Abstract
Glycogen synthase kinase-3 (GSK-3) isoforms, GSK-3α and GSK-3β, are serine/threonine kinases involved in numerous cellular processes and diverse diseases, including Alzheimer disease, cancer, and diabetes. GSK-3 isoforms function redundantly in some settings, while, in others, they exhibit distinct activities. Despite intensive investigation into the physiological roles of GSK-3 isoforms, the basis for their differential activities remains unresolved. A more comprehensive understanding of the mechanistic basis for GSK-3 isoform-specific functions could lead to the development of isoform-specific inhibitors. Here, we describe a structure-function analysis of GSK-3α and GSK-3β in mammalian cells. We deleted the noncatalytic N and C termini in both GSK-3 isoforms and generated point mutations of key regulatory residues. We examined the effect of these mutations on GSK-3 activity toward Tau, activity in Wnt signaling, interaction with Axin, and GSK-3α/β Tyr279/216 phosphorylation. We found that the N termini of both GSK-3 isoforms were dispensable, whereas progressive C-terminal deletions resulted in protein misfolding exhibited by deficient activity, impaired ability to interact with Axin, and a loss of Tyr279/216 phosphorylation. Our data predict that small molecules targeting the divergent C terminus may lead to isoform-specific GSK-3 inhibition through destabilization of the GSK-3 structure.
Keywords: Enzyme Structure, Neurodegeneration, Phosphorylation Enzymes, Signal Transduction, Wnt Pathway
Introduction
Glycogen synthase kinase-3 (GSK-3)2 enzymes are serine/threonine kinases originally identified based on their activity toward glycogen synthase (1). Two mammalian GSK-3 isoforms exist as products of distinct genes, GSK-3α and GSK-3β (2), which are highly homologous within their internal kinase domains but diverge in sequence outside this region. Aberrant regulation and subsequent hyperactivity of GSK-3 has been linked to diseases such as Alzheimer disease, cancer, and diabetes (3). Thus, tight regulation of ubiquitous (4) and constitutive (5) GSK-3 activity is important for maintaining normal cellular function. Control of GSK-3 activity occurs by a combination of mechanisms, including prerequisite phosphorylation of GSK-3 substrates (priming), phosphorylation of GSK-3 itself, and localization of GSK-3 activity through protein interactions.
With >40 putative substrates (6, 7), GSK-3 isoforms are broadly influential, and their activity toward substrates is commonly influenced by a priming phosphorylation mechanism (8). Recognition of a primed substrate by GSK-3 requires a triad of conserved residues, Arg159/96, Arg243/180, and Lys268/205 of GSK-3α/β, which form a phosphate-binding pocket and facilitate optimal alignment of GSK-3 into a catalytically active conformation (9–11). Although priming enhances the efficiency of GSK-3 phosphorylation, it is not a strict requirement. For example, the microtubule-associated protein Tau has both primed and unprimed sites targeted by GSK-3 activity (12).
Phosphorylation of Tyr279 in GSK-3α and Tyr216 in GSK-3β has been reported to facilitate GSK-3 activity by inducing rotation of the tyrosine side chain, which moves out of the substrate-binding groove, promoting substrate accessibility (9, 11, 13). Though other mechanisms have been reported (7, 14, 15), phosphorylation of GSK-3β at Tyr216 is believed to occur through a post-translational and intramolecular autophosphorylation event during protein folding in an Hsp90-dependent manner (5, 16, 17).
Inhibition of GSK-3 occurs as a downstream event in several signaling cascades, including insulin and Wnt signaling. Insulin signaling inhibits GSK-3 through N-terminal serine phosphorylation, Ser21 in GSK-3α and Ser9 in GSK-3β (7, 14, 15), which causes a conformational transition of GSK-3 to a pseudosubstrate structure and precludes substrate binding (9, 10). Wnt signaling regulates GSK-3 activity by controlling GSK-3 localization and thus its accessibility to the primed substrate β-catenin (18–21). Interestingly, regulation of GSK-3 via Wnt signaling does not involve Ser21/9 phosphorylation, and likewise, Ser21/9 phosphorylation of GSK-3 does not affect Wnt signaling (22), indicating unique mechanisms of regulation likely due to insulated subcellular pools of GSK-3.
Recently, a new mechanism of GSK-3 regulation was reported. Persistent activation of p38 MAPK was shown to mediate inhibitory phosphorylation of Thr390 in GSK-3β, resulting in accumulation of β-catenin and increased expression of Wnt target genes (23). Interestingly, p38 MAPK specifically phosphorylated GSK-3β but not GSK-3α (23). Consistently, other evidence has also suggested that GSK-3 isoforms exhibit distinct biological roles (24–29), providing rationale for the development of GSK-3 isoform-specific inhibitors.
