Abstract
Pancreatic ductal adenocarcinoma (PDAC) has a near 100% mortality because it is generally detected at an advanced stage and responds poorly to existing therapeutics. This review summarizes current evidence suggesting important roles of neurotransmitter receptors in the regulation of this malignancy. Experimental evidence indicates that the α7-nicotinic acetylcholine receptor (α7nAChR) stimulates PDAC via stress neurotransmitter-mediated activation of β-adrenergic signaling while the α4β2nAChR inhibits PDAC via GABA-mediated inhibition of adenylyl cyclase activation. In analogy to molecular mechanisms that govern nicotine addiction, chronic exposure to nicotine or its nitrosated derivative nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone render the stimulatory α7nAChR hyperactive while desensitizing the inhibitory α4β2nAChR. Accordingly, PDAC intervention strategies should include the diagnosis of unphysiological neurotransmitter levels and aim to restore any imbalance in stimulatory and inhibitory neurotransmitters.
Keywords: β-adrenergic receptor, GABA receptor, nicotinic acetylcholine receptor, pancreatic cancer, stress neurotransmitters
Pancreatic cancer is one of the most deadly neoplastic diseases because it typically does not cause any symptoms until it has reached an advanced stage. At the time of diagnosis, most pancreatic cancers are therefore inoperable and have metastasized to distant organs. In addition, this malignancy is generally unresponsive to conventional radio- and chemotherapy, resulting in a mortality rate near 100% within 6 months of diagnosis [1]. Although it only ranks tenth in incidence among the most common human cancers, pancreatic cancer is therefore the fourth leading cause of cancer deaths in Western countries [2].
Pancreatic cancer arises from endocrine or exocrine pancreatic cells, with more than 95% of all pancreatic cancers demonstrating histological features of pancreatic ductal adenocarcinoma (PDAC). Smoking, diabetes mellitus and pancreatitis from any etiology, including alcohol abuse, are risk factors for PDAC [3,4]. However, the reasons for these etiological associations are poorly understood. Increases in inflammatory mediators observed in diabetes and pancreatitis are thought to be contributing factors, an interpretation supported by the frequent overexpression of cyclooxygenase (COX)-2 and members of the lipoxygenase family in PDAC [5,6]. On the other hand, the nicotine-derived carcinogenic nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (N-nitroso nicotine ketone [NNK]) causes PDAC in laboratory rodents, thus supporting a causative role of smoking [7,8].
The growth regulation of PDAC and its putative cells of origin, pancreatic duct epithelia, are poorly understood. The majority of PDACs (approximately 75%) harbor activating point mutations in K-ras while also overexpressing the EGF receptor (EGFR), leading to the generally accepted view that EGFR signaling via ras and its downstream effectors, ERK1/2, play an important role in the regulation of this cancer [9]. Studies in vitro and in mouse xenografts from human cancer cell lines have demonstrated significant reductions in tumor growth in response to inhibition of the EGFR pathway by agents such as farnesyltransferase inhibitors, EGFR tyrosine kinase inhibitors, ERK1/2 cascade inhibitors or inhibitors of COX-2 [10]. In addition, suppression of VEGF has demonstrated promising responses in vitro and in an orthotopic mouse model of PDAC [11]. However, therapeutics that target the EGFR pathway by inhibiting tyrosine kinases or ras as well as inhibitors of COX-2, VEGF or the combination of such agents have disappointed in clinical trials [10], suggesting that the growth regulation of this malignancy is more complex.
Studies pioneered by our laboratory suggest that neurotransmitter receptors of the nicotinic, β-adrenergic and GABA families act as central regulators of PDAC and their cells of origin. This article summarizes evidence in support of the hypothesis that malfunctions of these receptors create an environment that selectively stimulates the development and progression of PDAC while inhibiting tumor suppressor functions.
PDAC stimulating roles of nicotinic & β-adrenergic receptors
Nicotinic acetylcholine receptors (nAChRs) are located in the plasma membrane of cells and are comprised of five subunits (pentamers) that enclose a central ion channel [12]. They can either be comprised of five identical α-subunits (homomeric nAChRs) or a combination of α-subunits with β-, γ- or δ-subunits (heteromeric nAChRs). Binding of an agonist to an nAChR causes conformational changes that open its ion channel, leading to the influx of ions into the cell. In turn, this leads to a host of cellular responses, including the synthesis and release of neurotransmitters, growth factors and angiogenic factors, as well as the activation of diverse intracellular signaling cascades [13]. It was initially widely believed that nAChRs are restricted to the nervous system and neuromuscular junctions. However, more recent studies have identified homomeric and heteromeric nAChRs in a large variety of non-neuronal cells where they serve diverse functions [14].
