Abstract
Signaling events leading to mammalian sperm capacitation rely on activation/deactivation of proteins by phosphorylation. This cascade includes soluble adenylyl cyclase, an atypical bicarbonate-stimulated adenylyl cyclase, and is mediated by protein kinase A and the subsequent stimulation of protein tyrosine phosphorylation. Recently, it has been proposed that the capacitation-associated increase in tyrosine phosphorylation is governed by Src tyrosine kinase activity. This conclusion was based mostly on the observation that Src is present in sperm and that the Src kinase family inhibitor SU6656 blocked the capacitation-associated increase in tyrosine phosphorylation. Results in the present manuscript confirmed these observations and provided evidence that these inhibitors were also able to inhibit protein kinase A phosphorylation, sperm motility, and in vitro fertilization. However, the block of capacitation-associated parameters was overcome when sperm were incubated in the presence of Ser/Thr phosphatase inhibitors such as okadaic acid and calyculin-A at concentrations reported to affect only PP2A. Altogether, these data indicate that Src is not directly involved in the observed increase in tyrosine phosphorylation. More importantly, this work presents strong evidence that capacitation is regulated by two parallel pathways. One of them requiring activation of protein kinase A and the second one involving inactivation of Ser/Thr phosphatases.
Keywords: Serine Threonine Protein Phosphatase, Signal Transduction, Spermatozoa, Src, Tyrosine Protein Kinase (Tyrosine Kinase), Sperm Capacitation
Introduction
The capacitation process is the major prerequisite for mammalian sperm to fertilize. This highly complex phenomenon occurs in the female reproductive tract, and renders the spermatozoa capable of binding and fusing with the oocyte (1). The commonly accepted end point of capacitation is the time when sperm have obtained the ability to fertilize an egg. However, different physiological modifications of sperm have been correlated with the capacitated state. These include: cholesterol loss from the sperm plasma membrane, increased membrane fluidity, changes in intracellular ion concentrations (2), hyperpolarization of the sperm plasma membrane (3), and increased protein tyrosine phosphorylation (4).
Among ion fluxes that occur during capacitation, the transport of HCO3− into sperm promotes cAMP synthesis by the activation of an atypical soluble adenylyl cyclase (SACY)2 (5) and subsequent PKA activation. cAMP-dependent phosphorylation of Ser/Thr residues is known to be a key regulator of tyrosine phosphorylation events linked to the process of capacitation. In mouse sperm exposed to HCO3−, cAMP rises to a maximum in <60 s, followed immediately by an increase in PKA-dependent phosphorylation (2). However, tyrosine phosphorylation is only observed after incubations for at least 30 min in conditions conducive to capacitation (6). Despite the lack of temporal correlation of PKA-induced phosphorylation and the increase in tyrosine phosphorylation, it has been shown that PKA inhibition blocks the onset of tyrosine phosphorylation (7). The one or more tyrosine kinases responsible for the capacitation-associated increase in tyrosine phosphorylation have remained enigmatic. Recent proposals, however, have put forward the hypothesis that the Src family of protein tyrosine kinases (SFKs) mediates the increase in tyrosine phosphorylation in mouse and human sperm (8–10).
The action of SFKs on mammalian cells has been extensively studied and demonstrated to be broadly pleiotropic, including effects on cell adhesion, proliferation, migration, differentiation, cell morphology, and survival (11). Several lines of evidence indicate that c-Src (the prototypic member of the SFKs) is activated through dephosphorylation of Tyr-527 (or the corresponding tyrosine in other members of the SFKs) (12). The currently accepted model proposes that c-Src tyrosine kinase (Csk) phosphorylates Tyr-527 allowing its intramolecular interaction with SH2 that keeps the protein in an inactive closed state. However, in its open state, Tyr-416 in the c-Src activation loop (or the corresponding tyrosine in other SFKs) can undergo autophosphorylation inducing kinase activity (13).
It has been shown that c-Src is present in mouse sperm and that SU6656, a c-Src kinase family inhibitor is able to block the capacitation-associated increase in tyrosine phosphorylation as well as the change in motility pattern, known as hyperactivation (14). This inhibitor is competitive with respect to ATP and is capable of affecting all SFKs, suggesting a role for these kinases in the capacitation process. In addition, more recent studies have shown that SFK inhibitors abrogate human and bovine sperm acrosome reactions (10, 15). In the present work we have further characterized the involvement of SFKs in mouse sperm capacitation. Due to the importance of PKA in this process, we focused on the effect of SFK inhibitors on this kinase. Interestingly, our results indicate that, in addition to their abrogation of tyrosine phosphorylation, SFK inhibitors block PKA phosphorylation, sperm motility, and in vitro fertilization. Although these data suggest unspecific PKA inactivation by SFK inhibitors, in vitro activity assays show that this is not the case. Here, we provide evidence that Ser/Thr phosphatase inhibitors overcome the block by SFK inhibitors to all capacitation parameters, including in vitro fertilization. In addition, sperm from Src-null mice contained similar levels of capacitation-associated tyrosine phosphorylation as wild-type sperm. Taken together, these data have indicated that Src is not directly involved in the observed capacitation-associated changes in tyrosine phosphorylation, and further have strongly suggested that Ser/Thr protein phosphatase inactivation is necessary for sperm capacitation.
EXPERIMENTAL PROCEDURES
Materials
Chemicals were obtained from the following sources: Bovine serum albumin (BSA, fatty acid-free), and dibutyryl-cAMP (Bt2cAMP), 3-isobutyl-1-methylxanthine (IBMX), were purchased from Sigma. SU6656 was obtained from Calbiochem, and SKI606 (bosutinib) was purchased from Selleck Chemicals. H-89, okadaic acid, and calyculin-A were acquired from LC Laboratories (Woburn, MA). Heat-stable protein kinase A inhibitor (PKI) (myristoylated, 14-22, amide) was purchased from Biomol (Enzo Life Sciences, Inc.). Cdc2 (c-Src substrate), anti-phosphotyrosine (pY) monoclonal antibody (clone 4G10) and anti-Src monoclonal antibody (clone GD11) were obtained from Upstate Biotechnology (Lake Placid, NY). Rabbit monoclonal anti-phospho-PKA substrates (clone 100G7E) and anti-Src monoclonal antibodies (clone 32G6) were purchased from Cell Signaling (Danvers, MA). Anti-β-tubulin monoclonal antibody (clone E7) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of NICHD, National Institutes of Health, and maintained by The University of Iowa Dept. of Biological Sciences, Iowa City, IA. Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and Amersham Biosciences, respectively.
