Abstract
NO synthesis is a prerequisite for proper insulin sensitivity in insulin-targeted tissues; however, the molecular basis for this process remains unclear. Using a gain-of-function model of endothelial nitric-oxide synthase (eNOS)-transfected COS-7 cells, we have shown a critical role of NO in insulin responsiveness, as evidenced by an NO-dependent increase of tyrosine phosphorylation levels of the insulin receptor and its downstream effectors insulin receptor substrate-1 and PKB/AKT. We hypothesized that NO-induced inactivation of endogenous protein-tyrosine phosphatases (PTPs) would enhance insulin receptor-mediated signaling. To test this hypothesis, we devised a new method of the PTP labeling using a cysteine sulfhydryl-reacted probe. Under the acidic conditions employed in this study, the probe recognized the reduced and active forms but not the S-nitrosylated and inactive forms of endogenous PTPs. Our data suggest that phosphatases SHP-1, SHP-2, and PTP1B, but not TC-PTP, are likely S-nitrosylated at the active site cysteine residue concomitantly with a burst of NO production in signaling response to insulin stimulation. These results were further confirmed by phosphatase activity assays. We investigated further the role of NO as a regulator of insulin signaling by RNA interference that ablates endogenous eNOS expression in endothelial MS-1 cells. We have shown that eNOS-dependent NO production is essential for the activation of insulin signaling. Our findings demonstrate that NO mediates enhancement of insulin responsiveness via the inhibition of insulin receptor phosphatases.
Keywords: Diseases/Diabetes, Enzymes/Nitric-oxide Synthase, Hormones/Insulin, Phosphorylation/Kinases/Tyrosine, Signal Transduction/Nitric Oxide, Signal Transduction/Phosphoprotein Phosphatases/Tyrosine, Signal Transduction/Phosphotyrosine/Receptors
Introduction
The liver, muscle, and fat of obese people or people with type 2 diabetes are less responsive to insulin stimulation (1). This insulin resistance leads to abnormal hepatic glucose production and impaired glucose uptake in peripheral tissues (1). Attempting to uncover the underlying mechanism through which this process occurs, many studies have focused on the complex signaling networks activated by the insulin receptor, a receptor tyrosine kinase responsible for insulin action. Although the molecular basis is not fully understood, the data accumulated over the last decade have suggested that the inhibition of insulin receptor-mediated signaling contributes to the development of insulin resistance (2–4). It has been shown that dephosphorylation of insulin receptor and its substrates by protein-tyrosine phosphatases (PTPs)2 attenuates insulin action, leading to insulin resistance in otherwise insulin-responsive cells (3, 4). In principle, any cell signaling that could inactivate PTPs of the insulin receptor and its substrates should be able to enhance insulin responsiveness in tissues targeted by insulin.
Several PTPs identified as insulin receptor phosphatases are expressed in tissues responsive to insulin (3). For PTPs that are insulin receptor phosphatases to respond properly to physiological conditions, they might need to be inactivated by insulin stimulation (5). However, in metabolic disorders such as obesity and type 2 diabetes, the intrinsic driving force responsible for the inactivation of those PTPs may be impaired, resulting in the suppression of insulin receptor-mediated signaling. An understanding of the underlying mechanisms that control insulin-induced inhibition of endogenous PTPs targeting insulin receptor and its substrates would make possible the development of new therapeutic strategies for obesity and type 2 diabetes. In this study, we explored the possible role of intracellular NO in this process.
Over the past decade great progress has been made in understanding the important role that intrinsic NO plays in the maintenance of insulin responsiveness. Early observations showed that pharmacologic blockage of nitric-oxide synthase activity caused peripheral insulin resistance in rats (6, 7). Later, a series of studies demonstrated that NO donors could mimic the activity of insulin by stimulating glucose transport and metabolism in isolated rat muscle (8–10). Recent studies have reported that by disrupting the gene encoding endothelial nitric-oxide synthase (eNOS), mice became resistant to insulin-stimulated glucose uptake, and they displayed hypertension and hyperlipidemia, two characteristics commonly associated with insulin resistance (11–13). Paralleling these sophisticated genetic analyses, biochemical studies have also uncovered mechanisms underlying the phosphorylation-dependent signaling cascade found in insulin-stimulated activation of eNOS in insulin-responsive cells (14–16). Moreover, eNOS has been found not only in endothelia but also in muscle (17) and adipose tissues (18). Presumably because of the function of endogenous eNOS, NO production is increased in human preadipocytes in response to insulin stimulation (18). Although these studies shed light on the close link between the regulatory role of NO and the insulin responsiveness, little is known about what NO specifically targets in the signaling context response to insulin.
We (19, 20) and others (21) have found PTPs to be easily inactivated by NO-induced S-nitrosylation of the active site Cys residue. Their susceptibility to S-nitrosylation makes the inhibition of phosphatase activity reversible (20). This inactivation of phosphatase activity occurs not only when purified PTP is exposed to NO in vitro (19, 22) but also when endogenous PTP responds to increased levels of cellular NO in vivo (23–25). Thus, it has been proposed that S-nitrosylation may function as a general means of inactivating PTPs in signaling pathways associated with NO production (20, 23–25). Based on available knowledge, it is likely that some insulin receptor PTPs may undergo S-nitrosylation and inactivation when insulin-induced signaling occurs with the simultaneous activation of eNOS. Should this hypothesized process be proven, then our understanding of the mechanisms underlying insulin responsiveness would be greatly advanced.
In this study, we investigated whether endogenous PTPs, specifically those identified as insulin receptor phosphatases, are targeted and inactivated by the NO produced in response to insulin-activated eNOS. Rather than relying on indirect pharmacologic blockage of nitric-oxide synthase, we used a gain-of-function model, in which ectopic eNOS was expressed in cells lacking endogenous eNOS, and a loss-of-function model, in which endogenous eNOS was ablated in cells via RNA interference, to examine the effect of insulin-induced NO production on PTP activity and insulin responsiveness. During the course of this study, we developed a new method of visualizing NO-mediated modification of endogenous PTPs in response to insulin stimulation.