Currently, small molecule inhibitors of GSK-3 largely target the ATP-binding pocket, which contains two conserved lysine residues, Lys148/149 in GSK-3α and Lys85/86 in GSK-3β, that are responsible for binding ATP and transferring the γ-phosphate (3, 15). Such inhibitors lack isoform specificity due to high homology of GSK-3 isoforms in this region. Thus, the development of isoform-specific GSK-3 inhibitors necessitates a greater understanding of the importance of the divergent N- and C-terminal regions of each GSK-3 isoform. Thus, we performed a structure-function analysis of GSK-3 isoforms in HEK 293T cells by examining the effect of GSK-3α and GSK-3β deletion and point mutants on 1) activity toward Tau, 2) ability to suppress Wnt signaling, 3) interaction with an Axin fragment, and 4) Tyr279/216 phosphorylation. Our data demonstrate that the C termini of GSK-3 isoforms are important for activity, protein interactions, and Tyr279/216 phosphorylation and predict that the development of therapeutic modulators targeting the C terminus may result in isoform-specific GSK-3 inhibition.
EXPERIMENTAL PROCEDURES
Cell Culture
HEK 293T cells (ATCC) were maintained in Dulbecco's modified Eagle medium (Cellgro) supplemented with 10% bovine growth serum (Hyclone) and 1% penicillin-streptomycin solution (Cellgro) at 37 °C and 5% CO2. Cells were plated at 1.0 × 106 in 6-well plates (Corning) 24 h prior to transfection under reduced serum (2.5%) conditions. L cells (ATCC) and L cells stably expressing Wnt3a (ATCC) were maintained in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% bovine growth serum (Hyclone) and 1% penicillin-streptomycin solution (Cellgro) at 37 °C and 5% CO2. Conditioned media was prepared as described in the ATCC protocol.
Cloning and Site-directed Mutagenesis
Full-length and deletion mutant human GSK-3α (Origene, accession number NM_019884) and human GSK-3β (obtained from Peter Klein, University of Pennsylvania) were PCR-amplified and TA-cloned into the Gateway entry vector pCR8GW TOPO (Invitrogen). Subsequent directional cloning into the Gateway destination vector pDEST27 (Invitrogen) generated N-terminal GST-tagged proteins. Point mutant GSK-3 constructs were generated using site-directed mutagenesis (Stratagene) (supplemental Fig. S1). DNA integrity and mutations were confirmed by sequence analysis. The GSK-3 interaction domain (GID, amino acids 321–429) of Xenopus Axin (obtained from Peter Klein, University of Pennsylvania) was PCR-amplified and TA-cloned into Gateway entry vector pCR8GW TOPO (Invitrogen). Subsequent directional cloning into Gateway destination vector, pDEST-Myc (obtained from the Belgian Coordinated Collections of Microorganisms/LMBP plasmid collection), generated N-terminal 6× Myc-tagged protein. DNA integrity was confirmed by sequence analysis.
Plasmids
FLAG-tagged human Tau was obtained from Hemant Paudel, McGill University. Super 8× TOPFlash and Super 8× FOPFlash were obtained from Randall Moon, University of Washington. pMAX EGFP was obtained from Amaxa. Renilla luciferase plasmid pRL SV40 was obtained from Promega. The empty vector pcDNA3.1 was obtained from Invitrogen.
Transient Transfections
Subconfluent HEK 293T cells were transiently transfected under reduced serum (2.5%) conditions using polyethylenimine (Polysciences, Inc.) dissolved in 50 mm HEPES buffer, pH 7.05. All transfections were normalized with empty vector pcDNA3.1. Transfections for analyzing GSK-3 activity toward Tau included either 1 μg pcDNA3.1 or GSK-3, 1 μg FLAG-Tau, and 0.2 μg pMAX EGFP. Transfections for examining GSK-3 activity in suppressing Wnt signaling included either 1 μg pcDNA3.1 or GSK-3, 1 μg Super 8× TOPFlash or Super 8× FOPFlash, 0.01 μg pRL SV40, and 0.2 μg pMAX EGFP. Transfections for assessing the GSK-3 interaction with Axin GID included either 1 μg pcDNA3.1 or GSK-3, 1 μg Axin GID, and 0.2 μg pMAX EGFP. Transfections for evaluating Tyr279/216 phosphorylation included 1 μg pcDNA3.1 or GSK-3 and 0.2 μg pMAX EGFP.
Cell Lysis
Approximately 24 h after transfection, cells were collected with trypsin and pelleted by centrifugation. After one wash with 1× phosphate-buffered saline, cells were lysed in lysis buffer (137 mm NaCl, 10 mm Tris pH 7.4, 1% Nonidet P-40) supplemented with protease inhibitor mixture (Sigma). Lysate was cleared by high speed centrifugation, and protein was denatured by boiling in sample buffer (50 mm Tris, pH 6.8, 12% glycerol, 4% SDS, 0.1 m dithiothreitol, 0.01% Coomassie Blue R-250).
GST Pulldown Assay
Cleared cell lysate was incubated with glutathione-Sepharose beads (GE Healthcare) for 2 h at 4 °C. Subsequently, reactions were centrifuged to pellet beads, which were then washed four times with 2 volumes lysis buffer supplemented with protease inhibitor mixture. After the final wash, protein was eluted by boiling in sample buffer.