Mouse Sperm Preparation
Cauda epididymal mouse sperm were collected from CD1 retired male breeders (Charles River Laboratories, Wilmington, MA) or from young adult (7–8 weeks old) Src-null males and their wild-type littermates (16) and sacrificed in accordance to the Animal Care and Use Committee guidelines from the University of Massachusetts at Amherst or from Temple University School of Medicine. Each minced cauda epididymis was placed in 500 μl of a modified Krebs-Ringer medium (Whitten's HEPES-buffered medium containing 5 mg/ml BSA) (17). After 10 min, epididymides were remove, and the suspension was adjusted with non-capacitating medium to a final concentration of 1–2 × 107 cells/ml before dilution of four times in the appropriate medium depending on the experiment performed. For capacitation, 15 mm NaHCO3 was added, and sperm was incubated at 37 °C for at least 1 h. To test the effect of the different inhibitors of capacitation, sperm were preincubated with inhibitors in non-capacitating medium for 15 min prior to the beginning of the capacitating period. For in vitro fertilization assays, sperm were obtained and incubated for capacitation in Whitten's medium without HEPES containing 22 mm NaHCO3 and 5 mg/ml BSA, then equilibrated in a humidified atmosphere of 5% CO2 (18).
SDS-PAGE and Immunoblotting
After treatment, sperm were collected by centrifugation, washed in 1 ml of phosphate-buffered saline, resuspended in Laemmli sample buffer (19) without β-mercaptoethanol, and boiled for 5 min. After centrifugation, 5% β-mercaptoethanol was added to the supernatants, and the mixture was boiled again for 5 min. Protein extracts equivalent to 1–2 × 106 sperm per lane were subjected to SDS-PAGE and electro-transferred to PVDF membranes (Bio-Rad) at 250 mA for 60 min on ice. Membranes were blocked with 5% fat-free milk in TBS containing 0.1% Tween 20 (T-TBS). For anti-pY and anti-pPKA immunodetections, membranes were blocked with 20% fish skin gelatin (Sigma) in T-TBS. Antibodies were diluted in T-TBS as follows: 1/10,000 for anti-PY (clone 4G10), 1/5,000 for anti-pPKA (clone 100G7E), 1/1,000 for both anti-Src antibodies (clone GD11 and clone 32G6), 1/10,000 for anti-tubulin (clone E7), and anti-actin. Secondary antibodies were diluted 1/10,000 in T-TBS and developed using an enhanced chemiluminescence detection kit (ECL plus, Amersham Biosciences) according to the manufacturer's instructions. When necessary, PVDF membranes were stripped at 60 °C for 15 min in 2% SDS, 0.74% β-mercaptoethanol, 62.5 mm Tris, pH 6.5, and washed 6 × 5 min in T-TBS. In all experiments, molecular masses were expressed in kilodaltons.
Sperm Motility Analysis
Sperm suspensions were loaded on a 20-μm chamber slide (Leja Slide, Spectrum Technologies) and placed on a microscope stage at 37 °C. Sperm movements were examined using the CEROS computer-assisted semen analysis (CASA) system (Hamilton Thorne Research, Beverly, MA). Parameters used were as follows: 30 frames acquired, frame rate of 60 Hz, minimum cell size of 4 pixels, low average path velocity cutoff of 5 mm/s, static head size of 0.2–2.99, static head intensity of 0.26–1.31, and static head elongation lower than 100. At least 20 microscopy fields corresponding to a minimum of 200 sperm were analyzed in each experiment.
Mouse Eggs Collection and in Vitro Fertilization Assays
Metaphase II-arrested eggs were collected as described previously (18), from 6- to 8-week-old superovulated CD1 female mice (Charles River Laboratories) at 13 h after human chorionic gonadotrophin (Sigma) intraperitoneal injection. Cumulus cells were removed by brief incubation (<5 min) in Whitten's HEPES-buffered medium containing 7 mm NaHCO3, 5 mg/ml BSA, and 0.02% type IV-S hyaluronidase (Sigma). After cumulus cell removal, eggs were placed in a drop of Whitten's medium containing 22 mm NaHCO3 and 5 mg/ml BSA and allowed to recover for 30 min in an incubator with 5% CO2 at 37 °C.
Fertilization drops (200 μl each) containing 10–20 eggs were inseminated with capacitated sperm (final concentration of 2.5 × 106 cells/ml). After 4 h of insemination, eggs were washed through brief passages in three drops of Whitten's medium containing 22 mm NaHCO3 and 15 mg/ml BSA using a thin bore pipette to detach any loosely attached sperm. After 3 h of further incubation, eggs were fixed with 3.7% paraformaldehyde/phosphate-buffered saline for 15 min, washed, and stained with Hoechst 33342 (Sigma, 10 μg/ml) in phosphate-buffered saline for 10 min at room temperature. Fertilization was assessed by visualization of the formation of the male and female pronuclei.
Cell-free Assay of PKA Substrate Phosphorylation
Sperm (1–2 × 106 cells in 50 μl of final volume) were incubated in the presence of different inhibitors for 30 min at 30 °C in Whitten's media supplemented with: 1% Triton X-100, 40 μm ATP, 1 mm Bt2cAMP, 10 μm aprotinin, 10 μm leupeptin, 100 μm sodium orthovanadate, 5 mm p-nitrophenyl phosphate, 40 mm β-glycerophosphate, and 10 mm MgCl2. Samples were further subjected to SDS-PAGE and Western blotting followed by immunodetection with an anti-pPKA substrates antibody (clone 100G7E), as described above.
In Vitro Assay of PKA Activity
PKA activity was measured as previously described (20, 21). Briefly, the amount of 32P incorporated into the Kemptide (Leu-Arg-Arg-Ala-Ser-Leu-Gly, Sigma)-specific substrate was quantified. The assay mixture was either supplemented or not supplemented with inhibitors as described under “Results,” while maintaining constant DMSO concentration (≤0.5%).
c-Src Immunoprecipitation and Kinase Activity Assay
After sperm incubation in the appropriate conditions, samples were centrifuged at 1700 × g for 1 min. The resulting pellet was resuspended in radioimmune precipitation assay buffer (10 mm Tris-HCl, pH 7.2, 50 mm NaCl, 0.1% SDS, 1% Triton X-100, 1 mm EDTA, 1 mm sodium orthovanadate, and protease inhibitors), incubated on ice for 30 min, and centrifuged at 4 °C for 5 min at 2500 × g. Supernatants were incubated with anti-Src (4 μg of antibody for 1 × 107 cells in a final volume of 500 μl, clone GD11) and anti-tubulin antibodies (clone E7, same amount) as a negative control for 2 h at 4 °C with constant rocking. After adding 20 μl of protein G-Sepharose (Amersham Biosciences), the reactions were further rocked for 1 h at 4 °C. The immune complex was recovered by centrifugation, washed four times in radioimmune precipitation assay buffer, and subjected to a kinase assay. Equal immunoprecipitation of Src from different samples was controlled by Western blot analysis. To avoid the strong signal from the IgG heavy chain that could partially overlap with the Src signal, anti-IgG light chain-specific antibodies were used (Jackson Laboratories, West Grove, PA). These antibodies do not detect IgG heavy chain.