MATERIALS AND METHODS
Reagents
Biotin-HPDP, Iodoacetyl-LC-Biotin, and Iodoacetyl-PEO-Biotin probes were purchased from Pierce. c-PTIO, iodoacetic acid, and insulin were from Sigma. The nitric oxide assay kit we used was purchased from Calbiochem. The 21-nucleotide siRNA duplexes against mouse eNOS were purchased from Dharmacon Thermo Scientific. Two oligonucleotides, 5′-GAUCCUAACUUGCCCUGCAUU-3′ (J-040956-07) and 5′-GAAUGGAAGUGGUUCAGCUUU-3′ (J-040956-08), were chosen. S-Nitrosocysteine was prepared by incubating an equamolar mixture of S-nitroso-N-penicillamine (Sigma) and l-cysteine (Sigma) for 15 min at 37 °C. The following antibodies were purchased from various vendors: SHP-1, SHP-2, and insulin receptor β-chain (InRβ) from Santa Cruz; biotin, β-actin, and catalase from Sigma; caspase-7, p130CAS, eNOS, and Ser(P)1177-eNOS from BD; pYpY1162/1163-InRβ, Tyr(P)972-InRβ, and Tyr(P)612-IRS-1 from Invitrogen; caspase-3, Ser(P)473-PKB/AKT, and PKB/AKT from Cell Signaling; and caspase-6 and IRS-1 from Millipore. Antibodies to PTP1B (FG6) and TC-PTP (CF4) were generously provided by Dr. Nicholas K. Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). The full-length cDNA of human eNOS was a gift from Dr. Danny Ling Wang (Academia Sinica, Taipei, Taiwan).
Cell Culture, Transfection, and Immunoprecipitation
Monkey kidney epithelial COS-7 cells were routinely maintained in high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Human endothelial-derived EA.hy926 cells were maintained in low glucose Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Mouse endothelial MS-1 cells were maintained in high glucose Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. For transient transfection with the eNOS expression vector, COS-7 cells (5 × 105 cells/6-cm plate) were incubated with a mixture of plasmid DNA (1 μg/6-cm plate) and Lipofectamine 2000 (Invitrogen), according to the manufacturer's directions. For knocking down endogenous eNOS, MS-1 cells were mixed with siRNA (3 × 106 cells + 600 pmol of siRNA) and then electroporated by the NeonTM transfection system (Invitrogen) according to the manufacturer's directions. For immunoprecipitation, the cells were lysed in buffer containing 50 mm Tris-HCl (pH 8.0), 1% Nonidet P-40, 150 mm NaCl, 2 mm Na3VO4, and protease inhibitors. An aliquot of total lysate (1 mg) was reacted with 8 μg of anti-insulin receptor antibody (29B4, Santa Cruz) for 1 h at 4 °C and then incubated with protein G-Sepharose (GE Healthcare) for an additional 3 h at 4 °C.
Isolation of PEO Probe-labeled Proteins
Cell extracts were prepared in degassed buffer (20 mm NaPO4, pH 6.0, 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, 1 mm PEO probe, and protease inhibitors) and then incubated at 25 °C for 15 min. After removal of excess probe by a PD-10 column (GE Healthcare), an aliquot of total lysate was incubated with streptavidin paramagnetic particles (Promega) at 25 °C for 2 h. Probe-labeled proteins were eluted by boiling particles for 6 min. The eluted proteins were then subjected to immunoblotting with various antibodies.
Phosphatase Activity Assay
Insulin-stimulated cells were harvested in immunoprecipitation lysis buffer containing 15 mm iodoacetic acid. The excess iodoacetic acid was removed by a PD-10 column, and the processed total lysate was reconstituted in 1× phosphate-buffered saline containing 1% Nonidet P-40 and 10 mm DTT. Endogenous SHP-1, SHP-2, PTP1B, or TC-PTP was immunoprecipitated from an aliquot of total lysate (2 mg) by a specific antibody. The immunocomplex was incubated in phosphatase activity assay buffer (50 mm Hepes, pH 7.5, 1% Nonidet P-40, 10 mm DTT, and 20 mm p-nitrophenyl phosphate). The reaction was carried out at 37 °C for 12 h. After the reaction was terminated by 2 n NaOH, the phosphatase activity in the immunocomplex was revealed by spectrometric analysis at 405 nm.
RESULTS
eNOS-induced NO Production Enhanced Tyr(P) of Insulin Receptor in Signaling Response to Insulin Stimulation
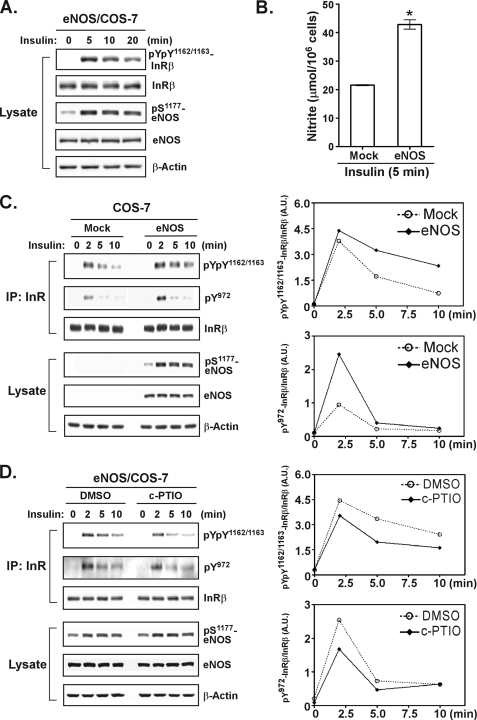
The primary goal of this study was to explore the mechanisms underlying NO enhancement of insulin sensitivity. To do this, we used COS-7 cells that do not express a detectable level of endogenous eNOS as a model (20, 26). We assumed that the use of the gain-of-function eNOS transfectants would help define the specific role of NO in the insulin-induced signaling pathway when compared with the parental COS-7 cells. We tested whether NO would be produced effectively in eNOS transfectants exposed to insulin. As shown in Fig. 1A, the ectopically expressed eNOS was activated in response to insulin stimulation, as evidenced by increased phosphorylation of Ser1177 in eNOS. This activation of eNOS was concomitant with a robust production of NO (Fig. 1B). To find out whether the NO produced by activated eNOS would enhance insulin responsiveness, we analyzed the phosphorylation level of specific tyrosine residues of the insulin receptor β-subunit (Tyr1162/1163 at the activation loop and Tyr972 at the juxtamembrane segment). Control mock cells and eNOS transfectants were stimulated with insulin, and the phosphorylation of immunoprecipitated insulin receptor β-subunit was monitored over time by immunoblotting with anti-phospho-insulin receptor-specific antibodies (Fig. 1C). We found the phosphorylation of the activation loop Tyr residues 1162 and 1163 to last longer and the phosphorylation of Tyr972 to be greater in insulin-treated eNOS transfectants than in control cells (Fig. 1C).
