Abstract
Targeted deletion of membrane guanylyl cyclases (GCs) has yielded new information concerning their function. Here, we summarize briefly recent results of laboratory generated non-photoreceptor GC knockouts characterized by complex phenotypes affecting the vasculature, heart, brain, kidney and other tissues. The main emphasis of the review, however, addresses the two GCs expressed in retinal photoreceptors, termed GC-E and GC-F. Naturally occurring GC-E (GUCY2D) null alleles in human and chicken are associated with an early onset blinding disorder, termed ‘Leber Congenital Amaurosis type 1’ (LCA-1), characterized by extinguished scotopic and photopic ERGs, and retina degeneration. In mouse, a GC-E null genotype produces a recessive cone dystrophy, while rods remain functional. Rod function is supported by the presence of GC-F (Gucy2f), a close relative of GC-E. Deletion of Gucy2f has very little effect on rod and cone physiology and survival. However, a GC-E/GC-F double knockout (GCdko) phenotypically resembles human LCA-1 with extinguished ERGs and rod/cone degneration. In GCdko rods, PDE6 and GCAPs are absent in outer segments. In contrast, GC-E-/- cones lack proteins of the entire phototransduction cascade. These results suggest that GC-E may participate in transport of peripheral membrane proteins from the endoplasmic reticulum (ER) to the outer segments.
Keywords: Membrane guanylate cyclase, targeted deletions, rod and cone photoreceptors, photoreceptor membrane protein transport
Soluble and membrane guanylate cyclases
Guanylate cyclases (GCs) synthesize cyclic GMP (cGMP), a secondary messenger in many pathways, in response to diverse signals, such as nitric oxide (NO), peptide ligands (hormones), and fluxes in intracellular Ca2+ mediated by Ca2+-binding proteins ([Ca2+]i) [1,2]. These signals use specific guanylate cyclase receptors and cofactors to initiate the conversion of cytosolic GTP to cGMP. Intracellular cGMP regulates cellular physiology by activating protein kinases, directly gating specific ion channels, or altering intracellular cyclic nucleotide concentrations through regulation of phosphodiesterases (PDEs). Guanylate cyclases are classified as either soluble or membrane (particulate), based on both their cellular distribution and structural domains [2,3]. Soluble guanylate cyclases are heterodimeric proteins consisting of α- and β-subunits, and are activated by nitric oxide, another secondary messenger. Soluble guanylate cyclases are present in various cells in vertebrate retina, and maybe involved in signal transmission/modulation between cells [4]. A role in photoreceptor physiology was envisioned earlier for soluble GCs [5,6], but no biochemical or genetic evidences are available for a role in modulation of cGMP in phototransduction. Based on phenotypes of photoreceptor GC double knockouts, a specific role for soluble GCs in phototransduction can safely be excluded.
Membrane GC isozymes (GC-A to GC-G, Table 1) exhibit highly conserved domain structures, an extracellular domain (ECD) which comprises a large part of the N-terminal part of the molecule, a single transmembrane (TM) region, an intracellular protein kinase-like homology domain (KHD), a dimerization (hinge) domain (DD), and a C-terminal catalytic domain (CAT). Based on their ligand specificities, membrane GCs have been subdivided into natriuretic peptide receptors (GC-A, GC-B), intestinal peptide-binding receptors (GC-C), olfactory uroguanylin- and guanylin-sensitive receptors (GC-D) and so-called ‘orphan’ receptors (GC-E/GC-F present in photoreceptors, and GC-G in testis). GC-E and GC-F have no known extracellular ligand (hence the term ‘orphan’), but are stimulated by intracellular ligands, the GC-activating proteins (GCAPs). Thus, currently the only real ‘orphan’ receptor is GC-G, a receptor of largely unknown distribution and function.
Table 1.
Nomenclature of GC enzymes and genes.
| Mouse (Gene) | Human (Gene) | Aliases | Ligand | Tissue/cells | Comments |
|---|---|---|---|---|---|
| GC-A (Npr1) | GCA, (NPR1) NPR-A | Natriuretic Peptide Receptor A | ANP BNP | vasculature | Regulates hypertension |
| GC-B(Npr2) | GCB, (NPR2) (NPR-B) | Natriuretic Peptide Receptor B | CNP | Brain, bone | Null alleles associated with dwarfism |
| GC-C (Gucy2c) | GCC (GUCY2C) | guanylin uroguanylin enterotoxin | Intestines | Null allele insensitive to enterotoxins | |
| GC-D (Gucy2d) | GCD (GUCY2E) | Olfactory neuroepithelia (ONE)-GC | guanylin uroguanylin | Subset of Olfactory neurons | Pseudogene in primates |
| GC-E (Gucy2e) | RetGC-1 (GUCY2D) | ROS-GC1, GC1 | GCAPs | rods and cones, pineal | Null alleles associated with LCA-1 |
| GC-F (Gucy2f) | RetGC-2 (GUCY2F) | ROS-GC2, GC2 | GCAPs | Rods | Mutant alleles in cancer cells |
| GC-G (Gucy2g) | GCG (GUCY2G) | unknown | mouse testis, kidney | Pseudogene in human |
Non-photoreceptor GCs and cGMP-signaling have been reviewed extensively [1-3,7]. However, within the last ten years, the generation of membrane GC knockouts have lead to important insights concerning their precise function (recent review: [8]). The following paragraphs attempt to briefly summarize the phenotypes of non-photoreceptor membrane GC knockouts and of naturally occurring null alleles in human.
Consequences of non-photoreceptor GC deletions
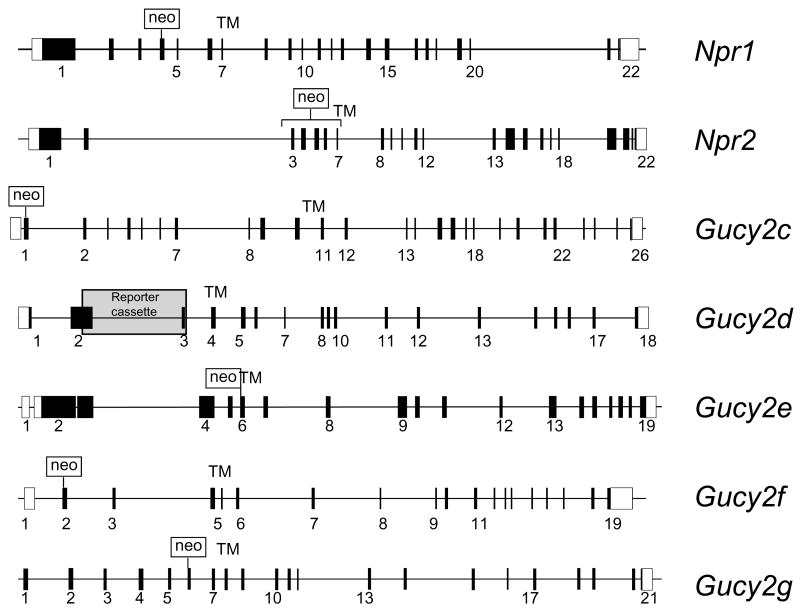
GC-A (natriuretic peptide receptor A, gene symbol Npr1, see Table 1) is expressed in the vasculature, heart, brain, testis and other tissues, and is stimulated by atrial natriuretic peptides secreted by heart muscles. Mice lacking the Npr1 gene, produced by placing a neo cassette in exon 4 [9] (Fig. 1), mimic many of the features of hypertensive heart disease in human patients. Npr1-/- mice showed multiple phenotypes, including elevated blood pressure, salt-resistant hypertension, progressive cardiac hypertrophy and sudden death, thereby demonstrating that GC-A is essential for the maintenance of normal blood pressure [9,10].
Figure 1.
Membrane guanylate cyclase knockout representations. White boxes, noncoding exons; black boxes or vertical lines, coding exons. TM, transmembrane domain. Neo cassettes indicate strategies for knockout constructs. References for knockout mice are: Npr1 [9]; Npr2 [11]; Gucy2c [97]; Gucy2d [18]; Gucy2e [82]; Gucy2f [31]; Gucy2g [21].
GC-B (natriuretic peptide receptor B, gene Npr2) is expressed in many different tissues, and its function had been unclear until a knockout was generated [11]. To generate the knockouts, exons 3-7 encoding a portion of the ECD and the transmembrane domain, were replaced by a neo cassette [11] (Fig. 1). Npr2-/- mice showed a dramatic impairment of endochondral ossification and an attenuation of longitudinal vertebra or limb-bone growth [11]. Female Npr2-/- mice were infertile, but male mice were not, due to the failure of the female reproductive tract to develop. Null mutations in the NPR2 gene in humans are associated with autosomal recessive skeletal dysplasia known as “acromesomelic dysplasia, type Maroteaux” (AMDM), characterized by reduced body height. In addition, heterozygous NPR2 mutations were found associated with short stature in humans [12]. Further, mutations in the Npr2 gene are responsible for dwarfism, short limbs and tail in the cn/cn and slw (short-limbed dwarfism) mouse [13,14].
Heat-stable enterotoxins activate GC-C (gene symbol Gucy2c) in intestines and increase levels of cGMP that activate downstream targets causing acute diarrhea. The Gucy2c deletion was generated by inserting a neo cassette into exon 1; the knockout mice developed normally and were resistant to enterotoxin-induced diarrhea [15]. GC-C is the major guanylate cyclase in the mammalian intestines, has been the focus of a link to colon cancer. However, Gucy2c null mice show reduced polyp formation and increased apoptosis, the opposite of proliferation [16].