Src activity of immunocomplexes was assayed using a synthetic peptide from the cdc2 sequence as substrate (KVEKIGEGTYGVVYK) in a final volume of 30 μl. The equivalent of 1 × 105 sperm was used per reaction in a buffer containing 25 mm HEPES, 1% Triton X-100, 0.5 mm dithiothreitol, 1 mg/ml BSA, 0.5 mm EGTA, 40 μm ATP, 1 μCi of [γ-32P]ATP, 10 μm aprotinin, 10 μm leupeptin, 100 μm sodium orthovanadate, 5 mm p-nitrophenyl phosphate, 40 mm β-glycerophosphate, and 10 mm MnCl2. Reactions were incubated for 30 min at 30 °C, and stopped by addition of 30 μl 20% trichloroacetic acid. Half the sample was subjected to 8% SDS-PAGE and transferred to PVDF. The membrane was developed first by autoradiography to detect autophosphorylation of c-Src and then by Western blot with anti-c-Src antibodies (clone 36D10).
The second half of each sample was centrifuged at room temperature for 3 min at 10,000 × g. Twenty microliters of the resultant supernatant was spotted onto Whatman P81 phosphocellulose paper (2 × 2 cm) and washed 5 × 5 min in 5 mm phosphoric acid with agitation. Phosphocellulose papers were dried, placed in vials with 2 ml of scintillation fluid, and subjected to liquid scintillation counting. In every case, assays were performed in sets of triplicates, to avoid experimental errors.
Statistical Analysis
Paired Student's t test was used for comparing mean values between control and tested groups. The difference between mean values of multiple groups was analyzed by one-way analysis of variance followed by Holm-Sidak test. Statistical significances are indicated in the figure legends.
RESULTS
Analysis of c-Src Involvement in the Capacitation-associated Increase in Tyrosine Phosphorylation.
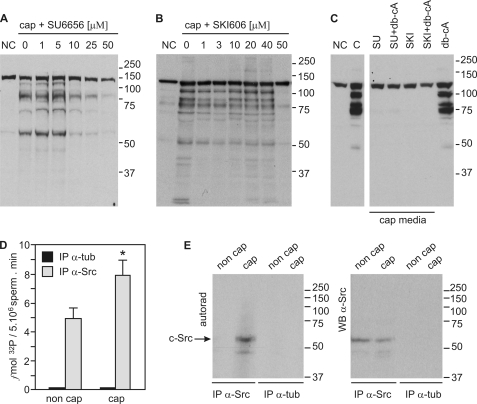
c-Src has been associated with the increase in protein-tyrosine phosphorylation during sperm capacitation (14). To validate a role for the SFKs in this signaling cascade, we used the SFK inhibitors SU6656 and SKI606. All SFK members are affected by SU6656, which is competitive with respect to ATP (22), whereas SKI606 has been shown to block the ATP binding site both in Src and Abl kinase families (23). Sperm were preincubated for 15 min with different inhibitor concentrations before exposure to conditions that support capacitation. In agreement with previous reports, SU6656 completely blocked the increase in tyrosine phosphorylation with an IC50 of ∼10 μm (Fig. 1A) (9, 14, 24). In the case of SKI606, a sharp inhibitory effect was observed at 50 μm (Fig. 1B). Because the increase in tyrosine phosphorylation is downstream of a cAMP/PKA pathway, we assessed whether cAMP agonists were able to overcome the inhibition of tyrosine phosphorylation. Although Bt2cAMP (1 mm) together with IBMX (100 μm) induced tyrosine phosphorylation in sperm incubated in the absence of HCO3− as previously described (6), cAMP agonists were not able to override the effect of SFK inhibitors (Fig. 1C).
FIGURE 1.
SFK inhibitors abrogate tyrosine phosphorylation associated with sperm capacitation. A, mouse sperm were incubated in the absence or in the presence of SU6656 for 60 min in capacitating (cap, with HCO3−) or non-capacitating media (NC, without HCO3−). Western blot analyses were performed with anti-pY antibodies. B, sperm were treated and processed as in panel A in the absence or in the presence of SKI606 (bosutinib). C, sperm were incubated for 60 min in capacitating medium containing 50 μm of either SU6656 or SKI606 in the presence or absence of 1 mm Bt2cAMP/0.1 mm IBMX (db-cA). Tyrosine phosphorylation was assessed as in A. D, c-Src immunokinase assay using a synthetic peptide derived from cdc2 as substrate. Capacitated and non-capacitated sperm were extracted with radioimmune precipitation assay buffer and immunoprecipitated with anti-Src (clone GD11) antibodies or anti-tubulin as controls. Kinase assays were performed on the immunocomplexes. Data are represented as the mean ± S.E. of three independent experiments, each performed in triplicate; *, p < 0.05. E, kinase reaction mixtures containing the immunocomplexes were subjected to 8% SDS-PAGE, transferred to PVDF, and developed by autoradiography. Only c-Src from capacitated sperm displayed autophosphorylation activity (left panel, arrow). The same PVDF membrane was probed with anti-Src antibodies (clone 36D10). All Western blots are representative of experiments repeated at least three times.
To directly measure c-Src activity, anti c-Src antibodies were used to immunoprecipitate c-Src from either capacitated or non capacitated mouse sperm extracts. c-Src activity assays were then performed as described under “Experimental Procedures” in the presence of [32P]ATP and the c-Src synthetic peptide substrate derived from p34cdc2 (25). Half the sample was centrifuged and 32P incorporation into the peptide substrate was quantified as described (Fig. 1D). Samples immunoprecipitated with anti-tubulin antibody were used as background activity and were not different from blank controls. The other half was boiled in sample buffer and subjected to SDS-PAGE, then transferred to PVDF. The PVDF membrane was first used for autoradiography (Fig. 1E, autoradiography), followed by Western blot analysis for c-Src (Fig. 1E, Western blot) to assess equal loading. In these assays, anti-tubulin immunoprecipitates were used as controls. Both incorporation of 32P to the specific substrate as well as c-Src autophosphorylation revealed an increase in c-Src activity in capacitated sperm.