FIGURE 1.
NO enhances insulin responsiveness. A, COS-7 cells ectopically expressing human eNOS were stimulated with insulin (20 nm). Aliquots of total lysates (25 μg) were subjected to immunoblotting with antibodies as indicated. B, COS-7 transfectants were treated with insulin for 5 min. Insulin-induced NO production was measured by the assay detecting the level of NO (represented by the concentration of nitrite) released to culture media. The data are presented as the means ± S.E. (n = 3). *, p < 0.05 versus mock transfectants. C, COS-7 transfectants were stimulated with insulin. Insulin receptor β-subunit (InRβ) was immunoprecipitated (IP) and then subjected to immunoblotting with antibodies as indicated. Aliquots of total lysate were also subjected to immunoblotting. The right panels show a densitometric analysis of the gel image as a ratio of phosphorylated InRβ relative to total InRβ. A.U., arbitrary unit. D, eNOS transfectants were treated with dimethyl sulfoxide (DMSO) or 300 μm c-PTIO for 30 min prior to insulin stimulation. The immunoprecipitated InRβ or aliquots of total lysates were subjected to immunoblotting with antibodies as indicated (left panel). The ratio of phosphorylated InRβ relative to total InRβ is shown in the right panels. Similar results (C and D) were observed in two independent experiments.
To address the critical role of NO in the regulation of insulin-mediated signaling, we used a synthetic NO scavenger, c-PTIO to treat eNOS transfectants prior to stimulation with insulin. In the presence of NO scavenger, the degree of the prolonged (Tyr(P)1162/Tyr(P)1163) and enhanced (Tyr(P)972) phosphorylation of insulin receptor β-subunit in eNOS transfectants was decreased (Fig. 1D). These results were consistent with the current hypothesis that NO may facilitate insulin sensitivity in insulin targeting cells, thus indicating that eNOS-transfected COS-7 cells may be suitable for investigating the role of NO in the context of insulin signaling. We therefore decided to explore whether insulin-induced NO production would lead to inactivation of insulin receptor phosphatases via Cys S-nitrosylation, using the above eNOS transfectant model.
Using Cys Sulfhydryl-reacted Probes to Determine Labeling Specificity on the Reduced Form of PTP
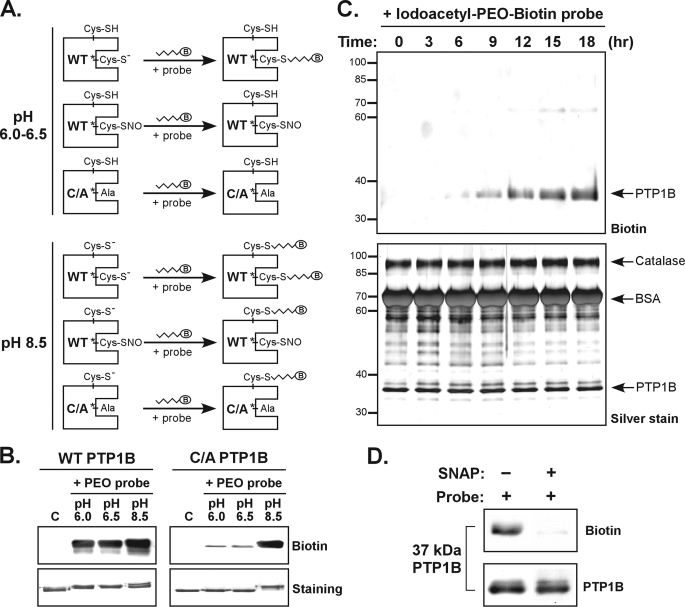
In a previous study, analyzing data from quantitative mass spectrometry and x-ray crystal structure, we found the active site Cys in PTPs to be the residue susceptible to S-nitrosylation (20). Therefore, a probe that could tag specifically the reduced but not S-nitrosylated form of the active site Cys could be used to detect NO-reacted cellular PTPs. Because the active site Cys of reduced PTPs has a low pKa (27), it bears a negative charge under acidic conditions (Fig. 2A). Under the same condition, however, the active site Cys of S-nitrosylated PTPs loses its negative charge (Fig. 2A). We proposed that reduced and S-nitrosylated PTPs could be measured with the use of chemical probes preferentially reacted with the negatively charged Cys residue. To test this hypothesis, we incubated purified PTP1B in either the wild type (WT) form or the C215A mutant form (mimicking the active site Cys S-nitrosylated PTP1B; Fig. 2A) with three commercially available Cys sulfhydryl-reacted probes (supplemental Fig. S1) under acidic or basic conditions. According to our results (supplemental Fig. S1), the Iodoacetyl-PEO-Biotin probe (referred to as the PEO probe hereafter) was selected for additional study. We found that the PEO probe worked well to label the WT form but not the C215A mutant form of PTP1B at pH 6.5 or pH 6.0 (Fig. 2B).
FIGURE 2.
The PEO probe recognizes the reduced form but not the S-nitrosylated form of PTP1B. A, schematic illustration of the PTP labeling workflow used to determine a condition for specific biotinylation of the active site Cys. The WT or C215A (C/A) mutant form of recombinant PTP1B was used in these experiments. Probes tested contain a biotin group (indicated as B in an oval). B, the Iodoacetyl-PEO-Biotin probe (PEO probe) was used for the analysis. The lanes C indicate control samples in which PTP1B was diluted in buffer (pH 7.5) without probe. For labeling reaction, the PEO probe (2 mm) was incubated with an aliquot of PTP1B (2 μg) at 25 °C for 90 min. The reaction was terminated by the addition of SDS-PAGE sample buffer followed by boiling for 6 min. An aliquot of PTP1B was subjected to immunoblotting with anti-biotin antibody or SDS-PAGE for staining with Coomassie Blue. C, the WT PTP1B (10 μg) was mixed with purified catalase (30 μg) and BSA (300 μg). This protein mixture was incubated with 1 mm PEO probe in a reaction buffer (pH 6.0) at 4 °C. At various times as indicated, an aliquot of protein mixture was harvested, mixed with SDS-PAGE sample buffer, and boiled. The samples were subjected to immunoblotting with anti-biotin antibody (upper panel), or SDS-PAGE for silver staining (lower panel). The arrowheads indicate the positions of proteins appearing in SDS-PAGE gels. D, the WT PTP1B (2 μg) was preincubated with 20 μm DTT for 10 min at 37 °C. The protein was left in the buffer containing DTT, or treated with the NO donor S-nitroso-N-penicillamine (SNAP, 1 mm) for 20 min at 37 °C. After removal of excessive DTT and S-nitroso-N-penicillamine by a PD-10 column, PTP1B was reconstituted in an acidic buffer (pH 6.0) and then incubated with the PEO probe (1 mm) for 30 min at 25 °C. An aliquot (375 ng) of PTP1B was subjected to immunoblotting with antibodies to biotin and PTP1B.