Immunoblotting and Antibodies
Approximately equal amounts of protein lysate were separated by Tricine-SDS-PAGE, transferred to nitrocellulose, and immunoblotted. The following primary antibodies were used: rabbit polyclonal anti-GST (Cell Signaling), mouse monoclonal anti-FLAG M2 (Sigma), mouse monoclonal PHF1 (obtained from Peter Davies, Albert Einstein College of Medicine), mouse monoclonal 9E10 (anti-Myc, developed by J. Michael Bishop and obtained from the Developmental Studies Hybridoma Bank, The University of Iowa) and mouse monoclonal anti-GSK-3β pY216 (BD Transduction Laboratories), which also recognized GSK-3α pY279. Corresponding secondary antibodies used were either horseradish peroxidase-linked anti-mouse or horseradish peroxidase-linked anti-rabbit (GE Healthcare). Detection was facilitated using ECL Western blotting Substrate (GE Healthcare).
Lef/TCF Luciferase Reporter Assay
Lef/TCF reporter constructs containing eight optimal (Super 8× TOPFlash) or eight mutant (Super 8× FOPFlash) Lef/TCF binding sites (30) driving firefly luciferase, were co-transfected into HEK 293T cells in conjunction with pRL SV40, and either empty vector, wild-type (WT) GSK-3α or GSK-3β, or mutant GSK-3α or GSK-3β. Twenty-four h later, each transfection was equally transferred to a 24-well plate. Twenty-four h after transfer, cells were treated in triplicate for 6 h with either 50% Wnt3a-conditioned media, to stimulate canonical Wnt signaling, or 50% L cell-conditioned media for control. Cells were then lysed with 1× passive lysis buffer and Firefly and Renilla luciferase activities were measured using Firefly and Renilla Luciferase Assay kit (Biotium) in a Veritas microplate luminometer (Turner Biosystems).
Densitometry
Chemiluminescence was quantified by measuring area density using UltraLum documentation system and software (UltraLum Inc., Claremont, CA).
RESULTS
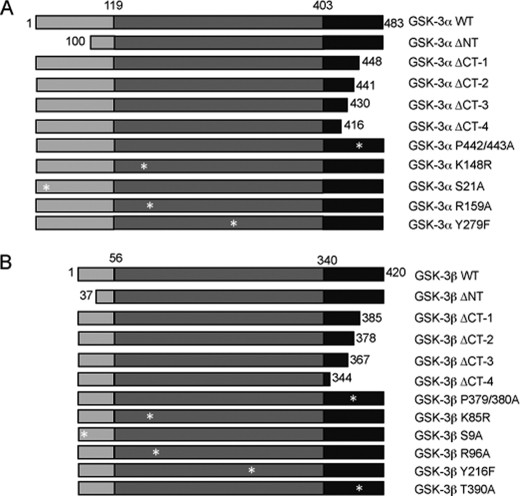
To investigate the functional significance of divergent regions and regulatory residues of GSK-3 isoforms, mammalian expression constructs containing N-terminal deletions, C-terminal deletions, or point mutations of human GSK-3α (Fig. 1A) and human GSK-3β (Fig. 1B) were generated. Deletion and point mutant GSK-3 expression constructs were tagged at the N terminus with GST.
FIGURE 1.
Schematic of GSK-3 mutants. Deletion and point mutants of GSK-3α (A) and GSK-3β (B) were created using PCR-based cloning and site-directed mutagenesis, respectively. GSK-3 enzymes were deleted from either the N terminus or consecutively from the C terminus. Point mutants were generated in the full-length enzyme, namely GSK-3α S21A (Ser21 → Ala), K148R (Lys148 → Arg), R159A (Arg159 → Ala), Y279F (Tyr279 → Phe), and P442A/P443A (Pro442/443 → Ala), and GSK-3β S9A (Ser9 → Ala), K85R (Lys85 → Arg), R96A (Arg96 → Ala), Y216F (Tyr216 → Phe), and P379/380A (Pro379/380 → Ala). The N-terminal domain is designated by light gray. The catalytic domain is colored dark gray, and the C-terminal domain is indicated in black. Numbering refers to amino acid residues of human GSK-3. Δ, deleted; NT, N terminus; CT, C terminus.
