Abstract
Rheumatoid arthritis (RA) is characterized by premature immune aging with accumulation of degenerate T cells deficient for CD28. Gene expression profiling of CD4+CD28− and CD4+CD28+ T cells to discover disease-promoting activities of CD28− T cells identified expression of CD70 as a most striking difference. Hence, CD70 was significantly more expressed in CD4 T cells from RA patients compared to age-matched controls (P<0.006). The underlying mechanism was a failure to repress CD70 expression after activation-dependent induction. This defect in RA was not related to differential promoter demethylation. CD70 on bystander CD4+CD28− T cells functioned by lowering the threshold for T-cell activation; admixture of CD4+CD28− T cells augmented TCR-induced responses of autologous naïve CD4+CD28+ T cells, particularly of low avidity T cells. The data support a model where CD70 expressed on T cells causes degeneracy in T-cell responses and undermines tolerance mechanisms that normally control T-cell autoreactivity.
Keywords: T cells, Rheumatoid Arthritis, Costimulation, Gene Regulation, Tolerance/Suppression/Anergy
Introduction
Rheumatoid arthritis (RA)4 is a systemic inflammatory disease which predominantly manifests in diarthrodial joints leading to structural damage of the joint architecture (1, 2). The main effector mechanisms causing cartilage degeneration and bony erosion include hyperplasia of synoviocytes, production of various cytokines—in particular TNF-α, and RANKL-mediated activation of osteoclasts (2–4). The nature of the defect upstream of these effector functions is a matter of debate (1, 5). In one model, the inflammation is considered to be the result of a misguided T-cell response. Several lines of indirect evidence have supported this notion (2, 6). The major disease-associated genetic risk factor is an HLA-DRB1 polymorphism that is important for the function of CD4+ T cells (7, 8). Also, newly defined genetic risk factors, such as CLTA-4 and PTPN22 are concerned with T-cell physiology (9, 10). Experiments in rheumatoid synovium/SCID mouse chimera animal models have shown that synovial inflammation was strictly T cell dependent (11). Moreover, many patients show evidence of lymphoid neogenesis in the synovium, which optimizes the recognition of Ag by B cells and T cells (2, 12). Finally, targeting the adaptive immune response has shown therapeutic benefits. The most obvious example is treatment with CTLA4-Ig, which is effective in RA (13). Also, the success of B-cell depletion in the treatment of rheumatoid arthritis has been attributed to the Ag-presenting function of B cells that take up and present Ag to tissue-infiltrating T cells (14).
T-cell activation is ultimately determined by positive signals from costimulatory molecules and negative signals from inhibitory receptors that are expressed on T cells and bind to ligands on APCs (15–18). The classical example for such a costimulatory pathway is the CD28-CD80/CD86 interaction which is particularly important in activating naïve T cells (15). Indeed, treatment with CTLA4-Ig that interferes with this receptor-ligand interaction reduces joint inflammation in RA(13). Another costimulatory pathway important for the activation of naïve T cells is the stimulation of CD27 that recognizes the CD70 molecule (16, 18–20). CD28 and CD27 are constitutively expressed, and costimulatory activities are controlled by the expression of the ligands CD80/CD86 and CD70, all of which are tissue-specific and spatially and temporally restricted (16, 19, 20).
In addition to CD28 and CD27, which mostly control the activation of naive CD4 T cells, a large array of molecules has been shown to regulate T-cell activation. One major variable in determining the profile of regulatory molecules on T cells is the age of the individual, or, possibly better stated, the replicative history of the T-cell population (2, 21). Many of the receptors that been found to control the function of senescent or end-differentiated T cells are primarily expressed on NK cells. Such molecules include members of the killer immunoglobulin-like receptor (KIR) family, NKG2D, fractalkine receptors, and immunoglobulin-like transcripts (21–24). In contrast, regulatory molecules that are usually associated with T-cell function, such as CD28, CD27, and CD40 ligand are frequently lost in such T cells (21). These changes appear to be of particular importance for the pathogenesis of RA, a disease of late adulthood with increasing age-related incidence (2). Moreover, the adaptive immune system in RA patients is pre-aged with premature appearance of many of these T-cell senescence markers as compared to age-matched healthy controls (2, 6, 21). Indeed, many of these aberrantly expressed T-cell regulatory molecules appear to be functionally important in RA and synovial inflammation. KIR2DS2 has been shown to be a genetic risk factor for extraarticular complications of rheumatoid arthritis (2, 25). NKG2D is expressed on end-differentiated CD4 T cells and provides a costimulatory signal recognizing a ligand expressed in the synovial tissue (22, 26). Similarly, fractalkine receptor is expressed on senescent CD4 T cells and communicates with synovial fibroblasts through the recognition of fractalkine (23). Aberrant expression of regulatory molecules on T cells may, therefore, be an important component enabling the rheumatic disease process.
