Abstract
Normal mammary development requires coordinated interactions of numerous factors, including prolactin (PRL) and insulin-like growth factor I (IGF-I), both of which have also been implicated in breast cancer pathogenesis and progression. We previously reported that PRL and IGF-I synergize in breast cancer cells to activate ERK1/2 and AKT, leading to increased proliferation, survival, and invasion. Intriguingly, PRL co-treatment with IGF-I augments IGF-I receptor (IGF-IR) phosphorylation 2-fold higher than IGF-I alone. Here, we showed the importance of the tyrosine phosphatase SHP-2 in this cross-talk using pharmacological inhibition and small interfering RNA. SHP-2 recruitment to IGF-IR was significantly attenuated by PRL co-treatment. Src family kinase activity was required for IGF-IR association with SHP-2, ligand-induced IGF-IR internalization, and PRL-enhanced IGF-IR phosphorylation. Inhibition of internalization, via knockdown of the GTPase, dynamin-2, prevented not only IGF-IR dephosphorylation, but also PRL-enhanced IGF-IR phosphorylation. Consistently, PRL diminished IGF-I-induced IGF-IR internalization, which may result from reduced SHP-2 association with IGF-IR, because we demonstrated an essential role for SHP-2 in IGF-IR internalization. Together, these findings describe a novel mechanism of cross-talk between PRL and IGF-I in breast cancer cells, with implications for our understanding of tumor progression and potential therapeutic strategies.
Keywords: Diseases/Cancer/Mammary, Growth Factors, Hormones/Growth Factors, Receptors/Membrane, Receptors/Phosphatases, IGF-I, SHP-2, Prolactin
Introduction
Prolactin (PRL)2 is a protein hormone essential for the proliferation and differentiation of lobuloalveolar cells during pregnancy and lactation, and recent evidence has revealed its importance in mammary tumorigenesis (for reviews, see Refs. 1–3). PRL exerts its biological effects by binding to the prolactin receptor (PRLR), a member of the type I cytokine superfamily of receptors that initiates downstream signaling events (2). Because the PRLR does not contain intrinsic tyrosine kinase activity, it relies on associated kinases, including Janus kinase 2 and members of the Src family of kinases (SFKs), to promote signals to downstream mediators, such as signal transducers and activators of transcription (STATs), extracellular signal-regulated kinases (ERKs), and AKT (2).
These signaling modulators are shared by numerous growth factor and cytokine receptors and can serve as important sites for PRL cross-talk. Indeed, PRL cooperates with several factors in vitro and in vivo, including estrogen, epidermal growth factor family members, and insulin-like growth factor I (IGF-I), resulting in signaling synergy and biological effects associated with neoplastic progression (4–9). These findings underscore the importance of understanding interactions between PRL and growth factors in breast cancer, where therapeutically targeting multiple hormones and/or growth factors shows considerable promise (10).
We have recently described cooperative cross-talk between PRL and IGF-I in breast cancer cells (7). Alone, IGF-I stimulates proliferation of mammary epithelial cells, contributing to ductal branching and elongation, and promotes survival of alveolar structures during lactation (11, 12). Like PRL, IGF-I also plays an important role in breast cancer (for reviews, see Refs. 13 and 14). IGF-I exerts its biological actions via binding to the IGF-I receptor (IGF-IR), a member of the insulin family of receptor tyrosine kinases, initiating signals to downstream pathways, which include many shared with PRL, such as ERK1/2 and phosphatidylinositol 3-kinase/AKT (14, 15).
IGF-IR is trafficked to the plasma membrane as a covalently linked α2β2 heterotetramer, and ligand binding induces conformational changes leading to IGF-IR autophosphorylation (15). The activation loop of the IGF-IR kinase domain contains three key tyrosine residues (Tyr1131, Tyr1135, and Tyr1136) that serve as receptor autophosphorylation sites. Mutation of all (16) or some (17) of these residues dramatically reduces the kinase activity, as well as the mitogenic and transforming capabilities of IGF-IR. Increased phosphorylation of IGF-IR at these residues is associated with poor clinical survival (18) and may contribute to resistance to antiestrogens, epidermal growth factor receptor/HER2 inhibitors, and some chemotherapeutic agents (19, 20). These data reveal IGF-IR phosphorylation at these sites to be an important determinant of tumor behavior. Thus, regulation of this dynamic process, including dephosphorylation, is of considerable interest. Protein-tyrosine phosphatases, such as PTP-1B and SHP-2, have been shown to dephosphorylate IGF-IR in other models (21, 22). However, phosphatases involved in IGF-IR dephosphorylation in breast cancer cells have not been determined.
We have previously shown that PRL and IGF-I synergistically activate ERK1/2 and AKT in breast cancer cells, leading to increased proliferation, survival, and invasion (7). This cooperation between IGF-I and PRL appears to be mediated primarily through IGF-IR, because PRL enhances IGF-I-induced phosphorylation of Tyr1135 and Tyr1136 within the IGF-IR kinase domain, but the converse effect on PRLR phosphorylation is not observed (7). In the current study, we investigated the molecular mechanism(s) by which PRL augments IGF-IR phosphorylation in MCF-7 breast cancer cells. We found that PRL-enhanced IGF-IR phosphorylation was dependent on SHP-2, but not PTP-1B. Our studies describe a novel SFK-dependent mechanism whereby PRL augments the phosphorylation of IGF-IR through decreased dephosphorylation, which is a result of reduced association with SHP-2 and diminished internalization. These findings contribute to our mechanistic understanding of cross-talk between PRL and IGF-I in breast cancer cells and have implications for the pathogenesis, progression, and treatment of breast cancer.
EXPERIMENTAL PROCEDURES
Reagents
Antibodies used for these studies were purchased as follows: p-Src Y418 (catalog number 44-660) from BIOSOURCE (Camarillo, CA); Dnm-2 (catalog number PC407) from Calbiochem; p-IGF-IR Tyr1135/Tyr1136-insulin receptor Tyr1150/Tyr1151 (catalog number 3024), p-ERK1/2 Thr202/Tyr204 (catalog number 9101), ERK1/2 (catalog number 9102), p-AKT S473 (catalog number 9271), and AKT (catalog number 9272) from Cell Signaling Technology (Beverly, MA); IGF-IRβ (sc-713), PRLR for immunoprecipitation (sc-20092), c-Src (sc-18), and protein A/G-Plus agarose (sc-2003) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); SHP-2 (catalog number 06-118) from Upstate Biotechnology (Lake Placid, NY); and PRLR-ECD (catalog number 35-9200) for immunoblotting from Zymed Laboratories Inc., Inc. (South San Francisco, CA). Inhibitors used for these studies were purchased as follows: PTP-1B inhibitor (catalog number 539741), SHP-2 inhibitor NSC-87877 (catalog number 565851), MEK1/2 inhibitor U0126 (catalog number 662005), and Src family kinase inhibitor SU6656 (catalog number 572635) from Calbiochem; Src family kinase inhibitor PP1 (catalog number EI275) from Biomol International, LP (Plymouth Meeting, PA); and phosphatidylinositol 3-kinase inhibitor LY294002 from Sigma- Aldrich. Recombinant hPRL (Lot AFP795) was obtained from Dr. A. F. Parlow (National Hormone and Pituitary Program, NIDDK, National Institutes of Health, Torrance, CA). Recombinant human IGF-I was purchased from Cell Sciences (Canton, MA). All other reagents were obtained from Sigma unless otherwise noted.
