Abstract
The 5-HT7 receptor has been suggested as a target for treating depression since inactivation or blockade of the receptor has an antidepressant-like behavioral effect. The present study investigated possible interactions between various classes of drugs with antidepressant properties and blockade or inactivation of the 5-HT7 receptor. Immobility despair in the tail suspension test and the forced swim test was evaluated in mice lacking the 5-HT7 receptor (5-HT7−/−) and in wild-type controls (5-HT7+/+) following acute drug treatments. Citalopram, a selective serotonin reuptake inhibitor and widely used antidepressant, dose-dependently reduced immobility in the tail suspension test in both 5-HT7+/+ and 5-HT7−/− mice. Combining doses of citalopram and the 5-HT7 receptor antagonist SB-269970 that by themselves did not affect behavior, reduced immobility in 5-HT7+/+ mice in both the tail suspension test and the forced swim test. No effect was seen in 5-HT7−/− mice. Desipramine and reboxetine, two norepinephrine reuptake inhibitors, dose-dependently reduced immobility in the tail suspension test in 5-HT7+/+ mice, but had no effect in 5-HT7−/− mice. A synergistic effect between desipramine and SB-269970 was found in both behavioral tests in 5-HT7+/+ mice. Reboxetine combined with SB-269970 had effect only in the forced swim test. GBR 12909, a dopamine reuptake inhibitor, dose-dependently reduced tail suspension test immobility in both genotypes. There was no interaction between GBR 12909 and SB-269970. Aripiprazole, an antipsychotic, reduced immobility in both tests in 5-HT7+/+ mice, but not in 5-HT7−/− mice. The results show that the 5-HT7 receptor is required for the observed interaction between this receptor and antidepressants such as citalopram. The data furthermore support the hypothesis that the 5-HT7 receptor might be a suitable target for treating depression.
Keywords: citalopram, desipramine, reboxetine, aripiprazole, forced swim test, tail suspension test, SB-269970, GBR 12909
1. Introduction
The 5-HT7 receptor, a member of a more recently discovered group of 5-HT receptors [1–6], has been found to modulate behaviors affected by antidepressant drugs. Behavioral models of significant value for evaluating putative antidepressants and for characterizing transgenic mice [7,8] include the forced swim test [9] and the tail suspension test [10]. In both of these tests, inactivation or blockade of the 5-HT7 receptor has been shown to lead to antidepressant-like behavior [11–13]. For inhibition of the 5-HT7 receptor, these studies used the selective 5-HT7 receptor antagonist SB-269970 [14,15]. Furthermore, inactivation or pharmacological blockade of the 5-HT7 receptor lead to changes in rapid eye movement sleep that are opposite to those seen in depression [12,15].
These findings have led to the hypothesis that the 5-HT7 receptor might be suitable new target for the treatment of depression [16–19]. It has been shown that certain antidepressants might exert some of their effects by acting directly at the 5-HT7 receptor [20]. More interestingly, recent studies have found a synergistic interaction between SB-269970 and various antidepressants [21,22]. One of the studies found that mice given an ineffective dose of citalopram, a selective serotonin reuptake inhibitor (SSRI), in conjunction with an ineffective dose of SB-269970 exhibited antidepressant-like behavior in the tail suspension test [21]. At a higher dose, it has been demonstrated that SB-269970 alone reduces immobility in the mouse tail suspension test [12,21]. A similar synergistic interaction between SB-269970 and citalopram has also been described in the mouse forced swim test [22]. This study also reported that interactions occur also between SB-269970 and other classes of antidepressants. Thus, ineffective doses of imipramine (a tricyclic antidepressant), desipramine (a tricyclic antidepressant acting mainly as a norepinephrine reuptake inhibitor), and moclobemide (a monoamine oxidase inhibitor) all reduced immobility in the mouse forced swim test when given in combination with SB-269970 [22]. The interaction between SB-269970 and imipramine has also been shown in the forced swim test using Wistar rats [23]. The prefrontal cortex has been suggested as an important region for these interactions [21,22], but the hippocampus has also been implicated in the effects of SB-269970 and imipramine on the rat forced swim test [24].
Although the monoamine hypothesis of depression with its focus on 5-HT and norepinephrine has been very important for the understanding of this disorder, there are remaining issues, suggesting that other mechanisms might also be involved [25]. One significant pharmacological issue is that reuptake inhibition following treatment with an antidepressant occur rapidly, but its clinical effects require much longer time [26]. The present study aims to further investigate the relationships between the 5-HT7 receptor and monoamine reuptake inhibition, and also to investigate possible relationships with other factors deemed to be relevant for the understanding of depression. Specifically, we have evaluated possible modulations of the 5-HT7 receptor on dopamine reuptake, on the 5-HT1A receptor, on the antidepressant properties of the antipsychotic aripiprazole, and on corticosterone in models of depression.
Dopamine has been implicated in depression as it has been suggested that reduced availability of dopamine, in a similar way as for 5-HT and norepinephrine, might contribute to the pathophysiology of depression. In fact, dopaminergic mechanisms have gained increased interest with the development of combined norepinephrine and dopamine reuptake inhibitors and even triple reuptake inhibitors [27–30]. Selective dopamine reuptake inhibitors have been shown to reduce immobility in the forced swim test in rats. Nomifensine at low doses, which significantly reduced the immobility time, had no significant effect on open field locomotor activity. At higher doses nomifensine increased overall locomotor activity as well as decreased immobility in the forced swim test [31]. GBR 12909 is another dopamine reuptake inhibitor that has recently been shown to reduce immobility in both the tail suspension test and the forced swim test, but also to increase overall locomotor activity [32].
Interestingly, it has recently been found that the dopamine D2/D3 antagonist amisulpride, an antipsychotic with known antidepressant properties [33,34], most likely exerts its antidepressant effect through the 5-HT7 receptor and not, as previously thought, through dopaminergic mechanisms [35]. Thus, amisulpride was shown to have high affinity for the human 5-HT7 receptor and to reduce immobility in both the tail suspension test and the forced swim test in 5-HT7+/+ mice, but not in 5-HT7−/− mice. To extend these findings we have in the present study investigated aripiprazole, an atypical antipsychotic with high affinity for the 5-HT7 receptor [36] that is approved as augmentation therapy to antidepressants [37] and that has recently has been shown to potentiate the effect of fluoxetine [38].
The 5-HT1A receptor and the 5-HT7 receptor have been shown to modulate the action of each other. After the discovery of the 5-HT7 receptor it was found that 8-OH-DPAT (8-hydroxy-2(di-n-propylamino)tetralin), originally thought to be a selective agonist for the 5-HT1A receptor, had high affinity for the 5-HT7 receptor [1]. It has subsequently been demonstrated that 5-HT1A and 5-HT7 receptors both are involved in mechanisms such as thermoregulation [39]. Mice lacking the 5-HT1A receptor show reduced immobility in the forced swim test [40], but it has not been possible to induce such an effect with antagonists for this receptor [41]. Nevertheless, 5-HT1A receptor antagonists such as pindolol have been successfully used to reduce the time needed until clinical effect is achieved with antidepressants [42].
For decades, it has been known that individuals under sustained, elevated stress levels are at a higher risk of developing mental illnesses, especially depression. Stressors induce the secretion of glucocorticoids such as corticosterone in rodents and cortisol in humans through the hypothalamic-pituitary-adrenal axis. The serotonergic system has been shown to modulate the effects of glucocorticoids in mood disorders as well as regulate the expression of glucocorticoid and mineralocorticoid receptors [43,44]. An increase in 5-HT7 mRNA in the hippocampus was observed following blockade of corticosterone synthesis in male Wistar rats likely due to a reduction in glucocorticoid receptor transmission in the hippocampus [45].
