Abstract
Objectives
To investigate the role of matrix metalloproteinase (MMP)-13/collagenase-3 in osteoarthritis (OA).
Methods
Surgically-induced OA in knees of MMP-13 knock out (KO) and wild type (WT) mice was compared. Femoral and tibial cartilage aggrecan loss (0–3), erosion (0–7) and chondrocyte hypertrophy (0–1), as well as osteophyte size (0–3) and maturity (0–3) were histologically scored. Serial sections were stained for collagen type X and the MMP-generated aggrecan neo-epitope DIPEN.
Results
Following surgery, aggrecan loss and cartilage erosion were more severe in the tibia than femur (p<0.01) and tibial cartilage erosion increased with time (p<0.05) in WT mice. Cartilaginous osteophytes were present at 4 weeks and underwent ossification, with size and maturity increasing by 8 weeks (p<0.01). There was no difference between genotypes in aggrecan loss or cartilage erosion at 4 weeks. Tibial cartilage erosion in KO mice was less than WT at 8 weeks (p<0.02). Cartilaginous osteophytes were larger in KO at 4 weeks (p<0.01), but by 8 weeks osteophyte maturity and size were no different from WT. Articular chondrocyte hypertrophy with positive type X collagen and DIPEN staining occurred in both WT and KO joints.
Conclusions
These studies have confirmed that structural cartilage damage in mouse experimental OA is dependent on MMP-13 activity. Chondrocyte hypertrophy is not regulated by MMP-13 activity in this model and does not in itself lead to cartilage erosion. MMP-13 deficiency can inhibit cartilage erosion in the presence of aggrecan depletion, supporting the potential for therapeutic intervention in established OA with MMP-13 inhibitors.
Progressive erosion of articular cartilage is a significant determinant of prognosis and the need for joint replacement surgery in osteoarthritis (OA). Proteolysis of the principal cartilage extracellular matrix constituents, aggrecan and the type II/IX/XI collagen network, directly causes erosion as well as predisposing the tissue to mechanical disruption even with loading at physiological levels. Aggrecan proteolysis and loss precedes and may be prerequisite for subsequent collagenolysis (1). A distintegrin and metalloproteinase with thrombospondin repeat (ADAMTS) enzymes are responsible for pathological aggrecanolysis (2, 3). ADAMTS-5 is the predominant arthritis-associated enzyme in mice, since animals deficient in ADAMTS-5 activity are protected from cartilage erosion in OA and inflammatory arthritis (4–6). Ablating the ADAMTS cleavage site in the interglobular domain of aggrecan also blocks cartilage structural damage, confirming that the effect in ADAMTS-5-deficient mice is due to inhibition of aggrecanolysis (7).
The above studies demonstrate that inhibiting the initiation of aggrecan loss can prevent subsequent structural cartilage damage/erosion in arthritis. Clinically, it is likely that early cartilage damage with aggrecan loss will have occurred at least focally prior to presentation. Articular cartilage aggrecan can be replenished if the insult is removed prior to collagen damage (7, 8). Whether aggrecan-depleted cartilage can withstand mechanical load bearing adequately, and how important proteolysis of the collagen network is in progression to cartilage erosion in this situation is less clear. It is well recognised that cartilage collagenolysis in vitro depends on activity of members of the matrix metalloproteinase (MMP) family (2). Indeed, inhibitors with broad activity against MMP-1, -2, -3, -8, -9, -13 and -14 abrogate cartilage erosion in animal models of OA (9, 10). Since these compounds also have some activity against ADAMTS enzymes, inhibition of these or other metalloproteinases may be responsible at least in part for disease modification observed. Similar compounds have failed in clinical trials because of unwanted joint fibrosis due to their broad spectrum of activity at OA-modifying doses (11). Thus there is a clear need to identify the major collagenase in OA cartilage and to determine whether more specific inhibitors could be therapeutically beneficial.
MMP-13 is more active against type II collagen than other collagenases (12). Selective inhibitor studies suggest that MMP-13 is the predominant activity responsible for collagen release from OA human cartilage in vitro (13, 14). Chondrocyte mRNA expression of MMP-13 but not MMP-1, -8 or -14 is increased in late stage human OA cartilage in association with cartilage erosion (15–18). Cartilage-specific over-expression of MMP-13 in mice induces precocious arthritis with cartilage erosion (19). An inhibitor with high specificity for MMP-13 (although activity against other metal-dependent enzymes was not reported) prevents surface fibrillation in a surgical OA model in rabbits (20). Taken together the above studies strongly implicate MMP-13 as the major collagenolytic activity involved in OA cartilage degeneration. However, other enzymes such as cathepsin K, may also play a significant role in cartilage collagen breakdown in OA (21). Mice with constitutive deletion of MMP-13 have been described by several groups (22–24). These MMP-13 knock out (KO) mice show transient abnormalities in cartilage resorption in long bone growth (22–24) and fracture healing (25, 26). In the present study we utilized a model of surgically induced OA to determine the specific role of MMP-13 in the onset and progression of cartilage erosion and osteophyte development.
Materials and Methods
Surgical model of OA in mice
MMP-13 KO mice on an FVBN background developed by Stickens et al (23) were imported and maintained as a homozygous colony in a specific pathogen free facility. Wild type FVBN male mice were purchased from the Animal Resource Centre (Canning Vale, Western Australia) and maintained in the same facility as KO animals. All mice were caged in groups (n = 3–6/cage) and received water and complete pelleted food ad libitum. When WT and KO mice reached 10 weeks of age, medial meniscal destabilization (MMD) surgery of the right knee was done by a single surgeon (CBL) as previously described (7). This OA-model is well characterised and induces progressive cartilage degeneration with little synovial inflammation (27). Only male mice were used to avoid sex-related differences in disease severity (28). Mice were maintained in their pre-operative groups, allowed unrestricted cage exercise, and were weighed weekly until sacrifice at 4 (n = 12 WT and 15 KO) or 8 (n = 12 WT and 10 KO) weeks post-operatively. All procedures were approved by the Royal North Shore Hospital Animal Care and Ethics Committee.