A small subpopulation of olfactory neurons in the main olfactory epithelium express GC-D, together with CNGA3 (cGMP-gated channel α-subunit first identified in cones) and cGMP-specific PDE2 [17,18]. A Gucy2d knockout was generated by replacing exons 2 and 3 with a reporter cassette fused to a floxed neo gene [18] (Fig. 1). The knockout permitted the identification of uroguanylin and guanylin as extracellular ligands suggesting that GC-D expressing olfactory neurons may detect cues related to hunger and thirst [18,19]. The ‘orphan receptor’ GC-G is expressed in mouse testes [20] and kidneys [21], but its distribution in other tissues and possible function are largely unknown. A Gucy2g knockout suggested that in kidneys, GC-G may promote apoptotic and inflammatory responses in ischemia-reperfusion induced renal injury [21]. Interestingly, the primate GUCY2E gene (encoding GC-D) and the human GUCY2G gene (encoding GC-G) contain multiple inactivating sequence changes and are non-functional pseudogenes [22]. These data suggest that GC-D has been lost in primates, prior to the divergence of Old World monkeys from New World monkeys (more than 40 million years ago) [22].
These gene targeting experiments clearly demonstrate the power of gene knockouts in pinpointing the exact functions of membrane GCs in-vivo. Below, we will discuss the photoreceptor GCs (GC-E and GC-F) expressed in rods and cones, with particular focus on gene knockouts and their consequences for photoreceptor physiology, membrane protein transport and photoreceptor survival.
Photoreceptor Guanylate cyclases (GC-E and GC-F)
Membrane GCs are key components responsible for the production of cGMP, an essential secondary messenger of phototransduction. During phototransduction, cGMP is rapidly hydrolyzed by a membrane-associated PDE6 located on rod outer segment disk membranes, i.e., coin-like stacks of membranes unconnected to the plasma membrane. Disappearance of the ‘messenger’ closes cGMP-gated cation channels residing in the cell membrane, effectively hyperpolarizing the cell. In the dark-adapted photoreceptor, the basal GC activity (inhibited by Ca2+-bound GC-activating proteins, termed GCAPs) is balanced by the low basal activity of PDE6, adjusting cytoplasmic cGMP to about 1-10 μM. When the intracellular calcium ions decrease from about 500 nM (dark) to <50 nM in light, the GCs are activated by the Ca2+-free GCAPs, increasing the catalytic GC activity about 10-fold. Provided that all components of phototransduction (R*, G*, PDE*) are ‘silenced’ by returning to the inactive dark state, GC stimulation leads to restoration of dark cGMP levels [23-26].
GC-E and GC-F each possess the structural features characteristic of membrane GCs - a signal sequence preceding a large amino-terminal ECD, a single membrane-spanning region, a kinase-like homology domain and a carboxy-terminal catalytic domain. While the nomenclature of the GCs in various species is somewhat confusing (Table 1), generally accepted abbreviations are retGC-1 and retGC-2 in human [27,28], ROS-GC1 and ROS-GC2 in bovine [29,30], and GC1 and GC2 in mouse [31]. Quantitative determination of GC-E and GC-F in outer segment preparations by mass spectrometry showed a ratio of GC-E/GC-F of 3.5/1 [32], but biochemical quantization showed a much higher ratio of GC-E/GC-F of 25/1 [33].
Human GC-E (retGC-1) [34] and human GC-F (retGC-2) [35] show regional localization in the photoreceptor cell bodies and inner segments by in situ hybridization with antisense probes [27,36]. Human and monkey GC-E was associated with outer segments of rods and cones, but also to a lesser extent with IS and other cells [34,37]. The mouse GC-E and GC-F polypeptides were shown to be present in rod outer segments, likely as homomers [38]. Mouse GC-E is prominently immunolocalized in outer segments of rods and cones [39], whereas GC-F is detectable only in outer segments of rods (Fig. 2, and [31]). Neither GC-E nor GC-F is detected elsewhere in the mouse retina, at least under the conditions of figure 2 [31].However, GC-E was also detected in the synaptic terminals of bovine photoreceptors [40,41]. Outside the retina, GC-E was detected in the pineal gland [38,42], an organ developmentally related to the retina, in the olfactory bulb [43], spermatogenic cells of bovine testes [44], as well as the cochlear nerve and the organ of Corti [45]. Interestingly, GC-F was identified in several human cancer cell lines [46] where its function is unknown.
Figure 2.
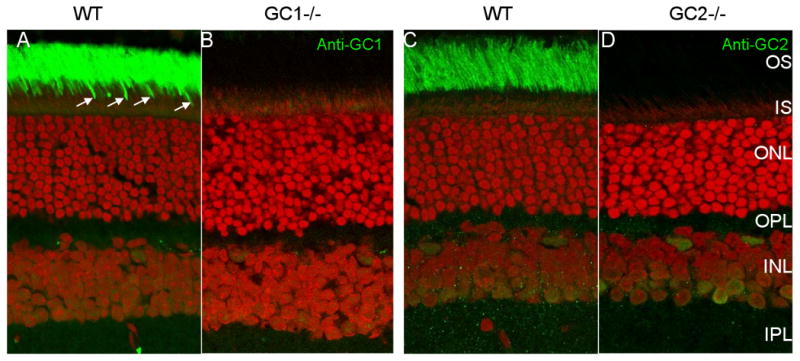
Distribution of GC1 and GC2 in WT (A,C) and GC knockout (B,D) mouse retina. Cryosections A,B were probed with anti-GC1, and C,D with anti-GC2 antibodies. GC1 is present in rod (intense green staining in the OS area) and cone (arrows) outer segments (A). GC2 is only detectable in WT rod outer segments (C). GC1 and GC2 are undetectable in the ONL or OPL (synaptic terminals). Faint immunofluorescence in the INL is nonspecific.
None of the membrane GCs has an affinity for Ca2+ ions, or conventional Ca2+-binding motifs. The activities of photoreceptor cyclases, however, are Ca2+-sensitive, a sensitivity that is mediated by guanylate cyclase-activating proteins (GCAPs) at low free Ca2+, as well as by S100B and neurocalcin at high free Ca2+. GCAPs are Ca2+-binding proteins with three high-affinity Ca2+-binding sites (EF hands) stimulating GC-E and GC-F by binding to cytoplasmic sites [47-50]. S100B and neurocalcin stimulate GC-E at 17-20 μM Ca2+ [51,52], a concentration which is too high for a physiological role in outer segments. The peptide ligands of non-photoreceptor GC receptors failed to activate either GC-E or GC-F [53]. GC enzymology, biochemistry, and history of cloning, is reviewed in detail in [29].
GUCY2D null alleles in human: Leber Congenital Amaurosis type 1 (LCA-1)
Leber Congenital Amaurosis type 1 (LCA-1), an autosomal recessive, early onset dystrophy defined as a “congenital stationary cone-rod dystrophy with high hypermetropia, panretinal degeneration and highly reduced visual acuity,” is a rare disease (prevalence approximately 1:100,000) [62]. Null mutations in the human GC-E gene (GUCY2D, on chromosome 17) are relatively frequent and distributed throughout the ECD, KHD, DIM and CAT domains (references in (Table 2)). Mutations in the GC-F gene (GUCY2F, on Xq22) [63] have not been linked to a retina disease phenotype. Since GUCY2F gene is located on X, only males would be affected by disease-causing mutations. Interestingly, several missense mutations in the peptide leader sequence, the KHD and CAT domains of GC-F have been identified in panels of colon, lung and breast cancer [64]. LCA is one of the most severe forms of inherited retinal dystrophies, accounting for approximately 5% of all inherited retinal dystrophies [55,65]. Children with LCA have greatly impaired vision and extinguished electroretinograms (ERG), despite seemingly normal fundi at birth or during the first months of life [66]. Immunofluorescent images of human GUCY2D-LCA retinas showed that all cones and rods lacked outer segments, as evidenced by the absence of labeling with anti-opsins and antirds/peripherin [59].
Table 2.
GC null alleles in human LCA-1 patients. Shaded mutations produce truncations. ECD, extracellular domain; KHD, kinase homology domain; DIM, dimerization domain; CAT, catalytic domain.
| Exon | Amino acid change | Domain | Enzyme activity | References |
|---|---|---|---|---|
| 2 | M1I | ECD | reduced | [54] |
| 2 | W21R | ECD | yes | [54] |
| 2 | L41F | ECD | yes | [55] |
| 2 | C105Y | ECD | [56] | |
| 2 | H109P | ECD | [54] | |
| 2 | N129K | ECD | yes | [55] |
| 3 | R313C | ECD | yes | [55] |
| 3 | L325P | ECD | [56] | |
| 4 | Y351C | ECD | [57] | |
| 4 | S448ter | ECD | no | [54] |
| 7 | R540C | KHD | [54] | |
| 8 | F565S | KHD | reduced | [58] |
| 10 | R660Q | KHD | [59] | |
| 12 | R768W | DIM | [57] | |
| 13 | Q855ter | DIM | no | [60] |
| 13 | P858S | DIM | [56] | |
| 15 | A934P | CAT | [61] | |
| 15 | L954P | CAT | [56] | |
| 15 | R976L | CAT | [54] | |
| 16 | R995W | CAT | no | [54] |
| 16 | M1009L | CAT | no | [61] |
| 17 | H1019P | CAT | no | [54] |
| 17 | Q1036ter | CAT | no | [62] |
| 18 | R1040ter | CAT | no | [61] |
A number of nonsense and frameshift mutations in the GUCY2D gene have been identified in LCA1 patients (Table 2). Missense mutations in the catalytic domain (e.g., R976L, R995W, M1009L, H1019P and Q1036Z) result in the total abolition of cyclase activity. Conversely, some missense mutations in the extracellular domain (e.g., W21R, L41F, N129K, R313C) do not affect cyclase activity in-vitro. Truncation mutations, e.g., (S448ter) remove the CAT domain, and prevent synthesis of cGMP. Some missense mutations in the extracellular domain (C105Y, L325P) have resulted in 50% decreased cyclase activity. L325 is highly conserved and C105 has been suggested to participate in intramolecular disulfide bond formation and oligomerization of the protein. Thus, GC mutations may alter the enzyme structure and stability [56], and may affect post-biosynthetic folding or retrograde transport of peripheral membrane-associated proteins along microtubules to the cilium (Figure 8).