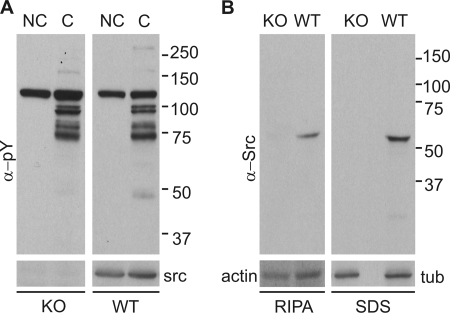
The previous data are consistent with the hypothesis that c-Src tyrosine kinase is directly involved in the capacitation-associated increase in tyrosine phosphorylation. To further investigate this possibility, the increase in tyrosine phosphorylation was evaluated in Src-null mice (16). Surprisingly, no differences in the capacitation-associated increase in tyrosine phosphorylation were observed between Src-null sperm and sperm from their wild-type siblings (Fig. 2A). Western blot analysis indicate that the anti-c-Src antibody used recognized only one band in wild type that is not present in c-Src null sperm (Fig. 2B). Moreover, this antibody was used to reprobe the anti-pY blots, showing the presence of c-Src in wild-type sperm but not in their null siblings. Thus, our unexpected result could have been the consequence of compensatory expression of other SFKs (26). However, the simplest explanation for these data is that c-Src is not directly responsible for the onset of tyrosine phosphorylation associated with the acquisition of fertilizing capacity by sperm.
FIGURE 2.
c-Src-KO mouse sperm undergo a capacitation-associated increase in tyrosine phosphorylation. A, capacitated and non-capacitated sperm extracts from Src-null (KO) and wild-type (WT) siblings were analyzed by Western blot with anti-pY (clone 4G10) antibodies. PVDF membranes were stripped and re-probed with anti-Src antibody (clone GD11), shown at the bottom. B, radioimmune precipitation assay and SDS extracts were further analyzed with a different anti-Src antibody (clone 32G6). A signal corresponding to c-Src was only detected in the WT siblings. Anti-tubulin and anti-actin immunodetections were used as loading controls.
Effect of SFK Inhibitors on PKA Phosphorylation of Sperm Proteins
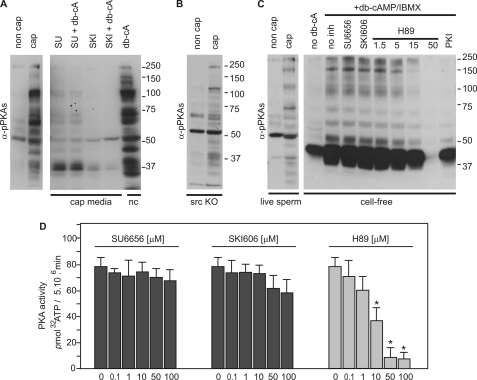
As mentioned above, the increase in protein-tyrosine phosphorylation is downstream of a cAMP/PKA pathway. This pathway can be analyzed using anti-phospho-PKA substrate antibodies (anti-pPKAs antibodies). These antibodies detect pSer or pThr at position RRXpS/pT (corresponding to a PKA consensus phosphorylation sequence) and have been previously used in sperm from different species, including mouse (27–30). To investigate whether SFK inhibitors were affecting PKA phosphorylation, sperm extracts were obtained after incubation in conditions that either support capacitation or do not and used in Western blot analyses with anti-pPKAs. As expected, a significant increase in phosphorylation of PKA substrates was detected in capacitated sperm (Fig. 3A). In addition, a similar pattern was observed when non-capacitated sperm were incubated in the presence of 1 mm Bt2cAMP and 0.1 mm IBMX. However, when either SU6656 or SKI606 inhibitors were present in the capacitation medium, the increase in PKA substrate phosphorylation was abrogated (Fig. 3A). Moreover, addition of cAMP agonists did not restore phosphorylation of PKA substrates. On the other hand, PKA phosphorylation was not affected in Src-null sperm (Fig. 3B).
FIGURE 3.
SFK inhibitors block phosphorylation of PKA substrates in live sperm but not in vitro. A, sperm were incubated for 60 min in capacitating medium containing 50 μm of either SU6656 or SKI606 in the presence or absence of 1 mm Bt2cAMP/0.1 mm IBMX (db-cA). Each condition was processed for Western blot analysis with a monoclonal anti-pPKAs antibody. B, Src-null (KO) mouse sperm displayed normal phosphorylation of PKA substrates. C, analysis of PKA activity in cell-free assays. Cell-free extracts were incubated for 30 min in medium containing 40 μm ATP and alternatively supplemented with1 mm Bt2cAMP, 0.1 mm IBMX, 50 μm SU6656, 50 μm SKI606, 10 μm PKI, and H-89 (as specified in micromolar). Each condition was processed for Western blot analysis and immunodetected with anti-pPKAs antibody. As control, the blot to the left shows live sperm incubated in conditions that support or do not support capacitation for comparison. D, sperm PKA activity was measured using Kemptide as substrate. Kinase buffer contained the indicated amounts of SU6656, SKI606, or H-89, maintaining a constant concentration of DMSO. Data represent mean ± S.E. of three independent experiments performed in triplicates; *, p < 0.01.
These unexpected results could be explained by unspecific effects of SFK inhibitors on PKA. To test this possibility, activity of PKA was assayed by two different approaches. In the first, sperm were lysed in 1% Triton X-100 buffer, and the whole lysate was incubated with ATP, Bt2cAMP, Mg2+, and a mixture of protease and phosphatase inhibitors (see “Experimental Procedures” for details). After 30 min, sample buffer was added to each experimental condition, and phosphorylation of PKA substrates was assayed by Western blot analysis using anti-pPKA antibodies. Under these conditions, H-89, a specific PKA inhibitor, as well as the inhibitory PKI peptide, blocked the cAMP agonist-induced increase in PKA substrate phosphorylation. However, contrary to observations in live sperm, SFK inhibitors did not block the increase in PKA substrate phosphorylation in cell-free assays when cAMP agonists were present in the incubation buffer (Fig. 3C). As a second approach, PKA activity was assayed by quantification of 32P incorporated into the Kemptide (Fig. 3D). Whereas H-89 inhibited the in vitro PKA activity from sperm extracts, neither SU6656 nor SKI606 used at concentrations as high as 100 μm showed inhibitory effects (Fig. 3D). The inability of the SFK inhibitors to affect PKA activity was confirmed using commercial PKA catalytic subunit from bovine heart (data not shown). These data ruled out the possibility that SFK inhibitors are directly blocking PKA. As an alternative hypothesis, SFK inhibitors could be acting in a pathway upstream or parallel to the activation of PKA.