In the present study we have further tested the hypothesis that the 5-HT7 receptor is a putative new target for the treatment of depressions [16–19]. We have performed an expanded series of experiments on the interaction between reuptake inhibitors for 5-HT, norepinephrine, and dopamine, and antagonists for the 5-HT1A and 5-HT7 receptors, as well as the antipsychotic aripiprazole. Furthermore, we have investigated possible influences on serum corticosterone levels. In all experiments, comparisons were made between wild-type mice (5-HT7+/+) and mice lacking the 5-HT7 receptor (5-HT7−/−) in order to test the hypothesis that this receptor is required for the observed interactions.
2. Materials and methods
2.1. Animals
Ten-to-twelve week old male 5-HT7−/− mice and their male 5-HT7+/+ sibling controls were used. The generation of the 5-HT7−/− mouse strain has been described previously [46]. The mice used in this study had been back-crossed on a C57BL/6J background for at least 16 generations. As the forced swim and tail suspension tests involve learning, the same animal was never used twice for the same behavioral method. A larger number of mice were exposed to the tail suspension test than the forced swim test. Thus, all mice tested in the forced swim test were also used for the tail suspension test, but not vice versa. A minimum of one week separated the two tests. A separate cohort of mice was used for evaluation of locomotor activity. All behavioral experiments were started at 09.00 h. The mice were housed in a 12-hour light/dark cycle (lights on at 06.00 and off at 18.00) and had free access to water and food pellets. All the experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health, and were approved by the Animal Care and Use Committee at The Scripps Research Institute. Every effort was made to reduce the number of animals used and to minimize potential suffering.
2.2. Tail suspension test
The tail suspension test was performed as previously described [12]. Briefly, mice were suspended from a metal rod mounted 50 cm above the surface by fastening the tail to the rod with adhesive tape. The duration of the test was 6 minutes and immobility was measured during the last 4 minutes to facilitate comparison with the forced swim test. Immobility was defined as the absence of any limb or body movements, except those caused by respiration.
2.3. Forced swim test
The forced swim test was performed as previously described [12]. Briefly, mice were gently placed in a clear plastic cylinder, diameter of 16 cm, height 25 cm, filled with 10 cm of clear water at 25°C. Test duration was 6 minutes and immobility was measured during the last 4 minutes. Immobility was defined as the absence of any horizontal or vertical movement in the water, but excluded minor movements required for the mouse to keep its head above the surface. The water was replaced before each animal.
2.4. Locomotor activity
Locomotor activity was measured in Plexiglas cages (42 × 22 × 20 cm) placed into frames (25.5 × 47 cm) mounted with two levels of photocell beams at 2 and 7 cm above the bottom of the cage (San Diego Instruments, San Diego, CA, USA). The two sets of beams allowed for the recording of both horizontal (locomotion) and vertical (rearing) behavior. A thin layer of bedding material was applied to the bottom of the cage. Mice were habituated to the activity boxes during three once daily 30-min sessions. The mice were then tested for drug for possible drug effects. For each treatment evaluated a mouse first had a 15-min session in the activity box followed by the injection. The mouse was then tested again 30 min later for 15 min.
2.5. Serum corticosterone
Blood was collected in heparinized capillaries through eye bleeds. Approximately 150 µl was collected from each animal sampled. The blood was transferred to 1.5 ml plastic tubes and allowed to coagulate. The samples were then centrifuged at 3000 rpm for 15 min. The serum was transferred to a new tube and stored at −80°C until analyzed. Serum corticosterone was measured using a radio-immuno assay kit following the instructions provided by the manufacturer (MP Biomedicals, Solon, OH).
2.6. Drug treatments
For the forced swim test, tail suspension test, and locomotor activity test, single intra-peritoneal injections were given 30 minutes prior to the test. Citalopram, desipramine, and WAY 100135 were obtained from Sigma (St. Louis, MO). GBR 12909 and SB-269970 were purchased from Tocris (Ellisville, MO). Reboxetine and aripiprazole was bought from Toronto Research Chemicals (North York, ON). All drugs were dissolved in 0.9% NaCl and administered in the doses indicated in a total volume of 8 ml/kg. Although long-term treatment with drugs like citalopram is required in the clinic to obtain therapeutic effect, acute single injections are sufficient when using animal models [7]. The vehicle 0.9% NaCl alone was used as control.
2.7. Data analysis
All values are expressed as means ± standard errors of the mean (S.E.M.). Possible differences between genotypes and/or drug treatments were analyzed using Student’s t-test or two-way analysis of variance (ANOVA) with genotype as one factor and drug treatment as the other factor. The ANOVA was followed by an appropriate Bonferroni posttest. All analyzes were performed using the GraphPad Prism (http://www.graphpad.com) software package. Differences were considered significant at P < 0.05.
3. Results
3.1. Tail suspension test and forced swim test
As previously reported [12], 5-HT7−/− mice showed lower immobility than 5-HT7+/+ mice in both the tail suspension test and the forced swim test when given vehicle only.
3.1.1. Serotonin reuptake inhibition
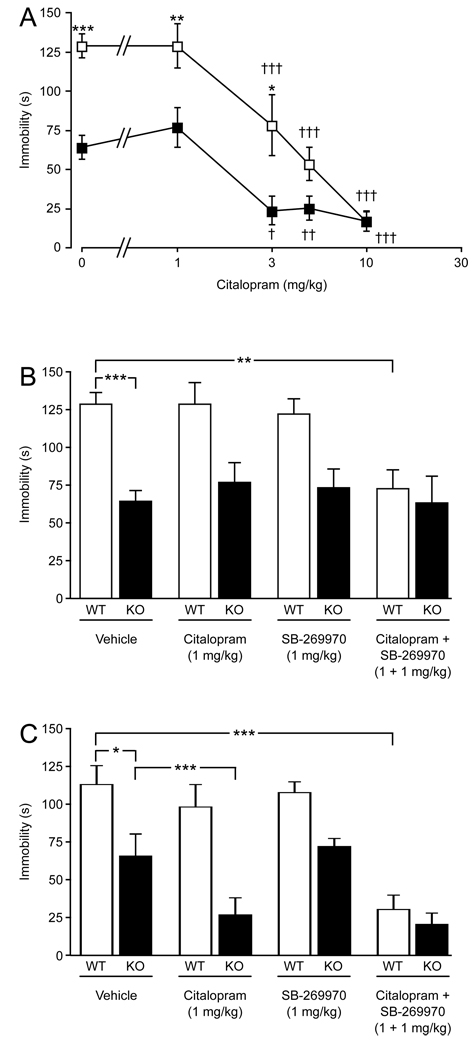
Citalopram dose-dependently reduced immobility in 5-HT7+/+ mice (Fig. 1A) in the tail suspension test. Citalopram also reduced immobility in 5-HT7−/− mice. A two-way ANOVA revealed significant effects for treatment (F(4, 58) = 35.56, P < 0.001), genotype (F(1, 58) = 40.80, P < 0.001), and interaction (F(4, 58) = 4.66, P < 0.05). A maximal effect (reduction) for the doses tested of approximately 20 s of immobility was reached at 10 mg/kg for 5-HT7+/+ mice and at 3 mg/kg for 5-HT7−/− mice. When an ineffective dose of citalopram (1 mg/kg) was combined with an ineffective dose of SB-269970 (1 mg/kg), immobility was reduced in 5-HT7+/+ mice (Finteraction(3, 34) = 3.48, P < 0.05; Fig. 1B). The dose of SB-269970 was chosen based on initial testing and earlier demonstrations that doses of 3 mg/kg and higher reduce immobility in wild-type mice [12,21]. This combination had no additional effect in 5-HT7−/− mice. A similar synergistic interaction between citalopram and SB-269970 was observed for 5-HT7+/+ mice in the forced swim test (Finteraction(3, 64) = 8.41, P < 0.001; Fig. 1C). Interestingly, in this test, the dose 1 mg/kg of citalopram had an effect of its own in 5-HT7−/− mice (Fig. 1C).