To examine the selectivity of the PEO probe among various Cys-containing proteins, we mixed the reduced PTP1B with excessive amounts of bovine serum albumin (BSA) and catalase, both of which contain unpaired free Cys residues with normal pKa (1 free Cys residue in BSA (28) and 3 free Cys residues in Aspergillus catalase; P55303 in UniProtKB), and then incubated the protein mixture with the PEO probe at pH 6.0 for various durations. As seen in Fig. 2C, the biotin signal that co-migrated with PTP1B in SDS-PAGE appeared at 6 h and then increased steadily over incubation time. The PEO probe, however, did not label BSA or catalase under the same conditions (Fig. 2C), despite the fact that there was a large quantity of proteins in the reaction (Fig. 2C). These results demonstrate that a low pKa Cys residue, which appears in PTP1B but not in BSA or Catalase, is required for labeling by the PEO probe.
We also tested whether the PEO probe would tag the reduced PTPs but not the S-nitrosylated PTPs (see the hypothesis in Fig. 2A). To do this, the probe was reacted with an aliquot of the WT form of PTP1B pre-exposed to a NO donor S-nitroso-N-penicillamine, which induces the active site Cys215 S-nitrosylation as evidenced by our previous studies (19, 20). As shown in Fig. 2D, whereas the reduced PTP1B was clearly biotinylated, the PEO probe did not recognize the S-nitrosylated form of PTP1B. These observations suggested that the same method could be used to compare the relative quantity of reduced PTPs and the active site Cys S-nitrosylated form of PTPs found in cells.
Exposure of Cells to NO Donor Leads to a Decreased Level of PTP Labeling
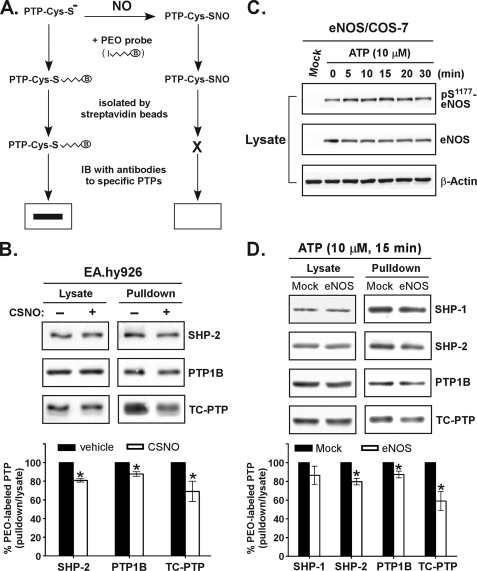
In the next phase of the study, we used the PEO probe to investigate whether cellular NO targets endogenous PTPs and affects phosphatase activity. To do this, we first tested the ability of the probe to recognize proteins in cell lysate. As expected, endogenous PTPs, but not other cellular proteins containing unpaired Cys residues with a normal pKa, were tagged by the probe (supplemental Fig. S2). As illustrated in Fig. 3A, we hypothesized that the probe would label reduced PTPs but it would not react with S-nitrosylated PTPs. This hypothesis was tested in EA.hy926 endothelial cells exposed to S-nitrosocysteine. After treatment, the cells were harvested in lysis buffer containing the PEO probe. An aliquot of lysates was then subjected to precipitation with the streptavidin matrix and analyzed by immunoblotting. The amount of probe-tagged PTPs, including SHP-2, PTP1B, and TC-PTP, was significantly diminished in cells with an increased level of NO (Fig. 3B). We further examined the effect of intracellularly produced NO on the regulation of endogenous PTPs. The gain-of-function eNOS-expressed COS-7 cells were stimulated with ATP, which transactivates eNOS for NO production in cells (20, 26). Consistent with previous observations, the phosphorylation level of Ser1177 in eNOS was rapidly increased after ATP stimulation (Fig. 3C), indicating the activation of eNOS. The labeling of PTPs by the PEO probe was further examined. Importantly, in response to ATP stimulation, endogenous PTPs expressed in eNOS transfectants were less susceptible to probe labeling compared with nontransfected cells (Fig. 3D), suggesting that intracellular NO-induced inactivation of PTPs had occurred. Based on these findings, we proposed that the PEO probe might be used to measure the relative degree of PTP S-nitrosylation in cells exposed to physiological ligands, including insulin.
FIGURE 3.
Application of the PEO probe labeling revealed that endogenous PTPs are susceptible to intracellular NO-induced S-nitrosylation and inactivation. A, a schematic illustration of the workflow to assess the NO-reacted, S-nitrosylated PTPs by the labeling of cellular proteins with the PEO probe. B, EA.hy926 cells were stimulated with S-nitrosocysteine (CSNO, 1 mm) for 15 min at 37 °C and then harvested in acidic lysis buffer (pH 6.0) containing 1 mm PEO probe. The biotinylated proteins were precipitated by streptavidin paramagnetic particles, eluted, and subjected to immunoblotting with antibodies as indicated (upper panel). The lower panel shows the results of the densitometric analysis of the gel image as the ratio of biotinylated PTP relative to total PTP in lysate. C, COS-7 cells ectopically expressing human eNOS were stimulated with ATP for the indicated times. Aliquots of lysate were subjected to immunoblotting with antibodies as indicated. D, COS-7 transfectants were stimulated with ATP for 15 min and then harvested at pH 6.0 in the presence of 1 mm PEO probe. The biotinylated proteins were precipitated and subjected to immunoblotting with antibodies as indicated (upper panel). The lower panel shows the results of the densitometric analysis of the gel image as the ratio of biotinylated PTP relative to total PTP in lysate. The data shown in B and D are presented as the means ± S.E. (n = 3). *, p < 0.05 when compared with untreated cells.