Deletion of GSK-3 C Termini Impairs Tau Phosphorylation
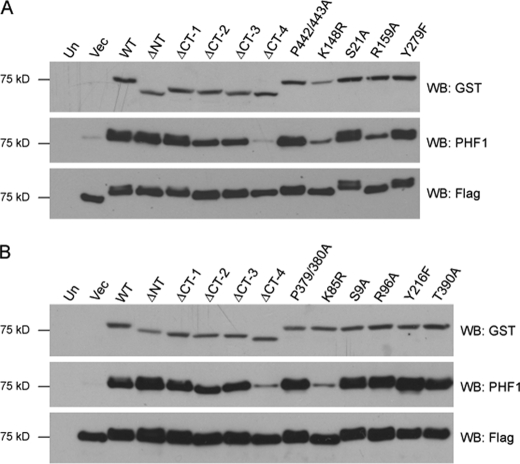
To assay the effects of mutations on the activity of GSK-3α (Fig. 2A) and GSK-3β (Fig. 2B), we examined the ability of WT and mutant enzymes to phosphorylate the well characterized GSK-3 substrate Tau in HEK 293T cells. Immunoblot analysis of total protein lysate revealed comparable expression levels of FLAG-Tau. Coexpression of WT GSK-3α and WT GSK-3β enhanced Tau phosphorylation at Ser396 and Ser404 as recognized by the phosphorylation-specific antibody PHF1 (31). Deletion of the N terminus did not affect the activity of either isoform toward Tau (αΔNT and βΔNT). However, when the C terminus was deleted (αΔCT-4 and βΔCT-4), GSK-3 isoforms lost nearly all activity, thus indicating amino acids 417–430 of GSK-3α and 345–367 of GSK-3β are essential for activity toward Tau. As anticipated, the point mutants resulting in GSK-3 inactivation (αK148R and βK85R) demonstrated little activity toward Tau. Surprisingly, we saw no impairment of activity when we mutated the tyrosine residues implicated in potentiating GSK-3 activity (αY279F and βY216F). Additionally, we observed a novel GSK-3α-specific requirement for priming exhibited by the impaired activity of αR159A activity, whereas βR96A activity was comparable with WT.
FIGURE 2.
C-terminal deletion of GSK-3 isoforms impairs GSK-3 activity toward Tau. Deletion and point mutants of GST-tagged GSK-3α (A) and GSK-3β (B) were coexpressed with FLAG-tagged human Tau in HEK 293T cells. Protein was separated by Tricine-SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies that recognize GSK-3 (GST, top panel), Tau specifically phosphorylated at Ser396 and Ser404 (PHF1, middle panel), and total Tau (FLAG, bottom panel). Results shown are representative of three independent experiments. Un, untransfected; Vec, vector; Δ, deleted; WB, Western blot; NT, N terminus; CT, C terminus.
Deletion of GSK-3 C Termini Abolishes Suppression of Wnt Signaling
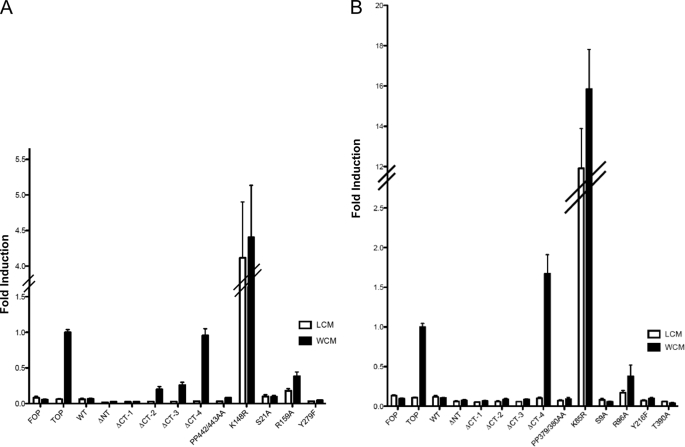
Next, we quantified the ability of GSK-3 mutants to regulate canonical Wnt signaling by measuring transcriptional activation of a β-catenin-inducible Lef/TCF luciferase reporter, Super 8× TOPFlash (30). HEK 293T cells were transfected with GST-GSK-3 constructs, Super 8× TOPFlash, and Renilla luciferase for normalizing, followed by treatment with either Wnt3a-conditioned media or control media (L cell-conditioned media) (32). Addition of Wnt3a ligand-enhanced luciferase reporter activity of Super 8× TOPFlash, without affecting the activity of a negative control, Super 8× FOPFlash. As expected, overexpression of WT GSK-3α (Fig. 3A) and WT GSK-3β (Fig. 3B) suppressed luciferase reporter activity. N-terminal deletion mutants (αΔNT and βΔNT) retained activity in the Wnt signaling pathway, whereas C-terminal deletion (αΔCT-4 and βΔCT-4) completely abolished GSK-3 activity, as noted by the lack of luciferase reporter suppression. Gene expression analysis of the Wnt target Axin2 by real-time quantitative PCR confirmed that αΔCT-4 and βΔCT-4 lack activity in the Wnt signaling pathway (supplemental Fig. S1). These data further argue that amino acids 417–430 in GSK-3α and 345–420 in GSK-3β are essential for GSK-3 activity. Point mutants αK148R and βK85R function as dominant-negatives in Wnt signaling by interfering with endogenous GSK-3 activities and activating luciferase reporter activity in the absence of the Wnt3a ligand, with the βK85R mutant producing a much more potent dominant-negative effect than the corresponding αK148R mutation. Expectedly, both αR159A and βR96A were impaired in their ability suppress the luciferase reporter, confirming that β-catenin is a primed GSK-3 substrate.
FIGURE 3.
