Abstract
A new cis-acting element, sterol regulatory element-binding protein-1 (SREBP-1) binding site, within the 5′-flanking human androgen receptor (AR) promoter region and its binding transcription factor, SREBP-1, was identified to regulate AR transcription in AR-positive human prostate cancer cells. We further characterized the molecular mechanism by which a novel anti-β2-microglobulin monoclonal antibody (β2M mAb), shown to induce massive cell death in a number of human and mouse cancer cell lines, interrupted multiple cell signaling pathways in human prostate cancer cells. β2M mAb decreased AR expression through inactivation of MAPK and SREBP-1. By inactivation of MAPK, β2M mAb decreased prostate cancer cell proliferation and survival. By inhibition of SREBP-1, β2M mAb reduced fatty acid and lipid levels, an integral component of cell membrane, cell signaling mediators, and energy metabolism. These results provide for the first time a molecular link between the β2M intracellular signaling axis mediated by MAPK and SREBP-1 and involving lipid signaling, which collectively regulates AR expression and function. Antagonizing β2M by β2M mAb may be an effective therapeutic approach simultaneously targeting multiple downstream signaling pathways converging with MAPK, SREBP-1, and AR, important for controlling prostate cancer cell growth, survival, and progression.
Keywords: Antibodies, MAP Kinases (MAPKs), Signal Transduction, Transcription Factors, Tumor, SREBP-1, Androgen Receptor, β2-microglobulin, Prostate Cancer
Introduction
β2-Microglobulin (β2M)3 is a co-receptor of a major histocompatibility complex class I antigen. β2M has been implicated in the regulation of the host immune mechanism and is essential for the recognition of foreign antigens by T-lymphocytes (1). Recent reports from our laboratory and others assigned additional biological functions to β2M as a diagnostic and prognostic indicator for multiple myeloma, prostate, and breast cancers (2–5); a growth factor and a signaling molecule (6, 7); a new androgen and androgen receptor (AR) target gene (8); and an attractive new therapeutic target for both liquid (9) and solid (10, 11) tumor malignancies. Blockade of β2M and its related signaling pathways by sequence-specific siRNA or antibody resulted in the inhibition of AR expression and activity and the induction of extensive prostate cancer cell death in vitro as well as prostate tumor regression in immune-compromised mice (7, 10). In addition, anti-β2M monoclonal antibody (β2M mAb) has been shown not to significantly affect the growth of normal cells, consistent with experimental observations where transgenic mice with a β2M deficit had normal organ function and life expectancy (9, 10, 12). Therefore, β2M and its signaling axis may offer an opportunity for improving the clinical targeting of prostate cancer.
AR is a key growth and survival regulatory transcription factor for androgen target organs during normal development and neoplastic progression. Recognition of the importance of the AR signaling axis, particularly in castration-resistant prostate cancer, has prompted discoveries targeting androgen biosynthetic pathways using abiraterone as an agent for a Phase III trial (13, 14). Novel strategies to target AR directly through AR gene transcription and translation (10) or interfering in the interaction between AR and its co-factors and their downstream functions in prostate cancer cells have also been successfully attempted (15–17). AR activity is regulated by a host of factors including steroid hormones, thyroid hormones, vitamin D3 (18), insulin-like growth factor I, insulin-like growth factor I receptor, keratinocyte growth factor, epidermal growth factor (19), interleukin-6 (20), and agents elevating and activating intracellular cAMP, G protein-coupled receptors, or a PKA signaling pathway (21, 22). The details of the transcriptional/translational mechanisms regulating AR within cancer cells remain unclear. Previous studies demonstrated that the 5′-flanking region of human AR promoter activity can be regulated by transcription factors Sp1 (23), cAMP-responsive element-binding protein (24), FOXO3a (25), and lymphoid enhancer-binding factor-1/T cell-specific factor (LEF-1/TCF) (26), whose activities are subjected to modulation by several known cell signaling pathways such as cAMP/PKA, PI3K/Akt, MAPK, and Wnt/β-catenin in prostate cancer cells. In this study, we identified an additional transcription factor, SREBP-1, which affected lipid metabolism and accumulation, as a new downstream transcription factor under regulation by β2M mAb in prostate cancer cells.
SREBP-1 belongs to the SREBP family, which is a basic helix-loop-helix leucine zipper transcription factor (27, 28). Three major isoforms of SREBP have been identified, SREBP-1a, SREBP-1c, and SREBP-2 (28). SREBP-1 has been determined to regulate genes involved in fatty acid and cholesterol biosynthesis (27, 29), whereas SREBP-2 is more specific in the control of cholesterol metabolism (30). Dysregulation of SREBPs and their downstream regulated genes such as fatty acid synthase (FAS), which has been proposed to be a metabolic oncogene (31, 32), was shown to be involved in the development and progression of prostate cancer (33, 34). The expression of SREBP-1 was observed to be highly elevated in clinical human prostate cancer specimens compared with nontumor prostate tissues, and this may be relevant to androgen-refractory progression (34).
The objective of this study is to determine the pleiotropic β2M-mediated signaling mechanism by which a novel monoclonal antibody, β2M mAb, inhibited AR mRNA and protein expression and its transcription activity in AR-positive human prostate cancer cell lines. The results of this study suggest that β2M regulated multiple growth and survival signaling pathways through the control of transcription factors and their modifiers such as AR, MAPK, and PI3K/Akt (7, 10, 35). In particular, we demonstrated that marked down-regulation of AR as the consequence of targeting β2M by β2M mAb was due to the inactivation of a lipogenic transcription factor, SREBP-1, known to be associated with androgen-refectory progression of clinical prostate cancer (34). Accompanying reduction of SREBP-1 expression in prostate cancer cells, β2M mAb also decreased FAS expression and fatty acid and lipid levels, which are the main components of cell membrane and energy storage. Our data reveal for the first time a lipogenic pathway through MAPK and SREBP-1 that is critical for controlling AR expression, activity, and function in prostate cancer cells.
EXPERIMENTAL PROCEDURES
Prostate Cancer Cell Lines, Cell Culture, and Reagents
The LNCaP (androgen-dependent) human prostate cancer cell line and the LNCaP lineage-derived C4-2B bone metastatic subline (androgen-independent) were cultured in T-medium (Invitrogen) supplemented with 5% fetal bovine serum, 100 IU/ml of penicillin, and 100 μg/ml of streptomycin. These prostate cancer lines were maintained in 5% CO2 at 37 °C. β2M mAb was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Human SREBP-1 expression vector and SREBP-1 siRNA were obtained from OriGene Technologies, Inc. (Rockville, MD) and Santa Cruz Biotechnology, respectively. The selective inhibitors for signaling pathways of MAPK/ERK, U0126; PI3K, LY294002; and PKA, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89) were purchased from Cell Signaling Technology, Inc. (Beverly, MA).
RT-PCR
Total RNA was isolated from prostate cancer cells using a RNeasy kit (Qiagen). Total RNA was used as the template for RT according to the manufacturer's instructions (Invitrogen). The oligonucleotide primer sets used for PCR analysis of cDNA were as follows: β2M, 5′-ACGCGTCCGAAGCTTACAGCATTC-3′ (forward) and 5′-CCAAATGCGGCATCTAGAAACCTCCATG-3′ (reverse); AR, 5′-ATGGCTGTCATTCAGTACTCCTGGA-3′ (forward) and 5′-AGATGGGCTTGACTTTCCCAGAAAG-3′ (reverse); PSA, 5′-ATGTGGGTCCCGGTTGTCTTCCTCACCCTGTC-3′ (forward) and 5′-TCAGGGGTTGGCCACGATGGTGTCCTTGATC-3′ (reverse); and GAPDH, 5′-ACCACAGTCCATGCCATCA-3′ (forward) and 5′-TCCACCACCCTGTTGCTGT-3′ (reverse). The thermal profiles for β2M, AR, PSA, and GAPDH cDNA amplification were 25–30 cycles starting with denaturation for 1 min at 94 °C, followed by 1 min of annealing at 64 °C (for β2M), 61 °C (for AR), 55 °C (for PSA), and 60 °C (for GAPDH), and 1 min of extension at 72 °C. RT-PCR products were analyzed by 1.2% agarose gel electrophoresis. Quantity one-4.1.1 Gel Doc gel documentation software (Bio-Rad) was used for quantification of mRNA expression.