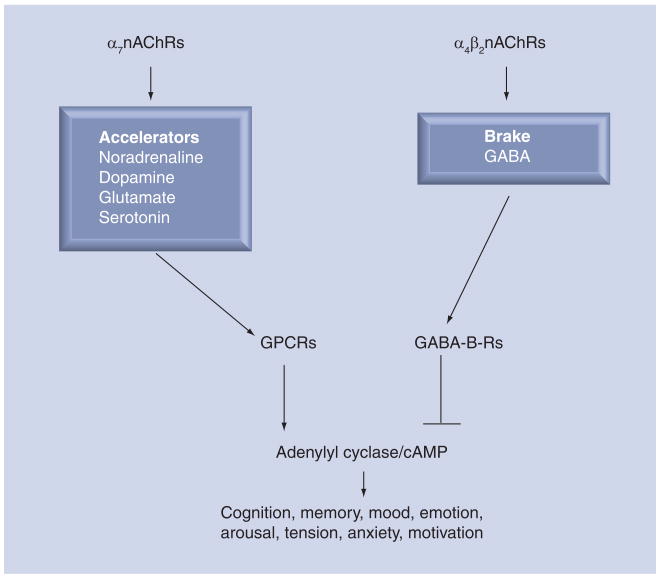
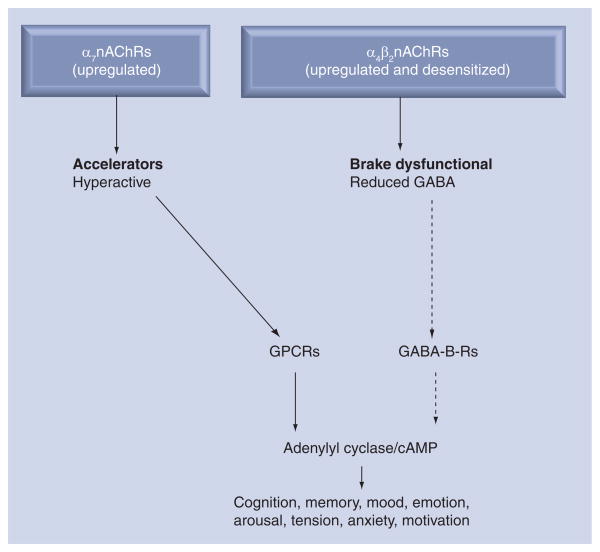
The homomeric α7nAChR and the heteromeric α4β2nAChR predominate in neurons of humans and other mammals and most of our current knowledge on the biology of nAChRs is derived from studies of these receptors in the brain. It has thus been demonstrated that the α7nAChR acts as the ‘accelerator’ in the brain (Figure 1) by stimulating the synthesis and release of excitatory neurotransmitters such as noradrenaline, dopamine, serotonin and glutamate [13,15,16]. By contrast, the α4β2nAChR acts as the ‘brake’ by regulating the synthesis and release of the most important inhibitory neurotransmitter, GABA [16]. The neurotransmitter acetylcholine is the physiological agonist for all cholinergic receptors, including nAChRs, and muscarinic acetylcholine receptors. By contrast, nicotine is a selective agonist for nAChRs to which it binds with significantly higher affinity than acetylcholine [12]. Studies in brain cells have demonstrated that chronic exposure to nicotine upregulates the protein expression of all nAChRs via post-transcriptional events that do not require an increase in receptor subunit RNA [17,18]. However, there are important differences in functional changes associated with nicotine-induced protein upregulation among nAChRs. While the heteromeric receptors, including the α4β2nAChR, increase their protein expression as a reaction to long-term desensitization caused by nicotine, no long-term desensitization is observed in the homomeric α7nAChR [12]. As a result, the production of inhibitory GABA is greatly reduced whereas the production of excitatory neurotransmitters increases (Figure 2). Symptoms of nicotine addiction and craving are generally thought to be caused by this imbalance in excitatory and inhibitory neurotransmitters [16,19].
Figure 1. Regulatory functions of the α7nAChR and the α4β2nAChR in the brain.
