Abstract
Background:
Nontuberculous mycobacteria (NTM) are environmental organisms associated with pulmonary disease without person-to-person transmission. Although genetic causes of disseminated NTM infection are well characterized, genetic causes for most human susceptibility to pulmonary NTM infection have not been determined.
Methods:
Family histories for relevant disease characteristics were obtained as part of an ongoing natural history study. Six families were identified in which at least two blood relatives had pulmonary NTM. A systematic review of medical records extracted data relevant to pulmonary infection and baseline demographics. Data were reconfirmed by telephone interviews.
Results:
Familial clustering of pulmonary NTM was proven in six families. Four of the families were white, and the majority of affected individuals were women. The average age at diagnosis was 56.4 ± 10.7 years, the average height was 167.5 ± 8.7 cm, and the mean BMI was 22.0 ± 2.98 kg/m2. Scoliosis was present in 31%. Five of 12 patients had cystic fibrosis transmembrane conductance regulator gene variations, but none had classic cystic fibrosis. Infections were caused by both slow and rapid growing mycobacteria, including Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium abscessus, and Mycobacterium massiliense. Family members were typically infected with different species of NTM.
Conclusion:
We identified six familial clusters of pulmonary NTM infection, suggesting that there are genetic factors contributing to host susceptibility to pulmonary infection with NTM among some individuals with nodular bronchiectatic disease.
Nontuberculous mycobacteria (NTM) are widespread environmental organisms capable of causing infection in people. Pulmonary NTM infection was initially reported among men with underlying lung disease.1 However, over the last 20 years, pulmonary NTM disease has been increasingly noted in thin, postmenopausal women without recognized predisposing factors.2,3 In the United States, the majority of these patients are white, with a higher than expected prevalence of scoliosis, pectus excavatum, and mitral valve prolapse.4,5 Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene have also been documented in a significantly higher percentage of patients than seen in the general population.3,5 This complex preexisting morphotype in women with pulmonary NTM indicates an underlying set of traits that antedate infection, thereby strongly implicating genetic predisposition. Familial clustering of pulmonary NTM disease would support the hypothesis of genetic predisposition.
Familial clustering of pulmonary NTM infections has been rarely reported. Kobashi et al6 reported a pair of siblings with Mycobacterium avium complex (MAC); however, both also had an underlying hematologic malignancy. Tanaka et al7 described two pairs of siblings with pulmonary NTM due to different strains of Mycobacterium avium, arguing against a common source exposure to a particularly virulent NTM. Person-to-person transmission of NTM has not been substantiated.8 Therefore, without a common exposure to account for disease, familial clustering suggests an underlying genetic predisposition for the development of pulmonary NTM.9
From a natural history cohort of pulmonary NTM patients followed at the National Institutes of Health (NIH), six families were identified with pulmonary NTM disease in two or more first-degree relatives. Although not conclusive, these familial clusters support the hypothesis of a genetic predisposition to pulmonary NTM infection among some individuals with nodular bronchiectatic pulmonary NTM disease.
Materials and Methods
As part of an Institutional Review Board-approved natural history study of mycobacterial disease at the NIH family histories for relevant disease characteristics were obtained. From a cohort of approximately 120 patients who met the American Thoracic Society disease criteria without known cystic fibrosis or primary ciliary dyskinesia, six families had at least two members affected with pulmonary NTM.8 A systematic review of the medical records was performed and patients were interviewed to confirm and complete the data. If a proband identified a family member with confirmed pulmonary NTM, the proband was asked to have the affected family member contact a study investigator to be interviewed. The patient’s demographics, species of NTM isolated, age at onset of symptoms, age and symptoms at diagnosis, medical comorbidities, antecedent lung disease (eg, cystic fibrosis, primary ciliary dyskinesia, TB), musculoskeletal abnormalities (eg, scoliosis or pectus excavatum), and detailed family history were obtained. WBC count with differential, quantitative immunoglobulins, α-1 antitrypsin, HIV status, and quantitative sweat chloride test results were noted. CFTR mutation status (Ambry; Aliso Viejo, CA) was recorded where available. Echocardiography was reviewed for mitral valve prolapse. A CT scan of the chest was reviewed for pulmonary parenchymal abnormalities and pectus excavatum. An experienced radiologist (S. C. H.) reviewed chest images for scoliosis.