Western Blot Analysis
The cell lysates were prepared from prostate cancer cells as described previously (10). The concentration of protein was determined by the Bradford method using Coomassie Plus protein reagent (Pierce). Western blot analysis was performed by a Novex system (Invitrogen). Primary antibodies against human β2M, AR, PSA, SREBP-1, SREBP-2, FAS, MAPK/ERK (Santa Cruz Biotechnology), Akt, phospho-Akt (Ser473), and phospho-p44/p42 MAPK (Thr202/Tyr204) (Cell Signaling Technology) were used. The corresponding secondary antibodies conjugated with horseradish peroxidase were purchased from GE Healthcare. Detection of protein bands was assayed by enhanced chemiluminescence Western blotting detection reagents (GE Healthcare).
Plasmid Construction
A luciferase reporter construct that contained the 5′-flanking region (−5400 to +580) of the full-length human AR (hAR) promoter was kindly provided by Dr. Donald J. Tindall (Mayo Clinic, Rochester, MN). The deletion constructs including, ΔA (deletion of −600 to −40), ΔB (deletion of −1100 to −600), and ΔC (deletion of −1600 to −1100) within the hAR promoter luciferase vector; and ΔEGR-1 binding site (5′-TCGCCCACGCTG-3′, −181 to −170), ΔSREBP-1 binding site (5′-CCTCGCCTCCAC-3′, −347 to −336), and ΔAP-1 binding site (5′-GCTTGGTCATG-3′, −475 to −465) within the hAR/SacI promoter (deletion of −4700 to −740) luciferase vector were generated by a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). All of the plasmid construct DNA sequences were confirmed by DNA sequencing.
Transfection and Luciferase Activity Assay
LNCaP and C4-2B cells were plated at a density of 1.5 × 105 cells/well in 12-well plates 24 h before transfection. Plasmid DNAs were transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Each transfection reaction contained 1.25 μg of tested DNA construct and 0.25 μg of a transfection efficiency control cytomegalovirus promoter β-galactosidase plasmid. After 6 h of incubation, DNA-liposome mixtures were replaced by fresh medium without fetal bovine serum. After overnight incubation, the transfected cells were treated with reagents or vehicles. After 24 h of additional incubation, the cells were harvested and lysed in 1× reporter lysis buffer (Promega). For luciferase activity assay, 20 μl of the lysate supernatant was mixed with 100 μl of the luciferase substrate (Promega) and detected by a luminometer (Monolight 3010 luminometer; PharMingen, San Diego, CA). For β-galactosidase activity assay, 100 μl of the supernatant was mixed with 100 μl of 2× β-galactosidase substrate (200 mm sodium phosphate buffer, pH 7.3, 2 mm MgCl2, 100 mm β-mercaptoethanol and 1.33 mg/ml o-nitrophenyl-β-d-galactopyranoside) and incubated at 37 °C for 30 min. β-Galactosidase activity was detected by a microplate reader (model 680; Bio-Rad) at 405 nm wavelength. The data were presented as the normalized luciferase activity (the means ± S.D.) defined as the luciferase activity normalized to internal control β-galactosidase activity. All of the experiments were performed as three independent experiments with duplicate assays.
Electrophoretic Mobility Shift Assay (EMSA)
LNCaP and C4-2B cells were cultured in T-medium with 5% fetal bovine serum until 80% confluence. The cells were then switched to 1-day complete serum-free condition and then treated with β2M mAb (5 μg/ml), β2M mAb preincubated with β2M or control IgG for an additional 24 h. The nuclear extracts were prepared by a NucBusterTM protein extraction kit (Novagen, San Diego, CA). The specific double-stranded oligonucleotide of SREBP-1 binding site within hAR promoter region used as a probe was 5′-TTCCTCCCTCCCTCGCCTCCACCCTGTTGGTT-3′. The double-stranded oligonucleotide was end-labeled with [γ-32P]ATP (3,000 Ci/mmol at 10 mCi/ml) using T4 polynucleotide kinase (New England BioLabs, Beverly, MA). Forty thousand cpm of the labeled probe and 5 μg of nuclear extracts were incubated with EMSA buffer containing 20 mm HEPES (pH 8.0), 100 mm KCl, 0.2 mm EDTA, 0.5 mm dithiothreitol, 20% glycerol, 500 ng of sonicated salmon sperm DNA, and 1 μg of poly(dI-dC) at 30 °C for 30 min. The samples were subjected to 6% nondenaturing DNA retardation gel and a Novex TBE system (Invitrogen). For the competition experiment, a 32P-unlabeled oligonucleotide probe (100×) was preincubated with nuclear extracts for 30 min at room temperature before the addition of the 32P-probe. After electrophoresis, the gels were dried with a Gel Dryer (model 583; Bio-Rad) and exposed to BioMax film (Kodak).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP was performed by a ChIP-IT kit (Active Motif, Inc., Carlsbad, CA). Briefly, LNCaP and C4-2B cells were serum-starved for 24 h and then treated with or without β2M mAb (5 μg/ml) for an additional 24 h. The formaldehyde-fixed chromatins were prepared from these prostate cancer cells and were sheared into 200–1,500 bp of DNA fragments by enzymatic shearing mixture for 10 min at 37 °C. A portion of the fixed and sheared chromatin fragments was reversed and used as input DNA. The other chromatin DNA fragments were immunoprecipitated by anti-SREBP-1 antibody (Santa Cruz Biotechnology), and DNA was extracted and purified from the immunoprecipitate. A PCR primer pair to amplify the SREBP-1 binding site is 5′-TGGCAGCCAGGAGCAGGTATT-3′ (forward) and 5′-TTTCCTGGAGGCCAGCACTCAC-3′ (reverse). A negative PCR primer pair included in the ChIP-IT kit was used as a negative control. To further quantify the PCR products of ChIP, we conducted quantitative real time PCR. Each purified DNA sample was mixed with Power SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a primer pair of the SREBP-1 binding site (see above), and quantitative PCR was performed using an iCycler iQ real time PCR detection system (Bio-Rad) for two independent experiments with duplicate assays. The results were normalized by input (assigned as 1.0-fold without treating β2M mAb) for each cell line.
Immunohistochemical Staining
Primary anti-AR antibody purchased from Santa Cruz Biotechnology (1:100 dilution) was used. Tissue specimens were deparaffinized, rehydrated, and subjected to pressure-cooking antigen retrieval at 125 °C and 20 p.s.i. for 30 s, 10 min of double endogenous enzyme block, 4 °C for overnight primary antibody reaction, and 30 min of EnVision+ dual link and streptavidin-peroxidase system incubation. The signals were detected by adding substrate hydrogen peroxide using diaminobenzidine as the chromogen and counterstained with hematoxylin. The staining reagents were obtained from Dako Corporation (Carpinteria, CA).
Statistical Analysis
The statistical analyses were performed as described previously (35). Student's t test and two-tailed distribution were applied in the analysis of statistical significance.