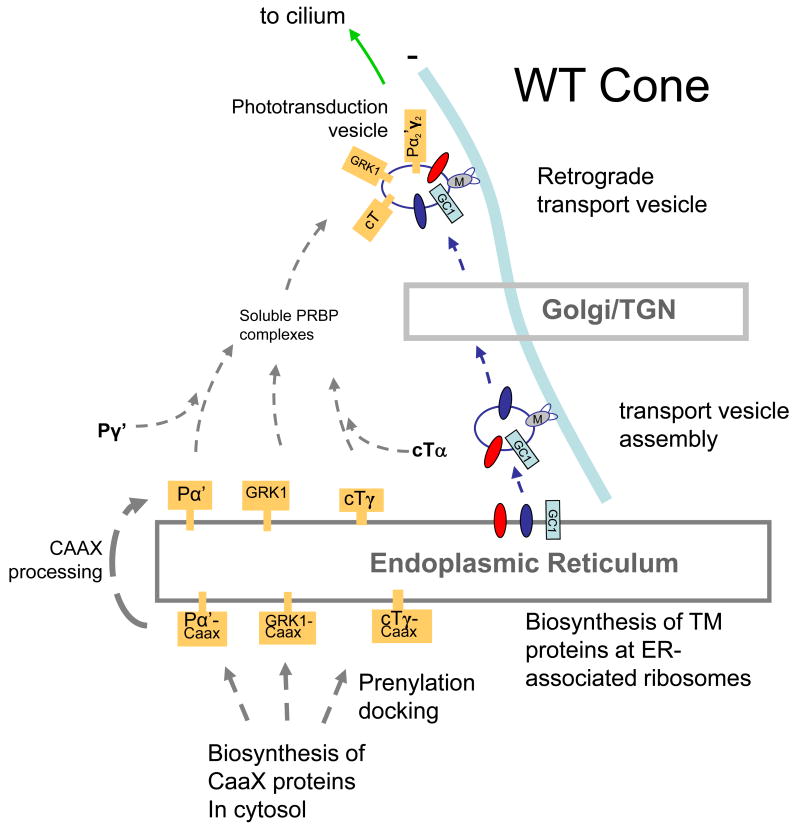
Figure 8.
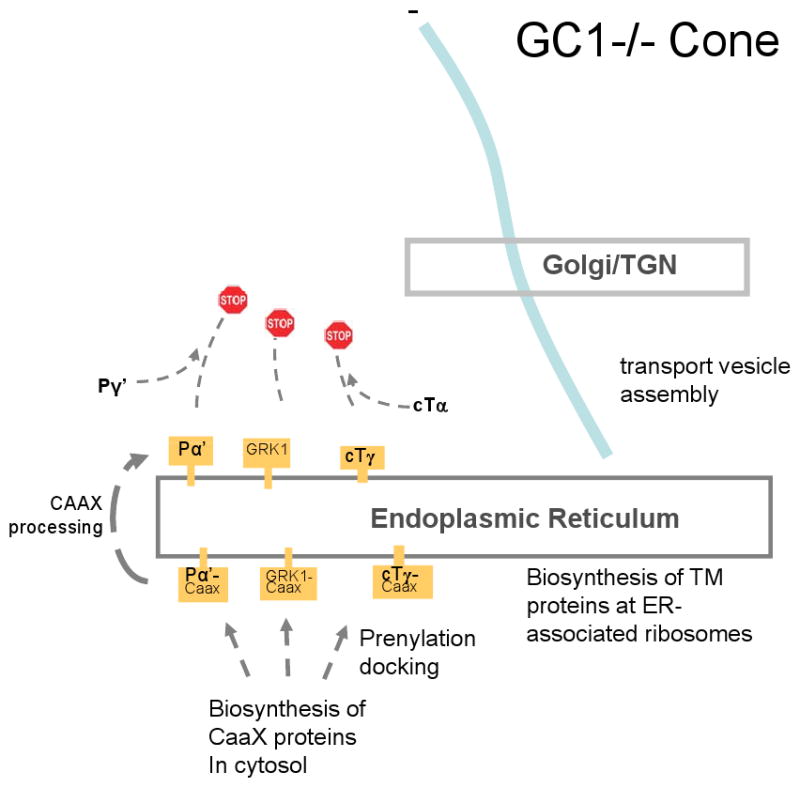
Putative model for transport of peripheral membrane proteins in cones. A, WT cone photoreceptor. B, GC-E knockout cone photoreceptor. In A, visual pigments and GC-E traffic from the ER to the Golgi/TGN by retrograde transport along microtubules. Vesicles emerge from the TGN and are charged with the peripheral membrane proteins PDEα′, cone T, GRK1, and GCAPs. Retrograde transport continues to the base of the cilium, followed by IFT to the outer segment. In B, GC-E is not produced. Peripheral proteins remain in the ER owing the absence of GC-E and are degraded.
Cone-rod dystrophy (CORD6)
Dominant cone-rod dystrophy (CORD6) also maps to the GC1 locus [67,68]. Mutations causing dominant cone-rod dystrophy are restricted to the dimerization domain. Cone-rod dystrophy is a progressive disorder, characterized by the initial degeneration of cone photoreceptor cells, causing early loss of visual acuity and color vision, followed by the degeneration of rods leading to progressive night blindness and peripheral visual field loss [67]. Some of the important missense mutations of the dimerization domain are E837D, R838A, R838H, R838C, T839M [69-71]. The three disease mutations at residue 838 are non-equivalent. They exhibit GC activity equal or superior to WT GC at low free [Ca]free in the order R838C< R838H< R838A and showed a higher affinity for GCAP1 than WT GC [71]. Interestingly, when expressed in HEK 293 cells, (R838C)GC1 showed a decreased response to GCAP2 suggesting distinct binding sites for the GCAPs [72]. Structural studies have shown that the residue at position at 838 is a key residue that determines the extent of coiled-coil structure responsible for maintaining the retGC1 dimer. Molecular dynamics-based modeling of RetGC1 coiled-coil suggests that Arg838 forms salt bridges which maintain the configuration of the helices producing repulsion between the polypeptide chains. This causes the coils to spread apart as they extend towards the catalytic domain which then determines the dimerization [73].
A naturally occurring GC-E knockout: the retinal degeneration (rd) chicken
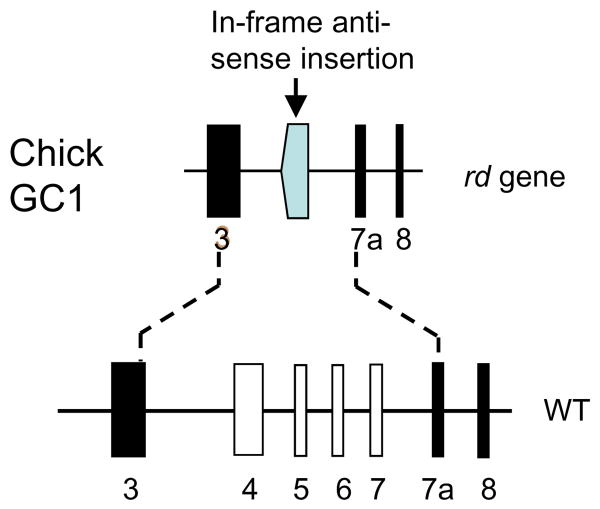
The rd (GUCY1*B) chicken is a naturally occurring blind mutant discovered in a Rhode Island Red flock about 30 years ago (reviewed in [74]). Photoreceptors in predegenerate rd/rd retina appeared normal at the ultrastructural level. Degeneration of rods and cones begins approximately 7 days post-hatch, and progresses relatively slowly over many months. The rd chicken carries a null mutation in the gene encoding photoreceptor GC-E exhibiting a disease phenotype resembling LCA [75,76]. The gene defect is described best as a deletion/insertion event in which a gene fragment containing exons 4-7 was replaced by an inverted portion of exon 9 without interruption of the open reading frame (Fig. 3). Deletion of exon 5, encoding the transmembrane region, is predicted to result in a soluble polypeptide that, if stably expressed, would fail to traffic to the outer segments. Consistent with the deletion of the GC-E gene, levels of cGMP in the mutant retina were very low, unable to sustain phototransduction, presumably leading to permanent closure of the cGMP-gated cation channels and elimination of the dark current (constitutive hyperpolarization). In chicken, as in human LCA type 1 (Gucy2e null alleles) [61], only GC-E appears to contribute to the pool of cGMP essential to support phototransduction. It is unclear whether a GC-F ortholog is expressed in chicken retina. A lentivirus-based gene transfer vector carrying a bovine GUCY2D cDNA, injected into early-stage GUCY1*B embryos, rescued retinal degeneration to a large part demonstrating convincingly that the rd chicken phenotype is caused by deletion of GC-E [77].