A Critical Role of NO-mediated Inactivation of Endogenous PTPs in Enhanced Insulin Responsiveness
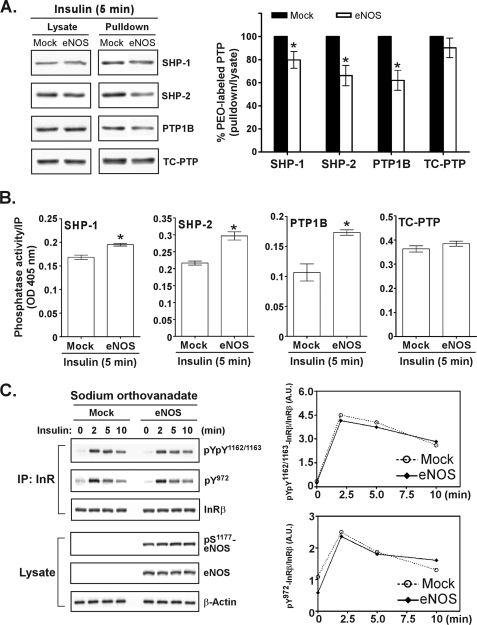
We further explored the role of eNOS-induced NO production in the regulation of endogenous PTPs in the signaling response to insulin stimulation. To do this, control mock transfectants and the eNOS transfectants of COS-7 cells were exposed to insulin for 5 min and then harvested in the presence of the PEO probe at pH 6.0. The probe-tagged proteins were then precipitated by the streptavidin matrix followed by immunoblotting analysis. Of the four PTPs identified as negative regulators of insulin signaling (29–32) being tested, three (SHP-1, SHP-2, and PTP1B) were found to be susceptible to NO-induced modification in insulin-treated cells expressing eNOS (Fig. 4A). These results strongly suggest that these PTPs were inactivated by S-nitrosylation of the active site Cys in signaling induced by insulin. This hypothesis was tested by a modified phosphatase activity assay. Both mock and eNOS transfectants were treated with insulin and then lysed in the presence of iodoacetic acid, which alkylates the reduced form of the active site Cys and then irreversibly inactivates this fraction of endogenous PTPs. After excess iodoacetic acid was removed, DTT was used to reduce S-nitrosylated PTPs, which were then subjected to immunoprecipitation for the analysis of phosphatase activity. We assumed that under this condition, the PTPs more reversibly inactivated by NO in cells would be found by in vitro assay to have higher phosphatase activity. The SHP-1, SHP-2, and PTP1B but not TC-PTP expressed in eNOS transfectants were found to be inactivated by insulin stimulation (Fig. 4B). The degree of PTP labeling by the PEO probe (Fig. 4A) was inversely correlated with the level of reversible inactivation (Fig. 4B) in the four phosphatases, suggesting that the two independent methods could be used reliably to monitor the status of endogenous PTPs.
FIGURE 4.
NO-mediated inactivation of endogenous PTPs is important for enhanced insulin responsiveness. A, COS-7 transfectants were stimulated with insulin for 5 min and then harvested at pH 6. 0 in the presence of 1 mm PEO probe. Biotinylated proteins were precipitated by streptavidin paramagnetic particles, eluted, and subjected to immunoblotting with antibodies as indicated (left panel). The right panel shows the results of a densitometric analysis of the gel image as the ratio of biotinylated PTP relative to total PTP in lysate. The data are presented as the means ± S.E. (n = 3). *, p < 0.05, versus insulin-treated mock transfectants. B, COS-7 transfectants were stimulated with insulin for 5 min and harvested in the presence of 15 mm iodoacetic acid. Endogenous PTPs were immunoprecipitated from an aliquot of total lysate (2 mg) and then subjected to in vitro phosphatase activity assay using p-nitrophenyl phosphate as a substrate. The data are presented as the means ± S.E. (n = 3). *, p < 0.05, versus insulin-treated mock transfectants. C, COS-7 transfectants were pretreated with 2 mm sodium orthovanadate for 60 min before insulin stimulation. Immunoprecipitated (IP) insulin receptor and aliquots of total lysate (25 μg) were subjected to immunoblotting with antibodies as indicated (left panel). The right panel shows the results of densitometric analysis of the gel image as the ratio of phosphorylated InRβ relative to total InRβ. A.U., arbitrary unit. Similar results were observed in two independent experiments.
We further investigated the role of NO in the enhancement of insulin responsiveness and asked whether insulin-induced NO production would primarily suppress PTP activity instead of promoting the kinase activity of the insulin receptor. To do this, we pre-exposed both mock transfected cells and eNOS transfectants to the PTP inhibitor orthovanadate before they were stimulated with insulin. We hypothesized that the orthovanadate-associated PTPs in eNOS transfectants would remain inactive when stimulated with insulin, and if this were the case, they could be considered insensitive to the level of NO in cells. Under these conditions, we observed that there was no difference in insulin responsiveness between control cells and eNOS transfectants in the presence of orthovanadate (Fig. 4C). The prolonged and enhanced phosphorylation of the insulin receptor in insulin-treated eNOS transfectants (Fig. 1) no longer existed when endogenous PTPs were inactivated by orthovanadate (Fig. 4C). Taken together, these findings suggest that one key determinate of NO-mediated enhancement of signaling in response to insulin stimulation is inactivation of PTPs.