Cell Culture
PRL-deficient MCF-7 cells were maintained as previously described (23). The cells were grown in phenol red-free RPMI 1640 containing 5% charcoal-stripped fetal bovine serum, penicillin, and streptomycin for 4–5 days prior to plating for all experiments. The cells were then serum-starved for 24–48 h prior to experiments in phenol red-free RPMI 1640. All of the hormone treatments were performed in this medium at the following concentrations: IGF-I, 20 ng/ml (2.6 nm); PRL, 100 ng/ml (4 nm).
Western Analysis and Immunoprecipitation
Immunoblotting was performed as previously described (23). All of the primary antibodies were used at 1:1000 dilutions except for p-ERK1/2 (1:5000), and the signals were visualized using enhanced chemiluminescence (Amersham Biosciences). The signals were quantified by scanning densitometry (ImageQuant software, v4.2a; Amersham Biosciences), and the phosphorylated protein levels were normalized to total protein levels as shown. The immunoprecipitations were performed as previously described (7). Briefly, the cells were lysed in IPA buffer (10 mm Tris, pH 8, 150 mm NaCl, 1 mm EDTA, pH 8, 1% Triton, 0.5% sodium deoxycholate, 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin), and 1 mg of protein was immunoprecipitated using 1 μg of the indicated antibody. Protein A/G Plus agarose slurry was added, and the mixture was allowed to tumble overnight at 4 °C. The beads were subsequently washed three times with 1× PBS, and the complexes were eluted via boiling in 2× Laemmli-SDS Buffer and prepared for electrophoresis. Associated proteins compared with total immunoprecipitated protein were quantified by scanning densitometry and expressed as fold change relative to vehicle-treated controls.
RNA Interference
siRNA targeting Dnm-2 was previously described (24). A predesigned siRNA targeting SHP-2 (catalog number AM51333), but not SHP-1 (25), was purchased from Ambion Inc. (Austin, TX). Nontargeting siRNA (NTsi, catalog number D-001910-03-05; Dharmacon, Lafayette, CO) was used as a control. All of the siRNA oligonucleotides were transfected in RPMI 1640 containing 5% charcoal-stripped fetal bovine serum using Lipofectamine 2000 (Invitrogen) at a ratio of 1 μl of transfection reagent/20 pmol of siRNA. The cells were incubated at 37 °C in the presence of the transfection complex for 24 h, then washed with serum-free RPMI 1640, and serum-starved for an additional 48 h prior to hormone stimulation. This protocol was optimal for knockdown of Dnm-2 and SHP-2 expression as determined by Western analysis.
Reversible Cell Surface Biotinylation Assay
Internalization experiments were performed using a cell surface labeling accessory pack (catalog number 1859419; Pierce) as previously described (24). Briefly, the cells were incubated with 0.25 mg/ml EZ-Link Sulfo-NHS-SS-Biotin in 1× PBS for 2 h at 4 °C. Quenching buffer was used to terminate the biotinylation reaction. The cells were then treated with vehicle, IGF-I, PRL, or IGF-I/PRL and warmed to 37 °C for the indicated times to allow for IGF-IR internalization. Upon termination of hormone treatment, the cells were incubated with 25 mm dithiothreitol in 1× PBS for two washes of 30 min at 4 °C to cleave biotin complexes remaining at the cell surface. After cell lysis, biotinylated proteins that had internalized were isolated from 1 mg of total protein using 50 μl of immobilized NeutrAvidin gel slurry overnight at 4 °C. NeutrAvidin-precipitated complexes were subsequently washed three times with 1× PBS, eluted via boiling in 2× Laemmli-SDS Buffer, and subjected to electrophoresis and Western analysis as described above.
Statistical Analyses
The statistical analyses were performed using Prism v4.00 (GraphPad Software, Inc., San Diego, CA).
RESULTS
PRL-mediated Enhancement of IGF-IR Phosphorylation Correlates with Augmented Downstream Activity
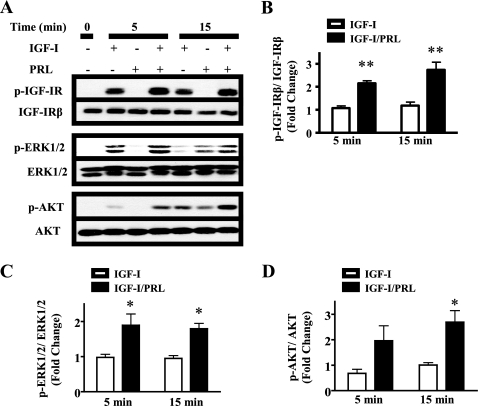
PRL cooperates with multiple oncogenic growth factors and hormones, including IGF-I (7). Interestingly, co-treatment of MCF-7 cells with PRL and IGF-I enhanced IGF-IR phosphorylation compared with treatment with IGF-I alone, as measured by phosphorylation with Tyr1135 and Tyr1136 (Fig. 1, A and B), two of the three tyrosines present in the kinase domain that are essential for IGF-IR activity (16). This increase was evident as early as 5 min and correlated with augmented signals to ERK1/2 and AKT (Fig. 1, A, C, and D), establishing the connection between enhanced phosphorylation of these residues and increased IGF-IR activation of downstream signaling pathways.
FIGURE 1.
PRL enhances IGF-I-induced IGF-IR phosphorylation and activation of downstream signaling pathways. Serum-starved MCF-7 cells were treated with vehicle, IGF-I, PRL, or IGF-I/PRL for the indicated times. The immunoblots were performed using cell lysates and the indicated antibodies. A, representative experiment. B, levels of p-IGF-IR/total IGF-IR in response to IGF-I alone, compared with IGF-I + PRL, quantified as described under “Experimental Procedures” (means ± S.D., n = 3). The asterisks denote significant differences compared with IGF-I treatment. ** p < 0.01 (two-way ANOVA, Bonferroni post-test). C and D, levels of phosphorylated ERK1/2 and AKT, respectively, compared with total kinase levels, quantified as described under “Experimental Procedures” (means ± S.D., n = 3). The asterisks denote significant differences compared with IGF-I treatment. *, p < 0.05 (paired t test).
PRL-mediated Enhancement of IGF-IR Phosphorylation Is Dependent upon SHP-2
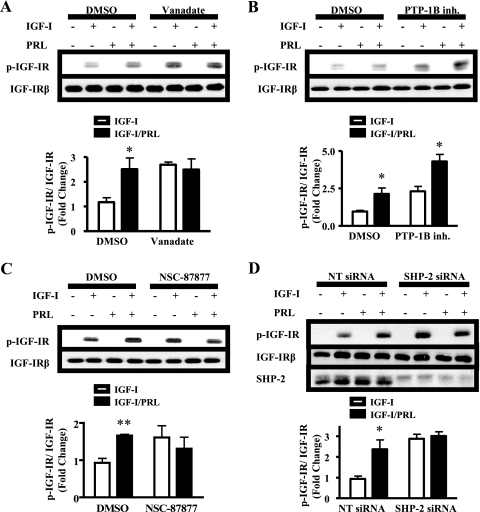
We hypothesized that the observed increase in p-IGF-IR levels caused by PRL co-treatment with IGF-I was a result of decreased dephosphorylation. To test this hypothesis, we treated MCF-7 cells with vanadate, a broad spectrum tyrosine phosphatase inhibitor. Levels of p-IGF-IR in response to IGF-I alone were significantly augmented in the presence of vanadate (p < 0.05), confirming the importance of tyrosine phosphatases (Fig. 2A). PRL was unable to further increase IGF-IR phosphorylation in the presence of this inhibitor (Fig. 2A), consistent with a tyrosine phosphatase mediator of PRL-enhanced IGF-IR phosphorylation.