Fig. 1.
Effects of citalopram on mouse behavior in the tail suspension and forced swim tests. (A) Dose-response effect of citalopram on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the tail suspension test. (B) Effects of individual and concurrent injections of 1 mg/kg citalopram and 1mg/kg SB-269970 in the tail suspension test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. (C) Effects of individual and concurrent injections of 1 mg/kg citalopram and 1 mg/kg SB-269970 in the forced swim test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. Values are mean ± SEM. n = 8–10 animals per genotype per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001 between the genotypes (A) or indicated groups (B, C); †P < 0.05, ††P < 0.01, †††P < 0.001 within a genotype compared to control; two-way ANOVA followed by Bonferroni's post-hoc test.
3.1.2. Norepinephrine reuptake inhibition
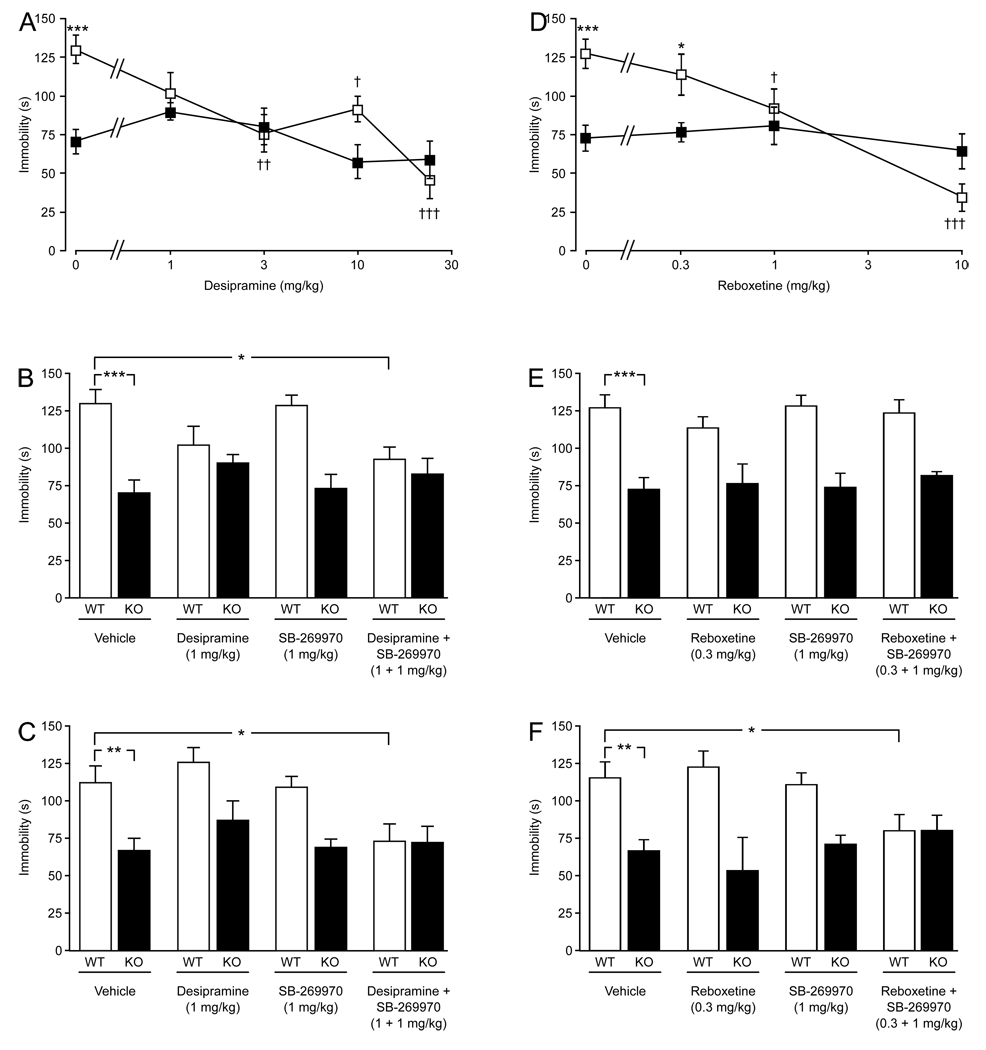
Desipramine dose-dependently reduced immobility in the tail suspension test for 5-HT7+/+ mice (Fig. 2A). For the doses tested desipramine did not further affect immobility in 5-HT7−/− mice. A two-way ANOVA showed significant effects for treatment (F(4, 71) = 7.75, P < 0.001), genotype (F(1, 71) = 7.21, P < 0.01), and interaction (F(4, 71) = 4.05, P < 0.01). When an ineffective dose of desipramine (1 mg/kg) was combined with an ineffective dose of SB-269970 (1 mg/kg), immobility was reduced in 5-HT7+/+ mice (Finteraction(3, 70) = 4.02, P < 0.05; Fig. 2B). Such an effect was also seen in the forced swim test where the combination of desipramine (1 mg/kg) and SB-269970 (1 mg/kg) reduced immobility in the 5-HT7+/+ mice (Finteraction(3, 56) = 3.00, P < 0.05; Fig. 2C). The dose 1 mg/kg of desipramine had no effect on immobility in the 5-HT7−/− mice.
Fig. 2.
Effects of desipramine and reboxetine on mouse behavior in the tail suspension and forced swim tests. (A) Dose-response effect of desipramine on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the tail suspension test. (B) Effects of individual and concurrent injections of 1 mg/kg desipramine and 1mg/kg SB-269970 in the tail suspension test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. (C) Effects of individual and concurrent injections of 1 mg/kg desipramine and 1mg/kg SB-269970 in the forced swim test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. (D) Dose-response effect of reboxetine on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the tail suspension test. (E) Effects of individual and concurrent injections of 0.3 mg/kg reboxetine and 1mg/kg SB-269970 in the tail suspension test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. (F) Effects of individual and concurrent injections of 0.3 mg/kg reboxetine and 1mg/kg SB-269970 in the forced swim test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. Values are mean ± SEM. n = 8–10 animals per genotype per treatment group. *P < 0.05, **P < 0.01, ***P < 0.001 between the genotypes (A, D) or indicated groups (B, C, E, F); †P < 0.05, ††P < 0.01, †††P < 0.001 within a genotype compared to control; two-way ANOVA followed by Bonferroni's post-hoc test.
In view of the results obtained with desipramine, especially its inability to reduce immobility in the 5-HT7−/− mice, the norepinephrine reuptake inhibitor reboxetine was also evaluated. The results for reboxetine were similar to those obtained with desipramine. Thus, reboxetine also dose-dependently reduced immobility in the tail suspension test for 5-HT7+/+ mice (Fig. 2D), but the doses tested did not further affect immobility in 5-HT7−/− mice. A two-way ANOVA showed significant effects for treatment (F(3, 49) = 10.09, P < 0.001), genotype (F(1, 49) = 6.39, P < 0.05), and interaction (F(3, 49) = 6.68, P < 0.001). When an ineffective dose of reboxetine (0.3 mg/kg) was combined with an ineffective dose of SB-269970 (1 mg/kg), immobility was not altered in the tail suspension test in any of the genotypes (Finteraction(3, 56) = 0.52, P = 0.67; Fig. 2E). However, in the forced swim test an interaction was observed where the combination of reboxetine (0.3 mg/kg) and SB-269970 (1 mg/kg) reduced immobility in the 5-HT7+/+ mice (Finteraction(3, 54) = 2.99, P < 0.05; Fig. 2F).