Activation of eNOS Enhanced Phosphorylation of IRS-1 and PKB/AKT in Signaling Response to a Physiological Level of Insulin
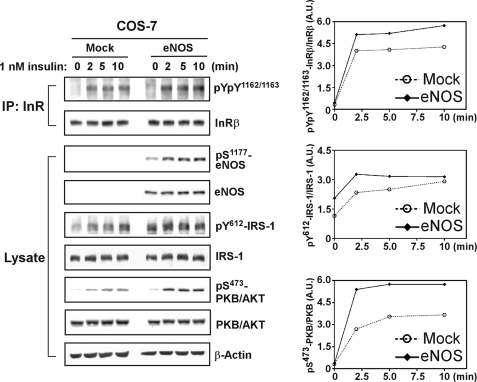
Having demonstrated that NO-induced inactivation of PTPs is a key mechanism in enhanced activation of insulin receptor (Figs. 1 and 4), we further examined the phosphorylation status of a downstream adaptor and a protein kinase. Insulin receptor substrate-1 (IRS-1) and PKB/AKT, both of which are critical effectors in the phosphatidylinositol 3-kinase pathway that mediates various intracellular responses to insulin (33), were analyzed in COS-7 cells ectopically expressing eNOS. The cells were stimulated with 1 nm insulin, a physiological concentration sufficient to promote maximal glucose transport in adipocytes (34). Under this condition, we observed that the activation loop Tyr residues 1162 and 1163 of the insulin receptor β-subunit were more highly phosphorylated in insulin-stimulated eNOS transfectants than in control cells (Fig. 5). This finding suggests that the signaling induced by a physiological level of insulin is capable of activating eNOS for NO-mediated inhibition of endogenous insulin receptor phosphatases. Other experiments made use of COS-7 transfectants treated with 1 nm insulin, where the phosphorylation of IRS-1 and PKB/AKT was monitored by immunoblotting with phospho-specific antibodies. As shown in Fig. 5, ectopic expression of eNOS enhanced the phosphorylation of IRS-1 and PKB/AKT in response to insulin, compared with the signaling response in the control cells. Interestingly, even though the ectopic expression level was lowered significantly, we observed a consistent function of eNOS in promoting the phosphorylation of PKB/AKT (supplemental Fig. S3). These results indicate a positive role for eNOS-produced NO in facilitating the activation of the insulin receptor and its downstream signaling cascade in response to physiological insulin stimulation.
FIGURE 5.
NO enhances phosphorylation of IRS-1 and PKB/AKT in signaling response to physiological levels of insulin. COS-7 cells ectopically expressing human eNOS were stimulated with insulin (1 nm). Insulin receptor β-subunit (InRβ) was immunoprecipitated (IP) and then subjected to immunoblotting with anti-Tyr(P)1162/Tyr(P)1163 and subsequently with anti-InRβ antibodies. Aliquots of total lysate (25 μg) were also subjected to immunoblotting with antibodies as indicated. The right panel shows a densitometric analysis of the gel image as a ratio of phosphorylated InRβ relative to total InRβ (top right panel), phosphorylated IRS-1 relative to total IRS-1 (middle right panel), and phosphorylated PKB/AKT relative to PKB/AKT (bottom right panel). A.U., arbitrary unit. Similar results were observed in two independent experiments.
Endogenous eNOS Is Essential for Activation of Insulin-induced Signaling in Endothelial Cells
Using the gain-of-function model, our data already link the function of eNOS to the regulation of signaling in response to insulin (Figs. 1–5). To explore the potential regulatory role of endogenous eNOS in insulin responsiveness, we examined the phosphorylation status of the insulin receptor and its downstream effectors in mouse endothelial MS-1 cells exposed to a physiological concentration of insulin (1 nm), effectively activating the insulin receptor-mediated signaling cascade (Fig. 6). MS-1 cells express a significant level of endogenous eNOS (supplemental Fig. S4), which was ablated by RNA interference via electroporation of cells with specific siRNA oligonucleotides (Fig. 6). Importantly, although the signaling cascade was induced significantly in the control MS-1 cells, the insulin receptor was only marginally activated in MS-1 transfectants treated with insulin, as monitored by a small increase in phosphorylation of Tyr residues 1162 and 1163 of the receptor (Fig. 6). Moreover, we also observed that the ablation of eNOS by RNA interference led to a lower degree of insulin-stimulated phosphorylation of IRS-1 and PKB/AKT in MS-1 transfectants, compared with the signaling response in the control cells (Fig. 6). Taken together, these results reveal an essential role for eNOS in insulin signaling and demonstrate that NO production mediated by the activation of endogenous eNOS is a quintessential mechanism in the promotion of insulin responsiveness.
FIGURE 6.
Ablation of eNOS by RNA interference suppresses insulin-induced activation of InRβ, IRS-1, and PKB in MS-1 endothelial cells. MS-1 cells were electroporated with scramble siRNA oligonucleotides (control) or siRNA oligonucleotides to eNOS (+siRNA). Two days after transfection, the cells were deprived of serum for 6 h and then stimulated with 1 nm insulin for the indicated times. Insulin receptor β-subunit (InRβ) was immunoprecipitated (IP) and then subjected to immunoblotting with antibodies as indicated. Aliquots of total lysate (25 μg) were also subjected to immunoblotting with antibodies as indicated. The right panel shows a densitometric analysis of the gel image as a ratio of phosphorylated InRβ relative to total InRβ (top right panel), phosphorylated IRS-1 relative to total IRS-1 (middle right panel), and phosphorylated PKB/AKT relative to PKB/AKT (bottom right panel). A.U., arbitrary unit. Similar results were observed in two independent experiments.
DISCUSSION
Previous findings have suggested that eNOS-mediated NO synthesis is a prerequisite for the maintenance of proper insulin sensitivity and carbohydrate metabolism in insulin-targeted tissues (11–13). Nevertheless, the molecular basis for the NO action that facilitates the insulin responsiveness remains an elusive unknown. In this study, we showed that insulin-induced NO targets endogenous PTPs, suppressing phosphatase activity in eNOS-expressed cells. These results demonstrate for the first time that as soon as the insulin receptor is activated, newly synthesized NO acts as a second messenger, rapidly inactivating the PTPs responsible for tyrosine dephosphorylation of insulin receptors. The inactivation of insulin receptor phosphatases occurs concomitantly with the enhanced activity of insulin receptor and downstream effectors IRS-1 and PKB/AKT. Importantly, the enhanced activity of insulin receptor was found to be dependent upon NO production and PTP inactivation, because the administration of NO scavenger and PTP inhibitor ablated the NO effect on insulin the signaling pathway. Moreover, using endothelial cells as a physiological model, we also showed that NO produced by endogenous eNOS is essential for promoting the activation of insulin receptor, IRS-1, and PKB/AKT in signaling cascade response to insulin stimulation. Thus, we have provided insight into the role of NO in the regulation of insulin sensitivity.