Case Series
Family 1:
1.II.1 is a 52-year-old South Korean woman diagnosed with pulmonary MAC at 47 years. She had a positive tuberculin skin test in the remote past, two prior episodes of pneumonia with unknown organisms in her 30s, and a right breast lumpectomy for a benign mass. She has lived in California since her teens and never smoked. Her lung disease was incidentally found by an elective whole-body screening CT scan; bronchoscopic culture grew MAC. She received appropriate antimicrobial therapy for more than 2 years with sputum sterilization and stabilization of radiographs.
1.II.2 is the 56-year-old sister of 1.II.1 who has lived in California since her teens and never smoked. She had a remote history of a positive tuberculin skin test and breast cancer diagnosed at the age of 41, successfully treated with mastectomy and axillary lymph node dissection. At 51 years, an elective whole-body CT scan showed an “old scar” in the lung. Two years later, repeat CT scanning showed bronchiectasis and small nodules in the right upper and middle lobes and lingula. Bronchoscopic culture grew M intracellulare. Four-drug therapy, including inhaled amikacin, was discontinued after 1 month because of hoarseness. She declined further therapy; her disease has remained clinically and radiographically stable for 2 years.
1.II.3, the 59-year-old brother of patients 1.II.1 and 1.II.2, was quarantined for suspected pulmonary TB at 23 years. He quit tobacco at age 30 and currently lives in China. He was not seen in this study; however, he and his physician provided pertinent information, including culture reports and CT images. Elective chest CT scan at 55 years showed multiple small nodules. As he was asymptomatic no action was taken. A repeat CT scan at 58 years showed a new cavitating nodule in the right lower lobe with increased bronchiolitis. Bronchoscopic culture grew MAC, and he started appropriate therapy.
Family history obtained from 1.II.1 and 1.II.2 revealed their father (1.I.1) had active pulmonary TB, and their mother (1.I.2) had a partial lobectomy in her 40s for a pulmonary nodule and was subsequently diagnosed with pulmonary MAC in her 60s.
Family 2:
2.I.1 is a 65-year-old white woman with osteopenia and scoliosis, diagnosed with pulmonary NTM at 55 years. She is a lifelong nonsmoker who lives in Maryland. A chronic cough developed 8 years before the diagnosis of pulmonary NTM. One year before diagnosis she had increasing dyspnea and intermittent fevers. Thoracoscopic lung biopsy showed necrotizing granulomata but was culture negative. Sputum grew both MAC and M kansasii. She had bronchiectasis predominantly involving the left lower lobe, multiple areas of small nodular densities, and mild pleural thickening. From sputum grew MAC X-cluster, M avium, and M massiliense, as well as Pseudomonas aeruginosa, Staphylococcus aureus, Nocardia spp, and Aspergillus spp, all of which have been treated appropriately.
2.I.2, the 65-year-old identical twin sister of 2.I.1, also has scoliosis, osteopenia, and is a lifelong nonsmoker. She lives in California. Chronic cough, shortness of breath, and easy fatigability began around 53 years of age. M intracellulare was recovered on bronchoscopy. P aeruginosa, Aspergillus spp, and Scedosporium spp have subsequently been isolated from respiratory samples. Therapy for her MAC is underway.
Family 3:
3.I.1 is a 75-year-old white woman with esophageal stricture who was diagnosed with pulmonary MAC at 72 years. She lives in Florida. She developed a dry cough, dyspnea, and weight loss 8 months before diagnosis with pulmonary NTM and one episode of hemoptysis the month preceding diagnosis. Her sputum grew M intracellulare, Nocardia nova, Aspergillus spp, and scant Fusarium. She is currently on treatment for MAC.