Ser/Thr Phosphatase Inhibitors Rescue the Effect of SU6656 and SKI606
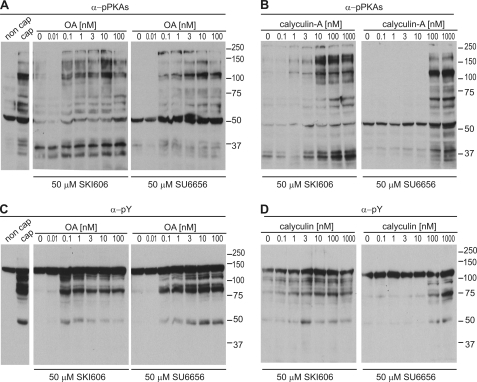
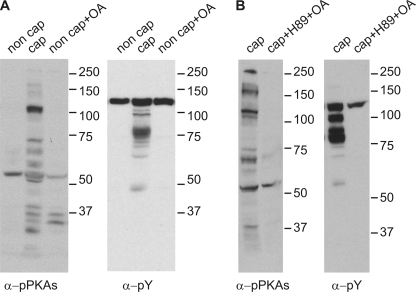
SFKs have been shown to down-regulate Ser/Thr phosphatases in other cell types (31, 32). Therefore, it is possible that the inhibitory action of SU6656 and SKI606 in sperm is due to up-regulation of Ser/Thr phosphatases. To investigate this hypothesis, sperm were incubated in capacitating media supplemented with either SU6656 or SKI606 in the absence or in the presence of Ser/Thr phosphatase inhibitors. Both okadaic acid and calyculin-A were able to rescue the inhibitory action of SU6656 and SKI606 on PKA (Fig. 4, A and B) and tyrosine phosphorylation (Fig. 4, C and D). These data indicate that inhibition of Ser/Thr phosphatases is sufficient to rescue the effect of SFK inhibitors when sperm are incubated in capacitating medium. It is noteworthy that 10 nm okadaic acid did not trigger tyrosine phosphorylation or phosphorylation of PKA substrates when used in non-capacitating medium (Fig. 5A). Interestingly, when H-89 was used, okadaic acid was not able to rescue the inhibition (Fig. 5B).
FIGURE 4.
Rescue of PKA and tyrosine phosphorylation by Ser/Thr phosphatase inhibitors. A and B, sperm were incubated in capacitating medium supplemented with SFK inhibitors and different concentrations of okadaic acid (OA) (A) or calyculin-A (B), before immunodetection of p-PKA substrates (clone 100G7E). C and D, PVDF membranes used in A and B were stripped as described and used for Western blot immunodetection with anti-PY antibodies (clone 4G10). All Western blots are representative of experiments repeated at least three times.
FIGURE 5.
Okadaic acid does not induce phosphorylation events in sperm incubated under non-capacitating conditions or when the sperm are in the presence of the PKA inhibitor H-89. A, mouse sperm were incubated under conditions that support or do not support capacitation either in the presence or absence of 10 nm okadaic acid. B, sperm were incubated with 50 μm H-89 in the presence of 10 nm okadaic acid. All Western blots are representative of experiments repeated at least three times.
Functional Studies
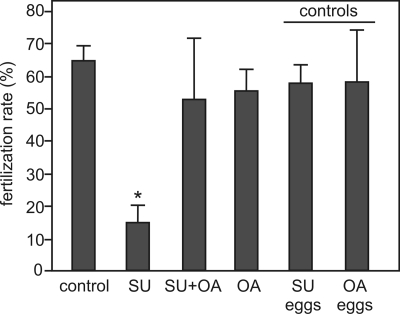
The cAMP/PKA pathway in sperm has been linked to activation of motility as well as to the ability of sperm to fertilize the egg in vitro. Therefore, it is expected that the inhibitory action of SU6656 and SKI606 on capacitation-associated phosphorylation would affect these functional parameters. Similar to the effect on PKA and tyrosine phosphorylation, 50 μm SU6656 significantly inhibited all motility parameters assessed with by CASA (Table 1) as well as in vitro fertilization (Fig. 6). The SU6656-induced inhibition on these parameters was overcome with 10 nm okadaic acid, indicating that the effect of this SFK inhibitor involved up-regulation of Ser/Thr phosphatase activity. To evaluate whether SU6656 or okadaic acid had any effect on the egg, these compounds were incubated with eggs and washed off before sperm addition, as controls. Under these conditions, no effect was observed in the in vitro fertilization assay.
TABLE 1.
Okadaic acid rescues sperm motility parameters affected by SU6656
Mouse sperm were incubated in the absence or presence of SU6656 (SU, 50 μm), okadaic acid (OA, 10 nm), or both inhibitors together (SU/OA) for 60 min in medium that either supports (with HCO3−) (cap) or does not support capacitation (without HCO3−) (non cap). After capacitation, sperm motility was examined using the CEROS computer-assisted semen analysis (CASA) system. Data represent mean ± S.E., n = 3. The effect of SFK and phosphatase inhibitors on mobility parameters of mouse sperm by CASA is shown. Mot, percent motility; VAP, velocity average path; VSL, velocity straight line; VCL, velocity curvilinear; prog, progressive motility.
| Treatmenta | Mot | VAP | VSL | VCL | Prog |
|---|---|---|---|---|---|
| % | μm/s | μm/s | μm/s | % | |
| Non cap | 75 ± 3.9 | 82 ± 4.9 | 63 ± 3.9b | 129 ± 5.2b | 27 ± 1.4b |
| Cap | 81 ± 6.5 | 77 ± 4.4 | 51 ± 2.8 | 160 ± 3.3 | 22 ± 1.7 |
| Cap+SU | 59 ± 4.1b | 23 ± 3.2b | 17 ± 2.1b | 43 ± 4.0b | 2 ± 0.5b |
| Cap+SU/OA | 67 ± 7.1 | 89 ± 9.1 | 71 ± 6.0 | 141 ± 5.3b | 24 ± 2.1 |
| Cap+OA | 77 ± 6.9 | 60 ± 5.0 | 42 ± 2.5b | 121 ± 4.6b | 33 ± 1.5b |
a SU = 50 μm SU6656; OA = 10 nm okadaic acid.
b p < 0.05 versus capacitated sperm.
FIGURE 6.
Okadaic acid rescues the inhibition of in vitro fertilization by SU6656. Sperm were capacitated in either the presence or absence of SU6656 (SU, 50 μm), okadaic acid (OA, 10 nm) or both. After capacitation, eggs were inseminated with 2.5 × 106 sperm/ml in 200-μl drops. As controls for the effect of the inhibitors on eggs, eggs were preincubated with inhibitors for 30 min and washed before insemination (where specified as SU eggs or OA eggs). Fertilization was assessed by microscopic visualization of pronuclei formation. Data represent mean ± S.E., n = 3; *, p < 0.01. At least 30 eggs were evaluated in each treatment.