FIGURE 2.
SHP-2 mediates PRL enhancement of IGF-IR phosphorylation. A–C, serum-starved MCF-7 cells were pretreated with dimethyl sulfoxide (DMSO) and either 200 μm vanadate (A), 10 μm PTP-1B inhibitor (B, inh.), or 50 μm NSC-87877 (C) for 1 h prior to treatment with vehicle, IGF-I, PRL, or IGF-I/PRL for 15 min. D, MCF-7 cells were transfected with nontargeting (NT) or SHP-2-specific siRNA duplexes and treated with vehicle, IGF-I, PRL, or IGF-I/PRL for 15 min. Cell lysates were immunoblotted with the indicated antibodies. Top panels, representative experiment; bottom panels, quantification of p-IGF-IR signal/total IGF-IR in response to IGF-I alone, compared with IGF-I + PRL, as described under “Experimental Procedures” (means ± S.D., n = 3 (A–C) or n = 4 (D)). The asterisks denote significant differences compared with IGF-I treatment. *, p < 0.05; **, p < 0.01 (two-way ANOVA, Bonferroni post-test (A, C, and D) or paired t test (B)).
Phosphatases known to dephosphorylate IGF-IR include PTP-1B and SHP-2 (21, 22). To determine whether PTP-1B was involved in PRL-enhanced IGF-IR phosphorylation, we utilized a pharmacological inhibitor of PTP-1B phosphatase activity (26). Although inhibition of PTP-1B significantly raised IGF-I-induced p-IGF-IR levels (p < 0.05), indicating that it played a role in IGF-IR dephosphorylation, it did not prevent PRL-enhanced IGF-IR phosphorylation (Fig. 2B). However, in contrast to PTP-1B, selective inhibition of SHP-2 with NSC-87877 blocked PRL-enhanced IGF-IR phosphorylation (Fig. 2C). To ascertain a requirement for SHP-2 protein and distinguish SHP-2 from the closely related SHP-1, we employed siRNA to knock down expression of SHP-2 (25). In the presence of SHP-2 siRNA, p-IGF-IR levels were significantly elevated in response to IGF-I (p < 0.05) but were not further increased by PRL (Fig. 2D). Similar results were obtained 5 min after the addition of ligands (mean fold change in p-IGF-IRβ/IGF-IRβ relative to NTsi/IGF-I ± S.D.: NTsi/IGF-I = 1; NTsi/IGF-I + PRL = 2.15 ± 0.25; SHP-2si/IGF-I = 1.94 ± 0.48; and SHP-2si/IGF-I + PRL = 1.90 ± 0.67, n = 3). Together, these data suggest that the ability of PRL to augment IGF-IR phosphorylation specifically requires SHP-2.
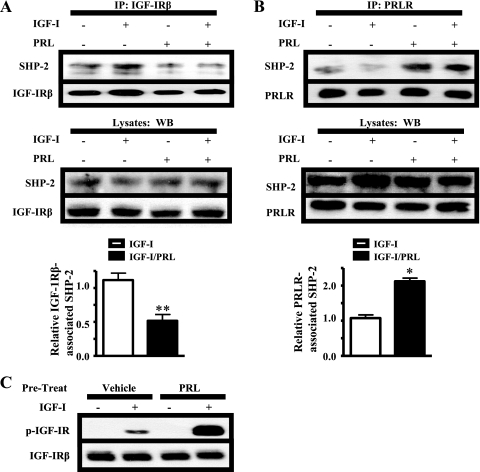
PRL Decreases SHP-2 Association with IGF-IR
In other cell lines, SHP-2 is recruited to both IGF-IR and PRLR in response to ligand (22, 27). Based on the involvement of SHP-2 in PRL-enhanced IGF-IR phosphorylation, we examined the effects of IGF-I and/or PRL treatment on the association of SHP-2 with both receptors. IGF-I/PRL co-treatment significantly reduced IGF-IR-associated SHP-2 compared with IGF-1 alone (Fig. 3A). Instead, higher levels of SHP-2 were associated with PRLR following IGF-I/PRL co-treatment (Fig. 3B), which correlated with augmented levels of p-IGF-IR. These findings predicted that activation of PRLR could reduce the pool of SHP-2 available to the IGF-IR. To test this, we pretreated the cells with vehicle or PRL for 5 min and then washed the cells to remove PRL from the media prior to IGF-I treatment. Under these conditions, IGF-I-induced IGF-IR phosphorylation was further enhanced (Fig. 3C), consistent with this model.
FIGURE 3.
PRL reduces association of SHP-2 with IGF-IR. A and B, serum-starved MCF-7 cells were treated with IGF-I, PRL, or IGF-I/PRL for 15 min. 1 mg of protein from cell lysates was immunoprecipitated (IP) with IGF-IRβ (A) or PRLR (B) antibodies and subjected to immunoblotting as shown. Top panel, immunoblot of representative immunoprecipitation results; middle panel, preimmunoprecipitation lysates; bottom panel, quantification of levels of SHP-2 in immunoprecipitates, compared with IGF-IRβ and PRLR in A and B, respectively, in response to IGF-I/PRL co-treatment compared with those induced by IGF-I alone (see “Experimental Procedures”; means ± S.D., n = 5 (A) or n = 4 (B)). The asterisks denote significant differences compared with IGF-I treatment (*p < 0.05, **p < 0.01, paired t test). C, serum-starved MCF-7 cells were pretreated with vehicle or PRL for 5 min, at which point medium containing vehicle or PRL was removed, and cells were washed with serum-free media. The cells were then exposed to medium containing IGF-I for an additional 15 min. The immunoblots were performed using cell lysates and the indicated antibodies (representative experiment shown). WB, Western blot.
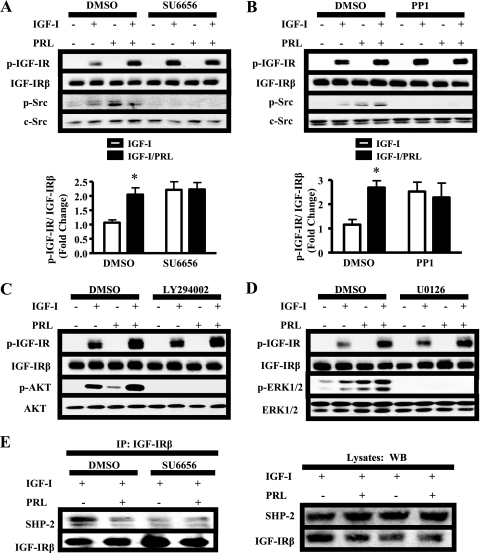
SFKs Are Required for PRL-enhanced IGF-IR Phosphorylation and SHP-2 Association with IGF-IR
Next, we aimed to identify which signal(s) mediated PRL-enhanced IGF-IR phosphorylation. SFKs were of particular interest because they associate with PRLR (24, 28) and also regulate SHP-2 recruitment to other membrane receptors, such as the platelet-derived growth factor receptor (29). To determine whether SFKs played a role in PRL-enhanced IGF-IR phosphorylation, we inhibited SFK activity using two distinct pharmacological inhibitors, SU6656 and PP1 (30). Although SFKs have been shown to phosphorylate IGF-IR (24), inhibition of SFK activity increased IGF-IR phosphorylation in response to IGF-I alone (Fig. 4, A and B; p < 0.05). This is similar to results obtained using phosphatase inhibitors (Fig. 2), suggesting that SFKs may play a role in the events leading to dephosphorylation of IGF-IR in these cells. However, both SU6656 and PP1 blocked further augmentation of IGF-IR by PRL, suggesting that SFK activity was required for PRL action. Because SFKs activate cascades leading to ERK1/2 and AKT in MCF-7 cells (24), we determined whether these downstream pathways altered ligand-induced IGF-IR phosphorylation. However, neither the MEK1/2 inhibitor U0126 nor the phosphatidylinositol 3-kinase inhibitor LY294002 impaired IGF-I-induced IGF-IR phosphorylation nor affected PRL-induced augmentation (Fig. 4, C and D), indicating that these pathways were not involved.