C-terminal deletion of GSK-3 isoforms impairs GSK-3 activity in suppressing the canonical Wnt signaling pathway. Deletion and point mutants of GST-tagged GSK-3α (A) and GSK-3β (B) were coexpressed with Renilla reporter and β-catenin-dependent luciferase reporter Super8× TOPFlash in HEK 293T cells. Cells were treated for 6 h with either control-conditioned media (L cell-conditioned media) or conditioned media containing the Wnt3a ligand (Wnt3a-conditioned media, WCM) to activate canonical Wnt signaling. Firefly and Renilla luciferase levels were measured from cell lysate. The mean and S.E. was determined from three independent experiments performed in triplicate (n = 9). FOP, Super 8× FOPFlash; TOP, Super 8× TOPFlash; Δ, deleted; NT, N terminus; CT, C terminus; LCM, L cell-conditioned media.
GSK-3 C-terminal Deletions Fail to Interact with Axin GID
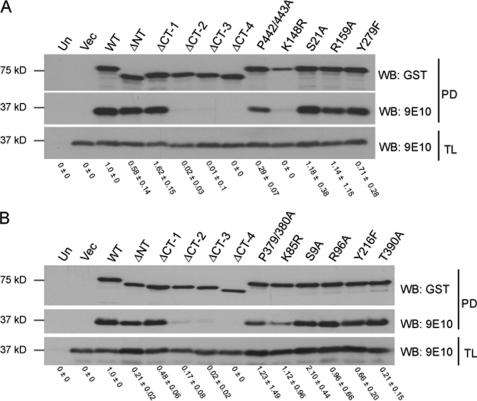
In the canonical Wnt signaling pathway, GSK-3 phosphorylation of β-catenin is regulated via interactions with the scaffold protein Axin (18, 19). To better understand why deletion of the C termini of GSK-3 isoforms resulted in a loss of activity toward β-catenin, we examined the ability of GSK-3 mutants to interact with the GID of Axin (33). We predicted that the inability of αΔCT-4 and βΔCT-4 to suppress Lef/TCF luciferase reporter activity might result from an impaired interaction with Axin GID. Following purification on glutathione-Sepharose, the interaction of GST-GSK-3 mutants with Myc-tagged Axin GID (residues 321–429) was assayed by immunoblotting. WT GSK-3α (Fig. 4A) and WT GSK-3β (Fig. 4B) strongly interacted with Axin GID, whereas αΔCT-4 and βΔCT-4 were greatly impaired. Unexpectedly, αΔCT-2, αΔCT-3, βΔCT-2, and βΔCT-3, which exhibited activity in Wnt signaling, were also greatly impaired in their ability to interact with Axin GID. Furthermore, despite their dominant-negative activity in Wnt signaling, αK148R and βK85R exhibited impaired ability to interact with Axin GID. These data suggest a potential Axin-independent mechanism of Wnt signaling modulation by GSK-3 isoforms.
FIGURE 4.
C-terminal deletion of GSK-3 isoforms abolishes GSK-3 interaction with Axin GID. Deletion and point mutants of GST-tagged GSK-3α (A) and GSK-3β (B) were coexpressed with 6× Myc-tagged Axin GID in HEK 293T cells. Total lysate (TL) and glutathione-Sepharose affinity-purified protein (PD) were separated by Tricine-SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies that recognize GSK-3 (GST, top panel) and Axin GID (9E10, middle and bottom panels). Results shown are representative of three independent experiments. Numerical values were derived from densitometry experiments, and error represents S.D. Un, untransfected; Vec, vector; Δ, deleted; NT, N terminus; CT, C terminus; PD, pull-down; WB, Western blot.
Mutating Conserved C-terminal Proline Residues Does Not Affect GSK-3 Activity
GSK-3β was crystallized in association with a minimal peptide derived from the Axin GID (13). Based on this crystal structure, the minimal Axin peptide occupies a hydrophobic groove in GSK-3β that is identical in sequence to GSK-3α (325–336 and 348–362 in GSK-3α and 262–273 and 285–299 in GSK-3β). This region was not deleted from our GSK-3 C-terminal deletion mutants. Thus, we speculated that GSK-3α and GSK-3β C-terminal deletion mutants were unable to bind Axin GID due to compromised structurally integrity. In silico sequence analysis revealed that two C-terminal proline residues were evolutionarily conserved (data not shown), Pro442/443 of GSK-3α and Pro379/380 of GSK-3β, suggesting a functional importance. Therefore, we sought to determine the structural important of these proline residues were by converting them to alanine in the context of full-length GSK-3. We found no difference between the proline mutants and WT GSK-3 in Tau phosphorylation (Fig. 2A, B) or Wnt suppression (Fig. 3A, B). A reduction in Axin GID interaction was noted with αP442/443A but not with βP379/380A (Fig. 4A, B). Taken together, the loss of activity observed with C-terminal deletion likely resulted from a general effect on protein structure, such as misfolding, and not from deletion of any particular residue(s).