We hypothesized here that comparing the gene expression profiles of CD4+CD28+ and CD4+CD28− T cells from patients would allow for identifying molecules related to accelerated immune aging in RA and involved in RA pathogenesis. The most striking difference in cell surface molecules identified in the arrays was the overexpression of the CD27 ligand CD70. Data presented here show that CD4+CD28− T cells have a defect in downregulating CD70, which leads to sustained expression after T-cell activation. Accordingly, expression of this molecule is increased on peripheral CD4 T cells from RA patients. Aberrant CD70 expression on bystander T cells lowers the T-cell receptor threshold necessary for the induction of primary T-cell responses, possibly leading to activation of self-reactive T cells and breaches in tolerance. Therapeutic interventions targeting the expression of CD70 and the CD27–CD70 interaction may, therefore, be of particular benefit for RA patients.
Material & Methods
Subjects
PBMCs were obtained from twenty two patients with rheumatoid factor-positive RA, aged 23 to 77 years, and twenty seven healthy volunteers, aged 25 to 81 years. The protocol was approved by the Mayo Clinic and the Emory University Institutional Review Boards, and all participants provided informed consent. Individuals with cancer, a history of chemotherapy, advanced atherosclerotic disease or congestive heart failure, poorly controlled diabetes mellitus, or chronic obstructive pulmonary disease were excluded; healthy volunteers did not have a chronic inflammatory disease.
Cell separation
CD4+CD28+ and CD4+CD28− T cells were purified from PBMCs by FACS vantage (BD Biosciences, San Jose, CA). Cells were stimulated with immobilized anti-CD3 (OKT3; Ortho Diagnostics, Rochester, NY) to generate short-term T cell lines for microarray studies and coculture experiments. Fresh CD4 T cells from PBMC were negatively enriched with a human CD4+ T-cell enrichment cocktail (RosetteSep; StemCell Technologies, Vancouver, Canada). Naive CD4 T cells were isolated by positive selection with anti-CD45RA magnetic beads (Miltenyi Biotec, Auburn, CA). In some experiments, CD4+ naive T cells were negatively selected by a CD4+/CD45RO− naive T-cell subset column kit (R&D System, Minneapolis, MN). Purity was between 92 and 97%.
Mature dendritic cells (DC) were generated from PBMCs as previously described (27). In brief, monocytes were isolated by either plastic adherence or anti-CD14 magnetic beads (Miltenyi Biotec), and were cultured for 6 days in RPMI-10% FCS supplemented with 800 U/ml GM-CSF and 1000 U/ml IL-4 (both from R&D System). At day 6, non-adherent immature DCs were harvested and stimulated with 1100 U/ml TNF-α (R&D System) and 1 µg/ml PGE2 (Sigma, St. Louis, MO) for 48 hrs.
Microarray analysis
Total RNA was extracted from CD4+CD28+ and CD4+CD28− T cell lines using a RNeasy Mini Kit (Qiagen, Valencia, CA). Preparation of biotinylated target RNA from total RNA and subsequent hybridization of cRNA to the Affymetrix Hu-95Av2 (Affymetrix Inc., Santa Clara, CA) probe-array cartridges were performed by Mayo Cancer Microarray Core Facility (Mayo Foundation, Rochester, MN).
FACS analysis
PBMCs or T cell lines were stained with FITC- or PE-conjugated anti-CD70, FITC-or PE-anti-CD27, FITC-anti-CD69, PerCP-anti-CD4, APC-anti-CD3, PE- or APC-anti-CD28, or APC-anti-CD25 (all from BD Biosciences), and PE-anti-TCR-Vβ2 mAbs (Beckman Coulter, Inc. Fullerton, CA). Stained cells were analyzed using FACSCalibur or FACSort (BD Biosciences). To examine CD70 kinetics, PBMCs from patients with high frequencies of CD4+CD28− T cells were stimulated with immobilized anti-CD3 (1µg/ml), and the expression patterns of CD70 on CD4+CD28+ and CD4+CD28− subsets were monitored using FACSCalibur or LSRII (BD Biosciences). In selected experiments, 5-aza-2'-deoxycytidine (5-Aza-dC) (Sigma-Aldrich, St. Louis, MO) at a final concentration of 1 µM or zebularine (10µM, Sigma-Aldrich) was added on day 3 to inhibit DNA methylation. In parallel experiments, the ERK pathway which had been shown to be involved in DNA methyltransferase (DNMT) control (28) was inhibited with PD98059 (25 µM, Calbiochem, San Diego, CA). CD70 expression on activated CD25+CD4+CD28+ and CD28− T cells was determined by flow cytometry.
Bisulfite sequencing and methylation-specific PCR
Genomic DNA was isolated with a QIAamp Blood Kit (Qiagen). 1 µg of the purified DNA was treated with sodium metabisulfite. 50 ng of bisulfite-modified DNA was used to amplify the middle (bp −281 to −609) and proximal portion of the CD70 promoter (bp +45 to − 301). The forward primers for the first and second round amplification of the −281 to -609 fragment were 5′-GGTGGATTATTTAAGGTTAGGAGTTT-3′ and 5′-GGAGTTTAAGTTTAGTTTGGTT-3′, the reverse primer was 5′-ACTATACACTAAAAATACAACA-3′ for both amplifications. The primers used to amplify the +45 to −301 fragment were 5′-GTTGTATTTTTAGTGTATAGTAT-3′ and 5′-TCTACTTACTTCAACCTATCAAA-3′ for the first round; and 5′-TAGGAAGATTGAATGTTTTTTGT-3′ and 5′-AAAAACMAACCTACCCCTCTCTAA-3′ for the second round. The PCR conditions were 95°C for 4 min, followed by 35 cycles of 95°C for 1 min, 50°C for 1 min and 72°C for 2 min. The resulting amplification products were cloned into pCR2.1-TOPO vector using a TOPO TA cloning kit (Invitrogen Life Technologies). Individual subclones were isolated and sequenced.