Figure 3.
The rd chicken gene defect. Top, digram of the mutant gene in which exons 4-7 are replaced by an inverted fragment of exon 9 (shaded). Bottom, predicted WT gene representing exons 3-8. Exon 7a is an additional exon not present in mammalian GC-E genes. Adapted from [75].
Transcription of several genes involved in the phototransduction cascade, including iodopsin and cone PDEα′, was shown to be normal [78,79]. However, protein levels of GCAP1 were reduced by more than 90% in predegenerate rd/rd retinas compared to age-matched control retinas, while GCAP2 levels were less affected [80], similarly as seen in GC-E/GC-F double knockouts (see below, and [31]). A regulatory mechanism at the gene level was excluded because transcript levels of both GCAP genes were near normal in the predegenerate rd retina [81]. The observed downregulation of GCAP1 was likely caused by mistrafficking of membrane-associated proteins which relies in part on the presence of GC-E (see below).
Photoreceptor GC knockouts
The genes encoding GC-E (Gucy2e, on mouse chromosome 11) and the GC-F (Gucy2f, on the X chromosome) genes [63] are closely related in structure, each consisting of 19 exons and featuring an untranslated exon 1. The GC-E knockout mouse was generated by the group of David Garbers at UT Southwestern [82]. His group deleted the Gucy2e gene by targeted recombination replacing exon 5, encoding the transmembrane domain, with a neo cassette. The neo cassette effectively disrupted translation in all reading frames, preserving the extracellular domain, but deleting cytoplasmic regions including the catalytic domain so no functional cyclase can be formed. It is unknown whether an extracellular polypeptide (encoded by exons 1-4) is formed in the Gucy2e-/- photoreceptor. At one month of age, mouse cone responses were undetectable while rods continued to function albeit with reduced a- and b-wave amplitudes suggesting a substantial loss of rod function ([82] and (Fig. 4)). The phenotype of the Gucy2e-/- mice therefore resembled a recessive cone dystrophy [82,83] while in human patients, GUCY2E null alleles are associated with rod/cone dystrophy (LCA-1).
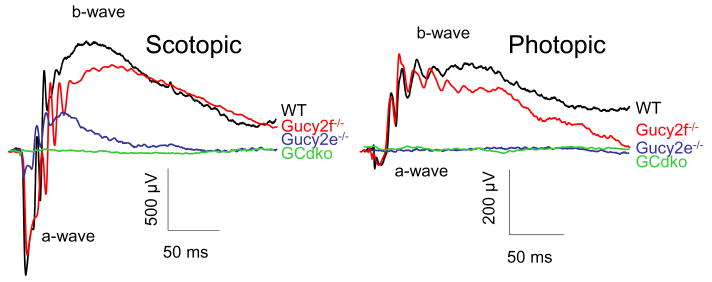
Figure 4.
Scotopic and photopic ERGs of WT, Gucy2e-/-, Gucy2f-/- and GCdko mice at 2.8 log cd s m-2. Note lack of response in GCdko scotopic ERGs and in Gucy2e-/- and GCdko photopic ERGs.
In Gucy2e-/- mouse rods, a second cyclase, GC-F, is obviously able to substitute to some extent for the loss of GC-E. To identify the precise function of GC-F in rods, we generated Gucy2f-/- by replacing exon 2 of the Gucy2f gene containing the translation start codon and the leader peptide with a neo cassette. Therefore, a functional GC-F cannot be expressed. The phenotype of Gucy2f-/- mice was very similar to WT mice (Table 3), but showed slower recovery from intense illumination (Fig. 3 in [31]). In scotopic ERG analyses, the Gucy2f-/- a-wave amplitude reflecting rod photoreceptor function was identical to WT, while the b-wave amplitude was reduced (Fig. 4). The photopic ERG responses in the Gucy2f-/- mice were very similar to those of WT mice. We proceeded to generate GC double knockout mice (GCdko) by breeding Gucy2f-/- mice with Gucy2e-/- mice. Both full-field ERG (Fig. 4) and rod single-cell recordings (Fig. 4 in [31]) showed that GCdko photoreceptors were insensitive to light.
Table 3.
Effect of GC-E deletion, GC-F deletion, and deletion of both GCs on photoreceptor physiology and outer segment constituents.
| Rod physiology | Cone physiology | ROS proteins | COS proteins | |
|---|---|---|---|---|
| Gucy2e-/- | Functional; ERG responses attenuated. | No response; cones degenerate slowly (autosomal recessive cone dystrophy) | GCAP1, GCAP2 downregulated; GC-F levels not affected | cone Tα, cone Tγ, GRK1, cone PDEα′ undetectable; COS unstable; very little |
| No degeneration | cone pigment present | |||
| Gucy2f-/- | Slower recovery from intense illumination | normal | normal | Normal GC-E levels not affected. |
| GCdko | No response Rods degenerate (recessive LCA) | No response Cones degenerate (recessive LCA) | PDEα, PDEβ, PDEγ, GCAP1, GCAP2 undetectable; Tα, Tγ, arrestin, rhodopsin not affected | cone Tα, cone Tγ, GRK1, cone PDEα′ undetectable; very little cone pigment present |
Consequence of GC-E /GC-F double knockout (GCdko) in rods
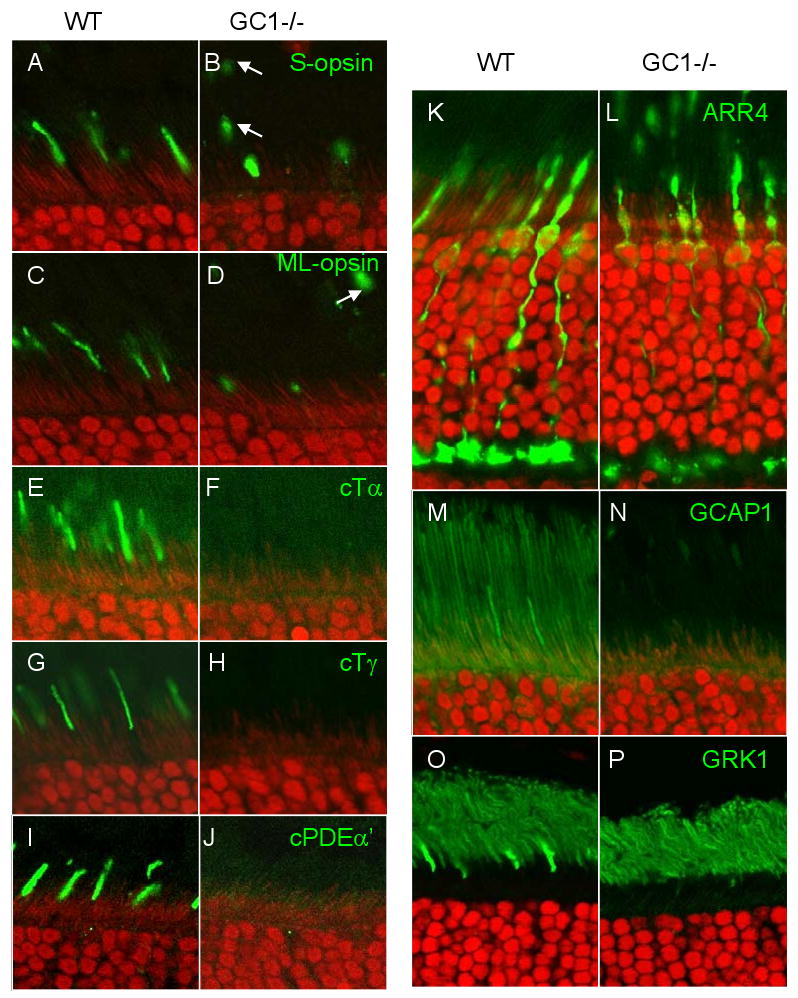
At one month, the GCdko photoreceptors are relatively stable but outer segments are slightly reduced in length. Immunolabeling patterns of rhodopsin (Fig. 5B), arrestin (Fig. 5D), Tα (Fig. 5F) and Tγ (Fig. 5H) were apparently normal and similar to WT, but the labeling pattern of rod outer segments was ‘patchy’, consistent with alternating bands of tubules and dense membranes observed by electron microscopy [31]. However, expression levels of several phototransduction polypeptides were reduced severely, including those of PDE6 subunits (Fig. 5J), GCAP1 (Fig. 5L) and GCAP2 (Fig. 5N). GCAPs activate GCs in low free Ca2+, are closely associated with GCs, and may be unstable in the absence of GCs. A similar downregulation of GCAP1 (but not GCAP2) was observed in the rd chicken carrying a GUCY2D null allele [81]. The diminished expression levels of GCAP1 and GCAP2 were confined mostly to the inner segments where biosynthesis occurs (Fig. 5L,N).
Figure 5.