RESULTS
Blockade of β2M Down-regulated AR and PSA Expression in Human Prostate Cancer Cells
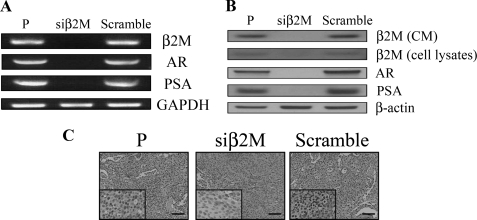
In support of our previous report (7), we observed that β2M knockdown can be achieved efficiently by genetic manipulation using β2M siRNA (siβ2M) in a human prostate cancer cell line, C4-2B. We observed that mRNA levels of β2M, AR, and PSA were dramatically decreased in β2M knockdown C4-2B cells compared with parental (P) and control scramble siRNA (Scramble) C4-2B cells (Fig. 1A). In addition to decreased mRNA levels of β2M, AR, and PSA, endogenous proteins of secreted (from conditioned media) and soluble (from cell lysates) β2M, AR, and PSA were also greatly reduced in siβ2M C4-2B cells compared with P and Scramble C4-2B cells (Fig. 1B). Furthermore, these results were supported by an in vivo subcutaneous C4-2B xenograft mouse model (7) where immunohistochemical staining confirmed that AR expression was greatly decreased in siβ2M-treated C4-2B tumors compared with P and Scramble siRNA-treated C4-2B tumors (Fig. 1C). Mouse serum PSA levels were also markedly decreased in siβ2M-treated C4-2B tumors (1.03 ± 0.52 ng/ml, n = 5, after 28 days of treatment) compared with Scramble siRNA-treated C4-2B tumors (19.70 ± 9.04 ng/ml, n = 5). These in vitro and in vivo data suggested that blockade of intracellular β2M by β2M siRNA greatly inhibited the expression of AR and PSA mRNA and protein in prostate cancer cells.
FIGURE 1.
β2M siRNA inhibited expression of AR and PSA mRNA and protein in prostate cancer cells in vitro and in vivo. A, siβ2M dramatically decreased expression of β2M, AR, and PSA mRNA in C4-2B prostate cancer cells analyzed by RT-PCR. Expression of GAPDH was used as a loading control. P, parental nontransfected C4-2B cells; Scramble, control scramble siRNA transfected C4-2B cells. B, siβ2M also markedly inhibited expression of secreted β2M (from conditioned media, CM), soluble β2M (from cell lysates), AR, and PSA protein in siβ2M C4-2B cells compared with P and Scramble C4-2B cells assayed by Western blot. β-Actin was used as an internal loading control. C, immunohistochemical staining analysis of AR in P (parental, untreated), siβ2M (β2M siRNA-treated), and Scramble (control scramble siRNA-treated) C4-2B tumor specimens from subcutaneous mouse xenografts (7). This result revealed that siβ2M greatly inhibited AR expression in C4-2B subcutaneous tumors in vivo. Scale bars, 100 μm.
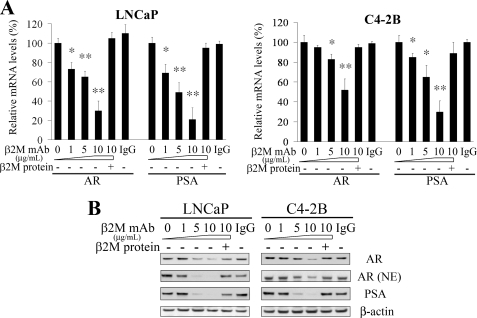
To test whether interrupting β2M from extracellular sources may also affect AR and PSA expression as well as cell growth of prostate cancer cells, we employed a new β2M mAb to neutralize extracellular β2M in an attempt to interrupt β2M downstream signaling. As shown in Figs. 2, β2M mAb (0 to 10 μg/ml) significantly decreased both steady-state mRNA levels and protein amounts of AR and PSA in LNCaP and C4-2B cells in a dose-dependent pattern determined by semi-quantitative RT-PCR and Western blot. Considering the specificity of the inhibitory effect of β2M mAb, purified β2M protein could rescue AR and PSA inhibition by β2M mAb in prostate cancer cells. Control IgG did not affect AR and PSA expression. In addition to decreasing endogenous total AR protein, β2M mAb also inhibited nuclear AR levels in LNCaP and C4-2B cells (Fig. 2B). These data suggested that antagonizing extracellular β2M by β2M mAb reduced AR and PSA transcription and translation in prostate cancer cells.
FIGURE 2.
β2M mAb decreased expression of AR and PSA in prostate cancer cells. A, β2M mAb decreased the steady-state mRNA levels of AR and PSA in a dose-dependent manner (0–10 μg/ml) in LNCaP and C4-2B AR-positive prostate cancer cells determined by semi-quantitative RT-PCR. The inhibitory effect was restored by preincubation of β2M mAb with purified β2M protein. Isotype control IgG (10 μg/ml) did not significantly affect AR and PSA mRNA expression. The relative mRNA levels of AR and PSA, normalized by GAPDH mRNA, were measured by Gel Doc gel documentation software (Bio-Rad). The relative mRNA levels (%) were assigned as 100% in the absence of β2M mAb treatment. *, p < 0.05; **, p < 0.005, significant differences from the β2M mAb-untreated group. The data represent the means ± S.D. of independent triplicate experiments. B, β2M mAb also inhibited total AR, nuclear AR (NE, nuclear extracts) and PSA protein expression in a dose-dependent pattern (0–10 μg/ml) in LNCaP and C4-2B cells assayed by Western blot. The inhibitory effect was abrogated by preincubation of β2M mAb with β2M protein. Control IgG (10 μg/ml) did not change AR and PSA protein expression. β-Actin was used as an internal loading control.
β2M mAb Blocked AR Transcription via Down-regulation of Sterol Regulatory Element-binding Protein-1 Activity
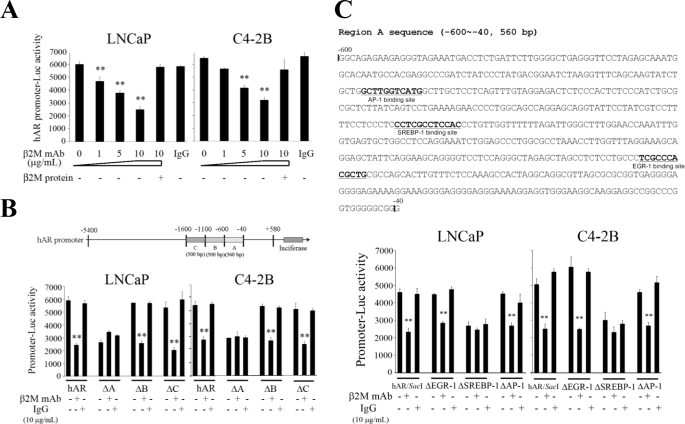
AR gene transcription was studied in LNCaP and C4-2B cells by transfecting these cells with either the full-length or the deletion constructs of the 5′-flanking region of hAR promoter luciferase reporters (i.e. from −5400 to +580). Consistent with previous RT-PCR and Western blot results (Fig. 2), β2M mAb (0–10 μg/ml) significantly decreased the full-length hAR promoter luciferase activity in a concentration-dependent pattern (Fig. 3A), and purified β2M protein was shown to restore the inhibition. Additionally, isotype-matched control IgG failed to decrease the hAR promoter luciferase activity in LNCaP and C4-2B cells. To further identify the responsible cis-acting element in the hAR promoter region, we conducted a hAR promoter deletion study. Three deletion constructs of hAR promoter fragment (ΔA, ΔB, and ΔC; Fig. 3B) were generated and confirmed the DNA sequence. After transfection into LNCaP and C4-2B cells, β2M mAb significantly inhibited the activities of the full-length hAR, ΔB (deletion of −1100 to −600), and ΔC (deletion of −1600 to −1100) promoter luciferase constructs (Fig. 3B). However, β2M mAb did not affect ΔA promoter luciferase activity (deletion of −600 to −40; Fig. 3B). Among the three deletion constructs, decreased basal AR promoter luciferase activity was observed only in the ΔA construct when tested in LNCaP and C4-2B cells. Control IgG did not significantly change the promoter activities of all these vector constructs. These results suggested that a potential cis-acting element mediating AR transcriptional activity by β2M mAb may reside at region A. Because the full-length hAR promoter reporter construct is ∼6 kb in length (from −5400 to +580), we further used a restriction enzyme, SacI, to generate a shorter promoter luciferase construct, a hAR/SacI vector (2 kb only, deletion of −4700 to −740), and tested this new reporter vector activity in LNCaP and C4-2B cells exposed to either β2M mAb or IgG. The basal luciferase activity of the truncated hAR/SacI promoter vector decreased slightly when compared with the full-length hAR promoter activity assayed in LNCaP and C4-2B cells (Fig. 3, B and C). These results indicated that the cis-elements spanning from −4700 to −740 of the hAR promoter were not responsible for AR transcriptional regulation in human prostate cancer cells.