Coculture experiments
Two assay systems were used to examine bystander effects of CD70-expressing T cells. CFSE-labeled (Molecular Probes, Eugene, OR) CD4 naive or memory T cells were stimulated with immobilized anti-CD3 (1µg/ml) and anti-CD28 (0.5ug/ml) in the presence of CD70-expressing CD4+CD28− or control CD4+CD28+ T cells; second, CFSE-labeled CD4 naive T cells were cocultured with mature DCs and pulsed with toxic shock syndrome toxin-1 (TSST-1; Toxin Technology, Sarasota, FL) in the presence or absence of CD4+CD28− T cells. Proliferation was assessed by CFSE dilution analysis using flow cytometry. In both assay systems, CD70 was blocked by 1 µg/ml anti-CD70 mAb (Ki-24) or isotype control mAb (J606; both from BD Biosciences).
Statistical Analysis
Data were analyzed using a nonparametric Mann-Whitney U test (SigmaStat; SPSS).
Results
Overexpression of CD70 on CD4+CD28− T cells
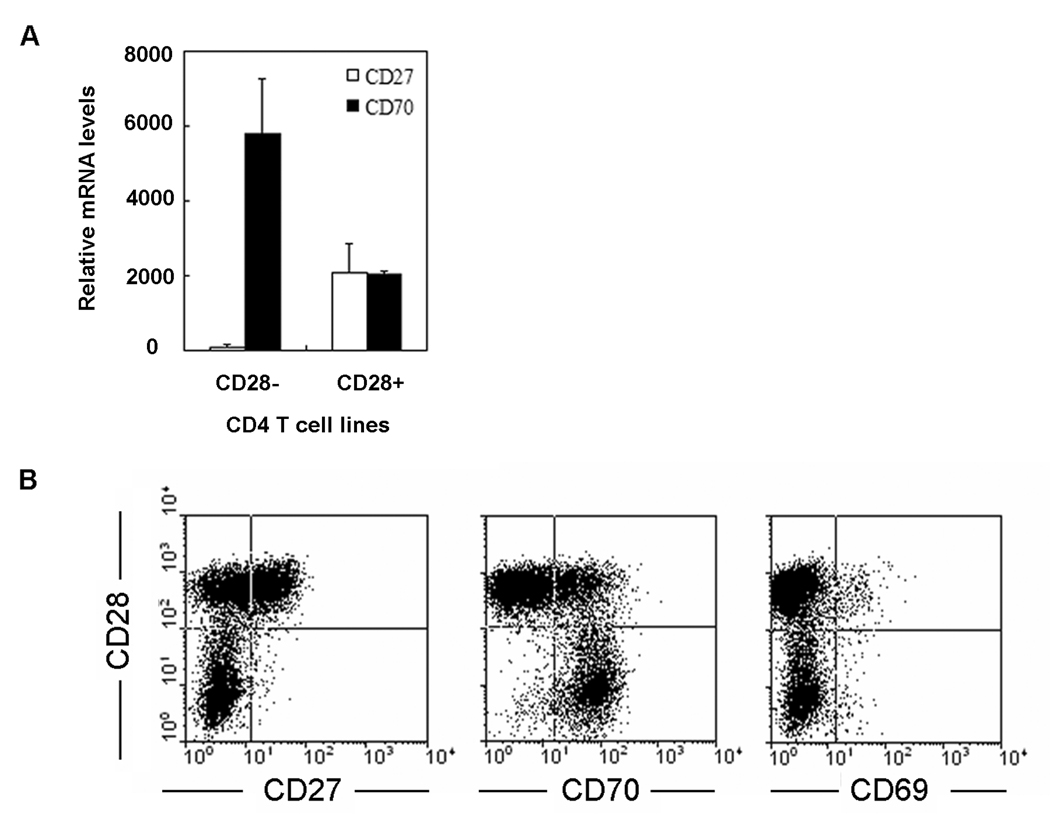
Previous studies have demonstrated that transcriptional inactivation of CD28 on CD4 T cells is closely correlated with a gain of new regulatory cell surface molecules (21, 26). We and others have proposed that costimulatory signals delivered through aberrantly expressed stimulatory receptors perturb peripheral tolerance and sustain autoimmune responses (22, 23). Aberrantly expressed molecules have so far been identified using a candidate gene approach. To more systematically screen for changes in gene expression of potential regulatory receptors, we compared gene expression profiles of CD4+CD28− and CD4+CD28+ short-term T cell lines from three patients using Affymetrix microarrays. Among regulatory cell surface molecules, differential gene expression was most impressive for the CD27–CD70 receptor-ligand pair. CD70 was markedly upregulated in all CD4+CD28− T cells as compared to in CD4+CD28+ T cells from the same donor. In contrast, CD27 transcripts were barely detected in the CD4+CD28− T cell lines (Figure 1A). This data was verified at the protein level by multicolor flow cytometry using CD4 T cell lines that included CD28-negative and positive T cells. As shown in Figure 1B, CD4+CD28− T cells were homogenous for the CD70+CD27− phenotype, whereas CD4+CD28+ T cells were heterogeneous; about 50% were CD27 positive and the majority CD70 negative. CD70 is known to be an inducible molecule that is transiently upregulated by T cells upon stimulation. To exclude that CD4+CD28− cells differ from CD4+CD28+ T cells in their activation state, cells were stained for the expression of CD69, a T-cell activating marker. CD69 was expressed on a minority of T cells and on fewer CD4+CD28− than CD4+CD28+ T cells, indicating that overexpression of CD70 was not related to differential activation (Figure 1B).