3.1.3. Dopamine reuptake inhibition
GBR 12909 dose-dependently reduced immobility in 5-HT7+/+ mice (Fig. 3A) in the tail suspension test. GBR 12909 also reduced immobility in 5-HT7−/− mice. A two-way ANOVA revealed significant effects for treatment (F(3, 54) = 46.76, P < 0.001), genotype (F(1, 54) = 16.25, P < 0.001), and interaction (F(3, 54) = 6.38, P < 0.001). A maximal effect (reduction) for the doses tested of approximately 15 s of immobility was reached at 10 mg/kg for both genotypes. The dose 1 mg/kg of GBR 12909 had no effect on immobility in either genotype. Interestingly, an intermediate dose (3 mg/kg) reduced immobility in 5-HT7+/+ mice, but had no effect in 5-HT7−/− mice (Fig. 3A). Combining the dose 1 mg/kg of GBR 12909 with 1 mg/kg of SB-269970 did not influence immobility (Finteraction(3, 59) = 1.33, P = 0.27; Fig. 3B). Comparable results were obtained in the forced swim test where 10 mg/kg of GBR 12909 reduced immobility in both genotypes (37.0 ± 14.1 s immobility, n = 9, for 5-HT7+/+ and 9.3 ± 6.2 s, n = 6 for 5-HT7−/−), but 1 mg/kg did not and there was no interaction with SB-269970 (Finteraction(3, 74) = 0.24, P = 0.87; Fig. 3C).
Fig. 3.
Effects of GBR 12909 on mouse behavior in the tail suspension and forced swim tests. (A) Dose-response effect of GBR 12909 on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the tail suspension test. (B) Effects of individual and concurrent injections of 1 mg/kg GBR 12909 and 1mg/kg SB-269970 in the tail suspension test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. (C) Effects of individual and concurrent injections of 1 mg/kg GBR 12909 and 1mg/kg SB-269970 in the forced swim test in 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. Values are mean ± SEM. n = 8–10 animals per genotype per treatment group. **P < 0.01, ***P < 0.001 between the genotypes (A) or indicated groups (B, C); †††P < 0.001 within a genotype compared to control; two-way ANOVA followed by Bonferroni's post-hoc test.
3.1.4. 5-HT1A receptor inhibition
WAY 100135 (10 mg/kg) did not influence immobility in the tail suspension test for 5-HT7+/+ or 5-HT7−/− mice. The dose of WAY 100135 was chosen based on its ability to inhibit 8-OH-DPAT induced hypothermia [39]. As expected, there was an effect for genotype (F(1,1) = 30.12, P < 0.001), but no effect for treatment (F(1, 1) = 0.31, P = 0.58). The observed immobility values (s) were 127.70 ± 7.81 (5-HT7+/+, vehicle, n = 10), 75.60 ± 5.16 (5-HT7−/−, vehicle, n = 10), 114.00 ± 12.72 (5-HT7+/+, WAY 100135, n = 8), and 80.56 ± 4.45 (5-HT7−/−, WAY 100135, n = 9).
3.1.5. Aripiprazole
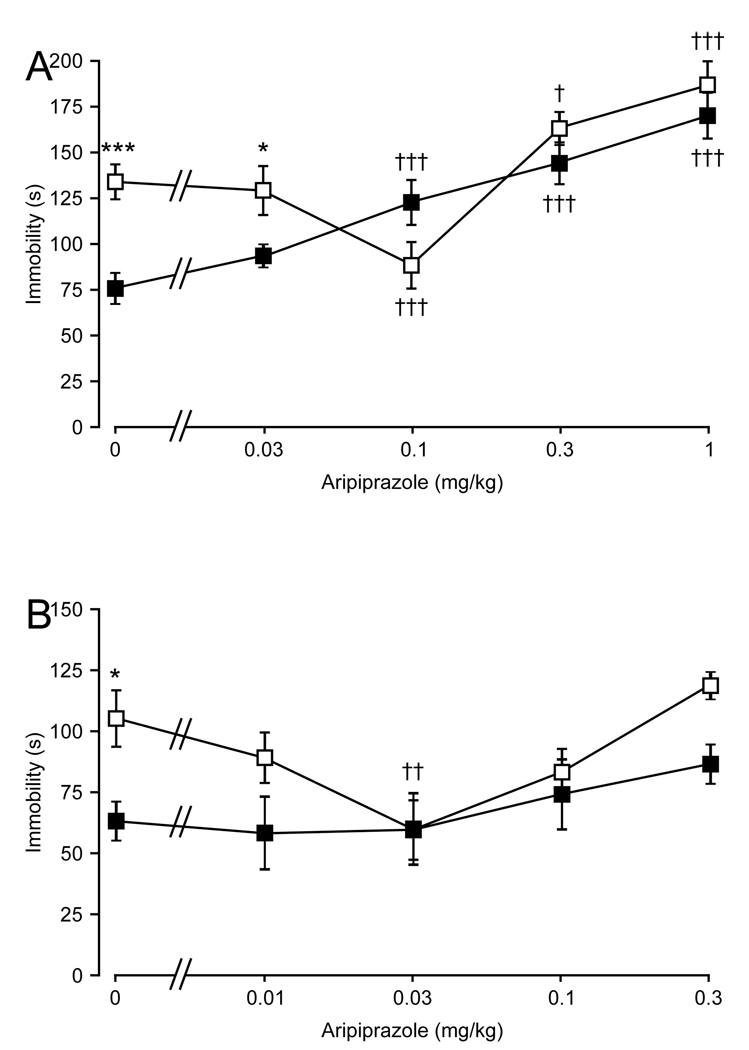
In 5-HT7+/+ mice aripiprazole showed a U-shaped dose-response curve (Fig. 4). In the tail suspension test immobility was reduced at 0.1 mg/kg, but then increased at higher doses to reach durations longer than for vehicle (Fig. 4A). In the forced swim test immobility was reduced at 0.03 mg/kg (Fig. 4B). In 5-HT7−/− mice aripiprazole dose-dependently increased immobility in the tail suspension test (Fig. 4A). Within the doses tested aripiprazole did not alter immobility in the 5-HT7−/− mice in the forced swim test (Fig. 4B). The two-way ANOVA values were for the tail suspension test for treatment (F(4, 58) = 27.53, P < 0.001), genotype (F(1, 58) = 11.17, P < 0.01), and interaction (F(4, 58) = 7.86, P < 0.001), and for the forced swim test for treatment (F(4, 69) = 3.51, P < 0.05), genotype (F(1, 69) = 9.8, P < 0.01), and interaction (F(4, 69) = 1.32, P = 0.27).
Fig. 4.
Effects of aripiprazole on mouse behavior in the tail suspension and forced swim tests. (A) Dose-response effect of aripiprazole on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the tail suspension test. (B) Dose-response effect of aripiprazole on the immobility profile of 5-HT7+/+ (□) and 5-HT7−/− (■) mice in the forced swim. Values are mean ± SEM. n = 8–10 animals per genotype per treatment group. *P < 0.05, ***P < 0.001 between the genotypes; †P < 0.05, ††P < 0.01, †††P < 0.001 within a genotype compared to control; two-way ANOVA followed by Bonferroni's post-hoc test.