The α7nAChR acts as an ‘accelerator by stimulating the synthesis and release of the excitatory neurotransmitters noradrenaline (from which adrenaline is formed), dopamine, glutamate and serotonin. Most effects of these neurotransmitters are mediated by GPCRs that activate the enzyme anenylyl cyclase. The α4β2nAChR acts as the ‘brake’ by stimulating the synthesis and release of the inhibitory neurotransmitter GABA, which blocks GPCR-mediated activation of adenylyl cyclase by Gi-mediated activity of the GABA-B-R.
GABA-B-R: GABA-B receptor; GPCR: G-protein-coupled receptor; nAChR: Nicotinic acetylcholine receptor.
Figure 2. Working model of changes in nAChR functions in the brain associated with nicotine addiction.
Chronic exposure to nicotine upregulates the stimulatory α7nAChR without concomittant receptor desensitization, whereas the inhibitory α4β2nAChR undergoes long-term desensitization accompanied by upregulation. The resulting predominance of excitatory neurotransmitters and relative deficiency in inhibitory GABA lead to symptoms associated with nicotine addiction and craving.
GABA-B-R: GABA-B receptor; GPCR: G-protein-coupled receptor; nAChR: Nicotinic acetylcholine receptor.
β-adrenergic receptors (βARs) consist of β1-, β2-, and β3-receptors and are members of the heptahelical G-protein-coupled cell membrane receptor family. The β3ARs are almost exclusively found in adipose tissue, whereas β1- and β2ARs are expressed in the majority of mammalian cells. The stress neurotransmitters adrenaline and noradrenaline are the physiological agonists for all βARs, with adrenaline preferentially binding to β2ARs while noradrenaline binds with higher affinity to β1ARs [20]. Owing to the fact that the synthesis and release of noradrenaline and adrenaline are stimulated by nAChRs, the βARs represent indirect downstream effectors of nAChRs (Figure 1–3). In addition to the pharmacological activation of nAChRs by nicotinic receptor agonists contained in tobacco products, the synthesis and release of noradrenaline and adrenaline are also stimulated by psychological stress via acetylcholine-induced activation of the α7nAChR in the nervous system as well as the heteromeric nAChRs containing the α3- and α5-subunits in the adrenal medulla. Binding of an agonist to βARs activates the adenylyl cyclase-stimulating G-protein, Gαs, resulting in the formation of cAMP and activation of protein kinase A (PKA), leading to phosphorylation of the transcription factor cAMP response element binding protein (CREB). It has been demonstrated that β1- and β2-adrenoreceptor stimulation can additionally transactivate the EGFR pathway in a PKA-dependent manner [21]. We have demonstrated that such transactivation occurs in immortalized human pancreatic duct epithelial cells [22] and in cell lines derived from human lung adenocarcinomas and immortalized small airway epithelial cells [23]. In addition to these direct effects on cancer cells, βARs stimulate the release of EGF [24,25] and VEGF [25–27] in a cAMP-dependent manner, thus indirectly contributing to the development and progression of numerous cancers, including PDAC. Indirect stimulation of cell proliferation in response to the release of arachidonic acid [28] and IL-6 [29] caused by noradrenaline or other β-adrenergic agonists in pancreatic cancer cells and pancreatic duct epithelial cells has also been reported.
Figure 3. Working model of the regulation of pancreatic ductal adenocarcinoma by neurotransmitters, their receptors and downstream effectors.
Chronic exposure to nicotinic agonists in tobacco products and in the human environment cause nAChR changes analogous to those in the nicotine-addicted brain (compare with Figure 2). The resulting predominance of stimulatory adenylyl cylase-dependent signaling and relative deficiency in inhibitory GABA leads to the selective activation of cell proliferation, migration and angiogenesis while inhibiting apoptosis. In addition, psychological stress activates this cancer-stimulating cascade by causing the release of acetylcholine that activates α7nAChRs in the nervous system and nAChRs containing the α3-or α5-subunits in the hypothalamus and adrenal medulla, resulting in the release of noradrenaline and adrenaline into the bloodstream.
AA: Arachidonic acid; AKT: Serine threonine protein kinase B;
βAR: β-adrenergic receptor; CREB: cAMP response element binding;
EGFR: EGF receptor; GABA-B-R: GABA-B receptor; nAChR: Nicotinic acetylcholine receptor; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone;
PKA: Protein kinase A.