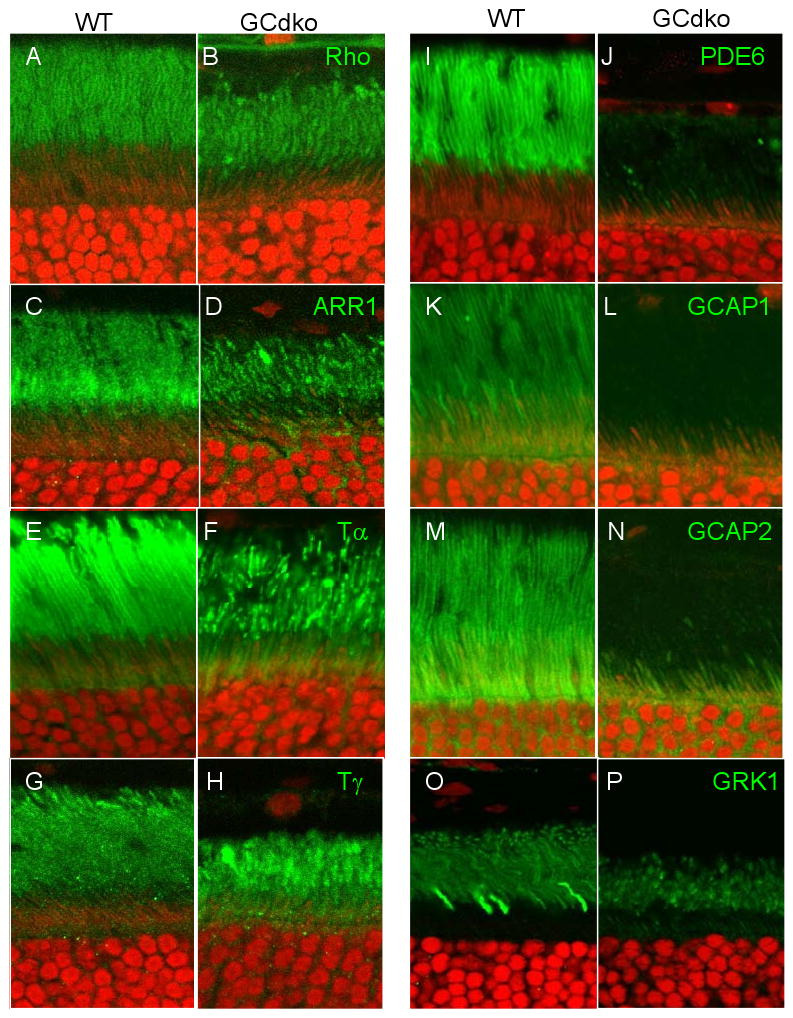
Distribution of phototransduction polypeptides in WT (A,C,E,G,I,K,M,O) and GCdko (B,D,F,H,J,L,N,P) mutant outer and inner segments. Antigens targeted by antibodies (green) are indicated top right in the GCdko panels. Nuclei of the ONL are counterstained with propidium iodide (red). The ‘patchy’ appearance of ROS indicates disorganization of the membrane structure [31].
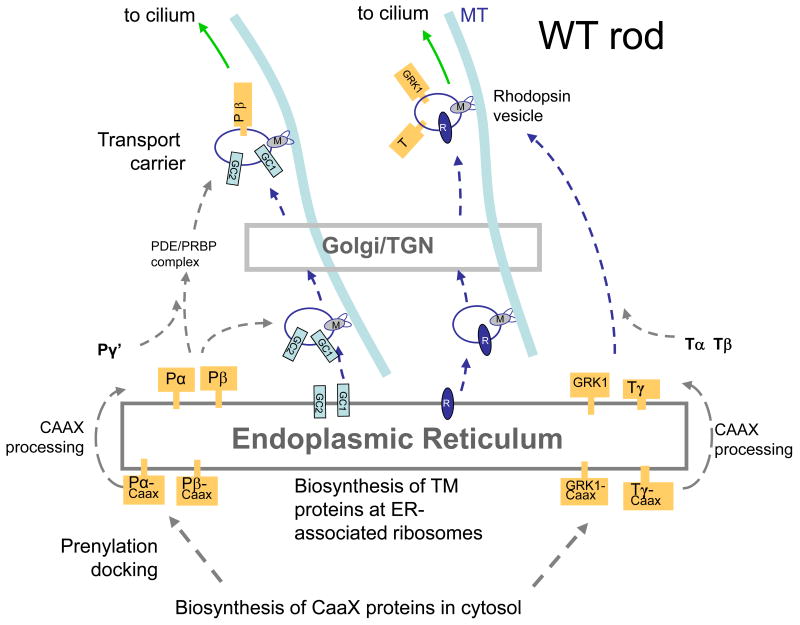
We were most surprised by absence of PDE6αβγ subunits in GCdko ROS (Fig. 5J). PDE6 is a peripheral membrane protein stably attached to the disk membrane surface by farnesyl and geranylgeranyl anchors. PDEα is known to be farnesylated and PDEβ geranylgeranylated at the C-terminal cysteines [84,85]. PDEαβ subunits are prenylated by cytosolic prenyltransferases and then dock to the ER. Once docked, they are insoluble and most likely require vesicular transport to reach their outer segment destination. In contrast to PDE6, transmembrane GCs are synthesized by ER-associated ribosomes, integrate in the ER membrane, and follow the secretory pathway from the ER to the plasma membrane, mediatedby IFT through the connecting cilium to the outer segments.
The posttranslational downregulation of PDE and GCAPs, observed by immunoblots [31], and the absence of photoreceptor immunoreactivity for GC-E and GC-F in GCdko retinas suggested a role in protein trafficking, comparable that of rhodopsin-beraring vesicles [86,87]. but in this case involving association of PDE6 and GCAPs with GC-bearing vesicles. Based on GC knockout results, enforced by PrBP/δ knockouts [88,89], we developed a model describing transport of GCs in vesicles to which PDE subunits are attached peripherally (Fig. 6A).
Figure 6.
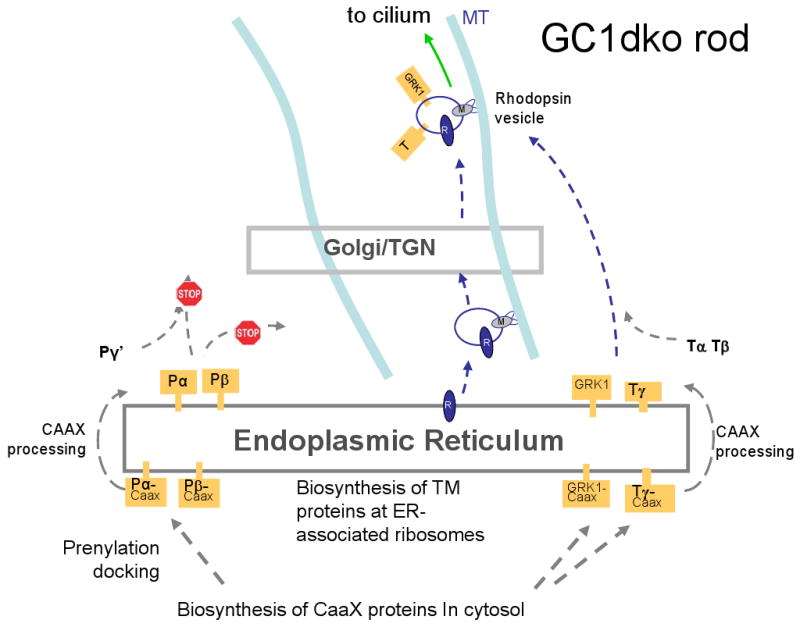
Putative model of GC and rhodopsin transport following synthesis at the ER. A, WT rod photoreceptor. B, GCdko photoreceptor. In A, integral membrane proteins traffic from the ER to the Golgi/TGN by retrograde transport. Vesicles emerge from the TGN and are charged with the peripheral membrane proteins PDE and GCAPs. Retrograde transport continues to the base of the cilium. In B, since GC-E and GC-F are not produced, GCAPs and PDE cannot traffic, and are degraded. Rhodopsin, transducin, and GRK1 transport is not affected. For details, see text.
Like other integral membrane proteins, GC-bearing vesicles are thought to traffic from the ER to the Golgi and emerge from the trans-Golgi network (TGN) (Fig. 6A). We previously observed that when the prenyl-binding protein PrBP/δ was deleted in rods, traffic of GRK1 and PDE6 to the outer segments was impeded while GC-E trafficked normally. We therefore propose that the GC-bearing vesicles are loaded with PDE6 subunits, aided by PrBP/δ (and possibly additional prenyl binding proteins) that transfer PDE6 from the ER to vesicles. For simplicity, in Figure 7 GC vesicles are charged with PDE post-TGN, but a pre-Golgi loading of the vesicles cannot be excluded. Results with either GC-E or GC-F knockouts indicate that one GC is sufficient for PDE6 (and GCAP) transport. GC-bearing vesicles loaded with peripheral membrane proteins continue retrograde transport along microtubules toward the minus end, powered by molecular motors (e.g., cytoplasmic dynein). Vesicles likely fuse with the plasma membrane at the base of the cilium where cargo is assembled for intraflagellar transport through the cilium. Heterotrimeric kinesin-2 was suggested to be the molecular motor for IFT in rods based on the observation that rhodopsin accumulated in the distal inner segment when KIF3A, the motor subunit of kinesin-2, was deleted. Intriguingly, GC-E was suggested to be mislocalized in mice expressing a hypomorphic mutation in IFT88, a protein associated with the axoneme and IFT complexes, but the fate of PDE6 was not investigated [90,91].
Figure 7.