FIGURE 4.
SFKs mediate PRL enhancement of IGF-IR phosphorylation and SHP-2 association with IGF-IR. A–D, serum-starved MCF-7 cells were pretreated with DMSO and either 10 μm SU6656 (A), 10 μm PP1 (B), 10 μm U0126 (C), or 10 μm LY294002 (D) for 1 h prior to treatment with vehicle, IGF-I, PRL, or IGF-I/PRL for 15 min. The immunoblots were performed using cell lysates and the indicated antibodies (representative experiments shown). p-IGF-IR signals induced by IGF-I/PRL co-treatment were quantified and compared with those induced by IGF-I alone as described under “Experimental Procedures” (means ± S.D., n = 5 (A) or n = 3 (B)). The asterisks denote significant differences compared with IGF-I treatment. *, p < 0.05; **, p < 0.01) two-way ANOVA, Bonferroni post-test). E, serum-starved MCF-7 cells were pretreated with dimethyl sulfoxide (DMSO) or 10 μm SU6656 for 1 h prior to hormone treatment for 15 min. 1 mg of protein from cell lysates was immunoprecipitated (IP) with 1 μg of IGF-IRβ antibody and subjected to immunoblotting as indicated. Top panel, immunoblots of representative immunoprecipitation results; bottom panel, preimmunoprecipitation lysates. WB, Western blot.
We hypothesized that the role for SFKs in IGF-IR dephosphorylation may involve the physical interaction between SHP-2 and IGF-IR. Indeed, in the presence of SU6656, SHP-2 association with IGF-IR was greatly reduced in response to IGF-I (Fig. 4E). The reduced association with this phosphatase may explain, at least in part, the increased phosphorylation of IGF-IR in response to IGF-I alone, as well as the inability of PRL to enhance IGF-IR phosphorylation in the presence of SFK inhibitors. SFK activity was also required for SHP-2 to associate with PRLR (data not shown).
IGF-IR Internalization Is Required for PRL-mediated Enhancement of IGF-IR Phosphorylation
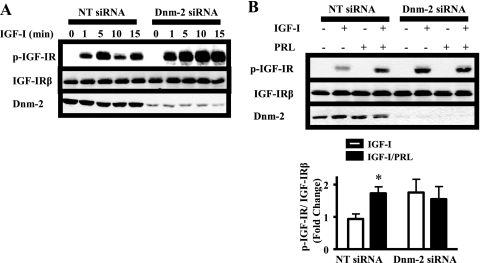
Because of the intimate relationship between internalization and dephosphorylation for many receptor tyrosine kinases (31, 32) and because of the importance of SFKs in the internalization of many membrane receptors (24, 33), we investigated the relationship between IGF-IR internalization and dephosphorylation. We observed an interesting time course of IGF-I-induced IGF-IR phosphorylation. Although IGF-I increased levels of p-IGF-IR at 5 min, we observed a consistent decrease in p-IGF-IR at 10 min, which subsequently rose again at 15 min (Fig. 5A). This pattern is consistent with reported recycling of the IGF-1Rβ (31, 34); the decrease at 10 min may reflect dephosphorylation of internalized IGF-IR, which is subsequently recycled to the cell surface where it can again bind ligand, generating the second rise in p-IGF-IR levels. To examine the requirement for internalization in dephosphorylation, we knocked down expression of the GTPase dynamin-2 (Dnm-2). Dnm-2 is critical for both clathrin-dependent and -independent endocytosis (35, 36), and we have previously shown that Dnm-2 siRNA blocks both pathways in MCF-7 cells (24). Upon Dnm-2 knockdown, the levels of p-IGF-IR were elevated at all times after exposure to IGF-I (Fig. 5A), consistent with a requirement for internalization in IGF-IR dephosphorylation. Next, we asked whether internalization was required for PRL to augment the p-IGF-IR levels. In the presence of Dnm-2 siRNA, PRL did not further raise p-IGF-IR levels above that induced by IGF-I alone (Fig. 5B), indicating that IGF-IR internalization is required for this phenomenon.
FIGURE 5.
IGF-IR internalization is required for dephosphorylation and PRL-enhanced phosphorylation. MCF-7 cells were transfected with nontargeting (NT) or Dnm-2-specific siRNA. Following transfection, the cells were treated with IGF-I for the times indicated (A) or with vehicle, IGF-I, PRL, or IGF-I/PRL for 15 min (B). Immunoblots were performed using cell lysates and the indicated antibodies (representative experiments shown). p-IGF-IR signals induced by IGF-I/PRL co-treatment were quantified and compared with those induced by IGF-I alone as described under “Experimental Procedures” (means ± S.D., n = 5). The asterisks denote significant differences compared with IGF-I treatment. *, p < 0.05 (two-way ANOVA, Bonferroni post-test).
PRL Attenuates IGF-I-induced IGF-IR Internalization
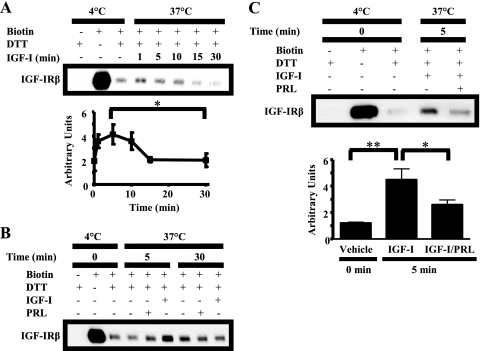
The requirement for internalization in IGF-IR dephosphorylation led us to hypothesize that PRL may alter IGF-IR endocytosis. To evaluate this hypothesis, we monitored internalization of endogenous IGF-IR using a reversible biotinylation assay. Internalization of biotinylated IGF-IR was induced by IGF-I treatment at 37 °C. The internalized subpopulation of IGF-IR was specifically detected following treatment with dithiothreitol to cleave biotin complexes remaining on the cell surface. We detected internalized IGF-IR as early as 1 min, which remained elevated for 10 min and then fell to base line after 15 min (Fig. 6A). This time course is consistent with IGF-IR recycling back to the plasma membrane and/or loss of the biotin label from the internalized IGF-1Rβ pool.
FIGURE 6.