FIGURE 3.
SREBP-1 binding site within the 5′-flanking promoter region of hAR gene is responsible for AR transcriptional activity mediated by β2M mAb. A, β2M mAb decreased the full-length hAR promoter (−5400 to +580) luciferase activity with a concentration-dependent pattern (0–10 μg/ml) in LNCaP and C4-2B cells. Purified β2M protein restored the inhibitory effect of hAR promoter activity regulated by β2M mAb. Control IgG did not suppress hAR promoter reporter activity. B, region A (−600 to −40) is responsible for hAR promoter luciferase activity mediated by β2M mAb in LNCaP and C4-2B cells. β2M mAb (10 μg/ml) significantly decreased the promoter luciferase activities of the deleted region B (ΔB, −1100 to −600) and C (ΔC, −1600 to −1100) in hAR promoter report constructs but did not affect the luciferase activity of the ΔA (−600 to −40) construct. Isotype control IgG (10 μg/ml) did not significantly change the promoter reporter activities of all deletion constructs. C, the DNA sequence of region A (560 bp) contains an EGR-1 binding site (−181 to −170), a SREBP-1 binding site (−347 to −336), and an AP-1 binding site (−475 to −465). Among the three deletion constructs (ΔEGR-1, ΔSREBP-1, and ΔAP-1 binding sites), the promoter luciferase activities of ΔEGR-1 and ΔAP-1 binding site constructs were significantly inhibited by β2M mAb in LNCaP and C4-2B cells. Only a slight decrease of promoter luciferase activity was observed in a ΔSREBP-1 binding site construct treated with β2M mAb in prostate cancer cells. Control IgG did not change the promoter reporter activities of these three deletion constructs. All of the promoter luciferase activity data (in Fig. 3) were normalized by internal control β-galactosidase activity and expressed as the means ± S.D. of three independent duplicate experiments. **, p < 0.005.
To determine the precise cis-acting elements in region A of the AR promoter responsible for β2M mAb-mediated regulation, we searched the data base and predicted three potential cis-acting elements in this region: the EGR-1 binding site (−181 to −170), SREBP-1 binding site (−347 to −336), and AP-1 binding site (−475 to −465) (Fig. 3C). Subsequently, we generated three respective deletion constructs and tested their luciferase reporter activities in prostate cancer cells exposed to either β2M mAb or control IgG. In resemblance to the truncated hAR/SacI luciferase construct, we found that β2M mAb inhibited ΔEGR-1 and ΔAP-1 but not ΔSREBP-1 binding site deletion construct activities in LNCaP and C4-2B cells (Fig. 3C). Decreased basal promoter luciferase activity was noted in the ΔSREBP-1 binding site deletion construct (Fig. 3C). Control IgG did not significantly change the promoter reporter activities of all deletion constructs. These data, taken together, demonstrated that the SREBP-1 binding site located within the 5′-flanking hAR promoter region is important for hAR promoter activity regulated by β2M mAb in AR-positive human prostate cancer cells.
Confirmation of Nuclear SREBP-1 Protein Interaction with the SREBP-1 Binding Site within the hAR Promoter by EMSA and ChIP Assay
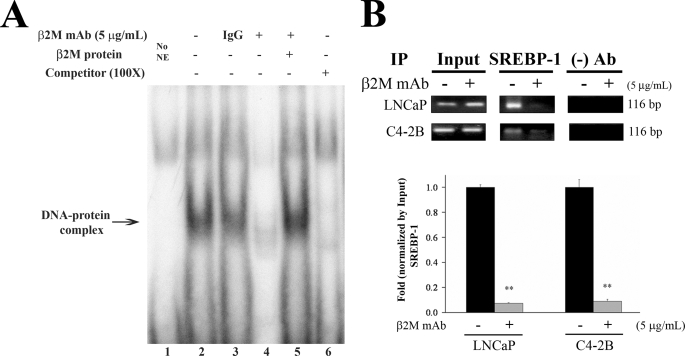
We conducted EMSA to further validate whether the SREBP-1 transcription factor is a key protein regulating AR transcriptional activity through β2M mAb in prostate cancer cells. As shown in Fig. 4A, nuclear extracts prepared from β2M mAb-treated LNCaP cells showed a greatly decreased the 32P-oligo-DNA and SREBP-1 binding complex (lane 4) compared with the DNA-nuclear protein complex without β2M mAb or control IgG treatment in LNCaP cells (lanes 2 and 3). Purified β2M protein was shown to abrogate the complex formation decreased by β2M mAb exposure (lane 5). The specificity of the binding of 32P-oligo-DNA probe with nuclear SREBP-1 protein in LNCaP cells was shown by the effective competition of 32P unlabeled oligo-DNA for this binding complex (lane 6).
FIGURE 4.
β2M mAb inhibited the interaction between SREBP-1 and its binding cis-acting element located in the 5′-flanking hAR promoter region in prostate cancer cells. A, EMSA. LNCaP cells were exposed to β2M mAb, control IgG (5 μg/ml), or vehicle for 24 h in serum-free conditions. The cells were harvested, and nuclear extracts were prepared. EMSA was performed by incubating nuclear extracts with the 32P-labeled SREBP-1 oligo-DNA probe and Novex TBE system (Invitrogen). Lane 1, no nuclear extracts (NE) added. Lanes 2 and 3, nuclear SREBP-1-oligo-DNA complex, not affected by control IgG. Lane 4, β2M mAb greatly inhibited this nuclear protein-DNA complex formation. Lane 5, purified β2M protein could rescue the inhibitory binding effect of β2M mAb. Lane 6, this complex was competed off by adding 100-fold 32P-unlabeled specific SREBP-1 oligo-DNA probe. B, ChIP assay. LNCaP and C4-2B cells were treated with or without β2M mAb (5 μg/ml) for 24 h. The chromatin and nuclear proteins were crossed-linked by formaldehyde and sheared by enzymatic shearing mixture (ChIP-IT kit, Active Motif, Inc.) and subjected to immunoprecipitation (IP) assay by anti-SREBP-1 antibody or without antibody as a negative control. In the PCR product from chromatin DNA fragments immunoprecipitated by anti-SREBP-1 antibody as templates, a predicted single DNA band (116 bp, top panel) was amplified and visualized in LNCaP and C4-2B cells. This PCR product was decreased by treatment with β2M mAb in prostate cancer cells. In addition, quantitative real time PCR of ChIP was performed (bottom panel). The results of quantitative PCR showed that β2M mAb significantly down-regulated the interaction between SREBP-1 and the SREBP-1 binding site in the 5′-flanking hAR promoter, with a 13.3 and 11.0-fold decrease in LNCaP and C4-2B cells, respectively. The quantitative PCR data were normalized by input and assigned as 1.0-fold without β2M mAb for each prostate cancer cell line. **, p < 0.005.