The first reports that implicated nAChRs in the regulation of cancer demonstrated that nicotine and the nicotine-derived carcinogenic nitrosamine NNK stimulated the proliferation of human small-cell lung cancer (SCLC) cells in vitro and that this response was blocked by the nAChR antagonist hexamethonium [30,31]. These experiments also demonstrated that the carcinogenic nitrosamine N-nitroso-diethylamine that is formed in numerous foods, beverages and cosmetics had similar effects as nicotine on SCLC cells. Studies by another laboratory additionally reported a year later that nicotine inhibited apoptosis in SCLC cells [32]. It was later discovered that NNK and N-nitroso-diethylamine are nAChR agonists that bind with high affinity to these receptors [33–35], thus explaining the nAChR-mediated effects on cancer cells. Since these early reports, numerous publications have described nAChR-mediated stimulatory effects via the activation of intracellular signaling pathways in a host of different cancers [13]. However, few investigators have explored a potential role of nAChRs and their effectors in the regulation of PDAC. It has thus been demonstrated that βARs, which are activated by noradrenaline and adrenaline in response to the nAChR-mediated synthesis and release of these neurotransmitters, cause a pronounced stimulation of cell proliferation and migration of human PDAC cells in vitro [36,37]. It was further demonstrated that the β2AR predominates in PDAC and immortalized pancreatic duct epithelial cells [22,36] and activates a signaling cascade that includes cAMP, PKA and the transcription factor CREB in these cells while also stimulating the release of arachidonic acid [36]. In addition, activated PKA transactivated the EGFR and its downstream effectors, the ras-dependent mitogen-activated protein kinases MEKK and ERK1/2 [22]. Interestingly, it was discovered that the tobacco carcinogen NNK is also a βAR agonist [38] that stimulates the proliferation of PDAC cells and pancreatic duct epithelial cells directly by binding to these receptors [22,36]. Treatment of pancreatic duct epithelial cells in vitro with ethanol additionally increased the levels of intra-cellular cAMP, thus enhancing cell proliferation in response to β-adrenergic activation of these signaling cascades [39]. These findings suggest that alcohol consumption may increase the risk for PDAC not only via alcohol-induced pancreatitis but additionally by enhancing PDAC stimulating cAMP-dependent signaling.
Investigations in hamsters have demonstrated that animals with ethanol-induced pancreatitis developed a high incidence of PDAC when additionally treated with NNK [8]. Studies in this animal model also demonstrated that the cyclooxygenase inhibitor ibuprofen significantly reduced the incidence of PDAC [40], while the β-blocker propranolol completely blocked the development of pancreatic tumors [41]. Moreover, the hamsters treated with NNK had significantly elevated serum levels of noradrenaline and adrenaline associated with increased cAMP levels in the cellular fraction of blood [41,42]. Western blots from pancreatic duct epithelia and PDACs harvested by laser capture microscopy revealed significant upregulation in PDAC cells of the α7nAChR that regulates the synthesis and release of these neurotransmitters [41,42]. At the same time, cAMP, phosphorylated (p)-CREB, p-ERK1/2, EGF and VEGF were all overexpressed in the PDAC cells. The observed overexpression α7nAChR protein as well as that of p-CREB, p-ERK1/2, EGF and VEGF were all inhibited by treatment of the hamsters with the βAR antagonist propranolol [41]. Collectively, these findings suggest an important regulatory role of α7nAChR-stimulated stress neurotransmitter production and the resulting βAR-activated cAMP-dependent signaling cascade (Figure 3). This interpretation was further corroborated by a study in nude mouse xenografts from a human PDAC cell line. This experiment demonstrated that xenografts of mice receiving nicotine in the drinking water for 30 days progressed significantly faster than the tumors in control animals, a response accompanied by elevated systemic levels of noradrenaline, adrenaline and cAMP as well as overexpression of cAMP, p-CREB and p-ERK1/2 in the xenograft tissues [43].
Inhibitory role of the GABA-B receptor in PDAC
The biological effects of the amino acid neurotransmitter GABA in the brain are mediated by GABA-A receptors, a family of ion channels, and by the GABA-B receptor (GABA-B-R), a receptor coupled to the inhibitory G-protein [44]. While GABA production was initially Gi believed to be restricted to the brain, more recent studies have demonstrated that GABA and its receptors are also expressed in numerous peripheral tissues and organs [45].
