Abstract
Background:
The article was designed to estimate the effect of active and passive household cigarette smoke exposure on asthma severity and obstetric and neonatal outcomes in pregnant women with asthma.
Methods:
We used a secondary observational analysis of pregnant women with mild and moderate-severe asthma enrolled in a prospective observational cohort study of asthma in pregnancy and a randomized clinical trial (RCT) comparing inhaled beclomethasone and oral theophylline. A baseline questionnaire detailing smoking history and passive household smoke exposure was given to each patient. Smoking status was confirmed in the RCT using cotinine levels. Data on asthma severity and obstetric and neonatal outcomes were collected and analyzed with respect to self-reported tobacco smoke exposure. Kruskal-Wallis and Pearson χ2 statistics were used to test for significance.
Results:
A total of 2,210 women were enrolled: 1,812 in the observational study and 398 in the RCT. Four hundred and eight (18%) women reported current active smoking. Of the nonsmokers, 790 (36%) women reported passive household smoke exposure. Active smoking was associated with more total symptomatic days (P < .001) and nights of sleep disturbance (P < .001). Among the newborns of active smokers, there was a greater risk of small for gestational age < 10th percentile (P < .001), and a lower mean birth weight (P < .001). There were no differences in symptom exacerbation or outcome between nonsmokers with and without passive household cigarette smoke exposure.
Conclusions:
Among pregnant women with asthma, active but not passive smoking is associated with increased asthma symptoms and fetal growth abnormalities.
Asthma is a common medical complication of pregnancy, affecting 6% to 8% of women.1,2 Both the prevalence and morbidity of asthma are increasing in the general population.3 The literature has been inconsistent regarding the relationship between asthma and adverse pregnancy outcomes. Increased risks of low birth weight (LBW), small for gestational age (SGA), and preterm delivery have been reported with asthma.4-8 Larger studies, however, have identified fewer significant adverse obstetric outcomes.1,9,10
A multicenter prospective observational cohort study performed by the Maternal-Fetal Medicine Units Network evaluated the relationship between asthma severity and maternal and fetal morbidity among pregnant women with asthma and matched controls.11 This study demonstrated a strong relationship between asthma severity and exacerbation during pregnancy, as well as associations with nonpulmonary pregnancy outcomes, including cesarean delivery, preeclampsia, gestational diabetes, and preterm birth.12 Oral corticosteroid use was also associated with an increased incidence of preterm birth and LBW, but not SGA.13
Cigarette smoking is known to aggravate asthma.14 Smoking is independently associated with both prematurity and intrauterine growth restriction,15,16 as well as fetal and neonatal mortality.17,18 A review of US national linked birth and infant death data for the 1997 birth cohort reported a prevalence of maternal smoking of 13.2%. The incidence of neonatal death was 40% higher among smokers, even after adjusting for confounding demographic variables.19 The effect of cigarette smoking on perinatal outcome is also dose dependent. Neonatal mortality associated with smoking increased from 22% to 68% between the lowest and highest exposure groups.19 The impact of smoking on pregnancy is usually underestimated, as maternal history underreports this exposure.12
Much less is known regarding the effect of passive environmental tobacco smoke (ETS) exposure on obstetric outcomes. Studies of ETS have revealed relatively modest effects on birth weight and length.20,21 There are few data available regarding the effects of ETS exposure on pregnant patients with a hyperresponsive respiratory disorder such as asthma. The goal of this investigation was to determine the effect of active and passive household ETS exposure on asthma severity and obstetric and neonatal outcomes in women with asthma.
Materials and Methods
This is a secondary analysis of pregnant women with mild, moderate, or severe asthma enrolled in two previously reported studies sponsored by the National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.11,12 The first was a multicenter prospective observational cohort study, and the second was a prospective randomized clinical trial (RCT) comparing inhaled beclomethasone and oral theophylline for the treatment of moderate asthma in pregnancy.22 The studies were undertaken simultaneously by the participating centers of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network, with recruitment occurring between December 1994 and February 2000. The severity was stratified based on the 1993 National Asthma Educational Program Working Group Report on Asthma and Pregnancy.14 The observational cohort included 906 women with mild asthma and 906 with moderate-severe asthma. The proportion of women who smoked (any in the past week) was prospectively balanced with the cohorts in the observational study (19% in both groups). In the RCT, women with moderate asthma were assigned to treatment with inhaled beclomethasone (n = 199) or oral theophylline (n = 199). Women with moderate asthma were offered entry into the observational cohort only if they declined the RCT. Patients in both studies were recruited by the same study nurses, and the same clinicians were involved in the patient management of both cohorts. Exclusion criteria for both studies included fetal demise, multiple gestation, gestational hypertension, congenital anomalies, pulmonary disease other than asthma, gestational age ≥ 26 weeks, or planned delivery elsewhere. Written informed consent was obtained from all participants. Institutional review board approval was obtained at all institutions.