3.2. Locomotor activity
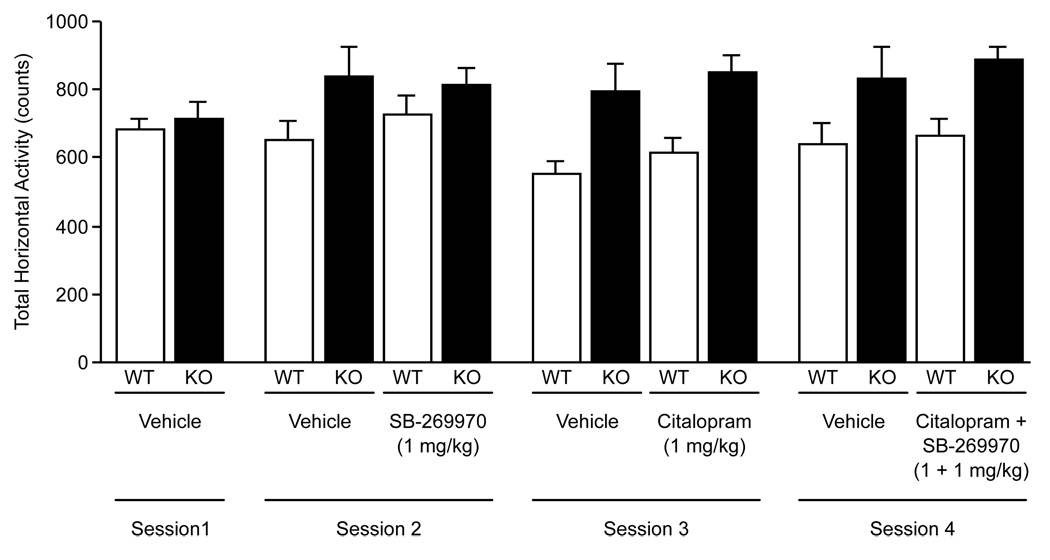
Citalopram, SB-269970, and their combination were assessed for any possible effect on locomotor activity as such an effect could influence behavior in the tail suspension or forced swim tests. The 1 mg/kg doses of citalopram or SB-269970 or their combination did not affect horizontal locomotor activity (Fig. 5). Rearing was also not affected (data not shown). As the limited availability of 5-HT7−/− mice required that the same animals were tested for all the different treatments it was noted that repeated testing increased locomotor activity in the 5-HT7−/− mice but not the 5-HT7+/+ (Fig. 5). The effect was however identical for vehicle treated animals and thus not a drug effect. The two-way ANOVA effects for genotype were F(1, 36) = 21.23, P < 0.001 for citalopram, F(1, 36) = 4.65, P < 0.05 for SB-269970, and F(1, 36) = 10.48, P < 0.01 for citalopram + SB-269970.
Fig. 5.
Effects of citalopram and SB-269970 on mouse locomotor activity. Mice were habituated to the test apparatus for three sessions (not shown). They were then tested in four separate sessions as indicated. In the first session all mice received vehicle. In the subsequent sessions half of the mice were randomly assigned to receive vehicle and the other half drug. Comparisons were made between 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice. There were no treatment-induced effects, but 5-HT7−/− mice increased their activity with repeated testing, see text for details. Values are mean ± SEM. n = 10 (20 in session 1) animals per genotype per treatment group.
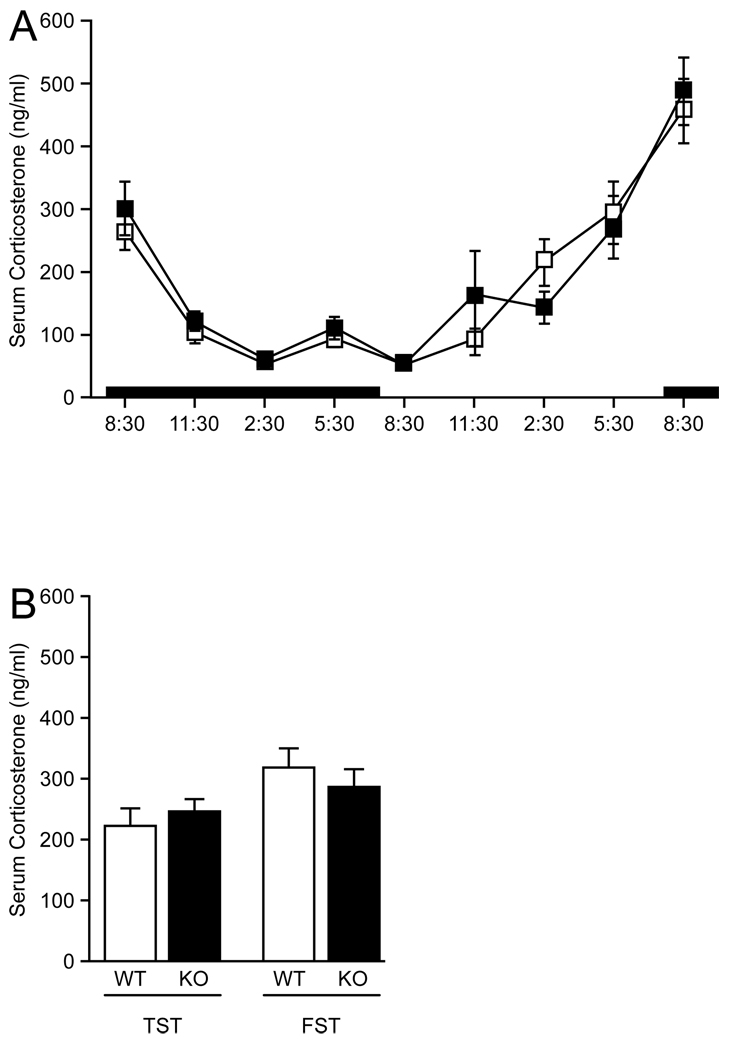
3.3 Serum corticosterone
Serum levels of corticosterone showed an expected circadian pattern in both 5-HT7+/+ and 5-HT7−/− mice that had not been exposed to any behavioral tests (Fig. 4A). Serum corticosterone was also measured in vehicle-treated mice exposed to the tail suspension test and the forced swim test (Fig. 4B). These measurements were taken at approximately 10.30 am during the light cycle. Thus, there appears to be an increase in serum corticosterone due to the stress of the behavioral tests, but this was not evaluated statistically as the aim was to determine any difference between the genotypes. As no such differences were observed, serum corticosterone levels were not studied further.
4. Discussion
The major finding of the present study was that although drugs representing various classes of antidepressants seem to act independently of the 5-HT7 receptor in reducing immobility in the tail suspension and forced swim tests, the interaction experiments clearly show a synergistic effect between certain monoamine uptake inhibitors, but not others, and a 5-HT7 receptor antagonist. Renewed evidence that atypical antipsychotics with high affinity for the 5-HT7 receptor mediate their clinically established antidepressant effect through the 5-HT7 receptor is also presented.
Citalopram represents the selective serotonin reuptake inhibitors and is widely used to treat depression [25]. It has been shown to reduce immobility in the forced swim test [47] and the tail suspension test [48]. At least in the tail suspension test the effect is dose-dependent [48]. Thus, the present results confirm and extend previous findings by showing a dose-dependent effect of citalopram in reducing immobility and that the effect is present in both 5-HT7+/+ and 5-HT7−/− mice. We have previously demonstrated that the selective 5-HT7 receptor antagonist SB-269970 reduces immobility in these test [12]. This has been confirmed for the tail suspension test by others [21]. The present results revealed a synergistic interaction between citalopram and SB-269970 when both compounds were given in ineffective doses in 5-HT7+/+ mice. The data also showed that the interaction affects both the tail suspension test and the forced swim test. The specificity of the synergism to 5-HT7 receptors and their requirement for this interaction was confirmed by the lack of such an effect in 5-HT7−/− mice. A synergistic interaction between citalopram and SB-269970 has previously been demonstrated for the forced swim test [22] and the tail suspension test [21]. The first group also showed similar synergism with imipramine (serotonin and norepinephrine reuptake inhibitor) where an ineffective dose of imipramine concurrently administered with SB-269970 resulted in significant reduction in immobility in the forced swim test.
