS (P < .001) recipients when compared with LLAS recipients (Fig 6). Freedom from BO did not differ across LAS groups (P = .469).
Figure 5.
Incidence of in-hospital complication by LAS. Incidence reported per 100 patients, except infection per 1,000 patients. PGF30 = primary graft failure < 30 days posttransplant. See Figure 1 legend for expansion of other abbreviation.* = P < 0.05 compared with group that has LAS < 50.
Figure 6.
Length of stay by LAS at the time of transplantation. See Figure 1 legend for expansion of the abbreviation.* = P < 0.05 compared with group that has LAS < 50.
Discussion
Despite advances in the medical and surgical management of advanced lung disease, lung transplantation remains an integral treatment of patients suffering from end-stage pulmonary disease.11 With the demand for lung transplantation continuing to outpace the supply of available organs, the LAS was developed to distribute organs in a manner that balances the degree of medical urgency with the probability of posttransplant survival. In this analysis, we use UNOS data to examine nearly 4,000 patients transplanted between April 2006 and December 2007.
Overall, an elevated LAS was associated with diminished survival as well as higher morbidity and a longer length of stay during the transplant hospitalization. Recipients in the HLAS group, with an LAS ≥ 75, had significantly worse actuarial survival when compared with recipients with an LLAS. When stratified by cause, these findings were only statistically significant for recipients with COPD and pulmonary fibrosis; however, there was a clear stepwise trend toward worse survival with increasing LAS in all disease categories. The diminished survival with increasing LAS appears to result from excess mortality in the first year posttransplant, because when comparing the IRD at 1 to 2 years and 2 to 3 years, there was no significant difference across groups.
Patients in the HLAS group spent nearly 40 days as inpatients during the transplant hospitalization alone. In addition, patients with an HLAS had a significantly higher incidence of infection and need for inpatient dialysis. While stroke, airway dehiscence, and postoperative graft failure at 30 days were not significantly higher in HLAS patients, there was a trend toward a stepwise increase in the incidence of these complications as the LAS increased.
Findings from this analysis demonstrate the importance of careful decision making when considering a patient with HLAS. Higher resource utilization and increased in-hospital complications highlight the potential shortcomings of a strategy where the sickest recipients are given preference in organ allocation. One of the primary goals of the LAS is to increase transplant benefit for lung recipients. However, this strategy may be preferentially allocating organs to recipients with unacceptably low posttransplant survival. This potential pitfall of the current allocation system is highlighted by additional analyses that examine outcomes of recipients with the highest scores more closely. For example, the median survival of recipients with an LAS from 80 to 89 and 90 to 100 were 2.28 and 1.56 years, respectively. No matter how poor these candidates’ expected survival on the waiting list, the additional survival gained by transplantation may not represent the optimal allocation of scare donor organs.
In an effort to limit the allocation of scare organs to candidates with a high likelihood of poor posttransplant outcomes and maximize the allocation to those with the greatest survival benefit, further modifications to the current allocation system may be needed for patients in the highest LAS group. One possible modification is to potentially place more weight on long-term posttransplant outcomes in future iterations of the LAS, incorporating three- or even five-year survival into the score rather than simply one-year survival, as is the current practice. Alternatively, it may be possible to incorporate a pretransplant risk stratification score based on pretransplant patient characteristics to estimate a candidate’s posttransplant risk and identify candidates with a risk exceeding a predetermined threshold.12 Candidates with a risk of posttransplant mortality that exceeds a predefined level could have their LAS scores significantly reduced, be inactivated, or be placed on an “alternate transplant” list—a strategy used by a number of heart transplant centers.13,14 Under alternate list strategies, high-risk recipients are only eligible for organs if the organ is not suitable for any potential standard recipient. As donor organs used by alternate list recipients would often otherwise not be transplanted, this strategy may expand the use of potential donor organs. Conversely, these lower-quality donor organs would likely be associated with further diminished outcomes. Considering the potential benefits and risks, outcomes such as survival, quality of life, and cost-effectiveness would have to be closely monitored to determine the effectiveness of such a strategy. A final possible strategy is to refer high-risk candidates to high-volume centers that may have superior outcomes.