Each patient completed a questionnaire at baseline detailing personal smoking history and passive household ETS exposure. The questionnaire asked: “Number of cigarettes smoked, per day, in the last week.” If the answer was “1 or more,” the patient was classified as a smoker. If on follow-up assessments the patient answered the above question with “1 or more,” the patient was recategorized as an active smoker. Information on the pack-year history was not collected. Passive ETS exposure was defined by a single yes/no question that read: “Does anyone else in the patient’s household smoke?” Smoking status was confirmed in |the RCT by measuring urinary cotinine levels at randomization. Obstetric outcomes obtained by chart review included the occurrence of preterm birth, preterm premature rupture of the membranes (PROM), preeclampsia, gestational diabetes, chorioamnionitis, placental abruption, oligohydramnios, and cesarean delivery. Neonatal outcomes included birth weight, SGA (defined as < 10th percentile),23 fetal sepsis, neonatal intensive care unit admission, transient tachypnea of the newborn, necrotizing enterocolitis, and intraventricular hemorrhage. Infant deaths included both stillbirths and neonatal demises.
In both studies, maternal asthma status was regularly evaluated with spirometry. FEV1 measurements ( > 4 h after bronchodilator use) were performed at enrollment and monthly thereafter. Information regarding symptom frequency was collected at each study visit from a symptom log diary, including nights with asthma symptoms, nights that asthma interfered with sleep, days that asthma interfered with activity, and asthma medication use. Asthma exacerbations were defined as symptoms severe enough to result in (1) hospitalization, (2) an unscheduled office visit, (3) an ED visit, or (4) treatment with oral corticosteroids. Study visits were scheduled monthly in the observational trial and biweekly in the RCT. Symptom frequency data were collected for the time period since the patient’s last study visit.
Data on asthma severity and obstetric and neonatal outcomes were analyzed with respect to self-reported cigarette smoking and household ETS. The Biostatistics Center of George Washington University analyzed the data using SAS 8.2 statistical software (SAS Institute; Cary, NC). Differences between the groups were compared using odds ratios (ORs) with 95th percentile CIs. Kruskal-Wallis and Pearson χ2 statistics were used for comparison of continuous and categorical variables, respectively, in Tables 1,2, and 3. Each application of these tests was to assess the hypothesis that there is no difference between the three smoking categories. Nominal statistical significance was defined as a P < .05. The Spearman correlation coefficient was used for analysis of the relationship between cotinine and subjective measures of asthma severity for women in the RCT only. The Wilcoxon P test was used to assess the relationship of cotinine as a continuous variable with dichotomous obstetric and neonatal outcome variables in Table 4.
Table 1.
—Demographic and Social Characteristics
| Characteristic | Nonsmokers/No Household ETS Exposurea (n = 1,011) | Nonsmokers/Household ETS Exposure (n = 790) | Smokers (n = 408) | P Valueb |
| Age, mean ± SD | 24.4 ± 5.9 | 21.5 ± 5.0 | 23.7 ± 5.6 | < .001 |
| Race | < .001 | |||
| African-American | 579 (57) | 514 (65) | 162 (40) | |
| White | 293 (29) | 196 (25) | 234 (57) | |
| Hispanic | 120 (12) | 67 (8) | 6 (1) | |
| Other | 19 (2) | 13 (2) | 6 (1) | |
| Private insurance | 196 (19) | 62 (8) | 25 (6) | < .001 |
| Marijuana use | 13 (1) | 24 (3) | 41 (10) | < .001 |
| Cocaine use | 3 (0.3) | 4 (1) | 17 (4) | < .001 |
| BMI (mean ± SD) | 27.8 ± 7.9 | 27.7 ± 7.8 | 27.4 ± 7.7 | .806 |
Data reported as No. (%) unless otherwise stated. ETS = environmental tobacco smoke.