PRL decreases IGF-I-induced IGF-IR internalization. Serum-starved MCF-7 cells were labeled with 0.25 mg/ml sulfo-NHS-biotin at 4 °C for 2 h. The cells were then treated for the times indicated with vehicle (B), IGF-I (A–C), PRL (B), or IGF-I/PRL (C) at 37 °C to allow for internalization of IGF-IR. Remaining plasma membrane-associated biotin was then cleaved with dithiothreitol (DTT). Biotinylated proteins that had internalized were immunoprecipitated using NeutrAvidin-conjugated beads and subjected to Western analysis (representative experiments shown). Internalized biotinylated IGF-IR was quantified using densitometry. A, means ± S.D. (n = 3). The asterisk denotes a significant difference in internalized biotinylated IGF-IR between 5 and 30 min. *, p < 0.05 (paired t test). C, means ± S.D. (n = 3). The asterisks denote significant differences in internalized biotinylated IGF-IR compared with IGF-I treatment. *, p < 0.05; **, p < 0.01 (one-way ANOVA, Neuman-Keuls post-test).
To evaluate the contributions of constitutive and PRL-induced IGF-IR internalization, we compared IGF-IR internalization in response to IGF-I with that induced by vehicle and PRL. At 5 min, both vehicle and PRL had little effect on internalized IGF-IR compared with IGF-I (Fig. 6B). After 30 min, the levels of internalized, biotin-labeled IGF-IR induced by IGF-I were reduced as expected and were indistinguishable from that following vehicle or PRL treatment (Fig. 6B). This indicates that the increased rate of IGF-IR internalization is ligand-specific.
We therefore examined the effect of PRL on IGF-I-induced IGF-IR internalization. IGF-I/PRL co-treatment significantly reduced IGF-IR internalization compared with IGF-I alone (Fig. 6C). Because IGF-IR internalization was required for dephosphorylation, the augmented levels of p-IGF-IR observed in response to IGF-I/PRL co-treatment may, at least in part, be explained by this diminished internalization. Consistently, endocytosis was required for PRL-enhanced p-IGF-IR levels (Fig. 5B), supporting a role for attenuated internalization in this process.
SFKs and SHP-2 Are Required for IGF-IR Internalization
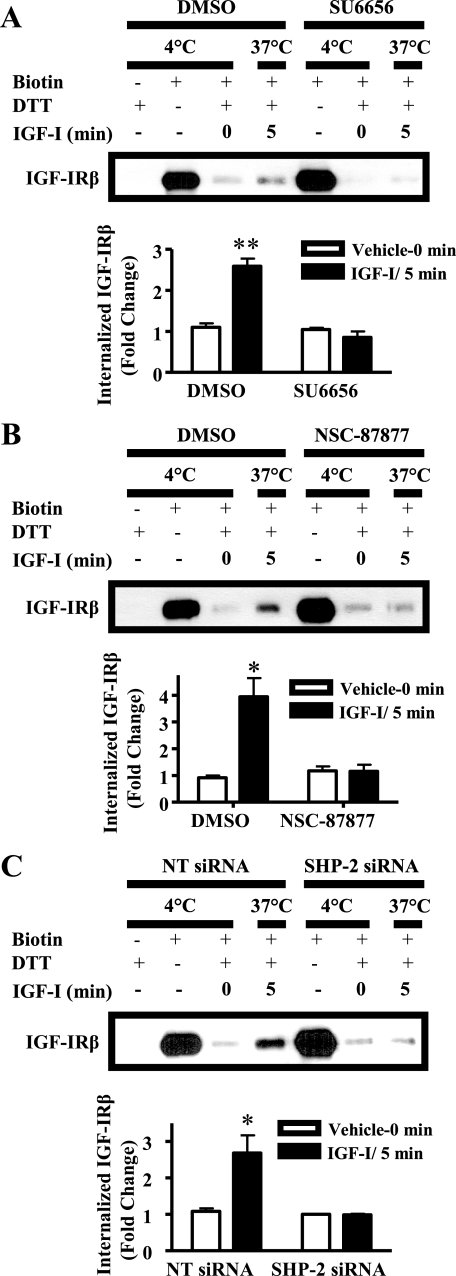
We subsequently investigated the roles of SFKs and SHP-2 in IGF-I-induced IGF-IR internalization. Inhibition of SFK activity using SU6656 markedly reduced IGF-IR internalization in response to IGF-I (Fig. 7A), indicating that SFK activity is required for ligand-induced IGF-IR internalization. The requirement of internalization for IGF-IR dephosphorylation may explain why SFK inhibition raised IGF-I-induced p-IGF-IR levels (Fig. 4, A and B). Interestingly, SU6656 elevated surface IGF-IR without altering the total IGF-IR levels (Figs. 4A and 7A), suggesting a role for SFKs in the constitutive trafficking of this receptor.
FIGURE 7.
SFKs and SHP-2 are required for IGF-IR internalization. Serum-starved MCF-7 cells were pretreated with dimethyl sulfoxide (DMSO) and either 10 μm SU6656 (A) or 50 μm NSC-87877 (B) or were transfected with nontargeting (NT) or SHP-2-specific siRNA duplexes (C) and labeled with 0.25 mg/ml sulfo-NHS-biotin at 4 °C for 2 h. The cells were then treated with IGF-I at 37 °C for 5 min to allow for internalization of IGF-IR. Plasma membrane-associated biotin was then cleaved with dithiothreitol (DTT). Biotinylated proteins that had internalized were immunoprecipitated using NeutrAvidin-conjugated beads and subjected to Western analysis. Top panels, representative experiment; bottom panels, quantitated internalized IGF-IRβ. The asterisks denote significant differences in internalized biotinylated IGF-IRβ compared with IGF-I treatment. *, p < 0.05; **, p < 0.01 (two-way ANOVA, Bonferroni post-test).
To investigate the role of SHP-2 in this process, we used pharmacological inhibition and siRNA. Both the SHP-2 inhibitor NSC-87877 and knockdown of SHP-2 expression blocked IGF-I-induced IGF-IR internalization evident at 5 min (Fig. 7, B and C). Moreover, like inhibition of SFKs, both inhibition and knockdown of SHP-2 elevated surface IGF-IR without altering the total IGF-IR levels (Figs. 2, C and D, and 7, B and C). Together, these data corroborate an essential role for SHP-2 in IGF-IR internalization.
DISCUSSION
PRL enhances IGF-I-induced phosphorylation of Tyr1135 and Tyr1136 of IGF-IR in MCF-7 breast cancer cells, increasing downstream signaling, proliferation, and invasion (7). In light of the consequences of augmented IGF-IR activity for neoplastic progression, understanding the underlying mechanism is critical. In our current studies, we demonstrated that SHP-2, but not PTP-1B, mediated PRL-enhanced IGF-IR phosphorylation. In the presence of IGF-I, PRL decreased SHP-2 association with IGF-IR, which correlated with increased IGF-IR phosphorylation. Additionally, PRL diminished IGF-I-induced IGF-IR internalization, an event required both for dephosphorylation of IGF-IR and for PRL-enhanced IGF-IR phosphorylation. Finally, we demonstrated an essential role for SHP-2 and SFKs in IGF-I-induced IGF-IR endocytosis, linking the reduction in IGF-IR- associated SHP-2 and attenuation of IGF-I-induced internalization mediated by PRL. Together, these studies describe a novel mechanism whereby PRL augments IGF-IR activity by decreasing its dephosphorylation resulting from the retention of IGF-IR at the plasma membrane. This mechanism of cooperation between PRL and IGF-I in breast cancer cells has important implications for the progression and treatment of breast cancer.