Distribution of cone phototransduction polypeptides in WT (A,C,E,G,I,K,M,O) and GC1-/- (B,D,F,H,J,L,N,P) mutant outer and inner segments. Antigens targeted by antibodies (green) are indicated top right in the GCdko panels. Arrows (B,D) indicate disconnected membrane packets containing pigments. Visual pigments, cone transducin, cone PDE, GCAP1 and GRK1 are undetectable in mutant COS.
Knockout of both GCs (Fig. 6B) prevents formation of GC-bearing vesicles at the ER and coordinately prevents transport of PDE6 and GCAPs. Since GC-deletion does not affect rhodopsin and transducin transport to the rod outer segment. GC transport and rhodopsin transport may occur independently. PDE docked and retained in the ER due to the absence of GC carriers, likely presents prolonged cellular stress leading to PDE6 degradation by eliciting the unfolded protein response (UPR) with removal by the proteasome/ubiquitination pathway.
Effect of GC-E deletion in cones
It was shown that Gucy2e-/- cone photoreceptors degenerate gradually [82,92]. Figure 7L shows that at one month of age, Gucy2e-/- cone outer segments are still present and of near-normal length, but S-opsin and M/L-opsin were present at reduced levels and mislocalized throughout the mutant cone cell. Further, acylated cone Tα (Fig. 7F), farnesylated cone Tγ (Fig. 7H), geranylgeranylated cone PDEα′ (Fig. 7J), and farnesylated GRK1 (Fig. 7P) were undetectable in Gucy2e-/- COSs. These results show that essential cone phototransduction proteins are either absent or below the threshold of detection in Gucy2e-/- COSs. At 6 months of age, cone cell remnants, particularly synaptic pedicles and somata, can be identified, but outer segments are absent. This finding is consistent with a postmortem study of an 11-year old LCA patient with a null mutation in the GUCY2D gene. The macula and peripheral retina of this patient revealed a substantial number of cones and rods, but both photoreceptor types lacked outer segments [59].
In contrast to GCdko rods where only GCAPs and PDE mistraffic, proteins of the entire phototransduction cascade are absent in Gucy2e-/- outer segments. Therefore the GC-E polypeptide, or its enzyme activity, must play a key role in retrograde trafficking of peripheral membrane proteins to the base of the cilium. An identical phenotype was observed in Lrat-/- and Rpe65-/- cones in which the retinoid cycle is blocked [93,94]. In these mutants, the 11-cis-retinal chromophore cannot be recycled and opsins are synthesized as apoproteins. Central Lrat-/- cones degenerate rapidly between 2 and 4 weeks postnatally, and immunocytochemistry showed that proteins of the entire phototransduction cascade, including GC-E, GCAPs, and R9AP, are absent in mutant cones. Thus, cone pigments require 11-cis-retinal as a cofactor to be competent for post-synthesis trafficking to the outer segments. The interdependence of GC-E, visual pigments, and peripheral membrane proteins supports the idea that a large cargo is assembled at the post-ER or post-TGN levels for co-transport and trafficking to cone outer segments. Further, we recently observed that cone-specific deletion of KIF3A, the obligatory motor subunit of heterotrimeric kinesin-2, prevents intraflagellar transport of visual pigments, GC1, R9AP, and peripheral membrane proteins through the cone cilium [95]. Thus, a common phenotype, i.e. absence of peripheral membrane protein transport, was replicated in four independent deletion models (Gucy2e-/-, cone Kif3a-/-, Lrat-/- and Rpe65-/- mice); this result argues for the coordinated insertion/assembly of several phototransduction-associated proteins into vesicular membrane for transport from the cone inner segment.
Our working model for cone transport is predicated on the interpretation that cone pigments and GC-E apparently traffic to the Golgi together (Fig. 8). That cone opsins and GC-E co-transport is supported by mislocalization of pigments in the GC-E knockout, and by mislocalization of GC-E in the LRAT/RPE65 knockouts. Emerging from the TGN, vesicles may be formed and loaded with peripheral membrane phototransduction polypeptides, which have been extracted from the ER by prenyl binding proteins. This mechanism is supported by a PrBP/δ knockout in which cone PDE and GRK1 are unable to traffic (they are permanently docked to the ER), while cone transducin traffics unimpeded. In WT cones, GC-E and pigment-bearing vesicles are then transported along microtubules to the cilium, where IFT-cargo is assembled. Intraflagellar transport through the cone cilium is powered by the heterotrimeric kinesin-2, an anterograde motor moving towards the distal end of the outer segment (the plus end of microtubules) [95]. Knockout of the motor subunit KIF3A specifically in cones leads to a phenotype mimicking the GC-E and LRAT/RPE65 knockout, thereby preventing integral membrane proteins and membrane-associated proteins of the phototransduction cascade from trafficking to the cone outer segment.
Conclusion
Deletion of one or both retinal GCs has very different effects on the physiology of rod and cone photoreceptors. Particularly, the cone phenotype argues that GC-E is essential for trafficking several transmembrane and peripheral membrane proteins to the outer segment. We postulate that absence of GC-E in GUCY2D-LCA accounts for weakening of the outer segment membrane structure and secondarily, that accumulation of peripherally-associated proteins at the ER (mistrafficked) constitutes stress that initiates apoptotic pathways mediated by UPR [96].
Acknowledgments
Supported by RO1 EY08123, RO1 EY019298, P30 EY014800 and a Center Grant form the Foundation Fighting Blindness.
Abbreviations
- ER
endoplasmic reticulum
- GC
guanylate cyclase
- GCdko
GC-E/GC-F double knockout
- GCAP
guanylate cyclase-activating protein
- LCA
Leber congenital amaurosis
- PDE6
cGMP-specific photoreceptor phosphodiesterase
- TGN
trans-Golgi network
- WT
wild-type
References
- 1.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 2.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 3.Potter LR. Domain analysis of human transmembrane guanylyl cyclase receptors: implications for regulation. Front Biosci. 2005;10:1205–1220. doi: 10.2741/1613. [DOI] [PubMed] [Google Scholar]
- 4.Sitaramayya A. Soluble guanylate cyclases in the retina. Mol Cell Biochem. 2002;230:177–186. [PubMed] [Google Scholar]
- 5.Noll GN, Billek M, Pietruck C, Schmidt KF. Inhibition of nitric oxide synthase alters light responses and dark voltage of amphibian photoreceptors. Neuropharmacology. 1994;33:1407–1412. doi: 10.1016/0028-3908(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, Blute TA, Eldred WD. Localization of heme oxygenase-2 and modulation of cGMP levels by carbon monoxide and/or nitric oxide in the retina. Vis Neurosci. 2000;17:319–329. doi: 10.1017/s0952523800173018. [DOI] [PubMed] [Google Scholar]
- 7.Garbers DL, Chrisman TD, Wiegn P, Katafuchi T, Albanesi JP, Bielinski V, Barylko B, Redfield MM, Burnett JC., Jr Membrane guanylyl cyclase receptors: an update. Trends Endocrinol Metab. 2006;17:251–258. doi: 10.1016/j.tem.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn M. Cardiac and intestinal natriuretic peptides: insights from genetically modified mice. Peptides. 2005;26:1078–1085. doi: 10.1016/j.peptides.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 10.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci U S A. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91:1229–1232. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- 13.Sogawa C, Tsuji T, Shinkai Y, Katayama K, Kunieda T. Short-limbed dwarfism: slw is a new allele of Npr2 causing chondrodysplasia. J Hered. 2007;98:575–580. doi: 10.1093/jhered/esm065. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem. 2005;280:14288–14292. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- 15.Schulz S, Lopez MJ, Kuhn M, Garbers DL. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest. 1997;100:1590–1595. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann EA, Steinbrecher KA, Stroup C, Witte DP, Cohen MB, Giannella RA. Lack of guanylyl cyclase C, the receptor for Escherichia coli heat-stable enterotoxin, results in reduced polyp formation and increased apoptosis in the multiple intestinal neoplasia (Min) mouse model. Int J Cancer. 2005;116:500–505. doi: 10.1002/ijc.21119. [DOI] [PubMed] [Google Scholar]