Using microdialysis, attempts have been made to find correlates between observed behavioral changes in response to antidepressants and possible changes in brain 5-HT concentrations [21,23,49]. A straight forward interpretation of the results has proved to be difficult, most likely due to different experimental designs. One study showed no effect of SB-269970 on 5-HT concentration in the frontal cortex [21]. Another study showed increases in 5-HT concentration in response to SB-269970 treatment in the prefrontal cortex, but this study was done already 24 h after surgery [23]. One possible explanation for the differing results is different stress levels at different post-surgical intervals. It has been shown that 5-HT7 receptor mRNA expression is upregulated in response to stress [50]. Possibly contradicting results were obtained in a study showing that a selective 5-HT7 receptor antagonist, SB-258741, could inhibit the increase in 5-HT levels induced by citalopram in the ventral hippocampus, but only if 5-HT1A receptors were simultaneously blocked by WAY 100635 [49].
Desipramine represents a different class of anti-depressants in two ways. It belongs to the so called tricyclic antidepressants and it acts mainly as a norepinephrine reuptake inhibitor [51]. In contrast to citalopram, desipramine only reduced immobility in 5-HT7+/+ mice even at higher doses. The highest dose of desipramine used in the current study has previously been shown to induce a maximal reduction in immobility in C57BL/6J mice [48,52]. A difference in action between citalopram and desipramine in 5-HT7−/− mice has previously been described in a sleep study [53]. This study showed that citalopram had a greater ability than desipramine to suppress rapid eye movement sleep in these mice. Nevertheless, a similar interaction as with citalopram was seen with ineffective doses of desipramine in the tail suspension test and the forced swim test. Again, that 5-HT7 receptors are required for this interaction was confirmed by the lack of such an effect in 5-HT7−/− mice. A synergistic interaction between desipramine and SB-269970 has previously been demonstrated for the forced swim test [22].
To verify and extend the results obtained with desipramine we also tested the selective norepinephrine reuptake inhibitor reboxetine, a clinically used antidepressant [54]. Reboxetine has been shown to reduce immobility in the tail suspension and forced swim tests including in mice on a C57BL/6J background [54,55]. The results were very similar compared with desipramine since both drugs reduced immobility in wild-type mice but not in 5-HT7−/− mice in the tail suspension test. A weak or absent interaction with SB-269970 was seen in the tail suspension test, whereas a more distinct interaction was observed for both drugs in the forced swim test. Thus, although both serotonin and norepinephrine reuptake are modulated by the 5-HT7 receptor there appears to be differences in how the different classes of drugs interact.
GBR 12909 represents a selective dopamine reuptake inhibitor. It has several hundred times higher affinity for the dopamine transporter than for the 5-HT transporter and no affinity for the norepinephrine transporter [56]. Thus, GBR 12909 should not affect synaptic 5-HT levels, at least not in animals with intact 5-HT transporters [57]. GBR 12909 has recently been shown to reduce immobility in both the tail suspension test and the forced swim test, but it is possible that this, at least in part, is due to an increase in overall locomotor activity [32]. The possible impact of changes in locomotor activity is, however, reduced by the fact that there is no difference in ambulatory or rearing activity over a 24-h period between 5-HT7+/+ and 5-HT7−/− mice [58]. The present data confirm the previous findings and show that the effects on immobility are dose-dependent. GBR 12909 (10 mg/kg) had an effect in both 5-HT7+/+ and 5-HT7−/− mice. Interestingly, however, the effect was not additive in 5-HT7−/− mice as 3 mg/kg of GBR 12909 reduced immobility in 5-HT7+/+ mice but had no additional effect in 5-HT7−/− mice. It is noteworthy that GBR 12909 did not interact with SB-269970 to modulate the immobility response in either test. Thus, even though to our knowledge the affinity of GBR 12909 for the 5-HT7 receptor is not known, the most probable interpretation of the present results is that GBR 12909 acts independently of the 5-HT7 receptor, and that as yet undescribed mechanisms in the 5-HT7−/− mice are responsible for the dose related differences seen between the genotypes. Even though there is evidence supporting the hypothesis that dopamine is involved in depression, the recent finding that the antidepressant effect of amisulpride is most likely mediated by the 5-HT7 receptor [35] might suggest the need for a reevaluation of some studies, especially those using antipsychotics with high affinity for the 5-HT7 receptor [59].
In a recent study it was found that the atypical antipsychotic amisulpride had high affinity for the 5-HT7 receptor and that it was able to reduce immobility in both the tail suspension and forced swim tests in 5-HT7+/+, but not 5-HT7−/− mice [35]. These findings provided the first rational explanation for the antidepressant properties of amisulpride [60–62]. Aripiprazole is another atypical antipsychotic with high affinity for the 5-HT7 receptor [63]. Aripiprazole is approved as augmentation therapy in depression [37,64]. Interestingly it was recently shown that aripiprazole potentiates the effect of fluoxetine in the mouse tail suspension test, an effect the authors were not able to fully explain [38]. With the present findings that aripiprazole reduced immobility in both the tail suspension and forced swim tests in 5-HT7+/+ mice but not in 5-HT7−/− mice it appears that also the antidepressant properties of aripiprazole are mediated by the 5-HT7 receptor.
To fully understand the mechanisms regulating the degree of immobility in the tail suspension test and the forced swim test has proven to be difficult. Recent studies have shown that there are pronounced strain differences in the behavioral response [52,65]. One notable finding is that C57BL/6J mice, the background strain for the 5-HT7−/− mice, generally have a high baseline immobility and do not respond to certain selective serotonin reuptake inhibitors (e.g. fluoxetine) [65]. There is a linear correlation between the amount of 5-HT transporter binding and immobility, but no such correlation exists for the norepinephrine transporter [52]. Interesting differences in response are seen also in the present study. Citalopram and GBR 12909 dose-dependently reduced immobility in the tail suspension test in both 5-HT7+/+ and 5-HT7−/− mice. In contrast, desipramine and reboxetine failed to further reduce immobility in the 5-HT7−/− mice. Citalopram, desipramine and reboxetine, but not GBR 12909, interacted synergistically with the 5-HT7 receptor antagonist SB-269970. Especially the interaction between citalopram and SB-269970 had an interesting profile that it might be possible to exploit therapeutically. With the norepinephrine reuptake inhibitors the interaction was most prominent in the forced swim test, whereas it was weak or absent in the tail suspension test. It should be noted that combining citalopram and SB-269970 synergistically increased prefrontal cortex levels of 5-HT [21], whereas the combination of imipramine and SB-269970 did not [23]. It even appears that blockade of the 5-HT7 receptor might lead to decreased 5-HT levels [49]. Nevertheless, an explanation based on a mechanism resulting in increased synaptic 5-HT (and possibly norepinephrine) levels for the enhanced antidepressant-like responses observed by combining antidepressants with 5-HT7 receptor blockade remains closest at hand. Inhibition of 5-HT7 receptors located on axon terminals might cause such an effect [21,66,67].
One factor that can influence the results in the tail suspension and the forced swim tests is changes in locomotor activity induced by drugs or genetic manipulations. We verified that the interactions seen between low doses of citalopram and SB-269970 were not due to changes in locomotion. This is in agreement with other studies [21,22]. Nevertheless, it was of importance to exclude this possibility since higher doses of citalopram has been shown to increase locomotor activity [68,69]. SB-269970 has been found not to affect locomotion [13]. Due to the increased activity seen in the 5-HT7−/− mice with repeated testing we did not test any of the other drugs used in this study. The cause and implications of this effect needs further more detailed studies as a previous 24 hour comparison between 5-HT7+/+ and 5-HT7−/− mice did no reveal any differences in locomotor activity [58]. It should be noted that desipramine has been found to reduce locomotor activity [69] and that reboxetine does not affect locomotion [70,69]. Thus, the observations made in the present study for these drugs are likely not due to changes in locomotion. On the other hand, the effects seen with GBR 12909 might be due to increased locomotor activity induced by this compound [71].