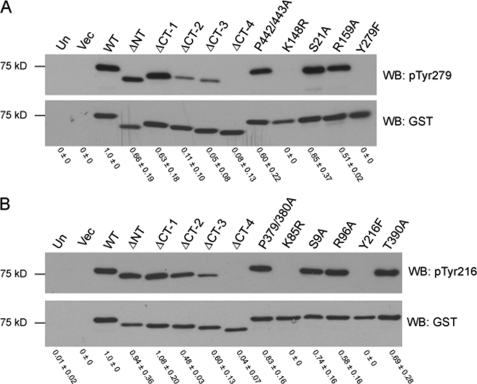
GSK-3 C-terminal Deletions Fail to Autophosphorylate Tyr279/216
To support our hypothesis that GSK-3 C-terminal deletion results in misfolding, we examined the phosphorylation status of Tyr279 in GSK-3α and Tyr216 in GSK-3β. Phosphorylation of GSK-3β Tyr216 has been shown to occur through an intramolecular autophosphorylation event during protein folding (5, 16, 17). We reasoned that if the C-terminal deletion mutants fail to fold properly, then phosphorylation at Tyr279/216 would be diminished. WT or mutant GST-GSK-3α or GST-GSK-3β were transfected into HEK 293T cells, and following purification on glutathione-Sepharose, the phosphorylation status of Tyr279/216 was analyzed by immunoblotting with a phosphorylation-specific antibody. WT GSK-3α (Fig. 5A) and WT GSK-3β (Fig. 5B) were phosphorylated on Tyr279 and Tyr216, respectively, whereas C-terminal deletion mutants exhibited impaired Tyr279/216 phosphorylation, which was completely abolished in αΔCT-4 and βΔCT-4. The lack of Tyr279/216 phosphorylation in catalytically inactive point mutants (αK148R and βK85R) further supports that an intramolecular autophosphorylation event mediates Tyr279/216 phosphorylation in both GSK-3 isoforms.
FIGURE 5.
C-terminal deletion of GSK-3 isoforms abolishes phosphorylation of GSK-3α Tyr279 and GSK-3β Tyr216. Deletion and point mutants of GST-tagged GSK-3α (A) and GSK-3β (B) were expressed in HEK 293T cells, affinity-purified on glutathione-Sepharose, separated by Tricine-SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies that recognize GSK-3 specifically phosphorylated at Tyr279/216 (pTyr279 and pTyr216, top panel) and GSK-3 (GST, bottom panel). Results shown are representative of three independent experiments. Numerical values were derived from densitometry experiments, and error represents S.D. Un, untransfected; Vec, vector; Δ, deleted; NT, N terminus; CT, C terminus; WB, Western blot.
DISCUSSION
Deletion of the C terminus in both GSK-3 isoforms resulted in a loss of activity, impaired ability to interact with Axin GID, and a concomitant lack of Tyr279/216 phosphorylation. Additionally, C-terminal deletion mutants exhibited perinuclear aggregated localization in HeLa cells (supplemental Fig. S2) and also demonstrated reduced protein half-lives (supplemental Fig. S3). These data strongly suggest that C-terminal deletion of GSK-3 isoforms resulted in protein misfolding.
Furthermore, GSK-3α appeared to have greater sensitivity to C-terminal deletion, becoming impaired in activity beginning with αΔCT-2 and progressing to complete loss of activity with αΔCT-4. GSK-3β, however, did not display impairment of activity until βΔCT-4. Thus, GSK-3α activity may require unique intramolecular interactions involving the C terminus that are inconsequential for GSK-3β activity.
Intriguingly, GSK-3α and GSK-3β deletion mutants, α/βΔCT-2 and α/βΔCT-3, possessed the ability to suppress Wnt signaling, yet failed to bind Axin GID. A similar observation was reported by Fraser and colleagues, who described a GSK-3β point mutant, V267G/E268R, which failed to interact with Axin GID, but retained the ability to repress TCF luciferase reporter activity (34). These data imply that an Axin-independent mechanism of GSK-3 modulation of Wnt signaling may exist but this requires further validation.
Consistent with other reports (35–37), αK148R and βK85R conferred dominant-negative activities in Wnt signaling but, surprisingly, both mutants exhibited considerably reduced affinity for Axin GID compared with WT GSK-3α and WT GSK-3β. Thus, the dominant-negative activity of αK148R and βK85R may not be due to displacement of endogenous GSK-3 from the Axin-mediated destruction complex as anticipated but rather, through an Axin-independent mechanism, perhaps similar to that of α/βΔCT-2 and α/βΔCT-3. However, we did not directly measure β-catenin levels, and we cannot discount β-catenin-independent activation of the Super 8X TOPFlash luciferase reporter.
As demonstrated previously (38), disruption of the primed phosphate-binding pocket in αR159A and βR96A point mutants conferred dominant-negative activity to GSK-3α and GSK-3β in the luciferase reporter assay, though to a much lesser extent than αK148R and βK85R. Further, αR159A and βR96A retained strong interaction with Axin GID, which may account for their ability to repress luciferase reporter activity upon activation of Wnt signaling, albeit to a slightly lesser extent than WT GSK-3α and WT GSK-3β.