In addition to its role in regulating glucose metabolism in liver, muscle, and fat, insulin also acts as a vasodilator by activation of eNOS and subsequent NO production in endothelia (12). It has been shown that a signaling cascade initiated from the insulin receptor, IRS-1, phosphatidylinositol 3-kinase, PKB/AKT, and eNOS is responsible for insulin-stimulated NO production (16). Intriguingly, in this study we further demonstrate that eNOS-dependent production of NO in endothelia has a positive regulatory role that promotes insulin receptor-directed signal transduction, presumably because of NO-mediated S-nitrosylation and inactivation of insulin receptor phosphatases. As a consequence, NO plays an essential role in inactivation of the PTPs, which provide the inhibitory constraint upon the system, thus facilitating insulin action in endothelia for a high level of NO production. We propose that such a regulatory mechanism is the key to boosting the vasodilator function of endothelia soon after stimulation by insulin.
Our observation that selective chemical labeling of Cys residues with low pKa by the PEO probe suggests that the active site Cys of endogenous PTPs is the primary target of NO (Figs. 2–4). We have shown previously that NO-mediated S-nitrosylation creates only the reversible modification but not highly oxidized and irreversible modification of the active site Cys in PTPs (20). The reversible S-NO form remains at the active site Cys even under severe nitrosative stress (20). Because insulin receptor-induced signal transduction is transient, strictly reversible Cys modification may be critical for the regulation of enzymatic activity of insulin receptor phosphatases. After the initial inhibition of PTP activity that leads to enhanced insulin receptor activity, cellular reductants may convert the S-nitrosylated Cys residue back to a reduced form, thus reactivating PTPs for tyrosine dephosphorylation of the insulin receptor and terminating the signaling response to insulin stimulation.
In this study, the key to visualizing several PTPs undergoing NO-mediated inactivation in insulin-induced signaling was the PEO probe. Although the biotin switch method is known to be a useful platform for detecting S-nitrosylated proteins in various biological systems (21, 35), including animal tissues (36) and cultured mammalian cells (37, 38), this method could not be used in our study because none of the endogenous PTPs with antibodies available could be identified in insulin-stimulated eNOS transfectants following the typical procedure of the biotin switch method. To date, only one member of the PTP family has been found by the biotin switch method to be a S-nitrosylated protein, although in that study the cells were exposed to an extremely high level of NO donor (37). Under severe nitrosative stress, the biotin switch method may have been sensitive enough to differentiate S-nitrosylated PTPs from the reduced PTPs in cell lysates. However, based on our own experience during the course of this study, it would be unlikely that the biotin switch method could be used directly to visualize S-nitrosylated PTPs in signaling response to a physiological stimulus, which in this study was insulin. For this task, the PEO probe labeling proved to be useful.
Previous literature has suggested that reactive oxygen species (ROS) (5, 39) and NO (14–16), both considered second messengers that rapidly react with a low pKa Cys residue of cellular protein, are produced shortly after insulin stimulation. Moreover, according to in vivo studies, ROS have been found capable of oxidizing and inactivating PTPs, which function as insulin receptor phosphatases (40, 41). Therefore, it would be difficult to define specifically the effect of ROS or NO on the regulation of insulin signaling through Cys modification of endogenous PTPs. To clarify the role of NO, COS-7 cells ectopically expressing eNOS were employed in this study. Although COS-7 cells have not been used typically as a model for investigation of insulin signaling and glucose metabolism, their lack of endogenous eNOS (26), inducible NOS, and neuronal NOS3 provides us with the opportunity to examine the role of insulin-induced NO production in the gain-of-function eNOS transfectants. Comparing control cells with eNOS-transfected cells, we showed unambiguously that NO induces the inactivation of SHP-1, SHP-2, and PTP1B, concomitant with the enhanced phosphorylation of insulin receptor in signaling response to insulin stimulation. In contrast, TC-PTP, which also functions as an insulin receptor phosphatase (5, 31, 42), was not targeted by NO in the experimental condition used in this study. TC-PTP may be oxidized and inactivated through a ROS-mediated mechanism in cells treated with insulin (5). Our findings combined with others suggest that NO and ROS may regulate specific fractions of endogenous PTPs in response to insulin stimulation. NO- and ROS-mediated inhibition of phosphatase activity may synergistically contribute to insulin sensitivity. Therefore, more research is needed to elucidate the fine-tuning process involved in the downstream signaling that is responsible for glucose uptake and metabolism controlled by NO or ROS.
Supplementary Material
Acknowledgment
We are grateful to Dr. Leonard Rabinow for critical reading of the manuscript.
This work was supported by Taiwan National Science Council Grants 97-3112-B-002-005 and 98-3112-B-001-028). This work was also supported by funding from Academia Sinica (to T.-C. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Y. C. Lai and T.-C. Meng, unpublished data.
- PTP
- protein-tyrosine phosphatase
- IRS
- insulin receptor substrate
- eNOS
- endothelial nitric-oxide synthase
- siRNA
- small interfering RNA
- DTT
- dithiothreitol
- WT
- wild type
- BSA
- bovine serum albumin
- ROS
- reactive oxygen species
- c-PTIO
- 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazole-1-oxyl-3-oxide.