Previous studies [11,12] and the present data show a strong correlation between effects seen in the tail suspension test and the forced swim test in 5-HT7−/− mice and by using a selective 5-HT7 receptor antagonists. Thus, one can be highly confident that the findings in 5-HT7−/− mice are due to the lack of 5-HT7 receptors. With the 5-HT1A receptor the situation is different. Mice lacking the 5-HT1A receptor exhibit reduced immobility in the forced swim test [40]. Interestingly, agonists for the 5-HT1A receptor, where 8-OH-DPAT is the most commonly used, also reduce immobility, an effect that can be blocked by relatively selective antagonists [72]. Selective antagonists for the 5-HT1A receptor by themselves do not influence immobility [41]. It has also been shown that there is no interaction between the 5-HT1A receptor and antidepressants in the forced swim test [41]. Although less studied, the results are similar for the 5-HT1A receptor and the tail suspension test [73]. Even though 8-OH-DPAT is also an agonist for 5-HT7 receptors it is unlikely that its ability to reduce immobility is mediated by this receptor since inactivation or blockade reduces immobility. In the present study, using the 5-HT1A receptor antagonist/partial agonist WAY 100135, no indication was found for a direct effect on immobility in the tail suspension test nor an interaction with 5-HT7 receptors.
Glucocorticoids are believed to play a major role in depression [25]. Several studies have also shown that the 5-HT7 receptor is involved in the central regulation of glucocorticoids. Serotonin-mediated upregulation of glucocorticoid receptors is mediated by 5-HT7 receptors [74]. Furthermore, both adrenalectomy and restraint stress has been shown to upregulate 5-HT7 receptor mRNA expression in the hippocampus [45,50]. Thus, we hypothesized that glucocorticoid homeostasis might be altered in 5-HT7−/− mice. However, the present data show that serum corticosterone levels were not altered in 5-HT7−/− mice, and that the serum levels had a normal circadian pattern. Serum corticosterone levels were also not differentially regulated in 5-HT7+/+ and 5-HT7−/− mice following the tail suspension test or the forced swim test.
The present findings support the hypothesis that the 5-HT7 receptor is a new target for disorders treated with antidepressants. Possibly a 5-HT7 receptor antagonist would be beneficial by itself as inactivation or blockade of the 5-HT7 receptor in itself is sufficient to induce antidepressant-like behavior. Alternatively, the combination of a 5-HT7 receptor antagonist with an antidepressant might prove to be a treatment with improved efficacy and reduced side-effects as lower doses most likely could be used.
Fig. 6.
(A) Corticosterone levels measured in blood serum. Expected corticosterone concentrations changes were observed during a 24-hour cycle. There were no differences between 5-HT7+/+ (□) and 5-HT7−/− (■) mice. The black bar represents the dark period of the day. (B) Serum corticosterone levels after the tail suspension test (TST) and the forced swim test (FST). These measurements were made at approximately 10:30 during the light phase and thus appear to be elevated as a result of the behavioral tests performed although this was not statistically evaluated as the objective was to determine possible changes between the genotypes. There were no differences in serum corticosterone levels between 5-HT7+/+ (WT) and 5-HT7−/− (KO) mice following either test. Values are mean ± SEM. n = 8 animals per genotype for the time-course data; n = 6 per genotype for the tail suspension test; n = 8 per genotype for the forced swim test.
Acknowledgments
This work was supported by National Institutes of Health grants MH73923 and GM32355, and by NARSAD. We thank Patria Danielson and Jeanette Helfers for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 2.Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- 3.Meyerhof W, Obermüller F, Fehr S, Richter D. A novel rat serotonin receptor: primary structure, pharmacology, and expression pattern in distinct brain regions. DNA Cell Biol. 1993;12:401–409. doi: 10.1089/dna.1993.12.401. [DOI] [PubMed] [Google Scholar]
- 4.Plassat JL, Amlaiky N, Hen R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- 5.Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, et al. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Y, Monsma FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- 7.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 9.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 10.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 11.Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, et al. Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48:492–502. doi: 10.1016/j.neuropharm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT(7) receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DR, Atkinson PJ, Ho M, Bromidge SM, Lovell PJ, Villani AJ, et al. a[3H]-SB-269970 - A selective antagonist radioligand for 5-HT7 receptors. Br J Pharmacol. 2000;130:409–417. doi: 10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, et al. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl.) 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millan MJ. Multi-target strategies for the improved treatment of depressive states: Conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther. 2006;110:135–370. doi: 10.1016/j.pharmthera.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Mnie-Filali O, Lambas-Señas L, Scarna H, Haddjeri N. Therapeutic potential of 5-HT(7) receptors in mood disorders. Curr Drug Targets. 2009;10:1109–1117. doi: 10.2174/138945009789735129. [DOI] [PubMed] [Google Scholar]
- 19.Mnie-Filali O, Lambás-Señas L, Zimmer L, Haddjeri N. 5-HT7 receptor antagonists as a new class of antidepressants. Drug News Perspect. 2007;20:613–618. doi: 10.1358/dnp.2007.20.10.1181354. [DOI] [PubMed] [Google Scholar]
- 20.Mullins UL, Gianutsos G, Eison AS. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- 21.Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007;321:690–698. doi: 10.1124/jpet.107.119404. [DOI] [PubMed] [Google Scholar]
- 22.Wesolowska A, Tatarczynska E, Nikiforuk A, Chojnacka-Wojcik E. Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur J Pharmacol. 2007;555:43–47. doi: 10.1016/j.ejphar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Wesolowska A, Kowalska M. Influence of serotonin 5-HT7 receptor blokade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol Rep. 2008;60:464–474. [PubMed] [Google Scholar]
- 24.Wesolowska A, Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol. 2006;553:185–190. doi: 10.1016/j.ejphar.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 25.Belmaker R, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 26.Invernizzi R, Bramante M, Samanin R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995;696:62–66. doi: 10.1016/0006-8993(95)00730-e. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Skolnick P. Triple uptake inhibitors: therapeutic potential in depression and beyond. Expert Opin Investig Drugs. 2007;16:1365–1377. doi: 10.1517/13543784.16.9.1365. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon S, Yang LPH, Curran MP. Spotlight on bupropion in major depressive disorder. CNS Drugs. 2008;22:613–617. doi: 10.2165/00023210-200822070-00006. [DOI] [PubMed] [Google Scholar]
- 29.Dailly E, Chenu F, Renard CE, Bourin M. Dopamine, depression and antidepressants. Fundam Clin Pharmacol. 2004;18:601–7. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 30.Papakostas GI. Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol. 2006;16:391–402. doi: 10.1016/j.euroneuro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, et al. Antidepressant-like effect of D2//3 receptor-, but not D4 receptor-activation in the rat forced swim test. Neuropsychopharmacology. 2005;30:1257–1268. doi: 10.1038/sj.npp.1300677. [DOI] [PubMed] [Google Scholar]