We performed ChIP to investigate whether the interaction between nuclear SREBP-1 and its binding cis-acting element is affected by β2M mAb in the chromatin environment in prostate cancer cells. An expected single DNA band (116 bp) was detected by a PCR primer set to amplify a SREBP-1 binding region within hAR promoter in LNCaP and C4-2B cells, whereas the chromatin DNA fragments immunoprecipitated by anti-SREBP-1 antibody were used as templates (Fig. 4B, top panel). The levels of this amplified PCR product were decreased by exposure to β2M mAb in prostate cancer cells (Fig. 4B). In addition, utilizing real time quantitative PCR as a readout, we observed that β2M mAb caused significant reduction of the interaction between SREBP-1 and its binding site within the AR promoter region, with 13.3- and 11.0-fold decreases in LNCaP and C4-2B cells, respectively (Fig. 4B, bottom panel). In summary, EMSA and ChIP data confirmed that β2M mAb inhibited the interaction between nuclear SREBP-1 and its cis-acting element within the AR promoter region, which accounts for the down-regulated AR transcriptional activity in prostate cancer cells.
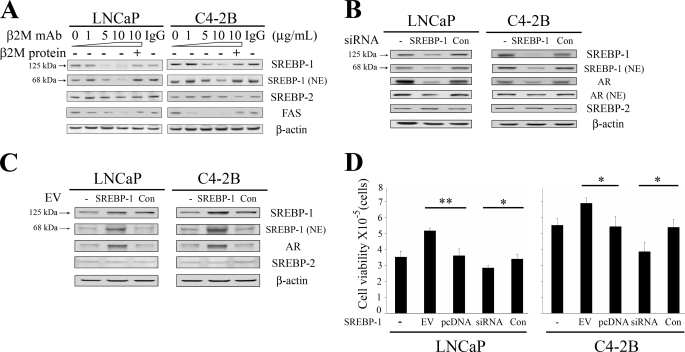
A Triad Relationship among β2M, SREBP-1, and AR Is Involved in the Regulation of Fatty Acid Levels and Cell Viability in Prostate Cancer Cells
We evaluated the potential triad relationship among the expression of β2M, SREBP-1, and AR in prostate cancer cells. As shown in Fig. 5A, β2M mAb (0 to 10 μg/ml) specifically inhibited expression of precursor (125 kDa) and mature nuclear (68 kDa) SREBP-1 proteins in a concentration-dependent manner but did not affect expression of SREBP-2, which is a SREBP-1 isoform. Purified β2M protein rescued the inhibitory effect of endogenous SREBP-1 expression by β2M mAb. Control IgG did not affect SREBP-1 and SREBP-2 expression. A fatty acid biosynthetic oncogene, FAS, which is a downstream target gene of SREBP-1, was also shown to be decreased by β2M mAb in a dose-dependent inhibition (Fig. 5A). In addition, we observed that β2M mAb (5 μg/ml, 24 h treatment) significantly decreased fatty acid levels in LNCaP (20.8 ± 2.9%) and C4-2B (26.6 ± 2.1%) cells. To investigate the role of SREBP-1 in regulating AR expression, we conducted functional studies to knock down and overexpress SREBP-1 in prostate cancer cells. A sequence-specific siRNA of SREBP-1 caused a marked decrease of both precursor and mature nuclear SREBP-1 proteins in LNCaP and C4-2B cells (Fig. 5B). Down-regulation of SREBP-1 by SREBP-1 siRNA also inhibited the expression of total AR and nuclear AR proteins in LNCaP and C4-2B cells (Fig. 5B). In testing the specificity of SREBP-1 siRNA, SREBP-2 expression was shown not to be affected by this siRNA. Control nonspecific siRNA did not inhibit expression of SREBP-1, SREBP-2, and AR. Conversely, overexpressing SREBP-1 by a SREBP-1 expression vector increased expression of precursor and nuclear SREBP-1 as well as AR but not SREBP-2 in LNCaP and C4-2B cells (Fig. 5C). In addition, overexpressing or knocking down SREBP-1 significantly increased or decreased cell viability (Fig. 5D) and fatty acid levels (data not shown) in prostate cancer cells. These data are consistent with previous reports and in aggregate reveal that β2M is a pleiotropic signaling molecule (6, 7, 36) and has a triad relationship with SREBP-1 and AR, which determines the growth and survival of prostate cancer cells. β2M-mediated signaling is important for the maintenance of SREBP-1 expression and SREBP-1 regulates AR expression and prostate cancer cell growth and survival (Fig. 5, B–D). Likewise, a reciprocal relationship has been reported between AR and β2M in which androgens and AR regulated β2M expression (8) and β2M mediated AR expression and prostate cancer cell growth and survival (Figs. 1 and 2) (10).
FIGURE 5.
A triad relationship among β2M, SREBP-1, and AR expression in prostate cancer cells. A, β2M mAb decreased the expression of precursor SREBP-1 (125 kDa), mature nuclear SREBP-1 (68 kDa), and FAS in a concentration-dependent pattern (0–10 μg/ml) in LNCaP and C4-2B cells as determined by Western blot. The inhibitory effect of β2M mAb on endogenous SREBP-1 and FAS expression was restored by preincubation of β2M mAb with purified β2M protein. Isotype control IgG (10 μg/ml) did not affect expression of these proteins. β2M mAb did not change the expression of SREBP-2, which is an isoform of SREBP-1. β-Actin was used as a loading control. B, a sequence-specific siRNA of SREBP-1 decreased the expression of precursor and nuclear SREBP-1 proteins in LNCaP and C4-2B cells. Because of down-regulation of SREBP-1 by SREBP-1 siRNA, total AR, and nuclear AR (NE) expression was also inhibited in LNCaP and C4-2B cells. To test the specificity of SREBP-1 siRNA, SREBP-2 expression was not changed by this siRNA. Control nonspecific siRNA (Con) did not affect SREBP-1, AR, and SREBP-2 expression. C, overexpressing SREBP-1 by a SREBP-1 expression vector (EV) increased the expression of precursor and nuclear SREBP-1 as well as endogenous AR protein in LNCaP and C4-2B cells. SREBP-1 EV did not affect SREBP-2 expression. Con, control empty expression vector. D, overexpressing or knocking down SREBP-1 by SREBP-1 EV or SREBP-1 siRNA significantly increased or decreased LNCaP and C4-2B cell viability after a 4-day transfection. Empty expression vector (pcDNA 3.1; Invitrogen) or nonspecific siRNA (Con) were used as control groups. The cell numbers (cell viability) were measured by a hemacytometer (Hausser Scientific, Horsham, PA) and crystal violet staining method. *, p < 0.05; **, p < 0.005, significant differences from the control groups. The data represent the means ± S.D. of two independent experiments replicated three times.