REFERENCES
- 1.Saltiel A. R., Kahn C. R. (2001) Nature 414, 799–806 [DOI] [PubMed] [Google Scholar]
- 2.Tang S., Le-Tien H., Goldstein B. J., Shin P., Lai R., Fantus I. G. (2001) Diabetes 50, 83–90 [DOI] [PubMed] [Google Scholar]
- 3.Cheng A., Dubé N., Gu F., Tremblay M. L. (2002) Eur. J. Biochem. 269, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 4.Asante-Appiah E., Kennedy B. P. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E663–E670 [DOI] [PubMed] [Google Scholar]
- 5.Meng T. C., Buckley D. A., Galic S., Tiganis T., Tonks N. K. (2004) J. Biol. Chem. 279, 37716–37725 [DOI] [PubMed] [Google Scholar]
- 6.Baron A. D., Zhu J. S., Marshall S., Irsula O., Brechtel G., Keech C. (1995) Am. J. Physiol. 269, E709–E715 [DOI] [PubMed] [Google Scholar]
- 7.Roy D., Perreault M., Marette A. (1998) Am. J. Physiol. 274, E692–699 [DOI] [PubMed] [Google Scholar]
- 8.Balon T. W., Nadler J. L. (1997) J. Appl. Physiol. 82, 359–363 [DOI] [PubMed] [Google Scholar]
- 9.Young M. E., Radda G. K., Leighton B. (1997) Biochem. J. 322, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higaki Y., Hirshman M. F., Fujii N., Goodyear L. J. (2001) Diabetes 50, 241–247 [DOI] [PubMed] [Google Scholar]
- 11.Shankar R. R., Wu Y., Shen H. Q., Zhu J. S., Baron A. D. (2000) Diabetes 49, 684–687 [DOI] [PubMed] [Google Scholar]
- 12.Duplain H., Burcelin R., Sartori C., Cook S., Egli M., Lepori M., Vollenweider P., Pedrazzini T., Nicod P., Thorens B., Scherrer U. (2001) Circulation 104, 342–345 [DOI] [PubMed] [Google Scholar]
- 13.Cook S., Hugli O., Egli M., Ménard B., Thalmann S., Sartori C., Perrin C., Nicod P., Thorens B., Vollenweider P., Scherrer U., Burcelin R. (2004) Diabetes 53, 2067–2072 [DOI] [PubMed] [Google Scholar]
- 14.Zeng G., Quon M. J. (1996) J. Clin. Invest. 98, 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng G., Nystrom F. H., Ravichandran L. V., Cong L. N., Kirby M., Mostowski H., Quon M. J. (2000) Circulation 101, 1539–1545 [DOI] [PubMed] [Google Scholar]
- 16.Montagnani M., Chen H., Barr V. A., Quon M. J. (2001) J. Biol. Chem. 276, 30392–30398 [DOI] [PubMed] [Google Scholar]
- 17.Kapur S., Bédard S., Marcotte B., Côté C. H., Marette A. (1997) Diabetes 46, 1691–1700 [DOI] [PubMed] [Google Scholar]
- 18.Engeli S., Janke J., Gorzelniak K., Böhnke J., Ghose N., Lindschau C., Luft F. C., Sharma A. M. (2004) J. Lipid Res. 45, 1640–1648 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y. Y., Huang Y. F., Khoo K. H., Meng T. C. (2007) Methods 42, 243–249 [DOI] [PubMed] [Google Scholar]
- 20.Chen Y. Y., Chu H. M., Pan K. T., Teng C. H., Wang D. L., Wang A. H., Khoo K. H., Meng T. C. (2008) J. Biol. Chem. 283, 35265–35272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrester M. T., Foster M. W., Stamler J. S. (2007) J. Biol. Chem. 282, 13977–13983 [DOI] [PubMed] [Google Scholar]
- 22.Caselli A., Camici G., Manao G., Moneti G., Pazzagli L., Cappugi G., Ramponi G. (1994) J. Biol. Chem. 269, 24878–24882 [PubMed] [Google Scholar]
- 23.Li S., Whorton A. R. (2003) Arch Biochem. Biophys. 410, 269–279 [DOI] [PubMed] [Google Scholar]
- 24.Yu C. X., Li S., Whorton A. R. (2005) Mol. Pharmacol. 68, 847–854 [DOI] [PubMed] [Google Scholar]
- 25.Barrett D. M., Black S. M., Todor H., Schmidt-Ullrich R. K., Dawson K. S., Mikkelsen R. B. (2005) J. Biol. Chem. 280, 14453–14461 [DOI] [PubMed] [Google Scholar]
- 26.Iwakiri Y., Satoh A., Chatterjee S., Toomre D. K., Chalouni C. M., Fulton D., Groszmann R. J., Shah V. H., Sessa W. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19777–19782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z. Y., Dixon J. E. (1993) Biochemistry 32, 9340–9345 [DOI] [PubMed] [Google Scholar]
- 28.He X. M., Carter D. C. (1992) Nature 358, 209–215 [DOI] [PubMed] [Google Scholar]
- 29.Dubois M. J., Bergeron S., Kim H. J., Dombrowski L., Perreault M., Fournès B., Faure R., Olivier M., Beauchemin N., Shulman G. I., Siminovitch K. A., Kim J. K., Marette A. (2006) Nat. Med. 12, 549–556 [DOI] [PubMed] [Google Scholar]
- 30.Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A. L., Normandin D., Cheng A., Himms-Hagen J., Chan C. C., Ramachandran C., Gresser M. J., Tremblay M. L., Kennedy B. P. (1999) Science 283, 1544–1548 [DOI] [PubMed] [Google Scholar]
- 31.Galic S., Klingler-Hoffmann M., Fodero-Tavoletti M. T., Puryer M. A., Meng T. C., Tonks N. K., Tiganis T. (2003) Mol. Cell. Biol. 23, 2096–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouwens D. M., van der Zon G. C., Maassen J. A. (2001) Mol. Cell. Endocrinol. 175, 131–140 [DOI] [PubMed] [Google Scholar]
- 33.Whiteman E. L., Cho H., Birnbaum M. J. (2002) Trends Endocrinol. Metab. 13, 444–451 [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez E., McGraw T. E. (2006) Mol. Biol. Cell 17, 4484–4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffrey S. R., Erdjument-Bromage H., Ferris C. D., Tempst P., Snyder S. H. (2001) Nat. Cell Biol. 3, 193–197 [DOI] [PubMed] [Google Scholar]
- 36.Hao G., Derakhshan B., Shi L., Campagne F., Gross S. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuncewicz T., Sheta E. A., Goldknopf I. L., Kone B. C. (2003) Mol. Cell Proteomics 2, 156–163 [DOI] [PubMed] [Google Scholar]
- 38.Greco T. M., Hodara R., Parastatidis I., Heijnen H. F., Dennehy M. K., Liebler D. C., Ischiropoulos H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7420–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahadev K., Zilbering A., Zhu L., Goldstein B. J. (2001) J. Biol. Chem. 276, 21938–21942 [DOI] [PubMed] [Google Scholar]
- 40.Meng T. C., Fukada T., Tonks N. K. (2002) Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 41.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. (2005) Cell 120, 649–661 [DOI] [PubMed] [Google Scholar]
- 42.Galic S., Hauser C., Kahn B. B., Haj F. G., Neel B. G., Tonks N. K., Tiganis T. (2005) Mol. Cell. Biol. 25, 819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.