3.I.2 is the 74-year-old brother of 3.I.1 who was diagnosed with pulmonary NTM at 60 years. He is a lifelong nonsmoker who lives in Florida. Cavitary changes on CT scanning prompted bronchoscopy, which showed MAC. Two years after his original diagnosis, he had recurrent hemoptysis, and M chelonae group (likely M abscessus) was isolated on bronchoscopy. He has developed extensive bronchiectasis and multiple bilateral subcentimeter pulmonary nodules with waxing and waning sputum production and occasional hemoptysis. M abscessus and Aspergillus niger have been isolated from multiple sputa; therapy is associated with intermittent clinical improvement.
Family 4:
4.II.1 is a 65-year-old white woman diagnosed with pulmonary NTM at 58 years of age, who has lived predominantly in the Mid-Atlantic region. A chronic cough developed in her 3 years before diagnosis, progressing to night sweats, dyspnea, and hemoptysis. CT scanning showed a right cavitary lesion with nodular infiltrates on the left. Sputum grew M avium, mucoid Pseudomonas, and scant Aspergillus fumigatus.
4.I.1, the mother of patient 4.II.1, became acutely ill with cough, fever, weight loss, and dyspnea at 78 years. M kansasii was isolated from a bronchoscopic specimen at 79 years. She also had osteoporosis, kyphosis, and COPD; eosinophilic pneumonitis was diagnosed in her 60s. She had a 10 pack-year smoking history. Chest CT scanning showed extensive bronchiectasis, bilateral subcentimeter nodules, and cavitary changes in the left upper lobe. Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Serratia marcescens were isolated from her sputum on various specimens. She died at 85 years.
Family 5:
5.I.1 is a 49-year-old Hispanic woman diagnosed with pulmonary NTM at 36 years. She had bronchitis as a child and has asthma and allergic rhinitis. She lives in Texas and is a lifelong nonsmoker. She was diagnosed with pulmonary MAC on lung resection after fever, night sweats, and hemoptysis. Five years after her MAC diagnosis, recurrent hemoptysis led to the diagnosis of M abscessus pulmonary disease.
5.I.2 is the 56-year-old Hispanic sister of 5.I.1 with hypertension and mild scoliosis; she is a lifelong nonsmoker from Texas. She deveoped fever, night sweats, productive cough, and hemoptysis at 53 years. CT scan was abnormal, and culture from bronchoscopy grew MAC.
Family 6:
6.I.1 is an 80-year-old white woman with pulmonary MAC diagnosed at 55 years. She is a lifelong nonsmoker from North Carolina who has osteopenia and colitis. She underwent a right middle lobectomy and has received several courses of therapy for persistent MAC. Her disease has been clinically and radiographically stable for the past 5 years.
6.II.1 is the 50-year-old son of 6.1.1 who has mild scoliosis and Crohn's disease, never treated with tumor necrosis factor-α inhibitors. He is a lifelong nonsmoker living in Maryland. Chest pain, persistent cough, and fatigue developed in him at 49 years. CT scanning revealed nodular disease involving the right upper lobe and bronchoscopy cultures grew MAC.
Results
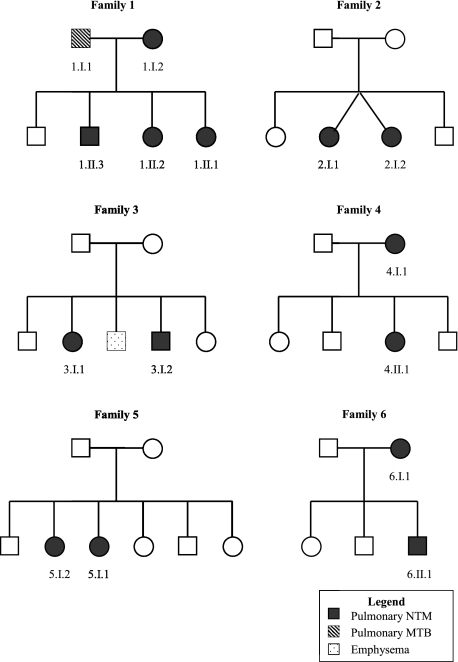
Sibling pairs were the most commonly affected family members (see Fig 1). However, two mother-child pairs were also identified, and an additional mother (1.I.2) was reported to have had pulmonary NTM disease. Four of the six families were white, and 10 of 13 patients (77%) were women (Table 1). All patients seen at NIH lived in coastal US states, but none of the relative pairs resided in the same household at the time of diagnosis and several lived in different states. The average age of diagnosis was 56.4 ± 10.7 years, the average height 167.5 ± 8.7 cm, and the mean BMI was 22.0 ± 2.98 kg/m2. As expected, the mean height and BMI are similar to values previously reported for the larger cohort from which these familial clusters were drawn.5 Scoliosis was present in 31%, a higher percentage than typically seen in the general population.5 Previous pulmonary NTM cohorts have reported up to a 52% prevalence of scoliosis among cases.4,5
Figure 1.