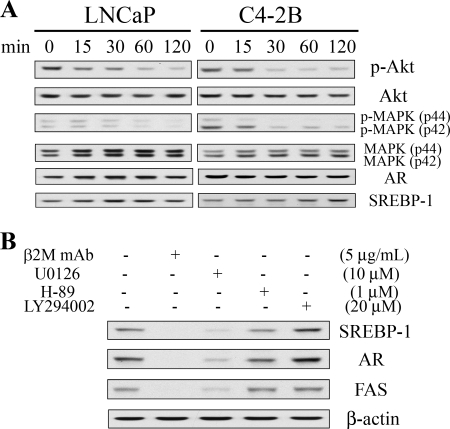
The Involvement of the MAPK/ERK Signaling Pathway in β2M mAb Inhibition of SREBP-1 and AR Expression in Prostate Cancer Cells
To determine the signaling mechanism by which β2M mAb inhibited SREBP-1 and AR expression in prostate cancer cells, we examined the β2M mAb-mediated status of MAPK/ERK and PI3K/Akt signaling pathways, which have been reported to regulate SREBP-1 and AR expression (25, 37, 38). The expression levels of both the phosphorylated and total proteins of Akt and MAPK were analyzed by Western blot. As shown in Fig. 6A, β2M mAb decreased expression of phospho-Akt (Ser473) and phospho-MAPK (Thr202/Tyr204) proteins in LNCaP and C4-2B cells within 2 h in a time-dependent pattern. However, β2M mAb did not suppress total Akt and MAPK expression. We also observed that AR and SREBP-1 proteins were not decreased by β2M mAb within 2 h of treatment (Fig. 6A). To further confirm the responsible signaling pathways regulating SREBP-1 and AR expression, we evaluated the effect of U0126 (a MAPK selective inhibitor), LY294002 (a PI3K selective inhibitor), and H-89 (a PKA selective inhibitor) on prostate cancer cells. β2M mAb and U0126 greatly inhibited expression of SREBP-1 and AR in LNCaP cells (Fig. 6B). FAS was also decreased by U0126 treatment. H-89 slightly decreased the expression of these proteins. However, LY294002 appeared to enhance SREBP-1 and AR expression in LNCaP cells. Interrupting the PI3K/Akt signaling pathway with LY294002 increased AR, and PSA protein expression in LNCaP cells has been reported (25). Similar results were observed when C4-2B cells were treated with these signaling pathway inhibitors (data not shown). These data suggested that a MAPK/ERK signaling pathway may play a dominant role in the regulation of SREBP-1 and AR expression through β2M mAb in prostate cancer cells.
FIGURE 6.
β2M mAb inhibited SREBP-1 and AR expression through a MAPK/ERK signaling pathway in prostate cancer cells. A, β2M mAb (10 μg/ml) decreased phospho-Akt (p-Akt, Ser473) and phospho-MAPK (p-MAPK, Thr202/Tyr204) protein expression but did not inhibit total Akt and MAPK (p44/p42) protein expression in LNCaP and C4-2B cells at different time points (0, 15, 30, 60, and 120 min) as assayed by Western blot. AR and SREBP-1 protein expression was not affected by β2M mAb within 2 h of treatment. B, β2M mAb (5 μg/ml) and U0126 (10 μm, a MAPK selective inhibitor) greatly inhibited expression of endogenous SREBP-1, AR, and FAS proteins in LNCaP cells with 24 h of treatment as determined by Western blot. H-89 (1 μm, a PKA selective inhibitor) slightly decreased SREBP-1, AR, and FAS expression. However, LY294002 (20 μm, a PI3K selective inhibitor) appeared to increase SREBP-1 and AR expression. β-Actin was used as an internal loading control.
DISCUSSION
This study investigated the pleiotropic signaling functions of β2M, which confer growth, survival, and metastasis benefits to prostate cancer cells. We focus here on the critical role of β2M in the regulation of AR through SREBP-1, which plays a key role in lipid homeostasis, regulating its downstream target genes, such as FAS, and accumulation of fatty acids and lipid droplets (supplemental Fig. S1) by Oil Red O staining (39) in prostate cancer cells. We identified a triad relationship among β2M, SREBP-1, and AR. In response to the β2M-mediated cell signaling, SREBP-1 regulated AR expression by altering AR gene transcription. Conversely, androgens and AR were also found to mediate SREBP-1 expression reciprocally in androgen-responsive prostate cancer cells (40, 41). These data, taken together with previous reports from our laboratory and others that documented the regulatory role of β2M on AR expression (10) and also androgens and AR regulation of β2M expression (8), support the triad relationship among β2M, SREBP-1, and AR.
There are several important clinical implications of this triad relationship. 1) β2M could be an important driver modulating SREBP-1 and AR expression in prostate cancer cells. It has been shown that upon androgen-refractory progression of human prostate cancer, dysregulated expression of β2M (2, 7, 42), SREBP-1 (34), and AR (43, 44) are observed. By employing β2M mAb as a therapeutic agent, we and others found that this antibody caused massive cell death in human prostate and renal cancers (10, 11), multiple myeloma, leukemia, and lymphoma (9) without affecting the growth of normal cells. We propose that the inhibitory action of β2M mAb could act via inhibition of SREBP-1 expression, which is linked to FAS expression and lipogenic pathways that are known to regulate cell membrane integrity, energy metabolism, lipid raft-mediated signaling in cancer cells (27, 29, 45, 46), and AR expression, which is regarded as a growth and survival factor for human prostate cancer cells (43). 2) The pleiotropic cell signaling network activated by β2M could have a mediatory action on lipid metabolism and storage and lipid raft-directed cell signaling pathways. In this study, we showed that β2M mAb inhibited a large number of cell signaling networks, including MAPK, SREBP-1, AR, and PI3K/Akt. It is conceivable that these signaling networks are interconnected through lipid raft complexes (47, 48). The inhibitory action exerted by β2M mAb could affect the lipid composition of the raft structures, hence altering domain interactions and how downstream cell signal networks can be assembled and interact in a coordinated manner (45, 49). 3) β2M-regulated downstream signaling is highly dynamic and could affect the function of cells without AR expression. β2M mAb was shown to block the growth and downstream signaling of cancer but not normal cells regardless of their endogenous levels of AR (10), implying that AR function is not obligatory for the triad relationship. These observations also suggest that AR function could be bypassed by other redundant cell signaling networks mediated by soluble factors such as insulin-like growth factor I, epidermal growth factor, keratinocyte growth factor, and interleukin-6 (19, 20, 50, 51). Additional studies may be warranted to define how the triad relationship would function in normal versus cancer cells and in clinical prostate cancer, which characteristically contains cells with heterogeneous arrays of AR, including AR gene amplification and mutation and AR protein overexpression and silencing (52–54). In addition, the data presented in this communication are collected from the study of established AR-positive human prostate cancer cell lines, and further investigation of this concept in primary prostate cancer cells might be of importance.
The precursors of SREBP family proteins are endoplasmic reticulum membrane-anchored with the mature amino-terminal forms being translocated to the cell nucleus responsible for the activation of their target genes (27). Blockade of SREBP-1, a crucial transcriptional regulator for fatty acid and lipid biosynthesis, by β2M mAb inhibited expression of both precursor and mature nuclear SREBP-1 and FAS (Fig. 5A) in prostate cancer cells and interrupted cell growth and promoted apoptosis (10). We also observed that β2M mAb significantly decreased fatty acid contents and lipid droplet accumulation in LNCaP and C4-2B cells. However, the signaling mechanism regulating SREBP-1 and its downstream target gene expression by β2M mAb in prostate cancer is still unclear. Reports in the literature suggest that SREBP-1 is induced by PI3K/Akt and/or MAPK signaling pathways in liver cells, macrophages, and mammary epithelial and breast cancer cells (38, 55, 56). The inhibitors of MAPK and PI3K signaling pathways were demonstrated to down-regulate SREBP-1 and FAS expression and inhibit fatty acid synthesis in MCF-7 and HCT166 carcinoma cells (38). In LNCaP and C4-2B prostate cancer cells, we observed that U0126 (a MAPK selective inhibitor) inhibited SREBP-1, FAS, and AR protein expression (Fig. 6B), similar to the action of β2M mAb. However, LY294002 (a PI3K selective inhibitor) increased SREBP-1 and AR protein expression in AR-positive prostate cancer cells. Blocking the PI3K/Akt signaling pathway by LY294002 in LNCaP cells resulted in induced AR expression through activation of a Forkhead transcription factor, FOXO3a (25). H-89, a PKA selective inhibitor, did not significantly affect SREBP-1 expression (Fig. 6B). In addition, we showed that β2M mAb decreased p-MAPK protein expression in LNCaP and C4-2B cells (Fig. 6A), and this inhibition coincides with decreased SREBP-1 and AR expression (Figs. 2 and 5A). Our data collectively suggest that MAPK signaling is the dominant pathway that positively controls SREBP-1, AR, and FAS expression in AR-positive prostate cancer cells and that this cell signaling network may be different in cancer and normal cells.