Data unavailable for one patient.
Kruskal-Wallis and Pearson χ2 tests used to assess the hypothesis that there is no difference between the three smoking categories.
Table 2.
—Objective and Subjective Symptoms of Asthma
| Nonsmokers/No Household ETS Exposurea (n = 1,011) | Nonsmokers/Household ETS Exposure (n = 790) | Smokers (n = 408) | P Valueb | |
| Total symptomatic days, mean ± SD | 69.3 ± 63.0 | 63.5 ± 59.5 | 78.8 ± 66.9 | < .001 |
| Total nights sleep disturbance, mean ± SD | 39.2 ± 50.5 | 36.5 ± 50.8 | 48.4 ± 59.0 | < .001 |
| Total days restricted activity, mean ± SD | 23.4 ± 40.1 | 21.8 ± 39.0 | 26.8 ± 41.8 | .006 |
| Asthma exacerbation during study, n (%) | 219 (23) | 146 (19) | 68 (17) | .062 |
| Hospitalization, n (%) | 54 (6) | 43 (6) | 16 (4) | .485 |
| Percentage predicted FEV1 at baseline, mean ± SD | 93.4 ± 14.1 | 94.0 ± 14.7 | 92.6 ± 13.9 | .296 |
| Percentage predicted FEV1 < 80% across pregnancy, n (%) | 174 (17) | 128 (16) | 70 (17) | .836 |
See Table 1 for expansion of the abbreviation.
Data unavailable for one patient.
Kruskal-Wallis and Pearson χ2 tests used to assess the hypothesis that there is no difference between the three smoking categories.
Table 3.
—Obstetric and Neonatal Outcomes
| Characteristic | Nonsmokers/No Household ETS Exposure (n = 972) | Nonsmokers/Household ETS Exposure (n = 760) | Smokers (n = 391) | P Valuea |
| Delivery < 37 wk | 153 (16) | 119 (16) | 74 (19) | NS |
| Delivery < 32 wk | 29 (3) | 25 (3) | 17 (4) | NS |
| Oligohydramnios | 62 (6) | 56 (7) | 18 (5) | NS |
| Abruption | 8 (0.8) | 12 (2) | 5 (1.3) | NS |
| Preeclampsia | 113 (12) | 102 (13) | 29 (7) | .01 |
| Perinatal death | 12 (1) | 6 (0.8) | 10 (3) | .04 |
| PROM | 110 (11) | 97 (13) | 59 (15) | NS |
| SGA < 10th percentile | 50 (5) | 54 (7) | 46 (12) | < .001 |
| Birthweight, mean ± SD | 3191 ± 692 | 3107 ± 665 | 2977 ± 712 | < .001 |
Data reported as n (%) unless otherwise stated. Obstetric and neonatal outcome data unavailable for 87 patients. NS = not significant; PROM = premature rupture of membranes; SGA = small for gestational age. See Table 1 for expansion of the other abbreviation.
Kruskal-Wallis and Pearson χ2 tests used to assess the hypothesis that there is no difference between the three smoking categories.
Table 4.