Pedigrees of familial clusters of pulmonary NTM disease. MTB = Mycobacterium tuberculosis; NTM = nontuberculous mycobacteria.
Table 1.
—Demographic Data and Pertinent Findings for Clusters of Nontuberculous Mycobacteria Pulmonary Disease Organized by Family
| Family/Patient | Ethnicity/ Gender | Age at Diagnosis, y | Height/ Weight, cm/kg | BMI,kg/m2 | Mycobacteria | Tobacco Use | Scoliosis | Sweat chloride, 0-40 mmol/L | CFTR Statusa |
| Family 1 | |||||||||
| 1.II.1 | Korean/F | 47 | 166/60 | 21.7 | M avium complex | No | No | 20 | No mutation |
| 1.II.2 | Korean/F | 53 | 165/65 | 23.9 | M intracellulare | No | No | 11 | Heterozygous, E681V |
| 1.II.3 | Korean/M | 60 | 175/63 | 20.6 | M avium complex | Yes | No | … | … |
| Family 2 | |||||||||
| 2.I.1 | White/F | 55 | 157 /50 | 20.1 | M avium, MAC X cluster, M kansasii, M massiliense | No | Yes | 46 | Heterozygous 5′UTR-680T > 6 |
| 2.I.2 | White/F | 57 | 158/57 | 22.6 | M intracellulare | No | Yes | … | Heterozygous 5′UTR-680T > 6 |
| Family 3 | |||||||||
| 3.I.1 | White/F | 72 | 168/81 | 28.6 | M intracellulare | No | No | 31 | Heterozygous V754M |
| 3.I.2 | White/M | 60 | 183/74 | 22.2 | M abscessus, M avium complex | No | No | 30 | Heterozygous V754M |
| Family 4 | |||||||||
| 4.II.1 | White/F | 58 | 173/68 | 22.7 | M avium | Yes | No | … | No mutation |
| 4.I.1 | White/F | 79 | 173/48 | 16.1b | M kansasii | Yes | Noc | … | No mutation |
| Family 5 | |||||||||
| 5.I.1 | Hispanic/F | 36 | 157/47 | 19.0 | M avium complex, M abscessus | No | No | 30 | No mutation |
| 5.I.2 | Hispanic/F | 53 | 160/54 | 21.4 | M avium complex | No | Yesd | 26 | No mutation |
| Family 6 | |||||||||
| 6.I.1 | White/F | 55 | 162/65 | 24.8 | M avium complex | No | No | … | No mutation |
| 6.II.1 | White/M | 49 | 180/73 | 22.5 | M avium complex | No | Yesd | 27 | No mutation |
CFTR = cystic fibrosis transmembrane conductance regulator; F = female; M = male; M = Mycobacterium; MAC = Mycobacterium avium complex.
CFTR genetic testing performed by Ambry Genetics (Aliso Viejo, CA).
Height not documented in record; estimated by daughter as approximately 173 cm.
Severe kyphosis present but no scoliosis.
Very mild lumbar scoliosis.