Limitations
There are several limitations to this analysis. First, large patient registries often have incomplete data entry. Fields contained within the UNOS database used for this analysis, however, were well populated, with a 90% to 99% data entry rate for the majority of variables. Patients with missing data were not included in the analysis; however, given the large sample size, it is unlikely that these excluded patients would have significantly altered the results. Furthermore, although the UNOS reporting system provides definitions for variables in data guidelines, there could be inaccuracies in individual center reporting to UNOS. Given that the major outcome variable in our analysis was mortality, which is used in center evaluation, it is unlikely our outcome would be altered by center reporting.
Second, because the LAS was developed in 2005, these data should be considered short term and will require further analysis for assessment of longitudinal trends. Also, diminished outcomes among higher-LAS recipients may be explained by inferior donors in the group. However, as summarized in Table 1, there was no clinically significant difference in donor age across groups, and HLAS recipients received more double lung transplants.
Third, for this analysis, recipients were censored at the time of death, retransplantation, or last known follow-up. Analysis of BO-free survival is limited because it assumes that the event of interest is only BO survival. If death occurred before this outcome, the patients were censored. Furthermore, the time period is early, so the expected BO survival rate would be low and differences masked. Therefore, future analysis of this end point should include longer follow-up and analysis of competing outcomes, including death.
Finally, 2-year survival in this cohort is lower than previously reported.15 This is likely because of follow-up data are entered by participating centers; follow-up data on recipients who die are entered when they expire, but for recipients who are alive, it is only updated at regular intervals. Nevertheless, outcomes in the LAS era must be closely monitored.
Conclusions and Implications
An increased LAS was associated with decreased survival following lung transplantation. In addition, an increased LAS was associated with increased length of stay following transplantation and higher rates of infection, renal failure, and stroke. Although statistically significant differences were observed only among recipients who had COPD and IPF, a stepwise trend toward worsening survival with increasing LAS was observed across all etiologies of disease. Given these findings in the setting of the critical scarcity of lungs available for transplantation, continued assessment of the LAS model must be carried out to ensure the optimal balance between medical urgency and posttransplant survival.
Acknowledgments
Author contributions: Dr Russo: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, participated in the study concept and design, acquired data, analyzed and interpreted data, drafted the manuscript, critically revised the manuscript for important intellectual content, and analyzed statistics.
Dr Iribarne: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, participated in the study concept and design, acquired data, analyzed and interpreted data, drafted the manuscript, critically revised the manuscript for important intellectual content, and analyzed statistics.
Ms Hong: participated in the study concept and design, acquired data, and provided administrative, technical, and material support.
Dr Davies: participated in the study concept and design, acquired data, and provided administrative, technical, and material support.
Dr Xydas: analyzed and interpreted data, critically revised the manuscript for important intellectual content, and provided administrative, technical, and material support.
Dr Takayama: analyzed and interpreted data, critically revised the manuscript for important intellectual content, and provided administrative, technical, and material support.
Dr Ibrahimiye: analyzed and interpreted data, drafted the manuscript, critically revised the manuscript for important intellectual content, and provided administrative, technical, and material support.
Dr Gelijns: analyzed and interpreted data, critically revised the manuscript for important intellectual content, and provided administrative, technical, and material support.
Dr Bacchetta: analyzed and interpreted data, and provided administrative, technical, and material support.
Dr D’Ovidio: analyzed and interpreted data, and provided administrative, technical, and material support.
Dr Arcasoy: analyzed and interpreted data, critically revised the manuscript for important intellectual content, provided administrative, technical, and material support, and provided study supervision.