The major difference we detected between GSK-3 isoforms was the distinct activities of αR159A and βR96A toward Tau phosphorylation at Ser396 and Ser404. Although αR159A and βR96A functioned similarly toward β-catenin, αR159A was greatly compromised in its ability to phosphorylate Tau, suggesting GSK-3α phosphorylates Ser396 and Ser404 by a primed mechanism. GSK-3β R96A, however, retained full ability to phosphorylate Tau, suggesting GSK-3β does not require prerequisite priming. Our data has extended a previous observation (12) and by comparison, has revealed a unique difference between GSK-3α and GSK-3β activity toward Ser396 and Ser404 in Tau. Phosphorylation of Ser396 has been implicated in the conversion of Tau to a pathological state in Alzheimer disease (39) and GSK-3 hyperactivity is believed to play an important role (reviewed in Ref. 40). Thus, GSK-3 serves as a target candidate for therapeutic intervention in Alzheimer disease, and based on our observation, targeting GSK-3 in an isoform-specific manner may be beneficial in preventing Ser396 phosphorylation, and the resulting pathological conversion of Tau and destabilization of microtubules as exhibited in Alzheimer disease.
Several reports have suggested that phosphorylation at Tyr216 enhances GSK-3β activity (5, 7, 14, 15). However, our data suggest that tyrosine phosphorylation is not essential for activity of GSK-3 isoforms as demonstrated by the retained activity of point mutants αY279F and βY216F, which were comparable with WT GSK-3α and WT GSK-3β, respectively. Our data correlates with crystal structure reports describing an active conformation of GSK-3β despite the lack of phosphorylation at Tyr216 (9, 11, 41). Thus, the importance of GSK-3α/β Tyr279/216 phosphorylation is unclear, and the functional significance of this modification requires further investigation.
Our data were collected under conditions that did not stimulate Ser21/9 phosphorylation or Thr390 phosphorylation and therefore, point mutants αS21A, βS9A, and βT390A did not exhibit any significant change in activity or Tyr279/216 phosphorylation compared with WT GSK-3α and GSK-3β. Therefore, the effects of αS21A, βS9A, and βT390A were not obvious in these assays.
The role of GSK-3 isoforms in numerous and diverse disease processes together with evidence suggesting GSK-3 isoforms exhibit distinct activities and control discrete biological processes advocates for the development of isoform-specific GSK-3 inhibitors for use in therapeutic intervention. Currently, isoform-specific GSK-3 inhibitors are unavailable, and their development requires a more comprehensive understanding of the variable N and C termini in GSK-3. As a step in this direction, the aim of this study was to gain insight into the functional significance of the divergent N and C termini of GSK-3 isoforms and to evaluate the importance of specific regulatory residues. Here, we have identified a novel deletion mutation in the C terminus of GSK-3 isoforms that abolishes activity of both GSK-3α and GSK-3β. Our data predict that targeting the divergent C terminus with modulators of GSK-3 activity may mimic C-terminal deletion and destabilize GSK-3 structure, resulting in isoform-specific inhibition. Future work directed at confirming and validating our hypothesis, particularly in an in vivo model system, may pave the way for new therapeutic approaches.
Supplementary Material
Acknowledgments
We thank Drs. Jing Yang, Yusen Liu, Scott Harper, and Jeffrey Kuret for helpful suggestions and discussions and Drs. Joanna Groden and Kirk McHugh for critical reading of the manuscript. We are also grateful to Dr. Peter Klein for the GSK-3β and Axin constructs, Dr. Hemant Paudel for the FLAG-Tau construct, Dr. Randall Moon for the Super 8× TOPFlash and Super 8× FOPFlash constructs, Dr. Nathan Lawson for the pCS Cherry plasmid, Dr. Peter Davies for the PHF1 antibody, and Drs. Brad Doble and James Woodgett for GSK-3α−/− β−/− embryonic stem cells.
This work was supported in part by National Institutes of Health Grant R01AG031883 (to C. J. P.) from NIA.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
- GSK-3
- glycogen synthase kinase-3
- MAPK
- mitogen-activated protein kinase
- HEK
- human embryonic kidney
- GST
- glutathione S-transferase
- GID
- GSK-3 interaction domain
- EGFP
- enhanced green fluorescent protein
- PHF
- paired helical filament
- Lef
- lymphoid enhancer factor
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- TCF
- T-cell factor
- WT
- wild type.