Histopathology
At sacrifice the operated knees (mid femur to mid tibia) of all mice, and non-operated knees from 3 animals, were harvested, and the skin and muscle removed. Specimens were fixed in 10% neutral buffered formalin for 24 hrs, decalcified for 3 days in 10% formic acid/5% formalin, and paraffin embedded. Serial 4μm sagittal sections were cut across the width of the medial femoro-tibial joint and mounted on superfrost plus glass slides (3 serial sections per slide) with heating at 85°C for 30 minutes then overnight at 55°C. Sections every 40μm were stained with 0.04% toluidine blue and counterstained with 0.1% fast green (12–15 slides per mouse).
Two observers (CBL, AB) blinded to genotype and post-operative time, scored cartilage aggrecan loss (0–3) and structural damage (0–7), with maximal and summed score (sum of all scores in all slides) recorded as previously described (7). Each slide received a single score for each parameter representing the maximal score in the three sections on the slide. The number of slides with scores for structural damage was recorded as a measure of the “stage” of OA (width of joint affected). The presence or absence of morphological chondrocyte hypertrophy (enlarged chondrocyte lacunae with lack of toluidine blue stain around a collapsed cell as typically observed in the growth plate or calcified cartilage) in the non-calcified articular cartilage was recorded. Osteophyte size (0 = none, 1 = small ~ the same thickness as the adjacent cartilage, 2 = medium ~ 1–3 × the thickness as the adjacent cartilage, 3 = large >3 × the thickness as the adjacent cartilage) and osteophyte maturity (0 = none, 1 = predominantly cartilaginous, 2 = mixed cartilage and bone with active vascular invasion and endochondral ossification, 3 = predominantly bone) were scored on coded digital images of the same location of the anterior-medial tibia in each animal.
Immunohistochemistry and TUNEL staining
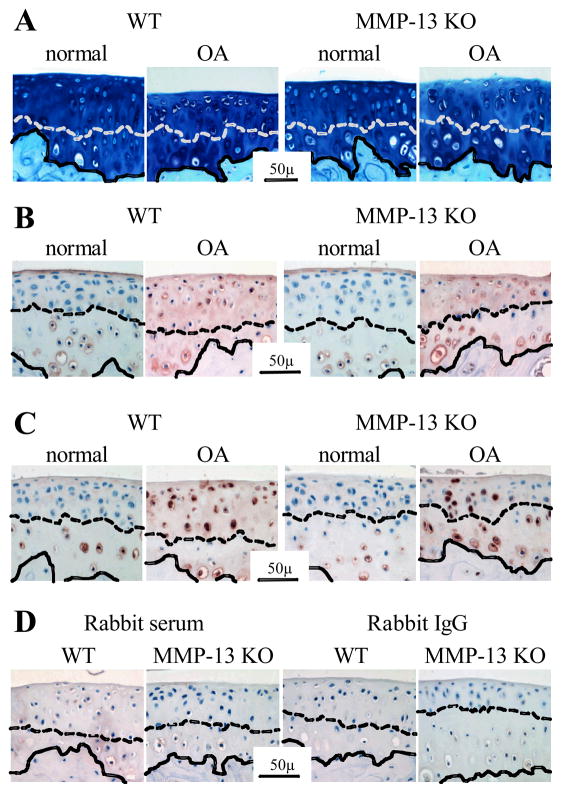
Serial sections from representative WT and MMP-13 KO mice 4 and 8 weeks after MMD-surgery (n = 3 for each genotype/time) were dewaxed in xylene and rehydrated through graded alcohols. Immunohistochemical evaluation was done essentially as previously described (29). Briefly, sections were pre-treated with protease-free Chondroitinase ABC (0.1 U/ml; Sigma-Aldrich) prior to blocking (Serum free protein block; Dako, Botany, Australia) and incubation overnight at 4°C with antibodies/antiserum recognising type X collagen (1:6000 LSL-LB-0092 whole rabbit serum; LSL, Tokyo, Japan), the MMP-generated aggrecan-neoepitope DIPEN (30) (1.36μg/ml), or MMP-14 (4μg/ml ab53712; Abcam, Cambridge UK). Equivalent dilutions/concentrations of normal rabbit serum (Dako X0902) or rabbit IgG (Dako X0936) were used as negative controls. Immunostaining was done with a biotinylated anti-rabbit IgG, horse-radish-peroxidase conjugated streptavidin, Nova Red color reagent (Vector Laboratories, CA, USA) and sections were counterstained with Mayers haematoxylin. Apoptosis was detected using TUNEL staining (ApopTag, Chemicon, Australia) according to the manufacturer’s instructions.
Statistics
All analyses were done using Stat View software for Macintosh (Acura, Berkeley, USA). Comparison of mean scores for aggrecan loss, cartilage structural damage, osteophyte maturity and osteophyte size in WT versus MMP-13 KO were analysed using the Mann-Whitney U test. The incidence of chondrocyte hypertrophy or growth plate closure in WT versus MMP-13 KO was compared by Chi squared analysis. Results are given as mean ± standard error and the alpha level was set at 0.05.