SHP-2 (also known as PTP1D, SHPTP-2, SHPTP-3, PTP2C, or Syp) is a nonreceptor tyrosine phosphatase expressed in many cells, including mammary epithelium (37, 38). Although phosphatases are generally thought to be negative regulators of signaling, the function of SHP-2 is complex, because it can both positively and negatively modulate signaling (39). This dual role has been established for IGF-IR; SHP-2 is required for some ligand-initiated signals (40, 41), but the accelerated recruitment of SHP-2 to IGF-IR negatively impacts signal activation and proliferation in multiple cell types (42–44). The latter has been postulated to result from dephosphorylation of IGF-IR, because association of SHP-2 with IGF-IR temporally correlates with decreased IGF-IR phosphorylation (22, 43). Consistent with this model, decreased recruitment of SHP-2 to IGF-IR has been linked to increased IGF-IR phosphorylation, enhanced activation of downstream signals to ERK1/2 and AKT, and augmented proliferation in several models (45–47).
In these studies, we investigated the interaction between SHP-2 and IGF-IR and consequences for IGF-IR phosphorylation. PRL attenuated ligand-induced SHP-2 association with IGF-IR by increasing its association with PRLR, which correlated with increased IGF-IR phosphorylation. This apparent competition between PRLR and IGF-IR for interaction with SHP-2 is intriguing, because both IGF-IR and PRLR reside in lipid raft microdomains of the plasma membrane in MCF-7 cells (24, 48), and SHP-2 is recruited to these domains in other cell types (49, 50). Although we were unable to demonstrate a physical association between IGF-IR and PRLR (data not shown), this spatial proximity may permit the competition for SHP-2 that we observed. SHP-2 interacts with membrane receptors through the immunoreceptor tyrosine-based inhibitory motif (ITIM), which has a consensus sequence of (S/V/I/L)XYXX(V/I/L) (51). Interestingly, the intracellular domain of PRLR contains four consensus ITIMs (surrounding Tyr283, Tyr290, Tyr406, and Tyr485), in contrast to the single ITIM present in the IGF-IR (surrounding Tyr943). This disparity may contribute to the preferential recruitment of SHP-2 to PRLR instead of IGF-IR when both receptors are activated by their respective ligands.
We demonstrated that SFKs are required for SHP-2 association with IGF-IR and that PRL decreases this association. It is well established that SFKs phosphorylate tyrosine residues within ITIMs, resulting in SHP-2 recruitment (51, 52), suggesting a mechanism by which SFKs mediate SHP-2 recruitment to IGF-IR and PRLR. Consistently, SFKs can phosphorylate the IGF-IR at Tyr943 (53) within the ITIM of IGF-IR. The role of SFKs in PRLR phosphorylation is less well defined. However, SFKs constitutively associate with PRLR in lipid rafts and are activated upon ligand binding in MCF-7 cells (24, 54). SFKs can also indirectly mediate recruitment of SHP-2 to membrane receptors; SFK-induced phosphorylation of afadin is required for SHP-2 to associate with and dephosphorylate platelet-derived growth factor receptor (29, 55), and SFKs can phosphorylate SHP-2 substrate-1 (56), a transmembrane protein involved in SHP-2 recruitment to IGF-IR in other cell types (22). However, SHP-2 substrate-1 is strongly down-regulated in several breast cancer cell lines, including MCF-7 cells (57), and is not absolutely required for SHP-2 association with IGF-IR (43).
Many receptor tyrosine kinases are dephosphorylated in endosomes (31, 58, 59). However, conflicting reports for the requirement of internalization in the dephosphorylation of insulin receptor (31, 60) led us to investigate the relationship between IGF-IR internalization and dephosphorylation in MCF-7 cells. We demonstrated that IGF-IR dephosphorylation was blocked in the presence of Dnm-2 siRNA, confirming the importance of internalization in this process. Our findings that PRL diminished IGF-I-induced IGF-IR internalization and that endocytosis was required for PRL to augment p-IGF-IR levels suggest that modulation of IGF-1Rβ trafficking plays a central role in PRL-enhanced IGF-IR phosphorylation.
PRL-mediated attenuation of ligand-induced IGF-IR internalization correlated with reduced SHP-2 interaction with IGF-IR. Both inhibition of SHP-2 activity and knockdown of SHP-2 expression blocked IGF-I-induced IGF-IR internalization, suggesting that the reduced SHP-2 association with IGF-IR in the presence of PRL caused retention of IGF-IR at the cell surface. Interestingly, the ITIM of the IGF-IR (residues 941–946) resides in the juxtamembrane region of the β subunit of IGF-IR that is critical for IGF-IR internalization, and mutations within the ITIM (Y943A and V946A) diminish ligand-induced IGF-IR internalization by up to 50% in Chinese hamster ovary cells (61). Although to date SHP-2 has not been reported to bind Tyr943, tyrosine residues essential for SHP-2 interaction with other receptors, including platelet-derived growth factor receptor (Tyr763 and Tyr1009) and gp130 (Tyr759), are contained within consensus ITIMs (62–65). However, the exact mechanism(s) by which SHP-2 contributes to ligand-induced IGF-IR internalization requires further investigation.
Our evidence for a role for SHP-2 in endocytosis of IGF-IR is novel; few reports have investigated the contributions of SHP-2 to membrane receptor internalization and/or trafficking. However, recently SHP-2 was implicated in poliovirus (PV) entry into human brain endothelial cells (66). In this model, PV engagement with the PV receptor stimulates tyrosine phosphorylation of PV receptor, which is required for association with SHP-2. Inhibition of SHP-2 activity or reduced SHP-2 protein retained PV on the cell surface and reduced the number of PV-infected cells (66). The similarity between our findings and this report suggests widespread roles for this phosphatase in membrane receptor endocytosis.
We also established an important role for SFKs in IGF-I-induced IGF-IR internalization. SFKs reside in lipid rafts in MCF-7 cells and are key players in clathrin-independent, lipid raft-mediated endocytosis (24, 33, 35). SFK inhibition blocked IGF-IR internalization, suggesting that IGF-IR internalizes through a clathrin-independent pathway in these cells. However, IGF-IR also can internalize through clathrin-dependent pathways in mouse embryonic fibroblasts (67), underscoring the importance of cell context. SFKs also may promote internalization of IGF-IR by mediating its association with SHP-2.
Growth hormone is also linked to breast cancer in women (68) and promotes mammary tumors in rat models (69). Primate growth hormones, unlike those of other mammals, are potent agonists at the PRLR, suggesting that they also may potentiate IGF-1R signals by the mechanism described herein. Moreover, the ability of growth hormone, like PRL, to increase IGFs (7, 68) underscores the potential synergy of these related hormones with IGF signals.
It is critical to understand how PRL enhances p-IGF-IR levels because of the striking consequences of increased IGF-IR activity in many epithelial cancers. Constitutive and/or enhanced activity of IGF-IR augments proliferation, survival, invasion, transformation, and the epithelial-to-mesenchymal transition in various cell culture models (14, 70, 71). Transgenic mouse models that increase mammary IGF-IR or its activity develop mammary lesions and eventually tumors (58, 72). In women, IGF-IR autophosphorylation and kinase activity are elevated in malignant compared with normal breast tissue (73), and high p-IGF-IR levels predict poor survival (18). Notably, increased IGF-IR activity has been implicated in resistance to some targeted therapeutics, including tamoxifen and trastuzumab, as well as several chemotherapies and radiotherapy (19, 20). Consequently, IGF-IR has emerged as a major therapeutic target in breast cancer and other malignancies, and various strategies are currently under clinical investigation (74, 75).