- 32.Smolders I, Clinckers R, Meurs A, De Bundel D, Portelli J, Ebinger G, et al. Direct enhancement of hippocampal dopamine or serotonin levels as a pharmacodynamic measure of combined antidepressant-anticonvulsant action. Neuropharmacology. 2008;54:1017–1028. doi: 10.1016/j.neuropharm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery SA. Dopaminergic deficit and the role of amisulpride in the treatment of mood disorders. Int Clin Psychopharmacol. 2002;17:S9–S15. [PubMed] [Google Scholar]
- 34.Racagni G, Canonico PL, Ravizza L, Pani L, Amore M. Consensus on the use of substituted benzamides in psychiatric patients. Neuropsychobiology. 2004;50:134–43. doi: 10.1159/000079104. [DOI] [PubMed] [Google Scholar]
- 35.Abbas A, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl.) 2009;205:119–128. doi: 10.1007/s00213-009-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu L, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–11. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 37.Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197–206. doi: 10.1017/s1092852900020216. [DOI] [PubMed] [Google Scholar]
- 38.Kamei J, Miyata S, Sunohara T, Kamei A, Shimada M, Ohsawa M. Potentiation of the antidepressant-like effect of fluoxetine by aripiprazole in the mouse tail suspension test. J Pharmacol Sci. 2008;108:381–384. doi: 10.1254/jphs.08201sc. [DOI] [PubMed] [Google Scholar]
- 39.Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser PC, Sanger DJ. 5-HT1A receptor antagonists neither potentiate nor inhibit the effects of fluoxetine and befloxatone in the forced swim test in rats. Eur J Pharmacol. 1999;372:127–134. doi: 10.1016/s0014-2999(99)00202-2. [DOI] [PubMed] [Google Scholar]
- 42.Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- 43.Seckl JR, Fink G. Antidepressants increase glucocorticoid and mineralocorticoid receptor mRNA expression in rat hippocampus in vivo. Neuroendocrinology. 1992;55:621–626. doi: 10.1159/000126180. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Li L, Tang S, Cao X, Li Z, Li W, et al. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behav Brain Res. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Yau JLW, Noble J, Widdowson J, Seckl JR. Impact of adrenalectomy on 5-HT6 and 5-HT7 receptor gene expression in the rat hippocampus. Mol Brain Res. 1997;45:182–186. doi: 10.1016/s0169-328x(97)00026-0. [DOI] [PubMed] [Google Scholar]
- 46.Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression Are they all alike? Psychopharmacology (Berl.) 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- 48.Ripoll N, David DJP, Dailly E, Hascoët M, Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 49.Bosker FJ, Folgering JHA, Gladkevich AV, Schmidt A, Hart MCGVD, Sprouse J, et al. Antagonism of 5-HT1A receptors uncovers an excitatory effect of SSRIs on 5-HT neuronal activity, an action probably mediated by 5-HT7 receptors. J Neurochem. 2009;108:1126–1135. doi: 10.1111/j.1471-4159.2008.05850.x. [DOI] [PubMed] [Google Scholar]
- 50.Yau JLW, Noble J, Seckl JR. Acute restraint stress increases 5-HT7 receptor mRNA expression in the rat hippocampus. Neurosci Lett. 2001;309:141–144. doi: 10.1016/s0304-3940(01)02054-7. [DOI] [PubMed] [Google Scholar]
- 51.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugimoto Y, Kajiwara Y, Hirano K, Yamada S, Tagawa N, Kobayashi Y, et al. Mouse strain differences in immobility and sensitivity to fluvoxamine and desipramine in the forced swimming test: Analysis of serotonin and noradrenaline transporter binding. Eur J Pharmacol. 2008;592:116–122. doi: 10.1016/j.ejphar.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Shelton J, Bonaventure P, Li X, Yun S, Lovenberg T, Dugovic C. 5-HT7 receptor deletion enhances REM sleep suppression induced by selective serotonin reuptake inhibitors, but not by direct stimulation of 5-HT1A receptor. Neuropharmacology. 2009;56:448–454. doi: 10.1016/j.neuropharm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Wong EH, Sonders MS, Amara SG, Tinholt PM, Piercey MF, Hoffmann WP, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- 55.Dziedzicka-Wasylewska M, Faron-Górecka A, Kuśmider M, Drozdowska E, Rogóz Z, Siwanowicz J, et al. Effect of antidepressant drugs in mice lacking the norepinephrine transporter. Neuropsychopharmacology. 2006;31:2424–2432. doi: 10.1038/sj.npp.1301064. [DOI] [PubMed] [Google Scholar]
- 56.Kula NS, Baldessarini RJ, Tarazi FI, Fisser R, Wang S, Trometer J, et al. [3H][beta]-CIT: a radioligand for dopamine transporters in rat brain tissue. Eur J Pharmacol. 1999;385:291–294. doi: 10.1016/s0014-2999(99)00695-0. [DOI] [PubMed] [Google Scholar]
- 57.Fox M, Jensen C, French H, Stein A, Huang S, Tolliver T, et al. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology (Berl.) 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts AJ, Krucker T, Levy CL, Slanina KA, Sutcliffe JG, Hedlund PB. Mice lacking 5-HT7 receptors show specific impairments in contextual learning. Eur J Neurosci. 2004;19:1913–1922. doi: 10.1111/j.1460-9568.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 59.Roth B, Craigo S, Choudhary M, Uluer A, Monsma FJ, Shen Y, et al. Binding of typical and atypical antipsychotic agents to 5- hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- 60.Lecrubier Y, Boyer P, Turjanski S, Rein W. Amisulpride versus imipramine placebo in dysthymia major depression. J Affect Disord. 1997;43:95–103. doi: 10.1016/s0165-0327(96)00103-6. [DOI] [PubMed] [Google Scholar]
- 61.Papp M, Wieronska J. Antidepressant-like activity of amisulpride in two animal models of depression. J Psychopharmacol. 2000;14:46–52. doi: 10.1177/026988110001400106. [DOI] [PubMed] [Google Scholar]
- 62.Smeraldi E. Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: A double-blind comparative study. J Affect Disord. 1998;48:47–56. doi: 10.1016/s0165-0327(97)00139-0. [DOI] [PubMed] [Google Scholar]
- 63.Davies MA, Sheffler DJ, Roth BL. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10:317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson JC, Pikalov A, Berman RM. Augmentation treatment in major depressive disorder: focus on aripiprazole. Neuropsychiatr Dis Treat. 2008;4:937–948. doi: 10.2147/ndt.s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl.) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 66.Harsing LG, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 heteroreceptor-mediated inhibition of [3H]serotonin release in raphe nuclei slices of the rat: evidence for a serotonergic-glutamatergic interaction. Neurochem Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- 67.Belenky MA, Pickard GE. Subcellular distribution of 5-HT1B and 5-HT7 receptors in the mouse suprachiasmatic nucleus. J Comp Neurol. 2001;432:371–388. doi: 10.1002/cne.1109. [DOI] [PubMed] [Google Scholar]
- 68.Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol Biochem Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 69.Weber M, Talmon S, Schulze I, Boeddinghaus C, Gross G, Schoemaker H, et al. Running wheel activity is sensitive to acute treatment with selective inhibitors for either serotonin or norepinephrine reuptake. Psychopharmacology (Berl) 2009;203:753–762. doi: 10.1007/s00213-008-1420-4. [DOI] [PubMed] [Google Scholar]
- 70.Szewczyk B, Poleszak E, Wlaz P, Wrobel A, Blicharska E, Cichy A, et al. The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:323–329. doi: 10.1016/j.pnpbp.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Davids E, Zhang K, Kula NS, Tarazi FI, Baldessarini RJ. Effects of Norepinephrine and Serotonin Transporter Inhibitors on Hyperactivity Induced by Neonatal 6-Hydroxydopamine Lesioning in Rats. J Pharmacol Exp Ther. 2002;301:1097–1102. doi: 10.1124/jpet.301.3.1097. [DOI] [PubMed] [Google Scholar]
- 72.Detke M, Wieland S, Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berl.) 1995;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- 73.Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptor mutant mice. J Pharmacol Exp Ther. 2001;298:1101–1107. [PubMed] [Google Scholar]
- 74.Laplante P, Diorio J, Meaney MJ. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Brain Res Dev Brain Res. 2002;139:199–203. doi: 10.1016/s0165-3806(02)00550-3. [DOI] [PubMed] [Google Scholar]