Results
Skeletal phenotype of MMP-13 KO mice
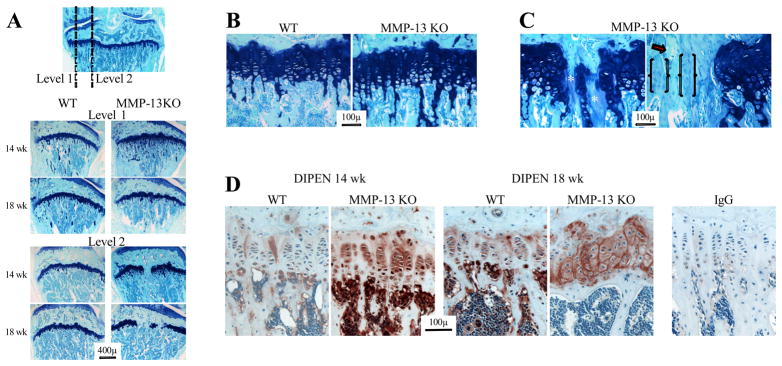
WT mice were grossly indistinguishable from KO animals. There was no difference in starting weight between genotypes (WT = 26.3±1.9g, MMP-13 KO = 25.8±3.4g), and both gained similar weight over the course of the experiment (weight 8 weeks post-MMD = 105–110% of starting weight). Three unoperated knees from WT and MMP-13 KO mice were examined at each post-operative time point. In serial sections towards the axial margin of the medial femoro-tibial joint, both the tibial and femoral growth plates in MMP-13 KO but not WT mice had focal regions of bony union (Fig 1A). When all operated joints were examined (n = 24 WT and 25 KO), focal closure was observed in 12.5% of WT and 96% of MMP-13 KO femoral growth plates, and 0% WT and 72% of MMP-13 KO tibial growth plates. The difference between genotypes in femoral and tibial growth plate closure frequency was significant at 4 and 8 weeks after surgery (p < 0.001 for all analyses), with no difference between time points in either genotype. In MMP-13 KO mice the width of the growth plate affected by closure increased from 14 to 18 weeks (Fig 1A).
Figure 1.
(A) Toluidine blue/fast green stained sections from wild type (WT) and MMP-13 knock out (KO) mice at 14 and 18 weeks of age. The frontal section (top) shows the axial levels from which sagittal sections were collected. Higher magnification images of the growth plate from level 1 (B) shows increased hypertrophic chondrocytes and retention of calcified cartilage trabeculae in the metaphysis of MMP-13 KO mice. In the axial area of MMP-13 KO mice (C) focal areas of growth plate closure had acellular regions with diminished toluidine blue staining (*), faintly stained areas with residual round chondroid-like cells (brackets), and cartilage with no toluidine blue and empty lacunae (arrow). (D) MMP-cleaved aggrecan (DIPEN), was localised to the lower hypertrophic chondrocytes in 14 week-old WT growth plates, but was throughout the growth plate of MMP-13 KO mice at the same age. There was increased DIPEN staining in both genotypes at 18 weeks of age, but it was still more intense in MMP-13 KO compared with WT animals. Minor non-specific staining was observed in sections localized with the same concentration of rabbit IgG as a negative control.
We examined serial sections of tibial growth plates from two additional mice at 4, 6, 8 and 10 weeks of age, and first found evidence of focal bony union at 8 weeks in the MMP-13 KO mice (data not shown). As previously reported (22, 23) there was expansion of the hypertrophic zone of the growth plate in young MMP-13 KO compared with WT mice, which resolved with time (data not shown). At 14 but not 18 weeks of age there was minimal expansion of the hypertrophic zone but some retention of calcified trabeculae in the metaphysis of MMP-13 KO compared with WT (Fig 1B). Surrounding the regions of bony union in MMP-13 KO growth plates, there were areas with loss of chondrocytes and a decrease of toluidine blue staining (Fig 1C*), islands of growth cartilage with no staining and empty lacunae (Fig 1C arrow) and tissue containing rounded chondrocyte-like cells but only faint toluidine blue staining (Fig 1C outlined by brackets). There was no difference in immunostaining for type X collagen in WT and MMP-13 KO growth plates (not shown). However, the MMP-cleaved aggrecan neoepitope DIPEN, was increased in MMP-13 KO growth plate cartilage compared with WT at both 14 and 18 weeks of age (Fig 1D).
We investigated whether growth plate closure in MMP-13 KO mice could be due to increased MMP-14 previously reported (22) or apoptosis of growth plate cells. Hypertrophic chondrocytes in the regions of the growth plate still with active vascular invasion at 14 weeks of age in both genotypes were immunopositive for MMP-14 (Supplementary Figure S1A). However no MMP-14 immunostaining was seen in the growth plate surrounding areas of bony fusion (Figure S1A). At 18 weeks of age active vascular invasion had ceased and there was no MMP-14 staining in the residual growth plate cartilage in either genotype (Figure S1A). TUNEL staining was variable but was generally less in MMP-13 KO compared with WT mice at 14 weeks of age (Figure S1B). TUNEL staining decreased by 18 weeks of age with no difference observed between genotypes (Figure S1B).
Effect of MMP-13 KO on cartilage degradation in OA
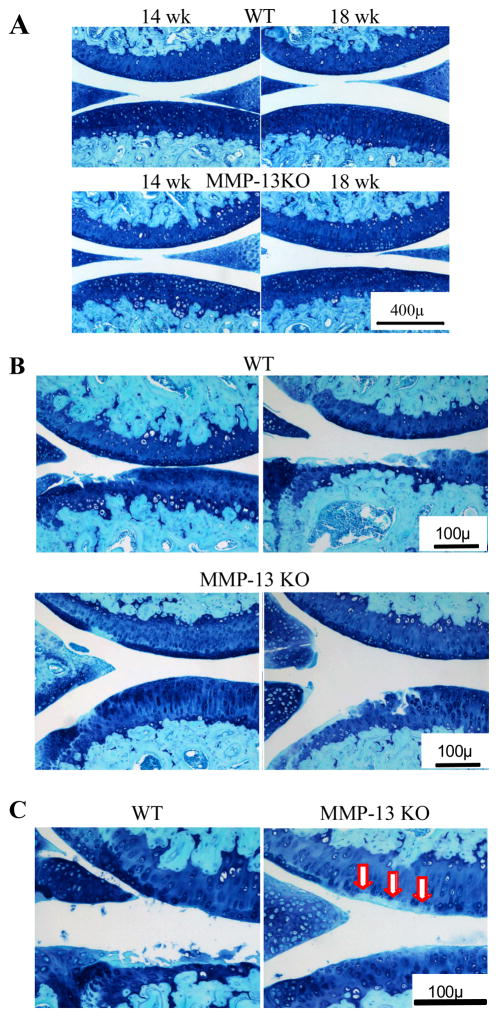
Articular cartilage, subchondral bone and joint morphology in unoperated joints was not different in adult MMP-13 KO compared with WT mice (Fig 2A), indicating that these animals would be suitable for OA studies. MMD-induced proteoglycan loss and cartilage structural damage in WT mice were more severe in the tibia compared with the femur (Fig 2B) as previously reported (7). Tibial cartilage lesions occurred in similar locations in MMP-13 KO mice but the extent of structural damage/erosion was less (Fig 2B). Despite the reduced structural damage in the tibia, there was pronounced focal aggrecan loss in MMP-13 KO mice that was particularly prominent 8 weeks post-operatively (Fig 2C arrows).