Inhibitory actions of GABA on certain cancers were first suggested by the observation that this neurotransmitter inhibited the noradrenaline-induced migration of colon cancer cells [46] and breast cancer cells in vitro [47]. Tumor suppressor function of the GABA-B-R for PDAC and lung adenocarcinomas under positive growth control by cAMP signaling was suggested by several recent publications. These investigations demonstrated that GABA reduced β-adrenergic agonist-induced DNA synthesis and migration of human pancreatic cancer cells in vitro below base levels observed in unstimulated cells [37]. The inhibitory effects of GABA were enhanced by transient transfection with the GABA-B-R and abolished by gene knockdown of this receptor, indicating an important role of this receptor in the observed effects of GABA. In support of this interpretation, the selective GABA-B-R agonist baclophen had similar inhibitory effects. GABA and baclophen also significantly reduced intracellular cAMP and the phosphorylation of ERK1/2 and CREB, the downstream effectors of β-adrenergic signaling. These findings are in accord with the GABA-B-R-mediated reduction in cAMP signaling via Gi-mediated inhibition of adenylyl cyclase reported in the brain [48]. Furthermore, immunohistochemical analysis of human tissue microarrays revealed overexpression of noradrenaline and activated PKA in the majority of investigated PDACs while GABA and its synthesizing enzyme GAD65 were suppressed [37]. Similarly, PDACs induced in hamsters by NNK and ethanol demonstrated suppressed GAD and GABA while p-CREB and p-ERK1/2 were overexpressed [42]. In addition, these animals had significantly elevated blood levels of noradrenaline, adrenaline and cAMP. Interestingly, protein expression of the α4β2nAChR that regulates the synthesis and release of GABA was overexpressed in the PDAC cells. In conjunction with the observed suppression of its effectors GAD and GABA, these findings suggest that the observed upregulation in receptor protein was a reaction to desensitization of the receptor. This interpretation is in accord with the nicotine-induced modulation of the α4β2nAChR and the GABA system reported in the brain that have been associated with nicotine addiction [16,18,19]. However, unlike the behavioral responses caused by nAChR and GABA neurotransmission in the brain, such changes in PDAC cells are associated with a significant stimulation of DNA synthesis and metastatic potential in the cancer cells. An experiment with PDAC xenografts in nude mice provided additional support for this hypothesis [43]. This study demonstrated that treatment of the mice for 30 days with nicotine in the drinking water significantly reduced the expression levels of GAD65, GAD67 and GABA. In addition, the noradrenaline-driven progression of the xenografts and associated upregulation of signaling proteins described in the previous section of this review was completely reversed by treatment of the animals with GABA [43], while GABA also significantly reduced xenograft growth in unstimulated mice.
While the discussed findings suggest that signaling of GABA via the GABA-B-R may be a promising target for the prevention and adjuvant therapy of PDAC, reports on the expression levels of GABA and its receptors in human PDAC tissues are controversial. By contrast to our immunohistochemical observation of suppressed GAD and GABA in PDAC tissue microarrays [37], another laboratory reported that GABA and the π-subunit of the GABA-A receptor (GABA-A-R) were overexpressed in the majority of investigated human PDAC cell lines and in surgical samples from PDACs [49]. In addition, these investigators reported a significant GABA-induced stimulation of PDAC cell proliferation in the cell lines that overexpressed the π-subunit of the GABA-A-R. These findings are contradicted by yet another publication that described a significant GABA deficiency in patients with pancreatitis and PDAC [50]. Differences in the smoking history of the investigated cell lines and PDAC patients may account for these discrepancies. Studies by proton magnetic resonance of the brain have thus demonstrated that smoking significantly reduced tissue GABA levels [51], a finding supported by our observation that chronic nicotine reduced GABA levels in PDAC xenografts [43].