—Relationship Between Cotinine (ng/mL) as a Continuous Variable and Obstetric and Neonatal Outcomes in the Randomized Clinical Trial22
| Characteristic | Outcome Present, N; Cotinine Means (SD) | Outcome Absent, N; Cotinine Means (SD) | Wilcoxon P Test for Significant Differences in Cotinine Levels, ng/mL |
| Asthma exacerbations on study | 85; 203 (459) | 290; 251 (542) | 0.293 |
| Asthma-related hospitalizations on study | 24; 173 (386) | 351; 245 (532) | 0.338 |
| Delivery < 37 wk | 66; 294 (590) | 309; 229 (509) | 0.547 |
| Delivery < 32 wk | 15; 119 (298) | 360; 245 (531) | 0.557 |
| Oligohydramnios | 21; 36 (153) | 354; 252 (536) | 0.025 |
| Preeclampsia | 31; 187 (452) | 344; 245 (531) | 0.540 |
| PROM | 51; 285 (604) | 324; 233 (511) | 0.688 |
| Perinatal death | Only six cases of perinatal death in RCT | ||
| SGA < 10th percentile | 27; 527 (719) | 345; 220 (502) | 0.035 |
| Abruption | Only two cases of abruption in RCT |
Results
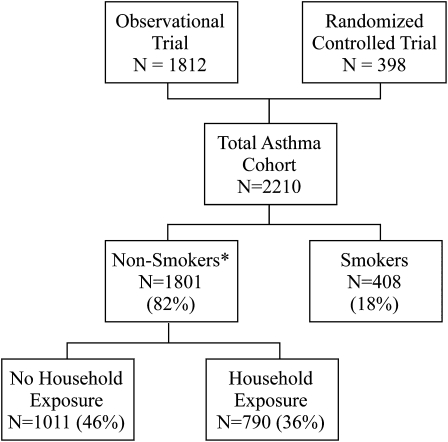
A total of 2,210 women with asthma were enrolled: 1,812 in the prospective observational cohort and 398 in the RCT (Fig 1). Eighty-seven patients were lost to follow-up (3.9%). Four hundred eight (18%) women reported current active smoking. The active smokers reported a median number of six cigarettes smoked per day, with a maximum of 40. Of the nonsmokers, 790 (36%) reported passive household ETS exposure. The remaining 1,011 women (46%) reported that they were nonsmokers and had no household ETS exposure. Information on active or passive ETS exposure was missing on a single patient. There were significant demographic and social differences between the three groups (Table 1). Smokers were more likely to be white and to report marijuana or cocaine use and less likely to have private insurance. The nonsmokers with household ETS exposure were younger than the women in either of the other two groups. These demographic differences remained significant when smoking exposure was stratified by asthma severity classification (data not shown).
Figure 1.
Enrollment of pregnant women with asthma from the observational cohort trial and the prospective RCT and their respective exposures to cigarette smoke. Data regarding ETS exposure were unavailable for one patient (*). ETS = environmental tobacco smoke; RCT = randomized clinical trial.
Table 2 presents subjective and objective measures of asthma severity. Smokers had significantly more symptomatic days, nights of sleep disturbance, and days of restricted activity. Nonsmokers with passive household ETS exposure had fewer symptomatic days or nights and fewer days of restricted activity than did the nonsmokers without ETS exposure, but these differences were not statistically significant. There were no significant differences between the total number of asthma exacerbations or hospitalizations between the smoking and nonsmoking groups. Nor were there any significant differences in the spirometric measures during pregnancy. A model of asthma exacerbations was constructed with the following covariates: asthma severity class, passive smoking, active smoking, passive smoking/asthma class interaction, and active smoking/asthma class interaction. The only significant term in the model was the asthma severity class, indicating that passive and active smoking or their interactions with asthma status did not make a difference.
A comparison of obstetric and neonatal outcomes is presented in Table 3. Women with asthma who were also current smokers had significantly more frequent SGA < 10th percentile and higher perinatal mortality. There were 10 (2.6%) perinatal deaths among the active smokers (eight stillbirths and two neonatal deaths). Among nonsmokers with passive household ETS exposure, there were six (0.8%) perinatal deaths (five stillbirths and one neonatal death), whereas the nonexposed nonsmokers had 12 (1.2%) perinatal deaths (six stillbirths and six neonatal deaths). When the perinatal mortality data are divided by asthma severity, the sample sizes are too small for meaningful analysis; however, the mortality rates remain about the same for each smoking exposure group between the women with mild (4 [1.0%], 3 [1.0%], and 4 [2.4%]) and moderate/severe asthma (8 [1.4%], 3 [0.7%], and 6 [2.6%]). Birth weight was significantly less (P < .001) for current smokers of one to nine cigarettes per day (2,994 ± 733 g) or ≥ 10 cigarettes per day (2,955 ± 684 g) compared with nonsmokers with (3,107 ± 665 g) or without (3,191 ± 692 g) passive household ETS exposure. Current smokers with asthma had lower rates of preeclampsia (7%) than either nonsmokers with (13%) or without (12%) passive household ETS exposure. The Spearman correlation coefficient for the relationship between birth weight and the number of cigarettes reportedly smoked per day was significant ( − 0.11; P = .03). Both preeclampsia (OR, 0.61; 95% CI, 0.40-0.94) and SGA (OR, 2.44; 95% CI, 1.61-3.72) remained significantly related to active maternal smoking, even after controlling for asthma severity at enrollment and race.