Figure 2.
Representative toluidine blue and fast green stained sections from wild type (WT) and MMP-13 knock out (KO) mice at 14 and 18 weeks of age. (A) Central weight-bearing region of the medial femoro-tibial compartment of unoperated joints. (B) Least (left image) and most (right image) severe cartilage erosion in the two genotypes at 8 weeks after surgery are shown. (C) Higher magnification images demonstrating the focal area of aggrecan depletion in the femoral cartilage opposite the tip of the destabilized meniscus in MMP-13 KO (arrows) but not in WT mice, where tibial cartilage erosion is evident.
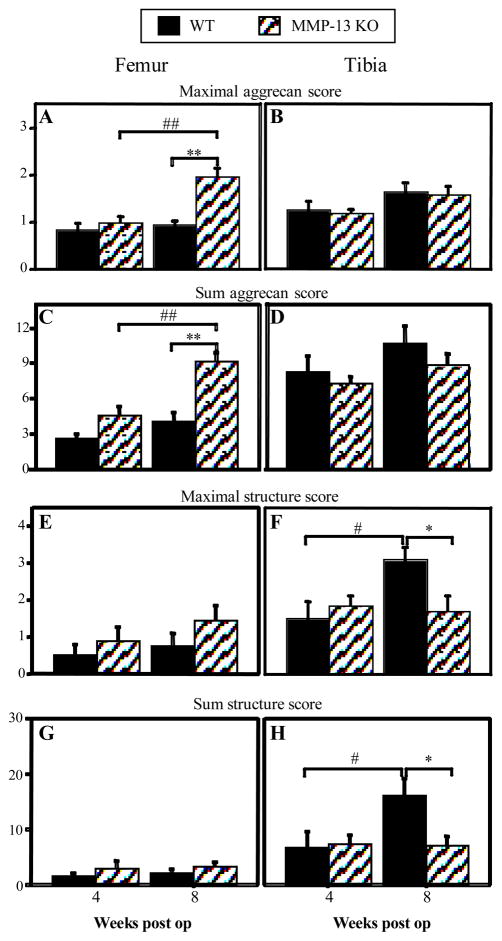
When femoral and tibial aggrecan loss (Fig 3A–D) and structural damage (Fig 3E–G) were scored, significant differences with post-operative time and between genotypes were identified. Aggrecan loss did not increase from 4–8 weeks in either femoral or tibial cartilage of WT mice. In contrast, maximal (Fig 3A) and summed (Fig 3C) femoral (but not tibial) aggrecan loss increased from 4 to 8 weeks in MMP-13 KO mice (p < 0.003 for both analyses), such that there was significantly greater aggrecan loss in KO compared with WT mice at 8 weeks (p < 0.001 for both analyses).
Figure 3.
Maximal (A, B, E, F) and summed (C, D, G, H) scores for aggrecan loss (A–D) and structural cartilage damage (E–H) in femoral and tibial cartilage of wild type (WT) and MMP-13 knock out (KO) mice 4 and 8 weeks after medial meniscal destabilization surgery (“weeks post op”). Graphs represent mean ± SEM; WT n = 12 at 4 and 8 weeks, MMP-13 KO n = 15 at 4 weeks and n = 10 at 8 weeks post op. *p<0.05 **p<0.01 for difference between WT and MMP-13 KO mice at the same time point; #p<0.05 ##p<0.01 for difference between 4 and 8 weeks within a genotype.
There was no significant increase in femoral cartilage structural damage with postoperative time in either genotype, and no difference between genotypes at either time (Fig 3E & G). However, structural damage in tibial cartilage increased significantly between 4 and 8 weeks post-operatively in WT but not MMP-13 KO mice (Fig 3F & H; p<0.02 for both analyses). As a result, tibial cartilage structural damage was significantly worse at 8 weeks after surgery in WT compared with MMP-13 KO mice (Fig 3F & H; p < 0.02 for both analyses). The increase in stage of disease (i.e. width of the joint surface affected) was reflected in the significant increase with post-operative time in the number of slides with a positive histopathological score in WT (2.9 to 7.2; p = 0.002) but not MMP-13 KO (4.5 to 4.7) animals.
Effect of MMP-13 KO on osteophyte development in OA
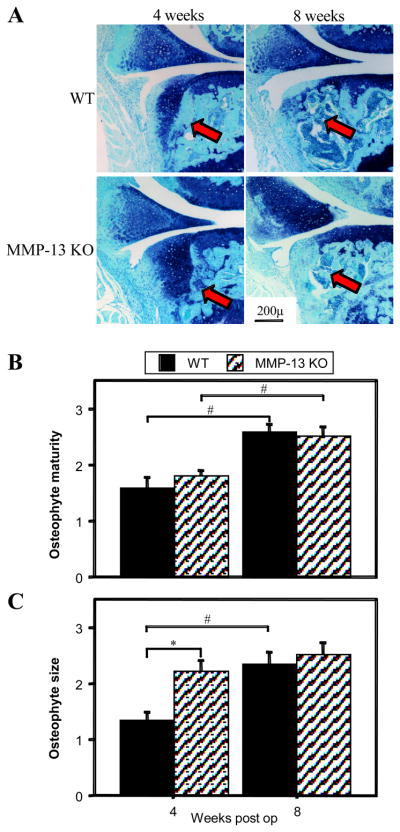
Osteophytes developed on the anterior-medial aspect of the tibial plateau following MMD surgery, initially being predominantly cartilaginous and undergoing endochondral ossification to become mainly bone by 8 weeks (Fig 4A). In both WT and MMP-13 KO mice osteophyte maturity increased with post-operative time (Fig 4B; p= 0.003 and 0.01 respectively) with no difference between genotypes at either 4 or 8 weeks. In MMP-13 KO mice however, the cartilaginous osteophytes were significantly larger than WT at 4 weeks (Fig 4C; p = 0.008), but by 8 weeks after surgery there was no difference between genotypes in osteophyte size.
