Abstract
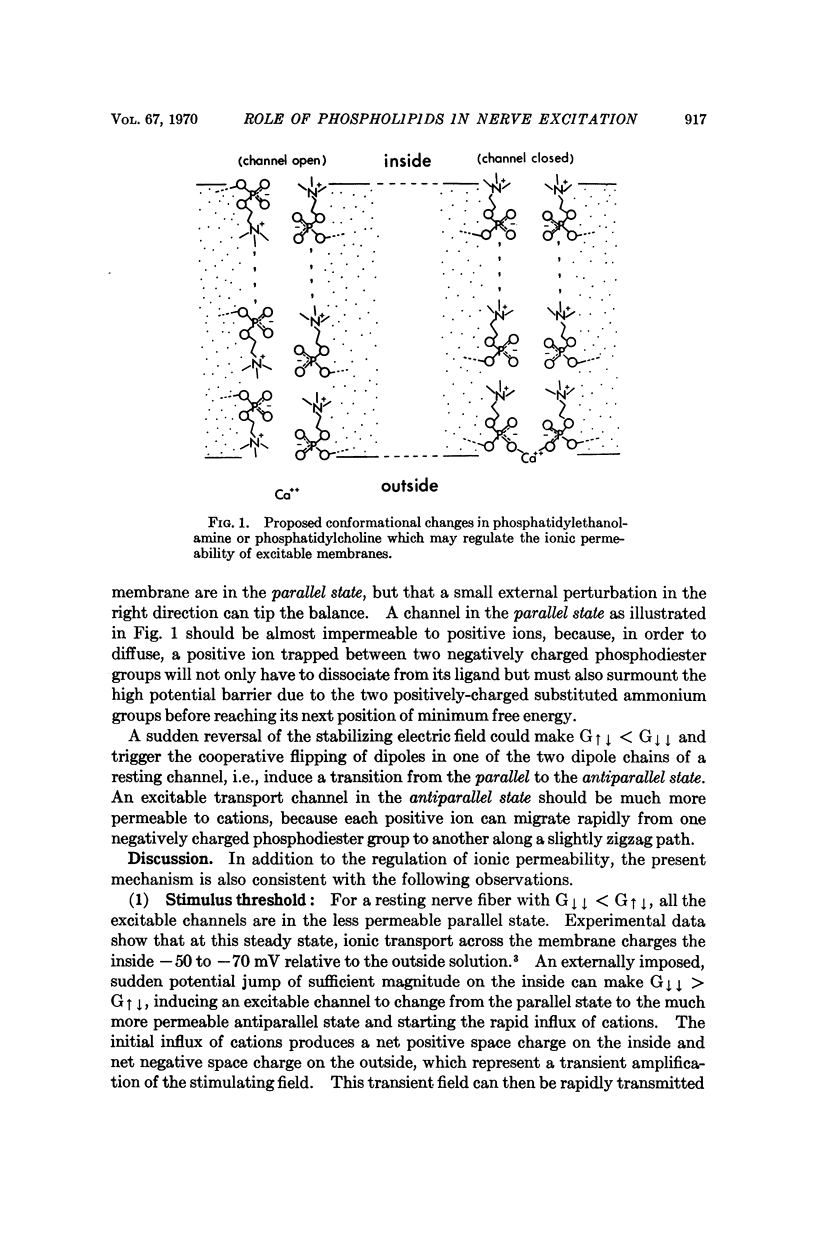
A possible role of phospholipid in regulating the ionic permeability of excitable membranes is proposed. It is assumed that each excitable transport channel in the nerve fiber is lined with two chains of phospholipid dipoles in either parallel or antiparallel directions; a molecular mechanism of nerve excitation is developed that is qualitatively consistent with most of the relevant observations reported in the literature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Hirtenstein M. D. Chemistry of the active site of rhodopsin. Biochem J. 1969 Nov;115(3):607–608. doi: 10.1042/bj1150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonting S. L., Bangham A. D. On the biochemical mechanism of the visual process. Exp Eye Res. 1967 Oct;6(4):400–413. doi: 10.1016/s0014-4835(67)80015-0. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D. Evidence for structural changes during the action potential in nerves from the walking legs of Maia squinado. J Physiol. 1968 Feb;194(2):85–6P. [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Frey A. H. Electromagnetic emission at micron wavelengths from active nerves. Biophys J. 1968 Jun;8(6):731–734. doi: 10.1016/S0006-3495(68)86517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- Kimbel R. L., Jr, Poincelot R. P., Abramhamson E. W. Chromophore transfer from lipid to protein in bovine rhodopsin. Biochemistry. 1970 Apr 14;9(8):1817–1820. doi: 10.1021/bi00810a022. [DOI] [PubMed] [Google Scholar]
- TASAKI I. Initiation and abolition of the action potential of a single node of Ranvier. J Gen Physiol. 1956 Jan 20;39(3):377–395. doi: 10.1085/jgp.39.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I. I., Carnay L., Watanabe A. Transient changes in extrinsic fluorescence of nerve produced by electric stimulation. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1362–1368. doi: 10.1073/pnas.64.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Watanabe A., Sandlin R., Carnay L. Changes in fluorescence, turbidity, and birefringence associated with nerve excitation. Proc Natl Acad Sci U S A. 1968 Nov;61(3):883–888. doi: 10.1073/pnas.61.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON I. B., BERGMANN F. Studies on cholinesterase. VII. The active surface of acetylcholine esterase derived from effects of pH on inhibitors. J Biol Chem. 1950 Aug;185(2):479–489. [PubMed] [Google Scholar]
- Wei L. Y. Role of surface dipoles on axon membrane. Science. 1969 Jan 17;163(3864):280–282. doi: 10.1126/science.163.3864.280. [DOI] [PubMed] [Google Scholar]