Figure 1. Preferential expression of CD70 on CD4+CD28− T cells.
(A) Gene expression in CD4+CD28+ and CD4+CD28− T cells was compared by Affymetrix Genechip Hu-95Av2. Amongst putative regulatory cell surface receptors, the largest difference was seen for the CD27–CD70 receptor-ligand pair. Data shown are the mean expression levels from three individuals. (B) Flow cytometric analysis of CD4+ T cell lines confirmed a CD70+CD27− phenotype for CD4+CD28− T cell lines. CD4+CD28− T cells lacked activation markers such as CD69, suggesting that CD70 expression is not due to constitutive activation.
Persistent CD70 expression on CD4+CD28− T cells after T-cell activation
While CD70 is clearly overexpressed on cultured CD4+CD28− T cell lines, and the expression is not lost with resting for 2 to 4 weeks after activation, CD70 is only expressed on a fraction of peripheral blood CD4+CD28− T cells (data not shown). We therefore hypothesized that CD4+CD28− and CD4+CD28+ T cells differ in the regulation of CD70 expression after stimulation. To address this question, PBMCs from patients who were known to have increased frequencies of CD4+CD28− T cells were stimulated, and CD70 expressions on T-cell subsets were monitored by multicolor flow cytometry. As shown in Figure 2, expression of CD70 was progressively gained on CD4+CD28− T cells for the first five days after stimulation and sustained throughout the 14-day culture. In contrast, only a subset of CD4+CD28+ T cells gained CD70 expression after stimulation and even these cells started to revert and lose it after one week, which is consistent with the current paradigm that the window of CD70 expression is tightly controlled. These data indicate that the defect in CD70 regulation lies in sustained expression after activation.
Figure 2. Kinetics of activation-induced CD70 expression on CD4+CD28+ and CD4+CD28− T cells.
PBMCs were stimulated with immobilized anti-CD3 and at the indicated time points stained with FITC-anti-CD70, PE-anti-CD28, PerCP-anti-CD4, and APC-anti-CD3 mAbs. Results are shown as histograms gated for CD4+CD28+ T cells (solid lines) and CD4+CD28− T cells (gray shaded areas). Dotted lines represent stains with isotype control mAb.
Correlation between CD70 promoter demethylation and CD70 transcription
To examine whether CD4+CD28+ and CD4+CD28− T cells differ in epigenetic control of CD70, we examined DNA methylation patterns. The CD70 promoter has 20 CpG dinucleotides between −9 to −512 bp. Bisulfite sequencing results shown in Figure 3A did not support the hypothesis that CpG islands in CD4+CD28− T cells are more demethylated than CD4+CD28+ T cells. Almost all CpG dinucleotides within the proximal promoter up to position −167 were demethylated in either cell type, while the remainder of the promoter was mostly methylated. These results are in contrast to patients with systemic lupus erythematosus (SLE) where CD70 overexpression has been correlated with demethylation within the region between −515 and −423 (28).
Figure 3. DNA demethylation does not account for increased CD70 promoter activity.
CpG methylation of the CD70 promoter of CD4+CD28+ and CD4+CD28− T cells from RA patients before (A) and 4 days after (B) stimulation with immobilized anti-CD3 was assessed. CD28+ and CD28− T cells showed similar methylation patterns; a representative example is shown. Closed circles (●) depict methylated CpG sites; open circles (○) represent unmethylated CpG sites. (C) Inhibition of DNMT activity (closed bars) with 1µM 5-Aza dC (left) or 10µM zebularine (middle) subsequent to anti-CD3 stimulation increased sustained CD70 expression, compared to untreated CD4 T cells (open bars). However, the relative difference between CD4+CD28+ and CD4−CD28− T cells was maintained. Results are shown as mean ± SD of 6 experiments. Inhibition of the ERK pathway with 25µM PD98059 did not have an effect on CD70 expression (right).
To examine whether the sustained expression of CD70 is caused by progressive promoter demethylation of CD4+CD28− T-cells upon activation and failure to remethylate, we examined promoters on day 4 after stimulation. Results did not demonstrate a significant difference between the two cell types. Overall activation-induced demethylation was minimal for CD4+CD28+ and CD4+CD28− T cells (only at positions −338, −383 and −423) (Figure 3B).