Conclusion
Pancreatic cancer research has mostly focused on the study of gene mutations and signal transduction pathways in PDAC cells, whereas the potential role of neurotransmitters and their receptors in the development and progression of this deadly neoplastic disease has been largely ignored. While it is well established that a host of different cancers are significantly influenced by hormones and their receptors, the concept of neurotransmitters and their receptors as central regulators of the most common human cancers, including PDAC, was only recently suggested [13,52]. However, similar to other cancers, PDAC is not a uniform disease and demonstrates instead considerable interindividual variations in environmental, lifestyle and genetic factors. There is therefore no ‘magic bullet’ that will prevent and/or cure PDAC. Instead, custom-tailored highly individualized approaches have to be pursued as PDAC intervention strategies. As is evident from the data summarized in this review, such approaches should include the monitoring of neurotransmitters, their receptors and downstream effectors, and attempt to restore and maintain a balance between stimulatory and inhibitory neurotransmission. Not only smoking but also numerous environmental and lifestyle factors, as well as psychological stress, can significantly modulate the systemic levels of stress neurotransmitters and GABA, resulting in modulations of their associated receptors. In light of the fact that patients diagnosed with pancreatic cancer demonstrated the highest levels of psychological disturbance and anxiety among 14 investigated cancer types [53], the potential negative impact of stress on the outcome of pancreatic cancer therapy may significantly contribute to the extremely poor prognosis of this malignancy. The appalling disconnect between the dismal clinical failure of most anti-PDAC agents that were highly effective in preclinical studies may be in part caused by the fact that preclinical studies are conducted in a carefully controlled stress-free environment. Neither the commonly used in vitro systems nor laboratory animal studies allow for the detection of neurotransmitter effects unless the neurotransmitters are deliberately added to the experimental environment. For example, when studying the signaling cascades that regulate PDAC (Figure 3), it is immediately apparent that agents that block EGFR signaling will be considerably less effective in the presence of high levels of noradrenaline, which stimulates multiple targets in addition to the EGFR. Similarly, antiangiogenesis therapy will be less effective in an individual whose angiogenesis is continually hyperstimulated by a hyperactive α7nAChR and/or hyperactive stress neurotransmitters and their receptors. On the other hand, PDAC intervention with GABA as suggested by data generated in our laboratory would obviously be contraindicated in individuals with upregulated π-subunits of the GABA-A-R that respond with PDAC stimulation to GABA treatment.
Future perspective
Neurotransmitters and their receptors are central regulators of all involuntary functions in the mammalian organism. Similar to numerous other cellular entities, the neurotransmitter receptors adapt to changes in their environment, thus altering their responsiveness to agonists. The fine-tuning of this intricate neurotransmission network enables all cells and organs in the mammalian organism to function in concert. Cancer is a disease characterized by the uncontrolled growth of cancer cells at the expense of healthy cells and tissues. The concept of malfunctioning neurotransmitter receptors as an important driving force in the development and progression of pancreatic cancer summarized in this review therefore applies to the disease complex ‘cancer’ in general. In support of this hypothesis, emerging research has identified an important stimulatory role of the stress neurotransmitter noradrenaline in cancer of the colon [46,54], prostate [55], mammary gland [47] and ovary [26]. While research on the role of neurotransmission in these cancers is centered on studies of nicotinic acetylcholine receptors, stress neurotransmitters and their receptors, as well as GABA and its receptors [13], additional neurotransmitters and their effectors may be involved in the regulation of other types of cancers. It is thus envisioned that efforts aimed to restore the distorted balance between stimulatory and inhibitory neurotransmitters and their receptors will become a focal point of future strategies for the development of more effective cancer intervention.
Executive summary
Inhibitors of established EGF-associated regulatory pathways in pancreatic ductal adenocarcinoma (PDAC) have failed in the therapy of this cancer although they were highly effective in preclinical tests. These findings suggest a lack of factor(s) in the preclinical testing systems that counteract the anticancer effects of these inhibitors in the patient.
PDAC stimulating roles of nicotinic & β-adrenergic receptors
Emerging research suggests that, analogous to molecular changes in the brain of nicotine addicts, an upregulated α7-nicotinic acetylcholine receptor causes increased levels of noradrenaline and adrenaline, resulting in hyperactivity of pancreatic cancer-stimulating signaling via β-adrenergic receptor-dependent activation of adenylyl cyclase.
Inhibitory role of the GABA-B receptor in PDAC
Simultaneous desensitization of the α7-nicotinic acetylcholine receptor results in a significant reduction of pancreatic cancer inhibiting GABA production.
Conclusion
Strategies for the prevention and therapy of pancreatic cancer should include the assessment of systemic noradrenaline and GABA levels and attempt to restore any detected imbalance among these stimulatory and inhibitory neurotransmitters.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Hildegard M Schuller, Email: hmsch@utk.edu, Experimental Oncology Laboratory, College of Veterinary Medicine, University of Tennessee, 2407 River Drive, Knoxville, TN 37996, USA, Tel.: +1 865 974 8217, Fax: +1 865 984 5616.