- 17.Juilfs DM, Fulle HJ, Zhao AZ, Houslay MD, Garbers DL, Beavo JA. A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci U S A. 1997;94:3388–3395. doi: 10.1073/pnas.94.7.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn M, Ng CK, Su YH, Kilic A, Mitko D, Bien-Ly N, Komuves LG, Yang RB. Identification of an orphan guanylate cyclase receptor selectively expressed in mouse testis. Biochem J. 2004;379:385–393. doi: 10.1042/BJ20031624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Cheng CF, Hou HH, Lian WS, Chao YC, Ciou YY, Djoko B, Tsai MT, Cheng CJ, Yang RB. Disruption of guanylyl cyclase-G protects against acute renal injury. J Am Soc Nephrol. 2008;19:339–348. doi: 10.1681/ASN.2007050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JM, Waters H, Dong C, Fulle HJ, Liman ER. Degeneration of the olfactory guanylyl cyclase D gene during primate evolution. PLoS ONE. 2007;2:e884. doi: 10.1371/journal.pone.0000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polans A, Baehr W, Palczewski K. Turned on by Ca2+! The physiology and pathology of Ca2+ binding proteins in the retina. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 24.Wensel TG. Signal transducing membrane complexes of photoreceptor outer segments. Vision Res. 2008;48:2052–2061. doi: 10.1016/j.visres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb TD, Pugh EN., Jr Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47:5138–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 26.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 27.Shyjan AW, de Sauvage FJ, Gillett NA, Goeddel DV, Lowe DG. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992;9:727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- 28.Garbers DL, Lowe DG. Guanylyl cyclase receptors. J Biol Chem. 1994;269:30741–30744. [PubMed] [Google Scholar]
- 29.Pugh EN, Jr, Duda T, Sitaramayya A, Sharma RK. Photoreceptor guanylate cyclases: a review. Biosci Rep. 1997;17:429–473. doi: 10.1023/a:1027365520442. [DOI] [PubMed] [Google Scholar]
- 30.Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. [PubMed] [Google Scholar]
- 31.Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helten A, Saftel W, Koch KW. Expression level and activity profile of membrane bound guanylate cyclase type 2 in rod outer segments. J Neurochem. 2007;103:1439–1446. doi: 10.1111/j.1471-4159.2007.04923.x. [DOI] [PubMed] [Google Scholar]
- 34.Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by Calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 35.Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Cloning and expression of a second photoreceptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imanishi Y, Li N, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci. 2002;15:63–78. doi: 10.1046/j.0953-816x.2001.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Seno K, Nishizawa Y, Hayashi F, Yamazaki A, Matsumoto H, Wakabayashi T, Usukura J. Ultrastructural localization of retinal guanylate cyclase in human and monkey retinas. Exp Eye Res. 1994;59:761–768. doi: 10.1006/exer.1994.1162. [DOI] [PubMed] [Google Scholar]
- 38.Yang RB, Garbers DL. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]
- 39.Haire SE, Pang J, Boye SL, Sokal I, Craft CM, Palczewski K, Hauswirth WW, Semple-Rowland SL. Light-Driven Cone Arrestin Translocation in Cones of Postnatal Guanylate Cyclase-1 Knockout Mouse Retina Treated with AAV-GC1. Invest Ophthalmol Vis Sci. 2006;47:3745–3753. doi: 10.1167/iovs.06-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duda T, Koch KW, Venkataraman V, Lange C, Beyermann M, Sharma RK. Ca(2+) sensor S100beta-modulated sites of membrane guanylate cyclase in the photoreceptor-bipolar synapse. EMBO J. 2002;21:2547–2556. doi: 10.1093/emboj/21.11.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkataraman V, Duda T, Vardi N, Koch KW, Sharma RK. Calcium-modulated guanylate cyclase transduction machinery in the photoreceptor--bipolar synaptic region. Biochemistry. 2003;42:5640–5648. doi: 10.1021/bi034025x. [DOI] [PubMed] [Google Scholar]
- 42.Venkataraman V, Nagele R, Duda T, Sharma RK. Rod outer segment membrane guanylate cyclase type 1-linked stimulatory and inhibitory calcium signaling systems in the pineal gland: biochemical, molecular, and immunohistochemical evidence. Biochemistry. 2000;39:6042–6052. doi: 10.1021/bi9929960. [DOI] [PubMed] [Google Scholar]
- 43.Duda T, Venkataraman V, Krishnan A, Nagele RG, Sharma RK. Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry. 2001;40:4654–4662. doi: 10.1021/bi0027985. [DOI] [PubMed] [Google Scholar]
- 44.Jankowska A, Burczynska B, Duda T, Warchol JB, Sharma RK. Calcium-modulated rod outer segment membrane guanylate cyclase type 1 transduction machinery in the testes. J Androl. 2007;28:50–58. doi: 10.2164/jandrol.106.000182. [DOI] [PubMed] [Google Scholar]
- 45.Seebacher T, Beitz E, Kumagami H, Wild K, Ruppersberg JP, Schultz JE. Expression of membrane-bound and cytosolic guanylyl cyclases in the rat inner ear. Hear Res. 1999;127:95–102. doi: 10.1016/s0378-5955(98)00176-2. [DOI] [PubMed] [Google Scholar]
- 46.Shao RX, Otsuka M, Kato N, Chang JH, Muroyama R, Taniguchi H, Moriyama M, Wang Y, Kawabe T, Omata M. No mutations in the tyrosine kinases of human hepatic, pancreatic, and gastric cancer cell lines. J Gastroenterol. 2005;40:918. doi: 10.1007/s00535-005-1661-5. [DOI] [PubMed] [Google Scholar]
- 47.Palczewski K, Polans AS, Baehr W, Ames JB. Calcium binding proteins in the retina: Structure, function, and the etiology of human visual diseases. BioEssays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 48.Sokal I, Li N, Verlinde CL, Haeseleer F, Baehr W, Palczewski K. Ca2+ -binding proteins in the retina: from discovery to etiology of human disease. Biochim Biophys Acta. 2000;1498:233–251. doi: 10.1016/s0167-4889(00)00099-9. [DOI] [PubMed] [Google Scholar]
- 49.Dizhoor AM, Hurley JB. Regulation of photoreceptor membrane guanylyl cyclases by guanylyl cyclase activator proteins. Methods. 1999;19:521–531. doi: 10.1006/meth.1999.0894. [DOI] [PubMed] [Google Scholar]
- 50.Koch KW. Target recognition of guanylate cyclase by guanylate cyclase-activating proteins. Adv Exp Med Biol. 2002;514:349–360. doi: 10.1007/978-1-4615-0121-3_21. [DOI] [PubMed] [Google Scholar]
- 51.Duda T, Goraczniak RM, Sharma RK. Molecular characterization of S100A1-S100B protein in retina and its activation mechanism of bovine photoreceptor guanylate cyclase. Biochemistry. 1996;35:6263–6266. doi: 10.1021/bi960007m. [DOI] [PubMed] [Google Scholar]
- 52.Kumar VD, Vijay-Kumar S, Krishnan A, Duda T, Sharma RK. A second calcium regulator of rod outer segment membrane guanylate cyclase, ROS-GC1: neurocalcin. Biochemistry. 1999;38:12614–12620. doi: 10.1021/bi990851n. [DOI] [PubMed] [Google Scholar]
- 53.Goraczniak RM, Duda T, Sitaramayya A, Sharma RK. Structural and functional characterization of the rod outer segment membrane guanylate cyclase. Biochem J. 1994;302:455–461. doi: 10.1042/bj3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozet JM, Perrault I, Gerber S, Hanein S, Barbet F, Ducroq D, Souied E, Munnich A, Kaplan J. Complete abolition of the retinal-specific guanylyl cyclase (retGC-1) catalytic ability consistently leads to leber congenital amaurosis (LCA) Invest Ophthalmol Vis Sci. 2001;42:1190–1192. [PubMed] [Google Scholar]
- 55.Perrault I, Rozet JM, Gerber S, Ghazi I, Ducroq D, Souied E, Leowski C, Bonnemaison M, Dufier JL, Munnich A, Kaplan J. Spectrum of retGC1 mutations in Leber's congenital amaurosis. Eur J Hum Genet. 2000;8:578–582. doi: 10.1038/sj.ejhg.5200503. [DOI] [PubMed] [Google Scholar]
- 56.Tucker CL, Ramamurthy V, Pina AL, Loyer M, Dharmaraj S, Li Y, Maumenee IH, Hurley JB, Koenekoop RK. Functional analyses of mutant recessive GUCY2D alleles identified in Leber congenital amaurosis patients: protein domain comparisons and dominant negative effects. Mol Vis. 2004;10:297–303. [PubMed] [Google Scholar]
- 57.Lotery AJ, Namperumalsamy P, Jacobson SG, Weleber RG, Fishman GA, Musarella MA, Hoyt CS, Heon E, Levin A, Jan J, Lam B, Carr RE, Franklin A, Radha S, Andorf JL, Sheffield VC, Stone EM. Mutation analysis of 3 genes in patients with Leber congenital amaurosis. Arch Ophthalmol. 2000;118:538–543. doi: 10.1001/archopht.118.4.538. [DOI] [PubMed] [Google Scholar]
- 58.Duda T, Venkataraman V, Goraczniak R, Lange C, Koch KW, Sharma RK. Functional consequences of a rod outer segment membrane guanylate cyclase (ROS-GC1) gene mutation linked with Leber's congenital amaurosis. Biochemistry. 1999;38:509–515. doi: 10.1021/bi9824137. [DOI] [PubMed] [Google Scholar]
- 59.Milam AH, Barakat MR, Gupta N, Rose L, Aleman TS, Pianta MJ, Cideciyan AV, Sheffield VC, Stone EM, Jacobson SG. Clinicopathologic effects of mutant GUCY2D in Leber congenital amaurosis. Ophthalmology. 2003;110:549–558. doi: 10.1016/S0161-6420(02)01757-8. [DOI] [PubMed] [Google Scholar]
- 60.El-Shanti H, Al-Salem M, El-Najjar M, Ajlouni K, Beck J, Sheffiled VC, Stone EM. A nonsense mutation in the retinal specific guanylate cyclase gene is the cause of Leber congenital amaurosis in a large inbred kindred from Jordan. J Med Genet. 1999;36:862–865. [PMC free article] [PubMed] [Google Scholar]