To further determine whether the sustained CD70 expression is due to a failure of CD4+CD28− T cells to remethylate, cells were activated, grown in the presence of the DNMT inhibitor 5-Aza-dC, and examined for CD70 expression. The response pattern was very similar for both T-cell types (Figure 3C). CD4+CD28− T cells had a higher and more sustained CD70 expression compared to CD4+CD28+ T cells, which is consistent with the data shown in Figure 2. Similar results were obtained with a second inhibitor, zebularine. DNMT inhibition increased CD70 expression in both cell types; however, the relative difference between CD4+CD28− and CD4+CD28+ T cells was maintained. In contrast, inhibition of the ERK pathway, which has recently been implicated in modifying methylation patterns of CD4 T cells in SLE patients (29), did not have any effects.
Increased frequencies of CD4+CD70+ T cells in RA patients
CD28 loss on memory T cells is a typical sign of immune aging, but is also disproportionately seen in several autoimmune diseases (2, 21). In RA, the frequency of CD4+CD28− T cells predicts severity of erosive disease and occurrence of extraarticular disease (30), suggesting that these cells are involved in disease pathogenesis. To examine whether the phenotypic changes in RA include increased CD70 expression on CD4 T cells, we compared 27 healthy individuals and 22 RA patients (Figure 4). CD70 was expressed on CD4+CD28− T cells and to a much lesser extent on CD4+CD28+ T cells (data not shown). The frequencies of CD4+CD70+ T cells in healthy individuals younger than 50 years old were low; these cells accounted for about 1% of all CD4 T cells. After the age of 50, frequencies increased about 2-fold (P=0.009). In contrast, RA patients had 4- to 6-fold higher frequencies of these cells compared to age-matched controls (P=0.004 and P=0.005, respectively for RA patients younger or older than 50 years). In RA patients, a trend for age-dependent CD70 expression was not significant (P=0.3).
Figure 4. Increased expression of CD70 on peripheral CD4 T cells from RA patients.
CD70 expression on peripheral blood CD4 T cells was examined in healthy individuals (n=27) and RA patients (n=22) by multicolor flow cytometry. Data are shown separately for two age strata as box plots displaying medians, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as whiskers.
CD70 on CD4+CD28− T cells acts as a bystander costimulatory signal
To explore the physiological implication of sustained CD70 expression, we tested the hypothesis that CD70 provides a costimulatory signal even if it is not expressed on the APC but on a third party T cell that provides a bystander signal. CD4+CD28+ and CD4+CD28− T cell lines were established from a patient with RA. Subsequently, PBMCs were obtained from the same patient whose disease was controlled at that time on weekly methotrexate, labeled with CFSE, and activated with immobilized anti-CD3 in the presence of either autologous CD4+CD28− or CD4+CD28+ T cells. Similar to what was shown in Figure 1, all cells in the CD4+CD28− T cell line were positive for CD70, while CD28+ T cells had a low or no CD70 expression. Adding CD4+CD28− T cells to the anti-CD3-stimulated T cells enhanced cell cycle entry and accelerated proliferation (Figures 5A) as compared to when CD4+CD28+ T cells were added (Figure 5D). In the presence of CD4+CD28− T cells, cocultured T cells had about two more population doublings within the culture period of 5 days. This proliferation-accelerating function of CD4+CD28− T cells was completely inhibited by adding an antibody to CD70 (Figure 5B), but not by adding a control antibody (Figure 5C). The phenomenon was not limited to patients with RA, but was also seen with CD4+CD28− T-cell lines from healthy donors who were selected to have expanded CD4+CD28− T-cell populations (data not shown). This result indicated that the observed enhanced proliferation is genuine to CD70-mediated bystander stimulation.
Figure 5. Costimulatory function of CD4+CD28− T cells.
PBMCs were labeled with CFSE and stimulated with immobilized anti-CD3 in the presence of irradiated autologous CD4+CD28− (A–C) or CD4+CD28+ T cells (D). Anti-CD70 mAb (B) or control Ig (C) was added to the CD4+CD28− T cells before culture initiation. Cells were examined on day 5 of culture. Results are shown as histograms of gated CD4 T cells and are representative for an RA patient. Similar CD70-dependent costimulatory activity was seen with PBMCs of normal controls who had expanded CD4+CD28− T-cell populations.
While expression of the CD70 receptor CD27 is variable on memory T cells depending on the age and immune status of the donor, CD27 is expressed on all naïve T cells (19). We wanted to examine whether CD70-mediated costimulation equally affects naïve and memory CD4 T cells. CD4 T cells were separated based on their CD45RA and CD45RO phenotypes and activated with suboptimal concentrations of anti-CD3 and anti-CD28 mAb in the presence or absence of CD28− T cells. Results are shown in Figure 6. The presence of CD4+CD28− T cells clearly facilitated cell cycle entry and proliferation of naïve CD4 T cells in response to suboptimal stimulation. The response again was sensitive to CD70 blocking, which decreased the frequency of cells that entered the cell cycle, although the costimulatory effects of CD28− T cells was only partially reversed. In contrast, no effects on the activation and proliferation of memory CD4 T cells were seen. Neither the addition of CD28− T cells nor the blocking with anti-CD70 mAb significantly influenced the proliferative behavior of CD4 memory T cells.
Figure 6. CD70-mediated costimulation preferentially targets naïve T cells.