Despite the association of PRL with the risk of breast cancer and treatment resistance (3), the mechanisms by which PRL promotes tumor development and progression are not well understood. In this report, we have characterized a novel mechanism whereby PRL enhances IGF-IR activity. Our findings suggest that it may prove beneficial to target both the PRL and IGF axes as part of a combinatorial therapeutic regimen in breast cancer patients.
Acknowledgment
We are grateful to Debra Rugowski for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants CA78312 and DK62783 (to L. A. S.) and T32 GM08349 (to K. C. C.).
- PRL
- prolactin
- PRLR
- PRL receptor
- IGF-I
- insulin-like growth factor I
- IGF-IR
- insulin-like growth factor I receptor
- SFK
- Src family of kinases
- ERK
- extracellular signal-regulated kinase
- Dnm
- dynamin
- siRNA
- small interfering RNA
- PTP
- protein-tyrosine phosphatase
- MEK
- mitogen-activated protein kinase/ERK kinase
- PBS
- phosphate-buffered saline
- ITIM
- immunoreceptor tyrosine-based inhibitory motif
- PV
- poliovirus
- ANOVA
- analysis of variance
- NT
- non-targeting.
REFERENCES
- 1.Arendt L. M., Schuler L. A. (2008) J. Mammary Gland Biol. Neoplasia 13, 29–40 [DOI] [PubMed] [Google Scholar]
- 2.Clevenger C. V., Furth P. A., Hankinson S. E., Schuler L. A. (2003) Endocr. Rev. 24, 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tworoger S. S., Hankinson S. E. (2008) J. Mammary Gland Biol. Neoplasia. 13, 41–53 [DOI] [PubMed] [Google Scholar]
- 4.Arendt L. M., Grafwallner-Huseth T. L., Schuler L. A. (2009) Am. J. Pathol. 174, 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendt L. M., Rose-Hellekant T. A., Sandgren E. P., Schuler L. A. (2006) Am. J. Pathol. 168, 1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arendt L. M., Schuler L. A. (2008) Am. J. Pathol. 172, 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver K. C., Schuler L. A. (2008) Mol. Cancer Res. 6, 634–643 [DOI] [PubMed] [Google Scholar]
- 8.Gutzman J. H., Nikolai S. E., Rugowski D. E., Watters J. J., Schuler L. A. (2005) Mol. Endocrinol. 19, 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., Li X., Jiang J., Frank S. J. (2006) Oncogene 25, 7565–7576 [DOI] [PubMed] [Google Scholar]
- 10.Johnston S. R., Martin L. A., Leary A., Head J., Dowsett M. (2007) J. Steroid Biochem. Mol. Biol. 106, 180–186 [DOI] [PubMed] [Google Scholar]
- 11.Marshman E., Streuli C. H. (2002) Breast Cancer Res. 4, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowzee A. M., Lazzarino D. A., Rota L., Sun Z., Wood T. L. (2008) J. Mammary Gland Biol. Neoplasia 13, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lann D., LeRoith D. (2008) J. Mammary Gland Biol. Neoplasia 13, 371–379 [DOI] [PubMed] [Google Scholar]
- 14.Samani A. A., Yakar S., LeRoith D., Brodt P. (2007) Endocr. Rev. 28, 20–47 [DOI] [PubMed] [Google Scholar]
- 15.Adams T. E., Epa V. C., Garrett T. P., Ward C. W. (2000) Cell. Mol. Life Sci. 57, 1050–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grønborg M., Wulff B. S., Rasmussen J. S., Kjeldsen T., Gammeltoft S. (1993) J. Biol. Chem. 268, 23435–23440 [PubMed] [Google Scholar]
- 17.Hernández-Sánchez C., Blakesley V., Kalebic T., Helman L., LeRoith D. (1995) J. Biol. Chem. 270, 29176–29181 [DOI] [PubMed] [Google Scholar]
- 18.Law J. H., Habibi G., Hu K., Masoudi H., Wang M. Y., Stratford A. L., Park E., Gee J. M., Finlay P., Jones H. E., Nicholson R. I., Carboni J., Gottardis M., Pollak M., Dunn S. E. (2008) Cancer Res. 68, 10238–10246 [DOI] [PubMed] [Google Scholar]
- 19.Casa A. J., Dearth R. K., Litzenburger B. C., Lee A. V., Cui X. (2008) Front. Biosci. 13, 3273–3287 [DOI] [PubMed] [Google Scholar]
- 20.Massarweh S., Osborne C. K., Creighton C. J., Qin L., Tsimelzon A., Huang S., Weiss H., Rimawi M., Schiff R. (2008) Cancer Res. 68, 826–833 [DOI] [PubMed] [Google Scholar]
- 21.Kenner K. A., Anyanwu E., Olefsky J. M., Kusari J. (1996) J. Biol. Chem. 271, 19810–19816 [DOI] [PubMed] [Google Scholar]
- 22.Maile L. A., Clemmons D. R. (2002) J. Biol. Chem. 277, 8955–8960 [DOI] [PubMed] [Google Scholar]
- 23.Schroeder M. D., Symowicz J., Schuler L. A. (2002) Mol. Endocrinol. 16, 45–57 [DOI] [PubMed] [Google Scholar]
- 24.Piazza T. M., Lu J. C., Carver K. C., Schuler L. A. (2009) Mol. Endocrinol. 23, 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N., Li Z., Ding R., Frank G. D., Senbonmatsu T., Landon E. J., Inagami T., Zhao Z. J. (2006) J. Biol. Chem. 281, 21878–21883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiesmann C., Barr K. J., Kung J., Zhu J., Erlanson D. A., Shen W., Fahr B. J., Zhong M., Taylor L., Randal M., McDowell R. S., Hansen S. K. (2004) Nat. Struct. Mol. Biol. 11, 730–737 [DOI] [PubMed] [Google Scholar]
- 27.Ali S., Ali S. (2000) J. Biol. Chem. 275, 39073–39080 [DOI] [PubMed] [Google Scholar]
- 28.Clevenger C. V., Medaglia M. V. (1994) Mol. Endocrinol. 8, 674–681 [DOI] [PubMed] [Google Scholar]
- 29.Nakata S., Fujita N., Kitagawa Y., Okamoto R., Ogita H., Takai Y. (2007) J. Biol. Chem. 282, 37815–37825 [DOI] [PubMed] [Google Scholar]
- 30.Bain J., McLauchlan H., Elliott M., Cohen P. (2003) Biochem. J. 371, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Guglielmo G. M., Drake P. G., Baass P. C., Authier F., Posner B. I., Bergeron J. J. (1998) Mol. Cell Biochem. 182, 59–63 [PubMed] [Google Scholar]
- 32.Haj F. G., Verveer P. J., Squire A., Neel B. G., Bastiaens P. I. (2002) Science 295, 1708–1711 [DOI] [PubMed] [Google Scholar]
- 33.Pelkmans L., Fava E., Grabner H., Hannus M., Habermann B., Krausz E., Zerial M. (2005) Nature 436, 78–86 [DOI] [PubMed] [Google Scholar]
- 34.Romanelli R. J., LeBeau A. P., Fulmer C. G., Lazzarino D. A., Hochberg A., Wood T. L. (2007) J. Biol. Chem. 282, 22513–22524 [DOI] [PubMed] [Google Scholar]