Dr Sonett: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, analyzed and interpreted data, critically revised the manuscript for important intellectual content, provided administrative, technical, and material support, and provided study supervision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Arcasoy receives grants for Astellas, Talecris, and APT Therapeutics for the study of various immunosuppressive therapies. The other authors have reported to CHEST that no potential conflicts of interest exist with the companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The content of this article is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, which provided some funding, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Other contributions: We thank UNOS for supplying the dataset used in this study and Katarina Anderson for assistance with our analysis.
Abbreviations
- BO
bronchiolitis obliterans
- GFR
glomerular filtration rate
- HLAS
high lung allocation score
- ILAS
intermediate lung allocation score
- IRD
incidence rate of death
- LAS
lung allocation score
- LLAS
low lung allocation score
- OPTN
Organ Procurement and Transplant Network
- PGF
primary graft failure
- UNOS
United Network of Organ Sharing
Footnotes
Funding/Support: This work was supported in part by Health Resources and Services Administration [contract 231-00-0115] and National Institutes of Health Training [Grant 5T32HL007854-13].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical methods and database design: implications for transplant researchers, 2005. Am J Transplant. 2006;6(5 Pt 2):1228–1242. doi: 10.1111/j.1600-6143.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 2.Travaline JM, Cordova FC, Furukawa S, Criner GJ. Discrepancy between severity of lung impairment and seniority on the lung transplantation list. Transplant Proc. 2004;36(10):3156–3160. doi: 10.1016/j.transproceed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.The American Society for Transplant Physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International Society for Heart and Lung Transplantation(ISHLT) International guidelines for the selection of lung transplant candidates. Am J Respir Crit Care Med. 1998;158(1):335–339. doi: 10.1164/ajrccm.158.1.15812. [DOI] [PubMed] [Google Scholar]
- 4.Egan TM, Kotloff RM. Pro/con debate: lung allocation should be based on medical urgency and transplant survival and not on waiting time. Chest. 2005;128(1):407–415. doi: 10.1378/chest.128.1.407. [DOI] [PubMed] [Google Scholar]
- 5.De Meester J, Smits JM, Persijn GG, Haverich A. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant. 2001;20(5):518–524. doi: 10.1016/s1053-2498(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 6.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 7.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg. 2008;135(1):166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 8.McCue JD, Mooney J, Quail J, Arrington A, Herrington C, Dahlberg PS. Ninety-day mortality and major complications are not affected by use of lung allocation score. J Heart Lung Transplant. 2008;27(2):192–196. doi: 10.1016/j.healun.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: impact on disease severity and survival. Chest. 2007;132(6):1954–1961. doi: 10.1378/chest.07-1160. [DOI] [PubMed] [Google Scholar]
- 10.Lingaraju R, Blumenthal NP, Kotloff RM, et al. Effects of lung allocation score on waiting list rankings and transplant procedures. J Heart Lung Transplant. 2006;25(9):1167–1170. doi: 10.1016/j.healun.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340(14):1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 12.Chen JM, Russo MJ, Hammond KM, et al. Alternate waiting list strategies for heart transplantation maximize donor organ utilization. Ann Thorac Surg. 2005;80(1):224–228. doi: 10.1016/j.athoracsur.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Kobashigawa JA, Laks H, Wu G, et al. The University of California at Los Angeles heart transplantation experience. Clin Transpl. 2005:173–185. [PubMed] [Google Scholar]
- 14.Felker GM, Hernandez AF, Rogers JG, et al. Cardiac transplantation at Duke University Medical Center. Clin Transpl. 2004:235–241. [PubMed] [Google Scholar]
- 15.Hachem RR, Trulock EP. The new lung allocation system and its impact on waitlist characteristics and post-transplant outcomes. Semin Thorac Cardiovasc Surg. 2008;20(2):139–142. doi: 10.1053/j.semtcvs.2008.04.004. [DOI] [PubMed] [Google Scholar]