Purified naive (upper panel) and memory (lower panel) CD4 T cells were labeled with CFSE and stimulated with 1µg/ml immobilized anti-CD3 and 0.5µg/ml anti-CD28 in the presence or absence of irradiated CD4+CD28− T cells. As indicated, CD4+CD28− T cells were preincubated with anti-CD70 or isotype-matched control mAb for 1 hr before coculture. On day 5, cell divisions were assessed by CFSE dilution analysis. Data are representative of 3 experiments.
CD70 on CD4+CD28− T cells facilitates recruitment of low-avidity naïve CD4+ T cells
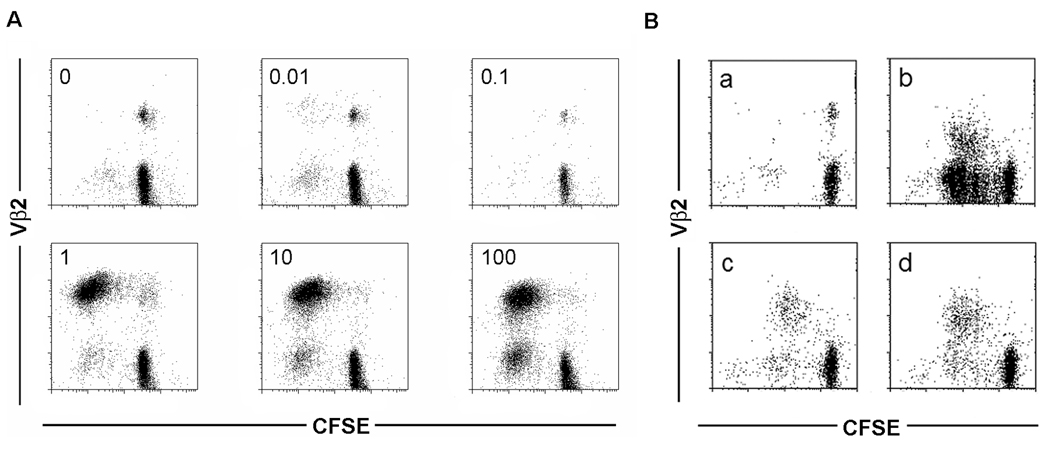
The data in Figure 5 and Figure 6 suggested that CD27 costimulation by CD70 on bystander T cells not only increased the cell cycle progression of stimulated cells, but also facilitated cell cycle entry of naïve T cells in response to suboptimal stimulation. To address this question in a more physiological system where stimulatory and costimulatory molecules are organized in an activation platform between T cells and APC, we used stimulation with the superantigen TSST in a T cell—DC system. TSST at low concentrations selectively activates Vβ2+ T cells (Figure 7A). At a concentration of 1 ng TSST/ml, the Vβ2+ T-cell response is maximized. With increasing TSST concentration, more and more Vβ2-negative T cells are stimulated and enter the cell cycle. We used a concentration of 1 ng/ml TSST to stimulate CFSE-labeled T cells cocultured with autologous DCs. In the presence of autologous CD4+CD28+ T cells, the majority of Vβ2+, but only very few Vβ2−, T cells proliferated (Figure 7B). In contrast, after the addition of CD4+CD28− T cells, a large fraction of Vβ2− T cells were recruited into the cycle, and the majority of proliferating cells were Vβ2−. This effect was completely reversible by the addition of an anti-CD70 antibody. These data showed that CD70 expressed on a third party cell is able to lower the activation threshold to antigen recognition presented by physiological APC.
Figure 7. CD70 costimulation favors recruitment of low avidity CD4 T cells.
(A) CFSE-labeled naïve CD4 T cells were stimulated with autologous DCs and increasing concentrations of TSST (0 to 100 ng/ml as indicated in the left-upper corner of each scatter plot). CFSE dilution was examined by flow cytometry for high (Vβ2+) and low (Vβ2−) avidity T cells. (B) CFSE-labeled naïve CD4+ T cells were cocultured with both autologous TSST-1-pulsed (1ng/ml) DCs and autologous CD4+CD28− T cells (b and c) or CD4+CD28+ T cells (d). A negative control without TSST-1 is shown in (a). To confirm that the costimulatory effect of CD4+CD28− T cells was related to their expression of CD70, cells were preincubated with anti-CD70 mAb (c) or with isotype control mAb (b). CFSE dilutions on Vβ2+ and Vβ2− T cells were examined on day 5.
Discussion
Our data show an abnormal regulation of CD70 expression in CD4+ T cells that have lost the expression of CD28. The regulatory defect leads to a sustained cell surface expression of CD70 after T-cell activation. CD4+CD70+ T cells provide third-party help in the initiation of T-cell responses through the CD27–CD70 pathway by lowering the threshold for the activation of low affinity T cells and, thereby, increasing the risk of autoreactivity. Because CD4+CD28− T-cell accumulation in RA patients is disproportionate for age, and the frequency of CD70 expression on CD4 T cells in RA is increased, the data provide a model for how CD4+CD28− T cells could influence RA pathogenesis.