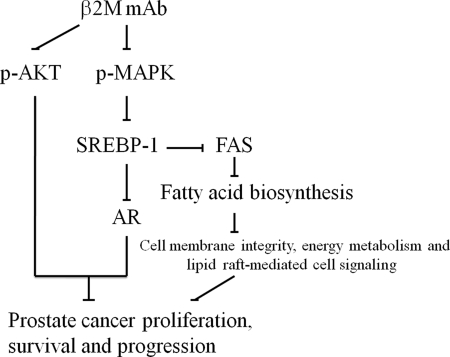
In conclusion, these results show for the first time that: 1) interrupting intracellular or extracellular β2M using sequence-specific siRNA or mAb resulted in decreased expression of AR and PSA at the transcriptional and translational levels in AR-positive prostate cancer cells; 2) β2M mAb inhibited AR expression by blocking a MAPK signaling pathway, decreasing the expression of precursor and nuclear SREBP-1 and reducing the binding between SREBP-1 and its cis-element binding site in the hAR promoter region in LNCaP and C4-2B cells; 3) β2M mAb decreased fatty acid and lipid accumulation by inhibition of SREBP-1 and FAS expression and may disrupt cell membrane integrity, intracellular lipid raft-mediated cell signaling and energy metabolism; and 4) functional studies of SREBP-1 demonstrated that SREBP-1 regulated endogenous AR expression and cell viability in LNCaP and C4-2B cells. Collectively, these results suggest that β2M mAb is a novel therapeutic antibody capable of inhibiting the pleiotropic cell signaling network converging on β2M in prostate cancer cells (Fig. 7). This study also expands our understanding of the key regulatory role of β2M, which controls intracellular signaling pathways through the regulation of transcription factors such as cAMP-responsive element-binding protein (7, 57), hypoxia inducible factor1-α (HIF1-α) (57), and SREBP-1.
FIGURE 7.
Proposed mechanism for β2M mAb inhibition of AR and cell proliferation, survival, and progression through a MAPK/SREBP-1 signaling pathway in prostate cancer cells. β2M mAb inhibited p-MAPK and p-AKT expression and decreased SREBP-1 expression in LNCaP and C4-2B cells. By the addition of U0126, a MAPK selective inhibitor, we observed down-regulation of SREBP-1 expression (see Fig. 6B). In addition, β2M mAb inhibited AR and FAS expression; the latter is known to regulate fatty acid biosynthesis, cell membrane integrity, energy metabolism, and lipid raft-regulated cell signaling (31, 32, 46), which ultimately control prostate cancer cell proliferation, survival, and progression.
Supplementary Material
Acknowledgments
We thank Dr. Donald J. Tindall (Mayo Clinic, Rochester, MN) for providing the hAR promoter luciferase reporter vector and Gary Mawyer for editing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 CA98912 (to L. W. K. C.). This work was also supported by Department of Defense Grants W81XWH-07-1-0172 (to L. W. K. C.) and W81XWH-08-1-0321 (to W.-C. H.) and Georgia Cancer Coalition-Cancer Research Awards (to W.-C. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Fig. S1.
- β2M
- β2-microglobulin
- mAb
- monoclonal antibody
- AR
- androgen receptor
- ChIP
- chromatin immunoprecipitation assay
- EMSA
- electrophoretic mobility shift assay
- ERK
- extracellular signal-regulated kinase
- FAS
- fatty acid synthase
- hAR
- human androgen receptor
- MAPK
- mitogen-activated protein kinase
- PKA
- protein kinase A
- PSA
- prostate-specific antigen
- siRNA
- small interfering RNA
- SREBP-1
- sterol regulatory element-binding protein-1
- PI3K
- phosphatidylinositol 3-kinase, reverse transcription
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- EGR
- early growth response gene
- siβ2M
- β2M siRNA
- AP-1
- activator protein-1.
REFERENCES
- 1.Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. (1986) Cell 44, 959–968 [DOI] [PubMed] [Google Scholar]
- 2.Abdul M., Hoosein N. (2000) Urol. Oncol. 5, 168–172 [DOI] [PubMed] [Google Scholar]
- 3.Klein T., Levin I., Niska A., Koren R., Gal R., Schachter J., Kfir B., Narinski R., Warchaizer S., Klein B. (1996) Eur. J. Immunogenet 23, 417–423 [DOI] [PubMed] [Google Scholar]
- 4.Bataille R., Grenier J., Commes T. (1988) Cancer Invest. 6, 271–277 [DOI] [PubMed] [Google Scholar]
- 5.Tsimberidou A. M., Kantarjian H. M., Wen S., O'Brien S., Cortes J., Wierda W. G., Koller C., Pierce S., Brandt M., Freireich E. J., Keating M. J., Estey E. H. (2008) Clin. Cancer Res. 14, 721–730 [DOI] [PubMed] [Google Scholar]
- 6.Nomura T., Huang W. C., Zhau H. E., Wu D., Xie Z., Mimata H., Zayzafoon M., Young A. N., Marshall F. F., Weitzmann M. N., Chung L. W. (2006) Clin. Cancer Res. 12, 7294–7305 [DOI] [PubMed] [Google Scholar]
- 7.Huang W. C., Wu D., Xie Z., Zhau H. E., Nomura T., Zayzafoon M., Pohl J., Hsieh C. L., Weitzmann M. N., Farach-Carson M. C., Chung L. W. (2006) Cancer Res. 66, 9108–9116 [DOI] [PubMed] [Google Scholar]
- 8.Yoon H. G., Wong J. (2006) Mol. Endocrinol. 20, 1048–1060 [DOI] [PubMed] [Google Scholar]
- 9.Yang J., Qian J., Wezeman M., Wang S., Lin P., Wang M., Yaccoby S., Kwak L. W., Barlogie B., Yi Q. (2006) Cancer Cell 10, 295–307 [DOI] [PubMed] [Google Scholar]
- 10.Huang W. C., Havel J. J., Zhau H. E., Qian W. P., Lue H. W., Chu C. Y., Nomura T., Chung L. W. (2008) Clin. Cancer Res. 14, 5341–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura T., Huang W. C., Seo S., Zhau H. E., Mimata H., Chung L. W. (2007) J. Urol. 178, 292–300 [DOI] [PubMed] [Google Scholar]
- 12.Koller B. H., Marrack P., Kappler J. W., Smithies O. (1990) Science 248, 1227–1230 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., Clegg N. J., Scher H. I. (2009) Lancet Oncol. 10, 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attard G., Reid A. H., Olmos D., de Bono J. S. (2009) Cancer Res. 69, 4937–4940 [DOI] [PubMed] [Google Scholar]
- 15.Molife L. R., Attard G., Fong P. C., Karavasilis V., Reid A. H., Patterson S., Riggs C. E., Jr., Higano C., Stadler W. M., McCulloch W., Dearnaley D., Parker C., de Bono J. S. (2010) Ann. Oncol. 21, 109–113 [DOI] [PubMed] [Google Scholar]
- 16.Taplin M. E. (2008) Expert Rev. Anticancer Ther. 8, 1495–1508 [DOI] [PubMed] [Google Scholar]