Seventy-seven percent of our patients were lifelong nonsmokers. Only one had a prior diagnosis of lung disease (COPD and eosinophilic pneumonitis). Five of 12 patients in whom CFTR gene analysis was performed had an identified mutation or novel variation. However, only a single allele was involved in each case. Only one patient had a borderline elevated sweat chloride. None of the patients tested had α 1-antitrypsin deficiency, abnormal immunoglobulin levels, or HIV.
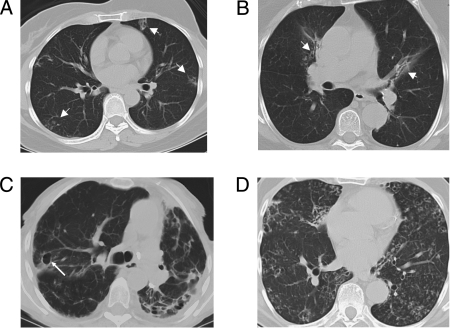
All patients had abnormalities on CT scans consistent with pulmonary NTM, ranging from localized nodules and bronchiectasis to extensive cavitary disease (Fig 2).
Figure 2.
Representative CT scans of the chest demonstrating characteristic radiographic manifestations of pulmonary nontuberculous mycobacteria disease, including: multilobar nodules and bronchiectasis (arrowheads), patient 1.II.2 (A) and patient 2.I.2 (B); extensive bronchiectasis and cavity formation (arrow), architecture distortion, calcified hilar nodes, pleural thickening with marked left volume loss, patient 4.I.1 (C); diffuse bronchiectasis, bronchiolectasis, and scattered nodules, patient 3.I.2 (D).
Discussion
We identified six unrelated families in which pulmonary NTM disease affects at least two first-degree relatives. The patterns of transmission are consistent with dominant (parent-child) and recessive (siblings only) modes of inheritance. The fact that in several families cases were infected with distinct species of NTM suggests that neither common source exposure nor patient-to-patient intrafamily transmission was responsible. Therefore, these patients support the hypothesis that some pulmonary NTM susceptibility is due to underlying heritable host factors.
This collection of familial cases of pulmonary NTM may be an underestimate of the true prevalence of familial clusters in pulmonary NTM, since the disease is usually chronic, indolent, and difficult to diagnose. The cohort from whence these familial clusters were identified is intentionally skewed toward more severe disease, which should increase the likelihood of finding underlying causes for pulmonary NTM disease. Nevertheless, our families were similar to those previously described in pulmonary NTM disease: the majority (77%) were lifelong nonsmokers, and only one had a prior diagnosis of lung disease (COPD and eosinophilic pneumonitis).1-5 Although three patients from the same family had positive tuberculin skin tests, all were Korean, had likely received bacille Calmette-Guérin vaccination, and came from a TB-endemic country; none had a history of documented active pulmonary tuberculosis. Forty-two percent of patients in whom CFTR gene analysis was performed had an identified mutation or novel variation involving a single allele and none had a diagnostic elevated sweat chloride. These findings argue against any of the patients having classic cystic fibrosis. However, the role of heterozygous CFTR mutations in pulmonary NTM is unclear. Ziedalski et al3 reported a 50% prevalence of CFTR mutations and Kim et al5 reported a 36.5% prevalence in pulmonary NTM. This increased prevalence of CFTR heterozygosity among cohorts of patients with pulmonary NTM suggests the CFTR gene may be somehow involved in disease susceptibility, perhaps as a modifying factor of host susceptibility. Although molecular strain relatedness of the mycobacteria was not determined in every case, speciation where available showed the isolates to be distinct. For instance, patient 2.I.2 was infected with M intracellulare, whereas her sister, 2.I.1, had been infected with MAC X-cluster, M avium, M kansasii, and M massiliense. Similarly, 4.II.1 was infected with M avium, whereas her mother, 4.I.1, had M kansasii infection. These findings are consistent with the report by Tanaka et al7 of two different strains of M avium infecting two brothers who lived in the same household.