Cotinine levels were available for 387 of the 398 patients in the RCT. Cotinine levels were highest among the 62 active smokers (1,109 ± 652 ng/mL; mean ± SD). Women who reported smoking one to nine cigarettes per day had cotinine levels of 1,107 ± 630 ng/mL (n = 32), while those who reported smoking 10 or more cigarettes per day had levels of 1,110 ± 686 (n = 30). The Spearman correlation between the number of cigarettes reported smoked and the cotinine levels was 0.24; P = .05. The mean cotinine level for the nonsmokers who reported passive household ETS exposure (n = 144) was more than twice as high (99 ± 315 ng/mL) as the non-smokers (n = 181) without household exposures (40 ± 188 ng/mL).
The serum cotinine levels were significantly higher (P < .001; Kruskal-Wallis) among the smokers compared with the nonsmokers with or without passive ETS exposure. The Spearman correlation coefficient demonstrated a significant association between cotinine levels and days of restricted activity (r = 0.11; P = .03), but no significant association with symptomatic days (r = 0.03; P = .55) or nights with sleep disturbances (r = 0.04; P = .43). There was a negative correlation between an increasing cotinine level and birth weight (r = − 0.11; P = .03). There were significant differences in cotinine levels between women with and without an SGA infant and in those with oligohydramnios (Table 4). The relationship between cotinine levels and perinatal death was not significant, but there were only six perinatal deaths in the RCT.
Discussion
Active smoking among women with asthma is associated with increased asthma symptoms, lower birth weights, an increased risk of SGA, perinatal death, and a decreased rate of preeclampsia compared with nonsmokers with or without passive household ETS exposure. The two studies on which this analysis is based represent the largest multicenter prospective studies of asthma during pregnancy. While active maternal smoking was associated with significant increases in all the subjectively reported measures of asthma severity, it was not associated with increased asthma exacerbations or hospitalizations compared with the nonsmokers with or without household ETS exposure. Nor were there differences in FEV1 values; however, these women may be too young, with too few pack-years of smoking to show significant group differences.
The association between active maternal smoking and impaired fetal growth has been well described15,16 and is also found in this cohort. There is also a lower risk of preeclampsia among active smokers, a phenomenon previously reported in obstetric populations without asthma. We did not find any increased adverse obstetric or neonatal outcomes among nonsmokers with passive household ETS exposure compared with unexposed nonsmokers. Studies of ETS exposure on birth outcomes revealed significant heterogeneity between studies, and their synthesized findings are not robust.20 A more recent retrospective study using interview data from the parents of 18,297 children born in the United Kingdom in 2000 and 2001 revealed that household ETS exposure reduced adjusted birth weight by 36 g (95% CI − 5 to − 67 g) compared with infants with no maternal ETS exposure.21 Eskenazi et al24 measured cotinine levels in maternal serum in 3,529 pregnant women at 27 weeks gestation. Based on the cotinine levels, nonsmokers were divided into those exposed (2-10 ng/mL) and unexposed ( < 2 ng/mL), while smokers were divided into tertiles based on cotinine levels. The infants of ETS exposed nonsmokers averaged 45 g less (P = .28) in birth weight compared with those of unexposed nonsmokers, while the infants of the active smokers averaged 78, 191, and 233 g less for the first, second, and third tertiles, respectively, which shows a significant inverse correlation between birth weight and serum cotinine levels (P < .001).24 As in Eskenazi and coworker’s investigation, even this current large cohort lacks adequate power to identify the 84-g-reduction in birth weight among the nonsmokers with ETS exposure as being statistically significant.
A weakness of this study is that the patients enrolled in the observational cohort study completed a questionnaire on household ETS exposure, but did not have cotinine levels measured. In obstetric patients, self-reported tobacco use is significantly underacknowledged. Only 66 women in the RCT (16.6%) reported active smoking; however, 116 women (29.1%) had simultaneously positive urinary cotinine levels.22 Acknowledging the probability of underreporting, it is unlikely that results would be significantly altered. Since active smoking among women with asthma is associated with both increased asthma symptoms and reduced birth weight, the misclassification of active smokers into the nonsmoking cohorts would only serve to increase the number of maternal complications in those groups, making them more similar to the active smoking cohort.