DISCUSSION
Increased tyrosine phosphorylation has been associated with the acquisition of sperm-fertilizing capacity in different species (6, 33, 34), and it is correlated with the onset of functional parameters such as hyperactivated motility and acrosomal responsiveness (35–38). In mammalian sperm, capacitation is a HCO3−-dependent process (39). A likely target for HCO3− is the regulation of cAMP metabolism through stimulation of a soluble adenylyl cyclase (SACY). Once activated, intracellular cAMP levels increase, promoting the activation of PKA through the release of its inhibitory subunits. As previously shown, inhibition of sperm PKA activity blocks the increase in tyrosine phosphorylation associated with capacitation. Recently, c-Src has been postulated to be the tyrosine kinase directly responsible for this increase in tyrosine phosphorylation. In addition, it has been proposed that c-Src is activated by a cAMP-mediated pathway via the inhibition of Csk, the tyrosine kinase responsible for the inactivation of c-Src in other cell types (14). Consistent with this model and with previous published results from different laboratories (8, 10, 14, 24), SU6656, an SFK inhibitor, blocked tyrosine phosphorylation in a concentration-dependent manner. Moreover, immunoprecipitated c-Src from capacitated sperm showed significantly higher activity than c-Src from non-capacitated sperm, suggesting that this kinase is activated during capacitation.
The initial aim of the present work was to further investigate the role of c-Src tyrosine kinase in mouse sperm capacitation. For this purpose, the increase in tyrosine phosphorylation was analyzed in sperm from mice in which c-Src had been eliminated by homologous recombination (16). Src-null mice have an osteopetrotic phenotype, are toothless, and normally succumb to various lesions (such as benign nasal polyps) by 15 weeks of age. Therefore, their reproductive phenotype has been difficult to analyze in detail. When the capacitation-associated increase in tyrosine phosphorylation was assessed in the Src-null sperm, no differences were found when compared with sperm from their wild-type littermates. These findings suggested that c-Src is not the enzyme that directly phosphorylates tyrosine residues as part of sperm capacitation, or, alternatively, that lack of c-Src can be compensated by other members of the SFKs.
As mentioned, activation of tyrosine phosphorylation is downstream of a cAMP/PKA pathway. Analysis of PKA phosphorylation using anti-PKA substrate antibodies revealed that this phosphorylation was unaffected in cSrc KO sperm. However, PKA phosphorylation was blocked in the presence of SFK inhibitors suggesting that SU6656 and SKI606 may not be specific at the concentrations used. This possibility was ruled out by directly measuring PKA activity in vitro. At concentrations as high as 100 μm, the SFK inhibitors did not block PKA activity in vitro. Because SU6656 as well as SKI606 are competitive inhibitors of the SFK ATP binding site, the conclusion from these experiments is that the effect of these inhibitors on sperm is not due to unspecific inhibition of PKA activity. Another possibility was that SFK inhibitors could be blocking the capacitation pathway upstream of PKA activation. This possibility was also ruled out, because addition of cAMP agonists did not restore phosphorylation of PKA substrates or the increase in tyrosine phosphorylation in the presence of SFK inhibitors.
In light of these data, we propose an alternative hypothesis in which SFK inhibitors act in a pathway parallel to PKA activation. The steady-state phosphorylation status of a protein is influenced by relative activities of both kinases and phosphatases acting on it. It has been shown that SFK members are able to phosphorylate the Ser/Thr phosphatase PP2A catalytic subunit in its C-terminal domain. This phosphorylation event results in the inactivation of PP2A (31, 32, 40–43). For example, rat cerebral ischemia has been associated with the SFK-induced down-regulation of PP2A activity through Tyr-307 phosphorylation (44). Taking this into consideration, the effect of the SFK inhibitors on PKA and tyrosine phosphorylation in sperm could be explained by the hypothesis that Ser/Thr phosphatases are released from inhibition by tyrosine kinase inhibition. Interestingly, both okadaic acid and calyculin-A, two potent inhibitors of Ser/Thr phosphatases, completely rescued the effect of the SFK inhibitors on PKA and tyrosine phosphorylation. However, neither PKA nor tyrosine phosphorylation was induced by okadaic acid when mouse sperm were incubated under non-capacitating conditions. This last set of experiments suggests that two parallel pathways need to be active to promote the capacitation-associated phosphorylation of PKA substrates and the consequent increase in tyrosine phosphorylation. One of the pathways is the well established HCO3−-dependent activation of cAMP synthesis by SACY; the second pathway depends on the inactivation of Ser/Thr phosphatases (Fig. 7). Consistent with this model, okadaic acid was unable to increase phosphorylation by PKA or tyrosine phosphorylation when sperm were incubated with the PKA inhibitor H-89.
FIGURE 7.
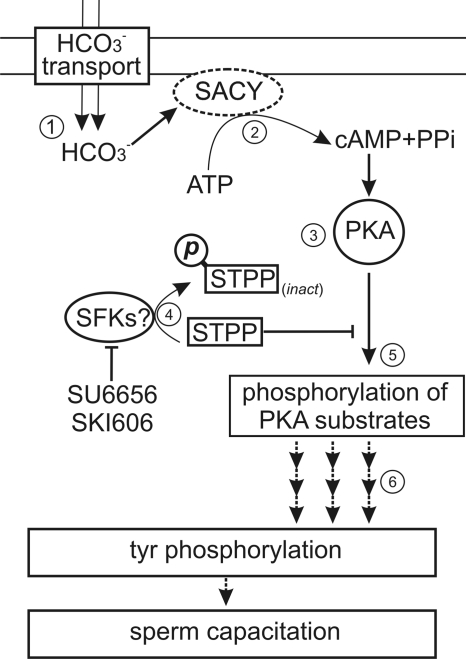
1, a model for the regulation of PKA activity by SFK inhibitors and Ser/Thr phosphatases. Influx of HCO3− stimulates the soluble adenylyl cyclase (SACY), increasing intracellular cAMP (2) and activating PKA (3). The activation of a kinase sensitive to both SU6656 and SKI606 down-regulates PP2A (4), which modifies the phosphorylated steady state of PKA substrates. As a consequence, the onset of PKA phosphorylation (5) is followed by the promotion of tyrosine phosphorylation associated with sperm capacitation (6).