- 61.Hanein S, Perrault I, Gerber S, Tanguy G, Barbet F, Ducroq D, Calvas P, Dollfus H, Hamel C, Lopponen T, Munier F, Santos L, Shalev S, Zafeiriou D, Dufier JL, Munnich A, Rozet JM, Kaplan J. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype-phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004;23:306–317. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 62.Perrault I, Hanein S, Gerber S, Lebail B, Vlajnik P, Barbet F, Ducroq D, Dufier JL, Munnich A, Kaplan J, Rozet JM. A novel mutation in the GUCY2D gene responsible for an early onset severe RP different from the usual GUCY2D-LCA phenotype. Hum Mutat. 2005;25:222. doi: 10.1002/humu.9304. [DOI] [PubMed] [Google Scholar]
- 63.Yang RB, Fulle HJ, Garbers DL. Chromosomal localization and genomic organization of genes encoding guanylyl cyclase receptors expressed in olfactory sensory neurons and retina. Genomics. 1996;31:367–372. doi: 10.1006/geno.1996.0060. [DOI] [PubMed] [Google Scholar]
- 64.Wood LD, Calhoun ES, Silliman N, Ptak J, Szabo S, Powell SM, Riggins GJ, Wang TL, Yan H, Gazdar A, Kern SE, Pennacchio L, Kinzler KW, Vogelstein B, Velculescu VE. Somatic mutations of GUCY2F, EPHA3, and NTRK3 in human cancers. Hum Mutat. 2006;27:1060–1061. doi: 10.1002/humu.9452. [DOI] [PubMed] [Google Scholar]
- 65.Perrault I, Rozet JM, Calvas P, Gerber S, Camuzat A, Dollfus H, Chatelin S, Souied E, Ghazi I, Leowski C, Bonnermaison M, Le Paslier D, Frezal J, Dufier JL, Pittler SJ, Munnich A, Kaplan J. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nature Genet. 1996;14:461–464. doi: 10.1038/ng1296-461. [DOI] [PubMed] [Google Scholar]
- 66.Yzer S, Leroy BP, De BE, de Ravel TJ, Zonneveld MN, Voesenek K, Kellner U, Ciriano JP, de Faber JT, Rohrschneider K, Roepman R, den Hollander AI, Cruysberg JR, Meire F, Casteels I, van Moll-Ramirez NG, Allikmets R, Van den Born LI, Cremers FP. Microarray-based mutation detection and phenotypic characterization of patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2006;47:1167–1176. doi: 10.1167/iovs.05-0848. [DOI] [PubMed] [Google Scholar]
- 67.Kelsell RE, Gregory-Evans K, Payne AM, Perrault I, Kaplan J, Yang RB, Garbers DL, Bird AC, Moore AT, Hunt DF. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant con-rod dystrophy. Hum Mol Genet. 1998;7:1179–1184. doi: 10.1093/hmg/7.7.1179. [DOI] [PubMed] [Google Scholar]
- 68.Perrault I, Rozet JM, Gerber S, Kelsell RE, Souied E, Cabot A, Hunt DM, Munnich A, Kaplan J. A retGC-1 mutation in autosomal dominant cone-rod dystrophy. Am J Hum Genet. 1998;63:651–654. doi: 10.1086/301985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Payne AM, Morris AG, Downes SM, Johnson S, Bird AC, Moore AT, Bhattacharya SS, Hunt DM. Clustering and frequency of mutations in the retinal guanylate cyclase (GUCY2D) gene in patients with dominant cone-rod dystrophies. J Med Genet. 2001;38:611–614. doi: 10.1136/jmg.38.9.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Downes SM, Payne AM, Kelsell RE, Fitzke FW, Holder GE, Hunt DM, Moore AT, Bird AC. Autosomal dominant cone-rod dystrophy with mutations in the guanylate cyclase 2D gene encoding retinal guanylate cyclase-1. Arch Ophthalmol. 2001;119:1667–1673. doi: 10.1001/archopht.119.11.1667. [DOI] [PubMed] [Google Scholar]
- 71.Wilkie SE, Newbold RJ, Deery E, Walker CE, Stinton I, Ramamurthy V, Hurley JB, Bhattacharya SS, Warren MJ, Hunt DM. Functional characterization of missense mutations at codon 838 in retinal guanylate cyclase correlates with disease severity in patients with autosomal dominant cone-rod dystrophy. Hum Mol Genet. 2000;9:3065–3073. doi: 10.1093/hmg/9.20.3065. [DOI] [PubMed] [Google Scholar]
- 72.Tucker CL, Woodcock SC, Kelsell RE, Ramamurthy V, Hunt DM, Hurley JB. Biochemical analysis of a dimerization domain mutation in RetGC-1 associated with dominant cone-rod dystrophy. Proc Natl Acad Sci U S A. 1999;96:9039–9044. doi: 10.1073/pnas.96.16.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramamurthy V, Tucker C, Wilkie SE, Daggett V, Hunt DM, Hurley JB. Interactions within the coiled-coil domain of RetGC-1 guanylyl cyclase are optimized for regulation rather than for high affinity. J Biol Chem. 2001;276:26218–26229. doi: 10.1074/jbc.M010495200. [DOI] [PubMed] [Google Scholar]
- 74.Semple-Rowland SL, Cheng KM. rd and rc chickens carry the same GC1 null allele (GUCY1*) Exp Eye Res. 1999;69:579–581. doi: 10.1006/exer.1999.0743. [DOI] [PubMed] [Google Scholar]
- 75.Semple-Rowland SL, Lee NR, Van-Hooser JP, Palczewski K, Baehr W. A null mutation in the photoreceptor guanylate cyclase gene causes the retinal degeneration chicken phenotype. Proc Natl Acad Sci U S A. 1998;95:1271–1276. doi: 10.1073/pnas.95.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Semple-Rowland SL, Lee NR. Avian models of inherited retinal disease. Methods Enzymol. 2000;316:526–536. doi: 10.1016/s0076-6879(00)16747-3. [DOI] [PubMed] [Google Scholar]
- 77.Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, Sokal I, Palczewski K, Jacobson SG, Semple-Rowland SL. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med. 2006;3:e201. doi: 10.1371/journal.pmed.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semple-Rowland SL, Green DA. Molecular characterization of the a′-subunit of cone photoreceptor cGMP phosphodiesterase in normal and rd chicken. Exp Eye Res. 1994;59:365–372. doi: 10.1006/exer.1994.1119. [DOI] [PubMed] [Google Scholar]
- 79.Semple-Rowland SL, Green DA. Molecular and biochemical analyses of Iodopsin in rd chick retina. Invest Ophthalmol & Vis Sci. 1994;35:2550–2557. [PubMed] [Google Scholar]
- 80.Semple-Rowland S, Gorczyca WA, Buczylko J, Ruiz CC, Subbaraya I, Helekar BS, Palczewski K, Baehr W. Expression of GCAP1 and GCAP2 in the retinal degeneration (rd) chicken retina. FEBS Letters. 1996;385:47–52. doi: 10.1016/0014-5793(96)00345-6. [DOI] [PubMed] [Google Scholar]
- 81.Semple-Rowland SL, Larkin P, Bronson JD, Nykamp K, Streit WJ, Baehr W. Characterization of the chicken GCAP gene array and analyses of GCAP1, GCAP2, and GC1 gene expression in normal and rd chicken pineal. Mol Vis. 1999;5:14. [PubMed] [Google Scholar]
- 82.Yang RB, Robinson SW, Xiong WH, Yau KW, Birch DG, Garbers DL. Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J Neurosci. 1999;19:5889–5897. doi: 10.1523/JNEUROSCI.19-14-05889.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coleman JE, Zhang Y, Brown GA, Semple-Rowland SL. Cone cell survival and downregulation of GCAP1 protein in the retinas of GC1 knockout mice. Invest Ophthalmol Vis Sci. 2004;45:3397–3403. doi: 10.1167/iovs.04-0392. [DOI] [PubMed] [Google Scholar]
- 84.Anant JS, Ong OC, Xie H, Clarke S, O'Brien PJ, Fung BKK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. [PubMed] [Google Scholar]
- 85.Qin N, Pittler SJ, Baehr W. In vitro isoprenylation and membrane association of mouse rod photoreceptor cGMP phosphodiesterase a and b subunits expressed in bacteria. J Biol Chem. 1992;267:8458–8463. [PubMed] [Google Scholar]
- 86.Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- 87.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision REs. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Li S, Doan T, Rieke F, Detwiler PB, Frederick JM, Baehr W. Deletion of PrBP/{delta} impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A. 2007;104:8857–8862. doi: 10.1073/pnas.0701681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karan S, Zhang H, Li S, Frederick JM, Baehr W. A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments. Vision Res. 2008;48:442–452. doi: 10.1016/j.visres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman JE, Semple-Rowland SL. GC1 deletion prevents light-dependent arrestin translocation in mouse cone photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46:12–16. doi: 10.1167/iovs.04-0691. [DOI] [PubMed] [Google Scholar]
- 93.Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65-/- and Lrat-/- mice: comparable models of Leber Congenital Amaurosis. Invest Ophthalmol Vis Sci. 2008;49:2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, Frederick JM, Crouch RK, Baehr W. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avasthi P, Le Y, Williams DS, Frederick JM, Baehr W. Kinesin-II Regulates Transport of Membrane-Associated Proteins to the Cone Photoreceptor Outer Segments. ARVO 2008; 2008. Abstract-1264. [Google Scholar]
- 96.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, LaVail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulz S, Lopez MJ, Kuhn M, Garbers DL. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest. 1997;100:1590–1595. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P, Watt CB, Williams DS, Le YZ, Li S, Chen CK, Mark RE, Frederick JM, Baehr W. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci. 2009;29:14287–98. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]