Another limitation may be that the ETS exposure questionnaire focused only on the home. Among 4,687 prenatal patients in Sweden, 267 were nonsmokers passively exposed only at work. Passive exposure in the home did not affect pregnancy outcome; however, workplace exposure was weakly associated with preterm birth, although not LBW.25 If occupational exposure of the nonsmoking/no-household-exposure group was significant, then that could have resulted in an overestimation of risk in the “nonexposed” nonsmokers. The restrictions against smoking in the workplace and public areas now prevalent in the United States should minimize the impact of nonhousehold exposure.
A final limitation is that all of the outcomes evaluated in this study were short term in nature. Five-year follow-up studies showed reductions in height but no differences in neurobehavior among children of ETS-exposed women.26,27 More recently, researchers performed detailed neurodevelopmental assessments on children delivered of nonsmoking, nonexposed mothers (n = 96); nonsmoking but ETS-exposed mothers (n = 16); and mothers who smoked during the final 6 months of pregnancy (n = 21).28 Both the smoking and ETS-exposed mothers had children with more severe psychologic problems, including attention deficit/hyperactivity disorder, aggressive behavior, defiance, and conduct disorders.
Because of the nature of the participating network centers, the patient population was heavily weighted toward single mothers, African-Americans, and those with subsidized insurance. This may limit the generalizability of the results, since African-American women have lower rates of smoking than most other racial and ethnic groups. However, this may also be a strength, as asthma is both more common and more severe among African-Americans and among patients of lower socio-economic status.29,30
The current study is reassuring in that passive household ETS exposure did not appear to have a significant impact on either subjective or objective measures of asthma severity during pregnancy. Further, passive ETS exposure did not appear to be associated with any increase in adverse obstetric or short-term neonatal outcomes. However, the strong evidence of an adverse effect of active cigarette smoking on maternal symptoms, fetal growth, and infant mortality among women with asthma as well as in the general obstetric population underscores the importance of this public health question. The lack of association between passive household ETS exposure and adverse outcomes may reflect an inadequate sample size to detect small differences between the exposed and unexposed nonsmokers, poor or inconsistent reporting of household ETS exposure, or the effect of noncaptured occupational ETS exposure. However, it may also reflect no actual biologic effect as the result of the household ETS exposure being below the threshold necessary to impact maternal or newborn outcomes. In the absence of a definitive answer to the above question, discouraging active smoking among women with asthma and emphasizing the National Asthma Educational Program recommendations to reduce or eliminate ETS exposure in both the home and workplace would seem to be the most appropriate clinical management approach.
Acknowledgments
Author contributions: Dr Newman: is a member of the NIH-NICHD Asthma Subcommittee of the Maternal-Fetal Medicine Units (MFMU) Network; participated in the conception of the primary study design and protocol development; provided the primary intellectual contribution to the drafting and revision of this secondary analysis with regard to its scientific content and form; coordinated the review of this manuscript at each stage of development by all the other authors and members of the Asthma Subcommittee; approved the final manuscript as submitted, and is the corresponding author.
Ms Momirova: as a member of the NIH-NICHD Asthma Subcommittee, participated in the conception of the study design and protocol development, and contributed to the critical aspects of the conduct of this research, including monitoring of recruitment and study progress, data-quality evaluation, and data analysis; contributed significantly to manuscript revisions, and provided the subsequent statistical analysis requested by the reviewers; provided significant intellectual contribution to the drafting and revision of this manuscript with regard to its scientific content and form; and approved the final manuscript as submitted.
Dr Dombrowski: as Chair of the NIH-NICHD Asthma Subcommittee for the Maternal Fetal Medicine Units Network, led the study design and protocol development and was responsible for overseeing the conduct of the research, including the monitoring of recruitment, study progress, data quality, and data analysis; has made significant intellectual contribution to both the drafting of this manuscript and to its revisions with regard to its scientific content and form; and approved the final manuscript as submitted.
Dr Schatz: as a member of the NIH-NICHD Asthma Subcommittee for the MFMU Network, participated in study design, and contributed to critical aspects of the conduct of this research including patient recruitment and data analysis; as a pulmonary specialist, provided significant intellectual contribution to the drafting of this manuscript and its revisions, and has contributed to its scientific content and form; and approved the final manuscript as submitted.
Dr Wise: as a member of the NIH-NICHD Asthma Subcommittee for the MFMU Network, participated in study design, and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; as a pulmonary specialist, provided significant intellectual contribution to the drafting of this manuscript and its revisions, and has contributed to its scientific content and form; and approved the final manuscript as submitted.