To further investigate the role of phosphatase inactivation in capacitation, the effect of SFK inhibitors and the reversal of their action with okadaic acid was tested in motility and in vitro fertilization assays. As expected, because of their action on phosphorylation of PKA substrates, SFK inhibitors significantly blocked motility and fertilization. The SU6656 inhibition was overcome with the addition of 10 nm okadaic acid indicating that phosphatase inactivation plays an essential role in the regulation of these processes. It has been shown that inhibition of phosphatase activity promotes initiation of caput sperm motility (45, 46), further supporting a role of phosphatases in the regulation of motility. The very low concentrations of okadaic acid (IC50: ∼1 nm) capable of rescuing the effect of SFK inhibitors points toward the participation of the phosphatase PP2A in this pathway, which is ∼100-fold more sensitive to okadaic acid than PP1 (43, 47–50). It is noteworthy that the two main Ser/Thr phosphatases present in the sperm are PP1γ2, a testis-specific splice variant of Ppp1cc (51), and PP2A, also present in other cell types. In bull sperm, the phosphorylation state of residue Thr-320 in PP1γ2 has been shown to be modulated during epididymal maturation, and involved in phosphatase activity regulation (52). This residue also appears to be phosphorylated in mouse sperm (53). Phosphatases PP1γ2 and PP2A neither co-localize (53) nor co-elute in chromatographically purified sperm protein fractions (54), suggesting the existence of distinct phosphatase pools with presumably different roles. The possibility that PP2A is inactivated during capacitation warrants more investigation. In addition, PP2A has been shown to be capable of hydrolyzing phosphotyrosine residues (55, 56). This finding might also be relevant to signaling pathways involving tyrosine phosphorylation in sperm.
In summary, the present study presents evidence that c-Src is not directly involved in the increase in tyrosine phosphorylation that occurs during sperm capacitation in the mouse. This conclusion is based on results using sperm from Src-null mice, as well as on the finding that Ser/Thr phosphatase inhibitors completely overcome the effect of SFK inhibitors used at concentrations in which no residual SFK activity should be observed. This last observation also suggests that at least one of the two sperm Ser/Thr phosphatases, presumably PP2A, must be inactive in capacitating sperm to allow pivotal phosphorylation events to occur. Thus, in sperm, c-Src could participate in this inactivation. In this case, PKA activity and subsequent tyrosine phosphorylation observed in sperm from Src-null mice could be explained by compensatory activity of another SFK member. Finally, we cannot rule out the possibility that SU6656 and SKI606 at the concentrations employed inhibited another tyrosine kinase different from c-Src. This alternative hypothesis is consistent with the high concentration of SU6656 and with the sharp inhibition observed with SKI606 at 50 μm on the capacitation-associated increase in tyrosine phosphorylation. These concentrations are at least one order of magnitude higher than the 0.3 μm IC50 expected for c-Src in vitro. If this is the case, we predict that this alternative kinase would act upstream of Ser/Thr phosphatases and would be involved in their inactivation.
This work was supported, in whole or in part, by National Institutes of Health Grants HD38082 and HD44044 (to P. E. V.).
- SACY
- soluble adenylyl cyclase
- SFK
- family of protein tyrosine kinase
- PP2A
- protein phosphatase 2A
- Bt2cAMP
- dibutyryl cyclic AMP
- CASA
- CEROS computer-assisted semen analysis
- PKA
- protein kinase A
- BSA
- bovine serum albumin
- IBMX
- isobutylmethylxanthine
- PVDF
- polyvinylidene difluoride
- pY
- anti-phosphotyrosine
- PKI
- heat stable protein kinase A inhibitor.
REFERENCES
- 1.Yanagimachi R. (1994) in Mammalian Fertilization (Knobil E., Neill J. eds) pp. 189–317, Raven Press, New York [Google Scholar]
- 2.Salicioni A. M., Platt M. D., Wertheimer E. V., Arcelay E., Allaire A., Sosnik J., Visconti P. E. (2007) Soc. Reprod. Fertil. Suppl. 65, 245–259 [PubMed] [Google Scholar]
- 3.Hernández-González E. O., Sosnik J., Edwards J., Acevedo J. J., Mendoza-Lujambio I., López-González I., Demarco I., Wertheimer E., Darszon A., Visconti P. E. (2006) J. Biol. Chem. 281, 5623–5633 [DOI] [PubMed] [Google Scholar]
- 4.Arcelay E., Salicioni A. M., Wertheimer E., Visconti P. E. (2008) Int. J. Dev. Biol. 52, 463–472 [DOI] [PubMed] [Google Scholar]
- 5.Litvin T. N., Kamenetsky M., Zarifyan A., Buck J., Levin L. R. (2003) J. Biol. Chem. 278, 15922–15926 [DOI] [PubMed] [Google Scholar]
- 6.Visconti P. E., Bailey J. L., Moore G. D., Pan D., Olds-Clarke P., Kopf G. S. (1995) Development 121, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 7.Visconti P. E., Moore G. D., Bailey J. L., Leclerc P., Connors S. A., Pan D., Olds-Clarke P., Kopf G. S. (1995) Development 121, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 8.Lawson C., Goupil S., Leclerc P. (2008) Biol. Reprod. 79, 657–666 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell L. A., Nixon B., Baker M. A., Aitken R. J. (2008) Mol. Hum. Reprod. 14, 235–243 [DOI] [PubMed] [Google Scholar]
- 10.Varano G., Lombardi A., Cantini G., Forti G., Baldi E., Luconi M. (2008) Hum. Reprod. 23, 2652–2662 [DOI] [PubMed] [Google Scholar]
- 11.Pawson T., Warner N. (2007) Oncogene 26, 1268–1275 [DOI] [PubMed] [Google Scholar]
- 12.Zheng X. M., Resnick R. J., Shalloway D. (2000) EMBO J. 19, 964–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roskoski R., Jr. (2005) Biochem. Biophys. Res. Commun. 331, 1–14 [DOI] [PubMed] [Google Scholar]