The CD27–CD70 receptor-ligand interaction is a well established pathway of costimulation (16, 18–20). CD27 is expressed on naïve CD4 and CD8 T cells, subsets of memory cells, NK cells, and primed B cells (31–34). Costimulatory activity is controlled by the expression of CD70, which is activation-dependent and mostly restricted to T cells, DCs, and B cells. CD27 stimulation occurs during T-cell priming when T cells recognize Ag on CD70-expressing DCs or B cells (19). An important role for CD27 stimulation has also been documented for the expansion of effector T cells and the accumulation of CD8+ effector T cells at the site of infection (35, 36). Stimulation of CD27 on primed B cells plays a role in the expansion of centroblasts and, at least in humans, promotion of plasma cell differentiation (37, 38). CD27 signals through TRAF-2/5 and NIK, leading to the activation of the NKκB and cJUN kinase pathways and promoting cell survival and differentiation (39). Recent experiments in CD27 knockout mice have emphasized the importance of CD27 stimulation for CD8 T-cell responses (40). In the absence of CD27 costimulation, the development of CD8 effector and memory T-cell responses is impaired; the likely mechanism is that CD27 stimulation inhibits the CD8 T-cell contraction that normally occurs after Ag-induced clonal expansion. Studies in transgenic mice that aberrantly express CD70 are of particular relevance for the findings presented here. Arens et al report the induction of protective immune responses against weakly immunogenic tumor cells that were lethal in wild-type mice, consistent with our finding that CD70 costimulation lowers the threshold for T-cell activation (40). Although autoimmunity was not a general finding in these transgenic mice, they had abnormal stimulation to self-Ag. CD70 transgenic mice had a loss of naïve T cells with an increased turnover of naïve to effector T cells even in the absence of exogenous antigenic stimulation (41). The resulting phenotype was that of clonal exhaustion and immunodeficiency. It should be noted that CD70 expression in these mice was targeted to APCs, namely B cells, and the effect is, therefore, likely more potent than under conditions where the aberrant expression occurs on bystander cells such as described here for senescent CD4 T cells.
In this context, it is of particular interest that RA patients combine a clinical picture of autoimmunity with evidence of age-inappropriate repertoire contraction and telomere shortening in naïve CD4 T cells as signs of premature immune aging (2, 21, 42, 43). The mechanisms underlying these findings and their relationship to developing autoimmunity are unclear. We have hypothesized that RA patients go through a stage of increased turnover, leading to repertoire contraction and selection of an autoimmune repertoire (44). It remains to be examined whether mice that constitutively express CD70 on cells other than APCs develop a phenotype with moderate clonal exhaustion and dominant features of autoimmunity, resembling the picture in RA.
We used a superantigen-driven system with TSST to probe naive CD4 T-cell responses in a semiphysiological setting. In this system, CD70 constimulation particularly favored the responses of Vβ2-negative T cells that are usually only minimally responsive to TSST. Lagenkamp et al have suggested that Vβ2− T cells responding to TSST represent low-affinity T cells (27). The system therefore allows for comparing high-affinity and low-affinity human T-cell responses, which is otherwise not possible because of the low frequency of human T cells to nominal peptides. Superantigen-mediated T-cell stimulation employs regular T-cell recognition synapse formation; however, recent studies have also shown that superantigens can bypass the Lck dependence of the TCR signaling pathways (45), suggesting that superantigen stimulation is an imperfect, although currently the best, experimental system to study human naive T-cell responses. In particular, TSST may be the best superantigenic model because of the unique structure of the MHC-TSST-TCR complex (46). With this caveat in mind, data obtained in this system appear to be informative for T-cell responses to nominal antigens.
Costimulatory molecules on T cells generally co-cluster with the T-cell activation complex induced by the recognition of antigenic peptide/MHC and costimulatory ligands expressed on the same APC. Our results show that CD27 stimulation in trans position to the TCR by encountering CD70 on cells other than the APC augments T-cell responses. The enhancing effect of third party CD70 was even observed when T cells were stimulated by DCs that expressed CD70 by themselves. Other studies have also shown bystander activity of CD70; application of soluble recombinant CD70 in vivo augmented CD8 T-cell responses after immunization with the ovalbumin peptide (47). It remains to be seen whether CD27 stimulatory signals in trans and cis positions relative to the T-cell receptor differ in their signaling cascades and functional outcomes.
CD70 overexpression is not only seen in RA patients; it has also been described in SLE (29, 48). The mechanisms are different, but the functional consequences appear to be very similar. Lu et al have proposed that CD70 overexpression in SLE is caused by DNA demethylation of the CD70 promoter between positions −515 and −423 (28). This region was only partially methylated in SLE patients, and demethylation of this region increased CD70 transcription. The mechanism of CD70 overexpression in CD4+CD28− cells is different; we have not found any differences in the demethylation patterns between CD4+CD28− and CD4+CD28+ T cells. The proximal CD70 promoter is fully demethylated in resting cells between position −9 and −167, which is apparently sufficient to allow activation-induced CD70 expression. Mechanisms that downregulate CD70 expression after activation are obviously important but remain undefined. Our experiments indicate that while DNMT inhibition can increase CD70 expression, activation-induced transcription does not involve DNA demethylation of the CD70 promoter, and DNA demethylation fails to explain why CD28− T cells have lost this timely ability to revert to CD70-negative cells.