Figure 4.
(A) Representative toluidine blue & fast green stained sections of wild type (WT) and MMP-13 knock out (KO) mice 4 & 8 weeks after medial meniscal destabilization surgery, demonstrating osteophyte development on the anterior tibial plateau (arrows). The mean scores of osteophyte maturity (B) and osteophyte size (C) in WT and MMP-13 KO mice 4 and 8 weeks after medial meniscal destabilization surgery (“weeks post-op”) are depicted graphically. *p<0.05 for difference between WT and MMP-13 KO mice at the same time point; #p<0.05 for difference between 4 and 8 weeks within a genotype.
Effect of MMP-13 KO on chondrocyte hypertrophy in OA
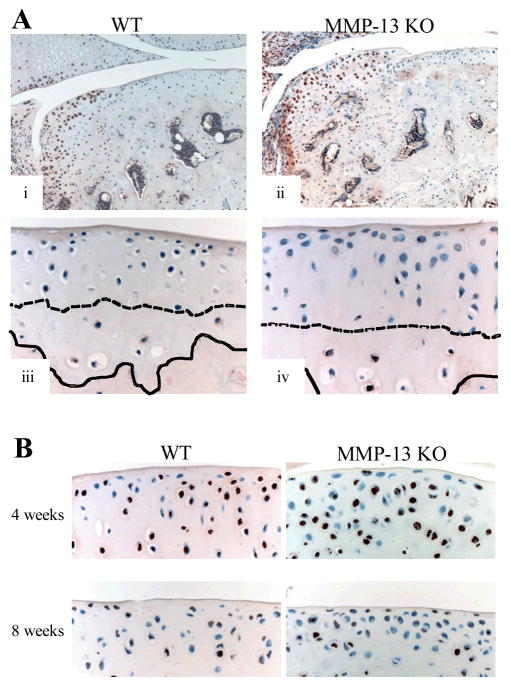
Hypertrophy of articular cartilage chondrocytes in OA has been well-described and implicated in the pathogenesis of cartilage degradation (31). We observed focal areas of non-calcified articular cartilage containing hypertrophic chondrocytes in of 70–90% of joints after surgery (Fig 5A), with no difference in frequency between genotypes or with post-operative time. In both operated and non-operated joints, hypertrophic chondrocytes in the calcified articular cartilage below but never above the tidemark, showed positive pericellular staining for type X collagen and the MMP-cleaved aggrecan neoepitope DIPEN (Fig 5B & 5C, respectively). At both 4 and 8 weeks after surgery, the hypertrophic chondrocytes and matrix in the non-calcified articular cartilage was positive for type X collagen in both WT and MMP-13 KO mice (Fig 5B). Similarly, cellular and pericellular DIPEN staining became evident in non-calcified cartilage above the tidemark in both genotypes at 4 and 8 weeks after surgery (Fig 5C). We examined whether hypertrophic articular chondrocytres in OA showed positive staining for MMP-14 and TUNEL as seen in terminal hypertrophic chondrocytes in the growth plate (Fig S1). While chondrocytes in the joint periphery and developing osteophytes were positive for MMP-14 in both WT and KO mice, no MMP-14 staining was observed in non-calcified articular cartilage chondrocytes at any time in either genotype (Fig. 6A). TUNEL-positive chondrocytes were observed in the articular cartilage following surgery, particularly at 4 weeks, but there was no difference between WT and MMP-13 KO mice (Fig. 6B).
Figure 5.
Representative sections of proximal tibial cartilage from non-operated (“normal”) joints and after medial meniscal destabilization induced osteoarthritis (“OA”) in wild type (WT) and MMP-13 knock out (KO) mice. (A) Toluidine blue & fast green stain; (B) immunolocalization of type X collagen and (C) immunolocalization of the MMP-generated aggrecan neoepitope DIPEN. Representative negative control sections using equivalent concentration of pre-immune rabbit serum or rabbit IgG in place of the primary antibody in WT and MMP-13 KO mice following surgical induction of OA (D). The dashed line demarcates the tidemark between calcified and non-calcified articular cartilage, while the solid line indicates the osteochondral junction.
Figure 6.
(A) Immunolocalization of MMP-14 (brown stain) in the articular cartilage of wild type (WT) and MMP-13 knock out (KO) mice. In both genotypes there was positive immunostaining of chondrocytes in the developing osteophyte (i & iii), and in calcified articular cartilage below the tidemark but not in the non-calcified articular cartilage even in areas with chondrocyte hypertrophy (ii & iv). The dashed line demarcates the tidemark between calcified and non-calcified articular cartilage, while the solid line indicates the osteochondral junction. (B) TUNEL staining (brown) of chondrocytes in the articular cartilage 4 and 8 weeks after surgery in WT and MMP-13 KO mice.