- 35.Lajoie P., Nabi I. R. (2007) J. Cell. Mol. Med. 11, 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungewickell E. J., Hinrichsen L. (2007) Curr. Opin. Cell Biol. 19, 417–425 [DOI] [PubMed] [Google Scholar]
- 37.Feng G. S. (1999) Exp. Cell Res. 253, 47–54 [DOI] [PubMed] [Google Scholar]
- 38.Mohi M. G., Neel B. G. (2007) Curr. Opin. Genet. Dev. 17, 23–30 [DOI] [PubMed] [Google Scholar]
- 39.Qu C. K. (2002) Biochim. Biophys. Acta 1592, 297–301 [DOI] [PubMed] [Google Scholar]
- 40.Kwon M., Ling Y., Maile L. A., Badley-Clark J., Clemmons D. R. (2006) Endocrinology 147, 1458–1465 [DOI] [PubMed] [Google Scholar]
- 41.Ling Y., Maile L. A., Lieskovska J., Badley-Clarke J., Clemmons D. R. (2005) Mol. Biol. Cell 16, 3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuemmerle J. F. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1194–G1202 [DOI] [PubMed] [Google Scholar]
- 43.Maile L. A., Clemmons D. R. (2002) Endocrinology 143, 4259–4264 [DOI] [PubMed] [Google Scholar]
- 44.Shen M. R., Hsu Y. M., Hsu K. F., Chen Y. F., Tang M. J., Chou C. Y. (2006) Carcinogenesis 27, 962–971 [DOI] [PubMed] [Google Scholar]
- 45.Edderkaoui M., Hong P., Lee J. K., Pandol S. J., Gukovskaya A. S. (2007) J. Biol. Chem. 282, 26646–26655 [DOI] [PubMed] [Google Scholar]
- 46.Kapur S., Mohan S., Baylink D. J., Lau K. H. (2005) J. Biol. Chem. 280, 20163–20170 [DOI] [PubMed] [Google Scholar]
- 47.Maile L. A., Clemmons D. R. (2003) Circ. Res. 93, 925–931 [DOI] [PubMed] [Google Scholar]
- 48.Sachdev D., Singh R., Fujita-Yamaguchi Y., Yee D. (2006) Cancer Res. 66, 2391–2402 [DOI] [PubMed] [Google Scholar]
- 49.Kim H. Y., Park S. J., Joe E. H., Jou I. (2006) J. Biol. Chem. 281, 11872–11878 [DOI] [PubMed] [Google Scholar]
- 50.Kiyan J., Haller H., Dumler I. (2009) Exp. Cell Res. 315, 1029–1039 [DOI] [PubMed] [Google Scholar]
- 51.Barrow A. D., Trowsdale J. (2006) Eur. J. Immunol. 36, 1646–1653 [DOI] [PubMed] [Google Scholar]
- 52.Ravetch J. V., Lanier L. L. (2000) Science 290, 84–89 [DOI] [PubMed] [Google Scholar]
- 53.Peterson J. E., Kulik G., Jelinek T., Reuter C. W., Shannon J. A., Weber M. J. (1996) J. Biol. Chem. 271, 31562–31571 [DOI] [PubMed] [Google Scholar]
- 54.Acosta J. J., Muñoz R. M., González L., Subtil-Rodríguez A., Dominguez-Caceres M. A., García-Martínez J. M., Calcabrini A., Lazaro-Trueba I., Martín-Pérez J. (2003) Mol. Endocrinol. 17, 2268–2282 [DOI] [PubMed] [Google Scholar]
- 55.Klinghoffer R. A., Kazlauskas A. (1995) J. Biol. Chem. 270, 22208–22217 [DOI] [PubMed] [Google Scholar]
- 56.Tsuda M., Matozaki T., Fukunaga K., Fujioka Y., Imamoto A., Noguchi T., Takada T., Yamao T., Takeda H., Ochi F., Yamamoto T., Kasuga M. (1998) J. Biol. Chem. 273, 13223–13229 [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki Y., Ito S., Tsunoda N., Kokuryo T., Hara K., Senga T., Kannagi R., Yamamoto T., Oda K., Nagino M., Nimura Y., Hamaguchi M. (2007) Biochem. Biophys. Res. Commun. 361, 7–13 [DOI] [PubMed] [Google Scholar]
- 58.Hadsell D. L., Bonnette S. G. (2000) J. Mammary. Gland Biol. Neoplasia 5, 19–30 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Barak L. S., Winkler K. E., Caron M. G., Ferguson S. S. (1997) J. Biol. Chem. 272, 27005–27014 [DOI] [PubMed] [Google Scholar]
- 60.Kouzmenko A. P., Takeyama K., Ito S., Furutani T., Sawatsubashi S., Maki A., Suzuki E., Kawasaki Y., Akiyama T., Tabata T., Kato S. (2004) J. Biol. Chem. 279, 40255–40258 [DOI] [PubMed] [Google Scholar]
- 61.Prager D., Li H. L., Yamasaki H., Melmed S. (1994) J. Biol. Chem. 269, 11934–11937 [PubMed] [Google Scholar]
- 62.Fukada T., Hibi M., Yamanaka Y., Takahashi-Tezuka M., Fujitani Y., Yamaguchi T., Nakajima K., Hirano T. (1996) Immunity 5, 449–460 [DOI] [PubMed] [Google Scholar]
- 63.Kazlauskas A., Feng G. S., Pawson T., Valius M. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6939–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lechleider R. J., Sugimoto S., Bennett A. M., Kashishian A. S., Cooper J. A., Shoelson S. E., Walsh C. T., Neel B. G. (1993) J. Biol. Chem. 268, 21478–21481 [PubMed] [Google Scholar]
- 65.Rönnstrand L., Arvidsson A. K., Kallin A., Rorsman C., Hellman U., Engström U., Wernstedt C., Heldin C. H. (1999) Oncogene 18, 3696–3702 [DOI] [PubMed] [Google Scholar]
- 66.Coyne C. B., Kim K. S., Bergelson J. M. (2007) EMBO J. 26, 4016–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monami G., Emiliozzi V., Morrione A. (2008) J. Cell. Physiol. 216, 426–437 [DOI] [PubMed] [Google Scholar]
- 68.Perry J. K., Mohankumar K. M., Emerald B. S., Mertani H. C., Lobie P. E. (2008) J. Mammary Gland Biol. Neoplasia. 13, 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Q., Lantvit D. D., Lin Q., Li Y., Christov K., Wang Z., Unterman T. G., Mehta R. G., Swanson S. M. (2007) Endocrinology 148, 4536–4544 [DOI] [PubMed] [Google Scholar]
- 70.Kim H. J., Litzenburger B. C., Cui X., Delgado D. A., Grabiner B. C., Lin X., Lewis M. T., Gottardis M. M., Wong T. W., Attar R. M., Carboni J. M., Lee A. V. (2007) Mol. Cell. Biol. 27, 3165–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sachdev D. (2008) J. Mammary Gland Biol. Neoplasia 13, 431–441 [DOI] [PubMed] [Google Scholar]
- 72.Jones R. A., Moorehead R. A. (2008) J. Mammary Gland Biol. Neoplasia 13, 407–413 [DOI] [PubMed] [Google Scholar]
- 73.Calvocoressi L., Sun A., Kasl S. V., Claus E. B., Jones B. A. (2008) Cancer 112, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sachdev D., Yee D. (2007) Mol. Can. Ther. 6, 1–12 [DOI] [PubMed] [Google Scholar]
- 75.Weroha S. J., Haluska P. (2008) J. Mammary Gland Biol. Neoplasia 13, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]