REFERENCES
- 1.Embi N., Rylatt D. B., Cohen P. (1980) Eur. J. Biochem. 107, 519–527 [PubMed] [Google Scholar]
- 2.Woodgett J. R. (1990) EMBO J. 9, 2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meijer L., Flajolet M., Greengard P. (2004) Trends Pharmacol. Sci. 25, 471–480 [DOI] [PubMed] [Google Scholar]
- 4.Lau K. F., Miller C. C., Anderton B. H., Shaw P. C. (1999) J. Pept. Res. 54, 85–91 [DOI] [PubMed] [Google Scholar]
- 5.Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. (1993) EMBO J. 12, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jope R. S., Johnson G. V. (2004) Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 7.Grimes C. A., Jope R. S. (2001) Prog. Neurobiol. 65, 391–426 [DOI] [PubMed] [Google Scholar]
- 8.Fiol C. J., Wang A., Roeske R. W., Roach P. J. (1990) J. Biol. Chem. 265, 6061–6065 [PubMed] [Google Scholar]
- 9.Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 10.Frame S., Cohen P., Biondi R. M. (2001) Mol. Cell 7, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 11.ter Haar E., Coll J. T., Austen D. A., Hsiao H. M., Swenson L., Jain J. (2001) Nat. Struct. Biol. 8, 593–596 [DOI] [PubMed] [Google Scholar]
- 12.Cho J. H., Johnson G. V. (2003) J. Biol. Chem. 278, 187–193 [DOI] [PubMed] [Google Scholar]
- 13.Dajani R., Fraser E., Roe S. M., Yeo M., Good V. M., Thompson V., Dale T. C., Pearl L. H. (2003) EMBO J. 22, 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frame S., Cohen P. (2001) Biochem. J. 359, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doble B. W., Woodgett J. R. (2003) J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole A., Frame S., Cohen P. (2004) Biochem. J. 377, 249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lochhead P. A., Kinstrie R., Sibbet G., Rawjee T., Morrice N., Cleghon V. (2006) Mol. Cell 24, 627–633 [DOI] [PubMed] [Google Scholar]
- 18.Logan C. Y., Nusse R. (2004) Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 19.Clevers H. (2006) Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 20.MacDonald B. T., Yokota C., Tamai K., Zeng X., He X. (2008) J. Biol. Chem. 283, 16115–16123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu G., Huang H., Garcia Abreu J., He X. (2009) PLoS ONE 4, e4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding V. W., Chen R. H., McCormick F. (2000) J. Biol. Chem. 275, 32475–32481 [DOI] [PubMed] [Google Scholar]
- 23.Thornton T. M., Pedraza-Alva G., Deng B., Wood C. D., Aronshtam A., Clements J. L., Sabio G., Davis R. J., Matthews D. E., Doble B., Rincon M. (2008) Science 320, 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goode N., Hughes K., Woodgett J. R., Parker P. J. (1992) J. Biol. Chem. 267, 16878–16882 [PubMed] [Google Scholar]
- 25.Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., Woodgett J. R. (2000) Nature 406, 86–90 [DOI] [PubMed] [Google Scholar]
- 26.Phiel C. J., Wilson C. A., Lee V. M., Klein P. S. (2003) Nature 423, 435–439 [DOI] [PubMed] [Google Scholar]
- 27.Liang M. H., Chuang D. M. (2006) J. Biol. Chem. 281, 30479–30484 [DOI] [PubMed] [Google Scholar]
- 28.Koivisto L., Jiang G., Häkkinen L., Chan B., Larjava H. (2006) Exp. Cell Res. 312, 2791–2805 [DOI] [PubMed] [Google Scholar]
- 29.MacAulay K., Doble B. W., Patel S., Hansotia T., Sinclair E. M., Drucker D. J., Nagy A., Woodgett J. R. (2007) Cell Metab. 6, 329–337 [DOI] [PubMed] [Google Scholar]
- 30.Veeman M. T., Slusarski D. C., Kaykas A., Louie S. H., Moon R. T. (2003) Curr. Biol. 13, 680–685 [DOI] [PubMed] [Google Scholar]
- 31.Otvos L., Jr., Feiner L., Lang E., Szendrei G. I., Goedert M., Lee V. M. (1994) J. Neurosci. Res. 39, 669–673 [DOI] [PubMed] [Google Scholar]
- 32.Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., 3rd, Nusse R. (2003) Nature 423, 448–452 [DOI] [PubMed] [Google Scholar]
- 33.Hedgepeth C. M., Deardorff M. A., Rankin K., Klein P. S. (1999) Mol. Cell Biol. 19, 7147–7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fraser E., Young N., Dajani R., Franca-Koh J., Ryves J., Williams R. S., Yeo M., Webster M. T., Richardson C., Smalley M. J., Pearl L. H., Harwood A., Dale T. C. (2002) J. Biol. Chem. 277, 2176–2185 [DOI] [PubMed] [Google Scholar]
- 35.He X., Saint-Jeannet J. P., Woodgett J. R., Varmus H. E., Dawid I. B. (1995) Nature 374, 617–622 [DOI] [PubMed] [Google Scholar]
- 36.Dominguez I., Itoh K., Sokol S. Y. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8498–8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce S. B., Kimelman D. (1995) Development 121, 755–765 [DOI] [PubMed] [Google Scholar]
- 38.Hagen T., Di Daniel E., Culbert A. A., Reith A. D. (2002) J. Biol. Chem. 277, 23330–23335 [DOI] [PubMed] [Google Scholar]
- 39.Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., Lee V. M. (1993) Neuron 10, 1089–1099 [DOI] [PubMed] [Google Scholar]
- 40.Avila J. (2006) FEBS Lett. 580, 2922–2927 [DOI] [PubMed] [Google Scholar]
- 41.Aoki M., Yokota T., Sugiura I., Sasaki C., Hasegawa T., Okumura C., Ishiguro K., Kohno T., Sugio S., Matsuzaki T. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 439–446 [DOI] [PubMed] [Google Scholar]
- 42.Deleted in proof
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.