Dr Landon: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; provided significant intellectual contribution to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Rouse: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; provided significant intellectual contribution to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Lindheimer: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Caritis: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Sheffield: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; was instrumental in developing the original concept for the secondary analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Miodovnik: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Wapner: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Varner: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr O’Sullivan: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Dr Conway: as a member of the NIH-NICHD Asthma Subcommittee of the MFMU Network, participated in the study design and contributed to critical aspects of the conduct of this research, including patient recruitment and data analysis; contributed to the drafting and revision of this manuscript with regards to its scientific content and form; and approved the final manuscript as submitted.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Schatz received pharmaceutical grant monies from Merck, GlaxoSmithKline, Aerocrine, Genetech, America’s Health Insurance Plans, and QualityMetric. The other authors have reported no potential conflicts of interest with any company/organization whose products or services may be discussed in this article.
Abbreviations
- ETS
5 environmental tobacco smoke
- LBW
low birth weight
- MFMU
Maternal-Fetal Medicine Units Network
- OR
odds ratio
- PROM
premature rupture of membranes
- RCT
randomized clinical trial
- SGA
small for gestational age
Appendix
In addition to the authors, other members of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows: R. Phillip Heine, M. Cotroneo, and E. Daugherty (University of Pittsburgh): W. Mabie, R. Ramsey, and B. Sibai (University of Tennessee); W. W. Andrews, R. L. Copper, S. Tate, and A. Northen (University of Alabama at Birmingham); G. Norman, Y. Sorokin, and A. Millinder (Wayne State University); N. Elder, T. A. Siddiqi, and V. Pemberton (University of Cincinnati); P. Meis, M. Harper, M. Swain, and A. Luper (Wake Forest University); A. H. Moawad, P. Jones, M. E. Brown, and G. Mallett (University of Chicago); F. Johnson, S. Meadows, and B. A. Collins (Ohio State University); S. Beydoun, C. Alfonso, and J. Potter (University of Miami); M. L. Sherman (University of Texas Southwestern Medical Center); S. Barker and O. Langer (University of Texas at San Antonio); D. J. Dudley and L. Reynolds (University of Utah); M. DiVito and K. Smith (Thomas Jefferson University); E. Thom, E. Rowland, and S. Brancolini (The George Washington University Biostatistics Center); D. McNellis and C. Catz (the Eunice Kennedy Shriver National Institute of Child Health and Human Development); and J. Kiley (National Heart, Lung, and Blood Institute).
Funding/Support: Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD21410, HD21414, HD21434, HD27869, HD 27917, HD27905, HD27889, HD27860, HD27861, HD27915, HD27883, HD34122, HD34116, HD34208, HD34136, HD36801] and the National Heart, Lung, and Blood Institute.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Alexander S, Dodds L, Armson BA. Perinatal outcomes in women with asthma during pregnancy. Obstet Gynecol. 1998;92(3):435–440. doi: 10.1016/s0029-7844(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 2.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13(5):317–324. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma . NHLBI. Washington, DC: NIH Publication No. 97-4051. April 1997. [Google Scholar]
- 4.Perlow JH, Montgomery D, Morgan MA, Towers CV, Porto M. Severity of asthma and perinatal outcome. Am J Obstet Gynecol. 1992;167(4 Pt 1):963–967. doi: 10.1016/s0002-9378(12)80020-2. [DOI] [PubMed] [Google Scholar]
- 5.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Net. 1998;158(4):1091–1095. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Wen SW, Demissie K, Marcoux S, Kramer MS. Maternal asthma and pregnancy outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2001;184(2):90–96. doi: 10.1067/mob.2001.108073. [DOI] [PubMed] [Google Scholar]
- 7.Doucette JT, Bracken MB. Possible role of asthma in the risk of preterm labor and delivery. Epidemiology. 1993;4(2):143–150. doi: 10.1097/00001648-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Källén B, Rydhstroem H, Aberg A. Asthma during pregnancy—a population based study. Eur J Epidemiol. 2000;16(2):167–171. doi: 10.1023/a:1007678404911. [DOI] [PubMed] [Google Scholar]