Hussein AN Al-Wadei, Experimental Oncology Laboratory, Department of Pathobiology, College of Veterinary Medicine, University of Tennessee, 2407 River Drive, Knoxville, TN 37996, USA and Sana’a University, Round Road, Sana’a, Yemen.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 2.ACS. Cancer Statistics 2009. American Cancer Society; 2009. [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P. Risk factors for pancreatic cancer. J Cell Biochem. 2005;95:649–656. doi: 10.1002/jcb.20461. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT. Risk factors for pancreatic cancer: a case–control study based on direct interviews. Teratog Carcinog Mutagen. 2001;21:7–25. doi: 10.1002/1520-6866(2001)21:1<7::aid-tcm3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9(4):411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Ding XZ, Hennig R, Adrian TE. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol Cancer. 2003;2:10. doi: 10.1186/1476-4598-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivenson A, Hoffmann D, Prokopczyk B, Amin S, Hecht SS. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res. 1988;48:6912–6917. [PubMed] [Google Scholar]
- 8.Schuller HM, Jorquera R, Reichert A, Castonguay A. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53:2498–2501. [PubMed] [Google Scholar]
- 9.Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6:412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas. 2005;31:301–316. doi: 10.1097/01.mpa.0000175893.04660.1b. [DOI] [PubMed] [Google Scholar]
- 11.Fukasawa M, Korc M. Vascular endothelial growth factor-trap suppresses tumorigenicity of multiple pancreatic cancer cell lines. Clin Cancer Res. 2004;10:3327–3332. doi: 10.1158/1078-0432.CCR-03-0820. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 13.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 14.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barik J, Wonnacott S. Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–628. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- 16.Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks MJ, Pauly JR, Gross SD, et al. Nicotine binding and nicotine receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harkness PC, Millar NS. Changes in conformational and subcellular distribution of nicotinic acetylcholine receptors α4β2 revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci. 2002;22:10172–10181. doi: 10.1523/JNEUROSCI.22-23-10172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai H, Berg DK. Nicotinic acetylcholine receptors containing the α 7 subunits on rat cortical neurons do not undergo long lasting inactivation even when upregulated by chronic nicotine exposure. J Neurochem. 2001;78:1367–1378. doi: 10.1046/j.1471-4159.2001.00526.x. [DOI] [PubMed] [Google Scholar]
- 20.Maki T, Kontula K, Harkonen M. The β-adrenergic system in man: physiological and pathophysiological response. Regulation of receptor density and functioning. Scand J Clin Lab Invest Suppl. 1990;201:25–43. [PubMed] [Google Scholar]
- 21.Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- 22.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through β-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laag E, Majidi M, Cekanova C, Masi T, Takahashi T, Schuller HM. NNK activates ERK1/2 and CREB/ATF-1 via β-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int J Cancer. 2006;119:1547–1552. doi: 10.1002/ijc.21987. [DOI] [PubMed] [Google Scholar]
- 24.Grau M, Soley M, Ramirez I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology. 1997;138:2601–2609. doi: 10.1210/endo.138.6.5183. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Wu WK, Yu L, et al. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via β-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem. 2008;5(1):53–60. doi: 10.1002/jcb.21802. [DOI] [PubMed] [Google Scholar]
- 26.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 27.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 28.Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218(4):437–445. doi: 10.1002/path.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan C, Lin H-J, Lin J. Stress-associated hormone, norepinephrine, increases proliferation and IL-6 levels of human pancreatic duct epithelial cells and can be inhibited by the dietary agent, sulforaphane. Int J Oncol. 2008;33:415–419. [PubMed] [Google Scholar]
- 30 ▪.Schuller HM. Cell type specific, receptor-mediated modulation of growth kinetics in human lung cancer cell lines by nicotine and tobacco-related nitrosamines. Biochem Pharmacol. 1989;38:3439–3442. doi: 10.1016/0006-2952(89)90112-3. First report to identify a regulatory function of nicotinic acetylcholine receptors in cancer cell proliferation. [DOI] [PubMed] [Google Scholar]