Discussion
Our results have demonstrated for the first time that cartilage structural damage in MMD-induced OA in mice is highly dependent on MMP-13 activity. There was no difference in cartilage damage between genotypes at 4 weeks, suggesting that early phases of OA are dominated by non-MMP-13-dependent events such as ADAMTS-driven aggrecanolysis. We hypothesise that the more severe localised aggrecan loss in the femur of KO mice 8 weeks postoperatively may result from focal compression of the displaced meniscal tip between the tibial and femoral cartilage, which does not occur in the WT mice due to erosion of the opposing tibial cartilage. The focal trauma and supra-physiological compression likely initiates a localised increase in ADAMTS activity. However even this focal area of severe aggrecan loss in KO mice did not progress to structural cartilage damage/erosion in the absence of MMP-13. Preservation of cartilage structure in MMP-13 KO mice despite aggrecan loss, demonstrates the importance of enzymatic cleavage of collagen to the actual loss of cartilage in OA, even in tissue with extensive aggrecan depletion. This suggests that normal joint use and loading of aggrecan-depleted cartilage, does not by itself lead to mechanical disruption of this tissue.
Whether our results in mice are transferable to humans depends in part on the relative contribution of MMP-13 to cartilage collagenolysis in the different species. Cells in adult mice (other than invading trophoblasts in the pregnant uterus), do not express an MMP-1 ortholog (32), and this enzyme may play some role in cartilage collagenolysis in humans (33). However, the predominant role for MMP-13 in OA cartilage collagenolysis in humans as for mice, is supported by MMP-13 being the most active type II collagenase, chondrocyte mRNA expression of MMP-13 but not MMP-1 being increased in late-stage human OA cartilage (15–18), and MMP-13 not MMP-1 being responsible for collagen release in cultured human OA human cartilage (13, 14). The mechanical loading forces experienced in mouse knee cartilage may be considerably different to those in larger species where physical disruption of aggrecan-depleted cartilage could be more important. Future studies could evaluate if chondroprotection is still evident in MMP-13 KO mice with increased exercise and/or time post injury. Nevertheless, the fact that cartilage erosion is inhibited despite ADAMTS-driven aggrecan depletion in MMP-13 KO mice, supports the potential for therapeutic intervention with collagenase inhibitors in established OA.
Our studies confirm the previously observed expansion of the growth plate hypertrophic zone that resolves with age in MMP-13 KO mice (22–24). By looking at older mice, we have identified focal closure of tibial and femoral growth plates in MMP-13 KO mice. Although this abnormality occurs in mice as young as 8 weeks, the focal nature of the lesions would make identification of growth plate closure difficult without serial sections across the width of the joint. As opposed to most other mammals, closure of long bone growth plates is not normally observed in rodents even into old age. Although the residual growth plates in mice >36 weeks old are no longer undergoing active hypertrophy as shown by the lack of type X collagen synthesis, the embedded chondrocytes remain viable and synthesise aggrecan and collagen-II (34). In vertebral growth plates in mice, vertical acellular calcified cartilage trabeculae bridge the residual growth plate and contribute to growth cessation (35). Whether a similar situation exists in residual appendicular growth plates has not been reported, but focal bridging by calcified elements has been seen by the authors in micro-CT analyses of 14–18 week old WT mice in unrelated studies (unpublished observation).
There is little published on the mechanisms of normal growth plate closure in non-rodent species. In human tibial growth plates, as in the MMP-13 KO mice in this report, the axial region closes before the periphery (36). Increased chondrocyte apoptosis occurs with growth plate closure in rabbits (37). We observed decreased TUNEL staining with age and growth plate quiescence, consistent with the reported increase in expression of anti-apoptotic factors in residual growth plates of 6-month-old mice (38). We did not find evidence for increased chondrocyte apoptosis or re-activation of hypertrophy, to explain the growth plate closure in MMP-13 KO mice. The increased DIPEN staining in residual growth plates of KO mice suggested increased MMP activity compared with WT, and confirmed that MMP-13 alone is not responsible for aggrecan cleavage in the growth plate (23). Inada et al (22) reported increased MMP-8, MMP-9 and MMP-14 expression in the hypertrophic zone and/or chondro-osseous junction in MMP-13 KO mice, all of which can generate DIPEN. We were unable to demonstrate increased MMP-14 in KO mice, suggesting that other MMPs are likely responsible for proteolysis and growth plate closure. In this regard, elevated MMP-3 has been associated with growth plate dissolution and closure in rats with collagen-induced arthritis, very similar to that observed in MMP-13 KO mice (39).
Increased chondrocyte MMP-13 expression in OA in humans (15–18) and mice (40–42), is associated with elevated expression of hypertrophy-associated genes such as type X collagen and runt-related transcription factor-2 (runx2) (41–43). It has been postulated that recapitulation of growth-plate-like hypertrophic differentiation of chondrocytes may play an important role in the pathogenesis of cartilage degradation in OA (31). We observed focal chondrocyte hypertrophy in cartilage following surgical-induction of OA as previously described (41–43), however this occurred equally in KO and WT mice with equivalent collagen X expression. While MMP-cleavage of aggrecan is not a hypertrophy-specific event, it is typically observed around hypertrophic chondrocytes in the growth plate and calcified articular cartilage. However, there was no difference between WT and MMP-13 KO mice in DIPEN staining in articular cartilage after surgery. These data suggest that it is not the hypertrophic differentiation of chondrocytes per se that leads to cartilage degradation (at least under the conditions examined in this study) but rather it is the hypertrophy-associated MMP-13 expression and activity that is critical for structural cartilage damage.
Over-expression of MMP-13 in adult cartilage leads to increased collagen X expression (19), and inhibition of MMP-13 reduces chondrocyte hypertrophy, collagen X and runx2 expression in vitro (44), suggesting that MMP-13 activity may drive the hypertrophic process. However, our results suggest that MMP-13 activity is down-stream of other hypertrophic changes in chondrocytes (e.g. type X collagen expression), consistent with the recognised runx2 transcriptional regulation of MMP-13 expression (45). Our data also indicate that MMP-13 activity is not critical for chondrocyte apoptosis associated with terminal hypertrophic-differentiation, and that chondroprotection in MMP-13 KO mice is likely not due to changes in chondrocyte death. The difference in our results to those reported with MMP-inhibition (44) could be explained by a more broad-spectrum activity of the MMP-inhibitor, and in vitro as opposed in vivo studies. It has been postulated that proteolytic generation of bioactive collagen peptides may drive chondrocyte hypertrophy (46), and our present studies cannot rule this out, as other collagenolytic enzymes (e.g. MMP-2, -8, -14, cathepsin K) may compensate in the MMP-13 KO mice. It is noteworthy, that mice in which the type II collagen triple helix is resistant to MMP cleavage, lack normal programmed cell death in hypertrophic chondrocytes in the growth plate (47), suggesting that MMP-derived collagen-II fragments might regulate terminal hypertrophic chondrocyte differentiation.