- 9.Schatz M, Zeiger RS, Hoffman CP, et al. Perinatal outcomes in the pregnancies of asthmatic women: a prospective controlled analysis. Am J Respir Crit Care Med. 1995;151(4):1170–1174. doi: 10.1164/ajrccm/151.4.1170. [DOI] [PubMed] [Google Scholar]
- 10.Stenius-Aarniala BS, Hedman J, Teramo KA. Acute asthma during pregnancy. Thorax. 1996;51(4):411–414. doi: 10.1136/thx.51.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatz M, Dombrowski MP, Wise R, et al. National Institute of Child Health and Development, Maternal-Fetal Medicine Units Network and the National Heart-Lung Blood Institute Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–288. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 12.Dombrowski MP, Schatz M, Wise R, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network and the National Heart, Lung, and Blood Institute Asthma during pregnancy. Obstet Gynecol. 2004;103(1):5–12. [Google Scholar]
- 13.Schatz M, Dombrowski MP, Wise R, et al. Maternal-Fetal Medicine Units Network, The National Institute of Child Health and Development; National Heart, Lung and Blood Institute The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113(6):1040–1045. doi: 10.1016/j.jaci.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 14.National Heart, Lung, and Blood Institute . National Asthma Education Program Report of the Working Group on Asthma and Pregnancy. Management of Asthma During Pregnancy. Bethesda, MD: National Institutes of Health; 1993. NIH publication 93-3279A. [Google Scholar]
- 15.US Department of Health and Human Services . Women and Smoking: A Report of the Surgeon General. Washington, D.C.: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 16.Higgins S. Smoking in pregnancy. Curr Opin Obstet Gynecol. 2002;14(2):145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Cnattingius S, Haglund B, Meirik O. Cigarette smoking as risk factor for late fetal and early neonatal death. BMJ. 1988;297(6643):258–261. doi: 10.1136/bmj.297.6643.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer MB, Jonas BS, Tonascia JA. Perinatal events associated with maternal smoking during pregnancy. Am J Epidemiol. 1976;103(5):464–476. doi: 10.1093/oxfordjournals.aje.a112248. [DOI] [PubMed] [Google Scholar]
- 19.Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7(4):219–227. doi: 10.1023/a:1027319517405. [DOI] [PubMed] [Google Scholar]
- 20.Windham GC, Eaton A, Hopkins B. Evidence for an association between environmental tobacco smoke exposure and birthweight: a meta-analysis and new data. Paediatr Perinat Epidemiol. 1999;13(1):35–57. doi: 10.1046/j.1365-3016.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 21.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81–86. doi: 10.1186/1471-2458-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dombrowski MP, Schatz M, Wise R, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network; National Heart, Lung, and Blood Institute Randomized trial of inhaled beclomethasone dipropionate versus theophylline for moderate asthma during pregnancy. Am J Obstet Gynecol. 2004;190(3):737–744. doi: 10.1016/j.ajog.2003.09.071. [DOI] [PubMed] [Google Scholar]
- 23.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126(5):555–564. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- 24.Eskenazi B, Prehn AW, Christianson RE. Passive and active maternal smoking as measured by serum cotinine: the effect on birthweight. Am J Public Health. 1995;85(3):395–398. doi: 10.2105/ajph.85.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantel N, Bodin L. Re: “Tobacco smoke exposure and pregnancy outcome among working women: a prospective study at prenatal care centers in Orebro County, Sweden”. Am J Epidemiol. 1992;135(7):837–838. doi: 10.1093/oxfordjournals.aje.a116376. [DOI] [PubMed] [Google Scholar]
- 26.Eskenazi B, Bergmann JJ. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. I. Effects on physical growth at age 5 years. Am J Epidemiol. 1995;142(9 Suppl):S10–S18. doi: 10.1093/aje/142.supplement_9.s10. [DOI] [PubMed] [Google Scholar]
- 27.Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5 years. Am J Epidemiol. 1995;142(9 Suppl):S19–S29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- 28.Gatze-Kopp LM, Beauchaine TP. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry and Human Development. 2007;38:255–269. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlieb DJ, Beiser AS, O’Connor GT. Poverty, race, and medication use are correlates of asthma hospitalization rates. A small area analysis in Boston. Chest. 1995;108(1):28–35. doi: 10.1378/chest.108.1.28. [DOI] [PubMed] [Google Scholar]
- 30.Castro M, Schechtman KB, Halstead J, Bloomberg G. Risk factors for asthma morbidity and mortality in a large metropolitan city. J Asthma. 2001;38(8):625–635. doi: 10.1081/jas-100107540. [DOI] [PubMed] [Google Scholar]