- 31.Schuller HM, Hegedus TJ. Effects of endogeneous and tobacco-related amines and nitrosamines on cell growth and morphology of a cell line derived from a human neuroendocrine lung cancer. Toxicol In Vitro. 1989;3:37–43. doi: 10.1016/0887-2333(89)90022-2. [DOI] [PubMed] [Google Scholar]
- 32 ▪.Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci USA. 1990;87:3294–3298. doi: 10.1073/pnas.87.9.3294. Provided first evidence for nicotinic acetylcholine receptors in the regulation of cancer cell apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33 ▪▪.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. Identified tobacco-specific carcinogenic nitrosamines as agonists for nicotinic acetylcholine receptors. [DOI] [PubMed] [Google Scholar]
- 34.Schuller HM. Nitrosamines as nicotinic receptor ligands. Life Sci. 2007;80:2274–2280. doi: 10.1016/j.lfs.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 36.Weddle DL, Tithoff P, Williams M, Schuller HM. β-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 37.Schuller HM, Al-Wadei HA, Majidi M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38 ▪.Schuller HM, Tithof PK, Williams M, Plummer H., 3rd The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a β-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via β-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–4515. Identified the carcinogenic nitrosamine NNK as β-adrenergic receptor agonist. [PubMed] [Google Scholar]
- 39.Askari MD, Tsao MS, Cekanova M, Schuller HM. Ethanol and the tobacco-specific carcinogen, NNK, contribute to signaling in immortalized human pancreatic duct epithelial cells. Pancreas. 2006;33:53–62. doi: 10.1097/01.mpa.0000226883.55828.e9. [DOI] [PubMed] [Google Scholar]
- 40.Schuller HM, Zhang L, Weddle DL, Castonguay A, Walker K, Miller MS. The cyclooxygenase inhibitor ibuprofen and the FLAP inhibitor MK886 inhibit pancreatic carcinogenesis induced in hamsters by transplacental exposure to ethanol and the tobacco carcinogen NNK. J Cancer Res Clin Oncol. 2002;128:525–532. doi: 10.1007/s00432-002-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the β-blocker propranolol. Anticancer Drugs. 2009;20:477–482. doi: 10.1097/CAD.0b013e32832bd1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42 ▪.Al-Wadei HA, Schuller HM. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J Pathol. 2009;218:437–445. doi: 10.1002/path.2542. Demonstrated, for the first time, that the carcinogen NNK increases systemic levels of stress neurotransmitters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43 ▪▪.Al-Wadei HA, Plummer HK, 3rd, Schuller HM. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter γ-aminobutyric acid. Carcinogenesis. 2009;30:506–511. doi: 10.1093/carcin/bgp010. Provided first evidence that GABA has potent tumor supressor function in pancreatic cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano T, Watanabe D, Kawaguchi SY, Pastan I, Nakanishi S. Roles of inhibitory interneurons in the cerebellar cortex. Ann NY Acad Sci. 2002;978:405–412. doi: 10.1111/j.1749-6632.2002.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 45.Gladkevich A, Korf J, Hakobyan VP, Melkonyan KV. The peripheral GABAergic system as a target in endocrine disorders. Auton Neurosci. 2006;124:1–8. doi: 10.1016/j.autneu.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 46 ▪.Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter γ-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467–6469. First report that showed cancer inhibiting activity of GABA. [PubMed] [Google Scholar]
- 47.Drell TLT, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 48.Hejnová L, Ihnatovych I, Novotny J, Kubová H, Mares P, Svoboda P. Modulation of adenylyl cyclase activity by baclofen in the developing rat brain: difference between cortex, thalamus and hippocampus. Neurosci Lett. 2002;13:9–12. doi: 10.1016/s0304-3940(02)00721-8. [DOI] [PubMed] [Google Scholar]
- 49.Takehara A, Hosokawa M, Eguchi H, et al. γ-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor π subunit. Cancer Res. 2007;67:9704–9712. doi: 10.1158/0008-5472.CAN-07-2099. [DOI] [PubMed] [Google Scholar]
- 50.Schrader H, Menge BA, Belyaev O, Uhl W, Schmidt WE, Meier JJ. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:416–421. doi: 10.1097/MPA.0b013e318194fc7a. [DOI] [PubMed] [Google Scholar]
- 51.Epperson CN, O’Malley S, Czarkowski KA, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19:655–671. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- 53.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via α7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Palm D, Lang K, Niggemann B, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]