The simplest explanation for inhibition of cartilage degradation in MMP-13 KO mice is reduced type II collagenolysis, however several alternative explanations deserve consideration. It is possible that reduced proteolysis of other MMP-13 substrates in cartilage (e.g. biglycan, fibromodulin, fibronectin, tenascin C and collagen IX (48, 49)) could contribute to inhibition of cartilage erosion. MMP-13 can activate other MMPs (50) and inhibition of such cascades may modulate inflammation, cytokine, chemokine and growth factor activity (51). MMP-13 may process secreted cytokines and chemokines, their receptors, and other cell surface molecules (52), and MMP-13 can modulate ERK signalling (53), thus its deficiency could directly alter chondrocyte metabolism. Osteoblast MMP-13 contributes to collagenolysis in bone necessary for osteoclastic activity (45). Increased subchondral bone thickness but higher turnover are features of OA and may contribute to cartilage degradation (54). MMP-13 KO mice have reduced bone turnover (23, 25) that could play a role in chondroprotection in OA. It is possible that MMP-13 is important for soft tissue remodelling following surgery, and increased fibrosis in KO mice could reduce joint motion and contribute to chondroprotection. We did not see histologic evidence of increased joint capsule fibrosis (although this was not quantified) and the equivalent osteophytosis in the genotypes suggest similar joint instability. Why the predominantly cartilaginous osteophytes 4 weeks after surgery are larger in MMP-13 KO mice is not clear. Previous reports have shown a delay in endochondral ossification in MMP-13 KO mice (25, 26). This would be consistent with retention of cartilage in the osteophytes, however the maturation was not different with both genotypes having mostly cartilage at 4 weeks. MMP-13 activity may normally acts to inhibit chondrogenesis, possibly by cleaving growth factors or releasing bioactive peptides, and in its absence cartilage accumulation may occur. As in the growth plate, other mechanisms ultimately compensate for the lack of MMP-13, and endochondral ossification proceeds such that the osteophytes in the two genotypes become similar by 8 weeks. The importance of osteophytes in OA pathogenesis is unclear, but the ultimate equality of osteophyte size and maturity in WT and MP-13 KO mice, demonstrates that this process is not linked with articular cartilage damage but rather other factors such as joint instability in this model.
In conclusion, we have demonstrated a significant inhibition of cartilage structural damage in surgically-induced OA in MMP-13 KO mice. This chondroprotection was not associated with any reduction in aggrecanolysis, nor a change in chondrocyte hypertrophy and apoptosis, or osteophyte development. These studies confirm that structural cartilage damage in OA in mice is dependent on MMP-13 activity. Furthermore, they suggest that cartilage erosion can be inhibited in the presence of ADAMTS-driven aggrecan depletion, supporting the potential of MMP-13 specific inhibitors for therapeutic intervention in established OA.
Supplementary Material
Acknowledgments
Grant funding to support this work was received from the National Health and Medical Research Council of Australia (NHMRC: project grants 502622, 400056 & 384414), The Rebecca Cooper Foundation, Arthritis Australia, The Ulysses Club and the National Institutes of Health, USA (AR046238).
References
- 1.Pratta M, Yao W, Decicco C, Tortorella M, Liu R, Copeland R, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278:45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 2.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5(2):94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–52. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 6.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. Eur Cell Mater. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 7.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, et al. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117(6):1627–36. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, Sondergaard BC. Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther. 2008;10(3):R63. doi: 10.1186/ar2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10(10):785–91. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 10.Sabatini M, Lesur C, Thomas M, Chomel A, Anract P, de Nanteuil G, et al. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52(1):171–80. doi: 10.1002/art.20900. [DOI] [PubMed] [Google Scholar]
- 11.Krzeski P, Buckland-Wright C, Balint G, Cline GA, Stoner K, Lyon R, et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther. 2007;9(5):R109. doi: 10.1186/ar2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minond D, Lauer-Fields JL, Cudic M, Overall CM, Pei D, Brew K, et al. The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J Biol Chem. 2006;281(50):38302–13. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- 13.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99(7):1534–45. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlberg L, Billinghurst RC, Manner P, Nelson F, Webb G, Ionescu M, et al. Selective enhancement of collagenase-mediated cleavage of resident type II collagen in cultured osteoarthritic cartilage and arrest with a synthetic inhibitor that spares collagenase 1 (matrix metalloproteinase 1) Arthritis Rheum. 2000;43(3):673–82. doi: 10.1002/1529-0131(200003)43:3<673::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44(12):2777–89. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46(10):2648–57. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 17.Davidson RK, Waters JG, Kevorkian L, Darrah C, Cooper A, Donell ST, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8(4):R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50(1):131–41. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 19.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson AR, Pavlovsky AG, Ortwine DF, Prior F, Man CF, Bornemeier DA, et al. Discovery and characterization of a novel inhibitor of matrix metalloprotease-13 that reduces cartilage damage in vivo without joint fibroplasia side effects. J Biol Chem. 2007;282(38):27781–91. doi: 10.1074/jbc.M703286200. [DOI] [PubMed] [Google Scholar]