Uncontrolled expression of CD70 could not only induce a chronic T-cell response, but also eventually compromise the naïve T-cell repertoire as seen in RA. CD70 therefore emerges as a therapeutic target in RA, e.g. by interfering with CD27–CD70 receptor-ligand interaction. Preferably, approaches can be developed to repress CD70 expression. In contrast to SLE, the defect in RA does not appear to involve epigenetic DNA methylation, suggesting that understanding the activation-induced transcriptional machinery in CD4+CD28− T cells will provide new therapeutic opportunities.
Acknowledgments
The authors thank Tamela Yeargin for manuscript editing.
Footnotes
Disclosures
The authors have no financial conflict of interest
This work was funded in part by grants from the National Institutes of Health (RO1 AR AR42527, RO1 AR 41974, and RO1 AI 44142)
Abbreviations used in this paper: RA, rheumatoid arthritis; KIR, killer immunoglobulin-like receptor; DC, dendritic cell; SLE, systemic lupus erythematosus.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 4.Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS. Mechanisms of Disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol. 2005;1:47–54. doi: 10.1038/ncprheum0036. [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. The Journal of clinical investigation. 2004;114:471–474. doi: 10.1172/JCI22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16:212–217. doi: 10.1097/00002281-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Nepom GT. Major histocompatibility complex-directed susceptibility to rheumatoid arthritis. Adv Immunol. 1998;68:315–332. doi: 10.1016/s0065-2776(08)60563-5. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Klimiuk PA, Goronzy JJ. Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol. 1998;20:5–22. doi: 10.1007/BF00831996. [DOI] [PubMed] [Google Scholar]
- 9.Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW, Wolfe F, Kastner DL, Alfredsson L, Altshuler D, Gregersen PK, Klareskog L, Rioux JD. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet. 2005;77:1044–1060. doi: 10.1086/498651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 11.Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM. BLyS and APRIL in rheumatoid arthritis. The Journal of clinical investigation. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker JC, Hagerty DT, Moreland LW. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 14.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 15.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 16.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 18.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 19.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27–CD70 interactions. Semin Immunol. 1998;10:491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- 21.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol. 2003;38:833–841. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 22.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawai H, Park YW, Roberson J, Imai T, Goronzy JJ, Weyand CM. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2005;52:1392–1401. doi: 10.1002/art.21140. [DOI] [PubMed] [Google Scholar]
- 24.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, Goronzy JJ. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–1167. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goronzy JJ, Henel G, Sawai H, Singh K, Lee EB, Pryshchep S, Weyand CM. Costimulatory pathways in rheumatoid synovitis and T-cell senescence. Ann N Y Acad Sci. 2005;1062:182–194. doi: 10.1196/annals.1358.022. [DOI] [PubMed] [Google Scholar]
- 27.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. European journal of immunology. 2002;32:2046–2054. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Wu A, Richardson BC. Demethylation of the same promoter sequence increases CD70 expression in lupus T cells and T cells treated with lupus-inducing drugs. J Immunol. 2005;174:6212–6219. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 29.Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, Richardson B. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–1860. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- 30.Goronzy JJ, Matteson EL, Fulbright JW, Warrington KJ, Chang-Miller A, Hunder GG, Mason TG, Nelson AM, Valente RM, Crowson CS, Erlich HA, Reynolds RL, Swee RG, O'Fallon WM, Weyand CM. Prognostic markers of radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2004;50:43–54. doi: 10.1002/art.11445. [DOI] [PubMed] [Google Scholar]
- 31.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 32.Amyes E, McMichael AJ, Callan MF. Human CD4+ T cells are predominantly distributed among six phenotypically and functionally distinct subsets. J Immunol. 2005;175:5765–5773. doi: 10.4049/jimmunol.175.9.5765. [DOI] [PubMed] [Google Scholar]
- 33.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, Kobayashi K, Ito M, Yagita H, Okumura K. CD27-mediated activation of murine NK cells. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 35.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 36.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 37.Jung J, Choe J, Li L, Choi YS. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. European journal of immunology. 2000;30:2437–2443. doi: 10.1002/1521-4141(2000)30:8<2437::AID-IMMU2437>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Xiao Y, Hendriks J, Langerak P, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172:7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 39.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN, van Oers MH. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27–CD70 interactions. Nat Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 42.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goronzy JJ, Weyand CM. Thymic function and peripheral T-cell homeostasis in rheumatoid arthritis. Trends Immunol. 2001;22:251–255. doi: 10.1016/s1471-4906(00)01841-x. [DOI] [PubMed] [Google Scholar]
- 45.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, Tsoukas CD, McCormick JK, Madrenas J. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Moza B, Varma AK, Buonpane RA, Zhu P, Herfst CA, Nicholson MJ, Wilbuer AK, Seth NP, Wucherpfennig KW, McCormick JK, Kranz DM, Sundberg EJ. Structural basis of T-cell specificity and activation by the bacterial superantigen TSST-1. The EMBO journal. 2007;26:1187–1197. doi: 10.1038/sj.emboj.7601531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowley TF, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 48.Han BK, White AM, Dao KH, Karp DR, Wakelan EK, Davis LS. Increased prevalence of activated CD70+CD4+ T cells in the periphery of patients with systemic lupus erythematosus. Lupus. 2005;14:598–606. doi: 10.1191/0961203305lu2171oa. [DOI] [PubMed] [Google Scholar]