- 14.Baker M. A., Hetherington L., Aitken R. J. (2006) J. Cell Sci. 119, 3182–3192 [DOI] [PubMed] [Google Scholar]
- 15.Etkovitz N., Tirosh Y., Chazan R., Jaldety Y., Daniel L., Rubinstein S., Breitbart H. (2009) Dev. Biol. 334, 447–457 [DOI] [PubMed] [Google Scholar]
- 16.Soriano P., Montgomery C., Geske R., Bradley A. (1991) Cell 64, 693–702 [DOI] [PubMed] [Google Scholar]
- 17.Moore G. D., Ayabe T., Visconti P. E., Schultz R. M., Kopf G. S. (1994) Development 120, 3313–3323 [DOI] [PubMed] [Google Scholar]
- 18.Wertheimer E. V., Salicioni A. M., Liu W., Trevino C. L., Chavez J., Hernández-González E. O., Darszon A., Visconti P. E. (2008) J. Biol. Chem. 283, 35539–35550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 20.Visconti P. E., Johnson L. R., Oyaski M., Fornés M., Moss S. B., Gerton G. L., Kopf G. S. (1997) Dev. Biol. 192, 351–363 [DOI] [PubMed] [Google Scholar]
- 21.Hao Z., Jha K. N., Kim Y. H., Vemuganti S., Westbrook V. A., Chertihin O., Markgraf K., Flickinger C. J., Coppola M., Herr J. C., Visconti P. E. (2004) Mol. Hum. Reprod. 10, 433–444 [DOI] [PubMed] [Google Scholar]
- 22.Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bantscheff M., Eberhard D., Abraham Y., Bastuck S., Boesche M., Hobson S., Mathieson T., Perrin J., Raida M., Rau C., Reader V., Sweetman G., Bauer A., Bouwmeester T., Hopf C., Kruse U., Neubauer G., Ramsden N., Rick J., Kuster B., Drewes G. (2007) Nat. Biotechnol. 25, 1035–1044 [DOI] [PubMed] [Google Scholar]
- 24.Baker M. A., Hetherington L., Curry B., Aitken R. J. (2009) Dev. Biol. 333, 57–66 [DOI] [PubMed] [Google Scholar]
- 25.Cheng H. C., Nishio H., Hatase O., Ralph S., Wang J. H. (1992) J. Biol. Chem. 267, 9248–9256 [PubMed] [Google Scholar]
- 26.Stein P. L., Vogel H., Soriano P. (1994) Genes Dev. 8, 1999–2007 [DOI] [PubMed] [Google Scholar]
- 27.Harrison R. A. (2004) Mol. Reprod. Dev. 67, 337–352 [DOI] [PubMed] [Google Scholar]
- 28.Kaneto M., Krisfalusi M., Eddy E. M., O'Brien D. A., Miki K. (2008) Mol. Reprod. Dev. 75, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 29.Morgan D. J., Weisenhaus M., Shum S., Su T., Zheng R., Zhang C., Shokat K. M., Hille B., Babcock D. F., McKnight G. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 20740–20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Flaherty C., de Lamirande E., Gagnon C. (2004) Mol. Hum. Reprod. 10, 355–363 [DOI] [PubMed] [Google Scholar]
- 31.Chen J., Martin B. L., Brautigan D. L. (1992) Science 257, 1261–1264 [DOI] [PubMed] [Google Scholar]
- 32.Chen J., Parsons S., Brautigan D. L. (1994) J. Biol. Chem. 269, 7957–7962 [PubMed] [Google Scholar]
- 33.Krapf D., Visconti P. E., Arranz S. E., Cabada M. O. (2007) Dev. Biol. 306, 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanichviriyakit R., Kruevaisayawan H., Weerachatyanukul W., Tawipreeda P., Withyachumnarnkul B., Pratoomchat B., Chavadej J., Sobhon P. (2004) Mol. Reprod. Dev 69, 356–363 [DOI] [PubMed] [Google Scholar]
- 35.Krapf D., O'Brien E. D., Cabada M. O., Visconti P. E., Arranz S. E. (2009) Biol. Reprod. 80, 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Si Y., Okuno M. (1999) Biol. Reprod. 61, 240–246 [DOI] [PubMed] [Google Scholar]
- 37.Visconti P. E., Stewart-Savage J., Blasco A., Battaglia L., Miranda P., Kopf G. S., Tezón J. G. (1999) Biol. Reprod. 61, 76–84 [DOI] [PubMed] [Google Scholar]
- 38.Krapf D., Vidal M., Arranz S. E., Cabada M. O. (2006) Biol. Cell 98, 403–413 [DOI] [PubMed] [Google Scholar]
- 39.Visconti P. E., Westbrook V. A., Chertihin O., Demarco I., Sleight S., Diekman A. B. (2002) J. Reprod. Immunol. 53, 133–150 [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan M., Begum N. (1994) J. Biol. Chem. 269, 12514–12520 [PubMed] [Google Scholar]
- 41.Villa-Moruzzi E., Puntoni F. (1996) Biochem. Biophys. Res. Commun. 219, 863–867 [DOI] [PubMed] [Google Scholar]
- 42.Mallozzi C., De Franceschi L., Brugnara C., Di Stasi A. M. (2005) Free Radic. Biol. Med. 38, 1625–1636 [DOI] [PubMed] [Google Scholar]
- 43.Mao L., Yang L., Arora A., Choe E. S., Zhang G., Liu Z., Fibuch E. E., Wang J. Q. (2005) J. Biol. Chem. 280, 12602–12610 [DOI] [PubMed] [Google Scholar]
- 44.Hu X., Wu X., Xu J., Zhou J., Han X., Guo J. (2009) BMC. Neurosci. 10, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith G. D., Wolf D. P., Trautman K. C., da Cruz e Silva EF, Greengard P., Vijayaraghavan S. (1996) Biol. Reprod. 54, 719–727 [DOI] [PubMed] [Google Scholar]
- 46.Vijayaraghavan S., Stephens D. T., Trautman K., Smith G. D., Khatra B., da Cruz e Silva E. F., Greengard P. (1996) Biol. Reprod. 54, 709–718 [DOI] [PubMed] [Google Scholar]
- 47.Bialojan C., Takai A. (1988) Biochem. J. 256, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. (1989) Biochem. Biophys. Res. Commun. 159, 871–877 [DOI] [PubMed] [Google Scholar]
- 49.Cohen P., Cohen P. T. (1989) J. Biol. Chem. 264, 21435–21438 [PubMed] [Google Scholar]
- 50.MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. (1990) FEBS Lett. 264, 187–192 [DOI] [PubMed] [Google Scholar]
- 51.Vijayaraghavan S., Chakrabarti R., Myers K. (2007) Soc. Reprod. Fertil. Suppl. 63, 111–121 [PubMed] [Google Scholar]
- 52.Huang Z., Vijayaraghavan S. (2004) Biol. Reprod. 70, 439–447 [DOI] [PubMed] [Google Scholar]
- 53.Goto N., Harayama H. (2009) J. Reprod. Dev. 55, 327–334 [DOI] [PubMed] [Google Scholar]
- 54.Cheng L., Pilder S., Nairn A. C., Ramdas S., Vijayaraghavan S. (2009) PLoS One 4, e4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chernoff J., Li H. C., Cheng Y. S., Chen L. B. (1983) J. Biol. Chem. 258, 7852–7857 [PubMed] [Google Scholar]
- 56.Janssens V., Goris J. (2001) Biochem. J. 353, 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]