- 21.Vinardell T, Dejica V, Poole AR, Mort JS, Richard H, Laverty S. Evidence to suggest that cathepsin K degrades articular cartilage in naturally occurring equine osteoarthritis. Osteoarthritis Cartilage. 2009;17(3):375–83. doi: 10.1016/j.joca.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A. 2004;101(49):17192–7. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131(23):5883–95. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takaishi H, Kimura T, Dalal S, Okada Y, D’Armiento J. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol. 2008;9(1):47–54. doi: 10.2174/138920108783497659. [DOI] [PubMed] [Google Scholar]
- 25.Behonick DJ, Xing Z, Lieu S, Buckley JM, Lotz JC, Marcucio RS, et al. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE. 2007;2(11):e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354(4):846–51. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 27.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Blanchet T, Morris E, Glasson S. Disease progression in surgically induced murine osteoarthritis is strain and sex dependent. Trans Orth Res Soc. 2005;30:1422. [Google Scholar]
- 29.Melrose J, Smith S, Little C, Kitson J, Hwa S, Ghosh P. Spatial and temporal localization of transforming growth factor-beta, fibroblast growth factor-2, and osteonectin, and identification of cells expressing alpha-smooth muscle actin in the injured anulus fibrosus: implications for extracellular matrix repair. Spine. 2002;27:1756–1764. doi: 10.1097/00007632-200208150-00014. [DOI] [PubMed] [Google Scholar]
- 30.Mercuri FA, Maciewicz RA, Tart J, Last K, Fosang AJ. Mutations in the interglobular domain of aggrecan alter matrix metalloproteinase and aggrecanase cleavage patterns. Evidence that matrix metalloproteinase cleavage interferes with aggrecanase activity. J Biol Chem. 2000;275(42):33038–45. doi: 10.1074/jbc.275.42.33038. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi H. Regulation of osteoarthritis development by Wnt-beta-catenin signaling through the endochondral ossification process. J Bone Miner Res. 2009;24(1):8–11. doi: 10.1359/jbmr.081115. [DOI] [PubMed] [Google Scholar]
- 32.Balbin M, Fueyo A, Knauper V, Lopez JM, Alvarez J, Sanchez LM, et al. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276(13):10253–62. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Billinghurst RC, Pidoux I, Antoniou J, Zukor D, Tanzer M, et al. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheum. 2002;46(8):2087–94. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 34.Chambers MG, Kuffner T, Cowan SK, Cheah KS, Mason RM. Expression of collagen and aggrecan genes in normal and osteoarthritic murine knee joints. Osteoarthritis Cartilage. 2002;10(1):51–61. doi: 10.1053/joca.2001.0481. [DOI] [PubMed] [Google Scholar]
- 35.Yokozeki K, Abe K, Watanabe S, Suda K, Kaneda K. Acellular calcified columns in the normal growth plate of mouse vertebrae. Arch Histol Cytol. 1998;61(3):269–76. doi: 10.1679/aohc.61.269. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki T, Ishibashi Y, Okamura Y, Toh S, Sasaki T. MRI evaluation of growth plate closure rate and pattern in the normal knee joint. J Knee Surg. 2002;15(2):72–6. [PubMed] [Google Scholar]
- 37.Aizawa T, Kokubun S, Tanaka Y. Apoptosis and proliferation of growth plate chondrocytes in rabbits. J Bone Joint Surg Br. 1997;79(3):483–6. doi: 10.1302/0301-620x.79b3.7221. [DOI] [PubMed] [Google Scholar]
- 38.Kinkel MD, Yagi R, McBurney D, Nugent A, Horton WE., Jr Age-related expression patterns of Bag-1 and Bcl-2 in growth plate and articular chondrocytes. Anat Rec A Discov Mol Cell Evol Biol. 2004;279(2):720–8. doi: 10.1002/ar.a.20063. [DOI] [PubMed] [Google Scholar]
- 39.Takahi K, Hashimoto J, Hayashida K, Shi K, Takano H, Tsuboi H, et al. Early closure of growth plate causes poor growth of long bones in collagen-induced arthritis rats. J Musculoskelet Neuronal Interact. 2002;2(4):344–51. [PubMed] [Google Scholar]
- 40.Flannelly J, Chambers MG, Dudhia J, Hembry RM, Murphy G, Mason RM, et al. Metalloproteinase and tissue inhibitor of metalloproteinase expression in the murine STR/ort model of osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):722–33. doi: 10.1053/joca.2002.0818. [DOI] [PubMed] [Google Scholar]
- 41.Kamekura S, Hoshi K, Shimoaka T, Chung U, Chikuda H, Yamada T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–41. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, et al. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54(8):2462–70. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 43.Tchetina EV, Squires G, Poole AR. Increased Type II Collagen Degradation and Very Early Focal Cartilage Degeneration Is Associated with Upregulation of Chondrocyte Differentiation Related Genes in Early Human Articular Cartilage Lesions. J Rheumatol. 2005;32(5):876–86. [PubMed] [Google Scholar]
- 44.Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, et al. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17(4):639–51. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 45.Jimenez MJ, Balbin M, Lopez JM, Alvarez J, Komori T, Lopez-Otin C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol. 1999;19(6):4431–42. doi: 10.1128/mcb.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26(4):247–58. doi: 10.1016/j.matbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 47.Gauci SJ, Golub SB, Tutolo L, Little CB, Sims NA, Lee ER, et al. Modulating chondrocyte hypertrophy in growth plate and osteoarthritic cartilage. J Musculoskelet Neuronal Interact. 2008;8(4):308–10. [PubMed] [Google Scholar]
- 48.Knauper V, Cowell S, Smith B, Lopez-Otin C, O’Shea M, Morris H, et al. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J Biol Chem. 1997;272:7608–7016. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 49.Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier JP, et al. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8(1):R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knauper V, Smith B, Lopez-Otin C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248(2):369–73. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 51.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8(3):245–57. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 53.Raggatt LJ, Jefcoat SC, Jr, Choudhury I, Williams S, Tiku M, Partridge NC. Matrix metalloproteinase-13 influences ERK signalling in articular rabbit chondrocytes. Osteoarthritis Cartilage. 2006;14(7):680–9. doi: 10.1016/j.joca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Karsdal MA, Leeming DJ, Dam EB, Henriksen K, Alexandersen P, Pastoureau P, et al. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage. 2008;16(6):638–46. doi: 10.1016/j.joca.2008.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.