These familial clusters of pulmonary NTM suggest that a heritable factor is involved in host susceptibility. Since many of these patients had other infections as well as NTM, including Gram-negative bacilli and fungi, this factor may predispose to more than one infection. However, whether this genetic factor is associated with immune dysfunction, such as mutations leading to TGF-β dysregulation, or one that predisposes to a mechanical airway clearance defect, such as cilia-related gene mutations, remains to be determined. The fact that the infection susceptibility tracks so closely with morphologic features suggests that there may be a causal association. However, until the gene or genes are identified, this remains hypothetical. The genes associated with the Marfanoid syndromes, such as the fibrillin or TGF-β receptor genes, may be attractive candidates for investigation given the overlapping phenotype between Marfan disease and nodular-bronchiectatic pulmonary NTM disease.10
Genetic predisposition to disseminated NTM infection is well established as being due to Mendelian mutations in the interferon-γ/interleukin-12 pathway.11 Unlike typical cases of pulmonary NTM infection, patients with disseminated infection manifest disease at an early age. Interestingly, none of the causes of Mendelian susceptibility to mycobacterial disease are associated with recognized morphotypes, further suggesting that lung and disseminated disease may not be related to the same susceptibility factors. Understanding the pathogenesis of pulmonary NTM susceptibility may lead to better understanding multiple pulmonary infections. It is to be hoped that insights into cause will allow us to interrupt the consequences of these severe, chronic, and debilitating infections.
Acknowledgments
Author contributions: Dr Olivier had full access to the data in this study and takes responsibility for the integrity of the data and accuracy of the analysis.
Dr Colombo: contributed to conducting the record review, contacting the participants to verify and obtain additional information, analyzing the data, creating the table and figures, and drafting the manuscript.
Dr Hill: contributed to reviewing and scoring all the scoliosis films, reviewing and interpreting selected chest CT scans for each patient and available family members, and editing and revising Figure 2.
Mr Claypool: contributed to assisting with data collection and contacting of the probands and selected family members. He provided data for and assisted in the creation of Table 1 and a critical review of the manuscript.
Dr Holland: contributed to evaluating many of the patients in this cohort, collecting key family history data that enabled this assessment, and conducting extensive editing and critical review of the manuscript. He is the Principal Investigator of the natural history study under which these patients were evaluated.
Dr Olivier: contributed to providing overall guidance for design and data collection, evaluating and collecting family history information from many of the patients included in this study, and critically editing and revising the manuscript, table, and figures.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This work was performed at the Clinical Center of the National Institutes of Health, Bethesda, MD.
Abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- MAC
Mycobacterium avium complex
- NIH
National Institutes of Health
- NTM
nontuberculous mycobacteria
Footnotes
Funding/Support: This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Falkinham JO. The changing pattern of nontuberculous mycobacterial disease. Can J Infect Dis. 2003;14(5):281–286. doi: 10.1155/2003/323058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- 3.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130(4):995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 4.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144(4):914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 5.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178(10):1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobashi Y, Yoshida K, Niki Y, Oka M. Sibling cases of Mycobacterium avium complex disease associated with hematological disease. J Infect Chemother. 2006;12(5):331–334. doi: 10.1007/s10156-006-0461-z. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka E, Kimoto T, Matsumoto H, et al. Familial pulmonary Mycobacterium avium complex disease. Am J Respir Crit Care Med. 2000;161(5):1643–1647. doi: 10.1164/ajrccm.161.5.9907144. [DOI] [PubMed] [Google Scholar]
- 8.Griffith DE, Aksamit T, Brown-Elliott BA, et al. ATS Mycobacterial Diseases Subcommittee. American Thoracic Society. Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 9.Mangione EJ, Huitt G, Lenaway D, et al. Nontuberculous mycobacterial disease following hot tub exposure. Emerg Infect Dis. 2001;7(6):1039–1042. doi: 10.3201/eid0706.010623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelb BD. Marfan’s syndrome and related disorders—more tightly connected than we thought. N Engl J Med. 2006;355(8):841–844. doi: 10.1056/NEJMe068122. [DOI] [PubMed] [Google Scholar]
- 11.Holland SM. Interferon gamma, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38(1-3):342–346. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]