- 17.Chang C. Y., McDonnell D. P. (2005) Trends Pharmacol. Sci. 26, 225–228 [DOI] [PubMed] [Google Scholar]
- 18.Zilliacus J., Wright A. P., Carlstedt-Duke J., Gustafsson J. A. (1995) Mol. Endocrinol. 9, 389–400 [DOI] [PubMed] [Google Scholar]
- 19.Culig Z., Hobisch A., Cronauer M. V., Radmayr C., Trapman J., Hittmair A., Bartsch G., Klocker H. (1994) Cancer Res. 54, 5474–5478 [PubMed] [Google Scholar]
- 20.Hobisch A., Eder I. E., Putz T., Horninger W., Bartsch G., Klocker H., Culig Z. (1998) Cancer Res. 58, 4640–4645 [PubMed] [Google Scholar]
- 21.Kasbohm E. A., Guo R., Yowell C. W., Bagchi G., Kelly P., Arora P., Casey P. J., Daaka Y. (2005) J. Biol. Chem. 280, 11583–11589 [DOI] [PubMed] [Google Scholar]
- 22.Sadar M. D. (1999) J. Biol. Chem. 274, 7777–7783 [DOI] [PubMed] [Google Scholar]
- 23.Takane K. K., McPhaul M. J. (1996) Mol. Cell. Endocrinol. 119, 83–93 [DOI] [PubMed] [Google Scholar]
- 24.Mizokami A., Yeh S. Y., Chang C. (1994) Mol. Endocrinol. 8, 77–88 [DOI] [PubMed] [Google Scholar]
- 25.Yang L., Xie S., Jamaluddin M. S., Altuwaijri S., Ni J., Kim E., Chen Y. T., Hu Y. C., Wang L., Chuang K. H., Wu C. T., Chang C. (2005) J. Biol. Chem. 280, 33558–33565 [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Chen M. W., Terry S., Vacherot F., Bemis D. L., Capodice J., Kitajewski J., de la Taille A., Benson M. C., Guo Y., Buttyan R. (2006) Oncogene 25, 3436–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown M. S., Goldstein J. L. (1997) Cell 89, 331–340 [DOI] [PubMed] [Google Scholar]
- 28.Hua X., Wu J., Goldstein J. L., Brown M. S., Hobbs H. H. (1995) Genomics 25, 667–673 [DOI] [PubMed] [Google Scholar]
- 29.Shimano H. (2001) Prog Lipid Res. 40, 439–452 [DOI] [PubMed] [Google Scholar]
- 30.Shimano H., Shimomura I., Hammer R. E., Herz J., Goldstein J. L., Brown M. S., Horton J. D. (1997) J. Clin. Invest. 100, 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez J. A., Decker J. P., Lupu R. (2005) J. Cell. Biochem. 94, 1–4 [DOI] [PubMed] [Google Scholar]
- 32.Baron A., Migita T., Tang D., Loda M. (2004) J. Cell. Biochem. 91, 47–53 [DOI] [PubMed] [Google Scholar]
- 33.Swinnen J. V., Heemers H., van de Sande T., de Schrijver E., Brusselmans K., Heyns W., Verhoeven G. (2004) J. Steroid Biochem. Mol. Biol. 92, 273–279 [DOI] [PubMed] [Google Scholar]
- 34.Ettinger S. L., Sobel R., Whitmore T. G., Akbari M., Bradley D. R., Gleave M. E., Nelson C. C. (2004) Cancer Res. 64, 2212–2221 [DOI] [PubMed] [Google Scholar]
- 35.Huang W. C., Xie Z., Konaka H., Sodek J., Zhau H. E., Chung L. W. (2005) Cancer Res. 65, 2303–2313 [DOI] [PubMed] [Google Scholar]
- 36.Chung L. W., Huang W. C., Sung S. Y., Wu D., Odero-Marah V., Nomura T., Shigemura K., Miyagi T., Seo S., Shi C., Molitierno J., Elmore J., Anderson C., Isotani S., Edlund M., Hsieh C. L., Wang R., Shehata B., Zhau H. E. (2006) Clin. Genitourin Cancer 5, 162–170 [DOI] [PubMed] [Google Scholar]
- 37.Yang L., Wang L., Lin H. K., Kan P. Y., Xie S., Tsai M. Y., Wang P. H., Chen Y. T., Chang C. (2003) Biochem. Biophys. Res. Commun. 305, 462–469 [DOI] [PubMed] [Google Scholar]
- 38.Yang Y. A., Han W. F., Morin P. J., Chrest F. J., Pizer E. S. (2002) Exp. Cell Res. 279, 80–90 [DOI] [PubMed] [Google Scholar]
- 39.Havel P. J. (2000) Proc. Nutr. Soc. 59, 359–371 [DOI] [PubMed] [Google Scholar]
- 40.Ngan S., Stronach E. A., Photiou A., Waxman J., Ali S., Buluwela L. (2009) Oncogene 28, 2051–2063 [DOI] [PubMed] [Google Scholar]
- 41.Heemers H. V., Verhoeven G., Swinnen J. V. (2006) Mol. Endocrinol. 20, 2265–2277 [DOI] [PubMed] [Google Scholar]
- 42.Gross M., Top I., Laux I., Katz J., Curran J., Tindell C., Agus D. (2007) Clin. Cancer Res. 13, 1979–1986 [DOI] [PubMed] [Google Scholar]
- 43.Heinlein C. A., Chang C. (2004) Endocr. Rev. 25, 276–308 [DOI] [PubMed] [Google Scholar]
- 44.Ruizeveld de Winter J. A., Janssen P. J., Sleddens H. M., Verleun-Mooijman M. C., Trapman J., Brinkmann A. O., Santerse A. B., Schröder F. H., van der Kwast T. H. (1994) Am. J. Pathol. 144, 735–746 [PMC free article] [PubMed] [Google Scholar]
- 45.Di Vizio D., Solomon K. R., Freeman M. R. (2008) Tumori 94, 633–639 [DOI] [PubMed] [Google Scholar]
- 46.Di Vizio D., Adam R. M., Kim J., Kim R., Sotgia F., Williams T., Demichelis F., Solomon K. R., Loda M., Rubin M. A., Lisanti M. P., Freeman M. R. (2008) Cell Cycle 7, 2257–2267 [DOI] [PubMed] [Google Scholar]
- 47.Oh H. Y., Lee E. J., Yoon S., Chung B. H., Cho K. S., Hong S. J. (2007) Prostate 67, 1061–1069 [DOI] [PubMed] [Google Scholar]
- 48.Cinar B., Mukhopadhyay N. K., Meng G., Freeman M. R. (2007) J. Biol. Chem. 282, 29584–29593 [DOI] [PubMed] [Google Scholar]
- 49.Freeman M. R., Solomon K. R. (2004) J. Cell. Biochem. 91, 54–69 [DOI] [PubMed] [Google Scholar]
- 50.Wu J. D., Haugk K., Woodke L., Nelson P., Coleman I., Plymate S. R. (2006) J. Cell. Biochem. 99, 392–401 [DOI] [PubMed] [Google Scholar]
- 51.Orio F., Jr., Térouanne B., Georget V., Lumbroso S., Avances C., Siatka C., Sultan C. (2002) Mol. Cell. Endocrinol. 198, 105–114 [DOI] [PubMed] [Google Scholar]
- 52.Golias Ch., Iliadis I., Peschos D., Charalabopoulos K. (2009) Exp. Oncol. 31, 3–8 [PubMed] [Google Scholar]
- 53.Devlin H. L., Mudryj M. (2009) Cancer Lett. 274, 177–186 [DOI] [PubMed] [Google Scholar]
- 54.Brooke G. N., Bevan C. L. (2009) Curr. Genomics 10, 18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuhrman B., Nitzan O., Karry R., Volkova N., Dumler I., Aviram M. (2007) Atherosclerosis 195, e108–e116 [DOI] [PubMed] [Google Scholar]
- 56.Fleischmann M., Iynedjian P. B. (2000) Biochem. J. 349, 13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D., Zhau H. E., Huang W. C., Iqbal S., Habib F. K., Sartor O., Cvitanovic L., Marshall F. F., Xu Z., Chung L. W. (2007) Oncogene 26, 5070–5077 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.