Abstract
8-Chloroadenosine (8-Cl-Ado) is a ribosyl nucleoside analog currently in phase I testing for the treatment of chronic lymphocytic leukemia (CLL). 8-Cl-Ado activity is dependent on adenosine kinase and requires intracellular accumulation of 8-Cl-Ado as mono-, di-, and tri-phosphates. In the current study with four mantle cell lymphoma cell lines, we report a new major metabolic pathway for 8-Cl-Ado intracellular metabolism, the formation of succinyl-8-chloro-adenosine (S-8-Cl-Ado) and its monophosphate (S-8-Cl-AMP). 8-Cl-AMP levels were highly associated with S-8-Cl-AMP levels and reached a steady-state prior to the secondary metabolites, 8-Cl-ATP and S-8-Cl-Ado. Consistent with fumarate as a required substrate for formation of succinyl-8-Cl-adenylate metabolites, the S-8-Cl-adenylate concentrations in multiple cell lines were associated with fumarate loss. The distribution of metabolites was also altered using the energy metabolism modifiers, metformin and oligomycin. The rates of succinyl-8-Cl-adenylate metabolism were enhanced by increasing the intracellular fumarate concentrations after metformin co-treatment. In addition, the S-8-Cl-AMP concentrations were increased after acute inhibition of ATP synthase by oligomycin. We conclude that 8-Cl-Ado metabolism not only affects intracellular purine metabolism; 8-Cl-Ado conversion to succinyl analogs ties its metabolism to the citric acid cycle by reduction of the fumarate pool.
Keywords: Anticancer Drug, Cancer Therapy, Energy Metabolism, Nucleoside Nucleotide Analogs, Nucleoside Nucleotide Biosynthesis, Nucleoside Nucleotide Metabolism, Tricarboxylic Acid (TCA) Cycle
Introduction
8-Chloroadenosine (8-Cl-Ado)3 is a purine nucleoside analog with reported preclinical activity in multiple hematological malignancies (1–3). Currently, 8-Cl-Ado is in a phase I clinical trial at the M. D. Anderson Cancer Center for the treatment of CLL. Compared with conventional genotoxic deoxyribose nucleoside analogs, 8-Cl-Ado is a cytotoxic ribosyl nucleoside analog with unique mechanisms of action (1–8): transcription inhibition of short-lived antiapoptotic proteins and reduction of intracellular ATP or dATP. For both mechanisms, 8-Cl-Ado actions are dependent on phosphorylation to its active ADP or ATP-analogs, 8-Cl-ADP or 8-Cl-ATP, respectively. Intracellular 8-Cl-ATP inhibits mRNA transcription of RNA polymerase II by directly incorporating into mRNA as a chain terminator (2, 3) or by incorporating into the poly(A)tail of mRNA transcripts, which is required for functionality and stability of the transcripts (9). Subsequent apoptosis is promoted by reduced expression of short-lived survival proteins, such as Mcl-1 in CLL and MET in multiple myeloma (4, 6). In addition, intracellular 8-Cl-ADP competes with ADP for mitochondrial ATP synthase and consequently reduces intracellular ATP concentrations (10). 8-Cl-Ado phosphorylated metabolites may also inhibit ribonucleotide reductase and reduce dATP levels causing an imbalance of the dNTP pool (1).
As previously described, the mechanisms of action for 8-Cl-Ado have focused on phosphorylated metabolites, in particular 8-Cl-ATP, as the primary active intracellular metabolites. However, as with normal adenosine metabolism, 8-Cl-Ado may be a precursor not just for its mono-, di-, and tri-phosphate nucleotides but for multiple metabolic conjugates including nicotinamide adenine dinucleotide (NAD), S-adenosyl methionine (SAM), and flavin adenine dinucleotide (FAD). In the current study, we identified two new major 8-Cl-Ado metabolites, but the metabolites were not the downstream products we predicted. Instead, 8-Cl-Ado was metabolized to adenosine precursor molecules, succinyl-conjugated metabolites: succinyl-8-chloro-adenosine (S-8-Cl-Ado) and succinyl-8-chloro-AMP (S-8-Cl-AMP). Whereas the loss of ATP has already been associated with 8-Cl-ATP formation (1, 2, 5), this newly described metabolic pathway connects 8-Cl-Ado metabolism directly to the citric acid cycle through fumarate. An understanding of this pathway may provide additional strategies for therapeutic applications of this compound.
EXPERIMENTAL PROCEDURES
Cell Culture
Granta 519, JeKo, Mino, and SP-53 mantle cell lymphoma (MCL) cell lines were a gift from Hesham Amin (M. D. Anderson) and were maintained as described previously (1). All cells were routinely tested for Mycoplasma infection using a MycoTect Kit (Invitrogen). The identities of all cell lines were verified using AmpF&STR Identifiler kit (Applied Biosystems, Foster City, CA). Data base information was not available for SP-53, but its STR profile confirmed SP-53 did not match any known cell line.
Chemicals
8-Cl-Ado, 8-Cl-AMP, 8-Cl-ADP, 8-Cl-ATP, and 8-Cl-IMP were purchased from BioLog (Bremen, Germany). 8-Cl-[2-3H]Ado was procured from Moravek Biochemicals, Inc. (Brea, CA). HPLC grade reagents were purchased from Fisher Scientific, and all other reagents were purchased from Sigma Aldrich.
Drug Treatment of Cells
Exponentially growing MCL cells (2 to 4 × 105/ml) were incubated with various concentrations of 8-Cl-Ado and/or other reagents. 8-Cl-Ado and metformin were dissolved in water. For oligomycin experiments, equal volumes of ethanol (0.04%) were added to the treated cells and the corresponding vehicle controls. Cell concentrations and median volumes were quantified using a Z2 Coulter Counter (Beckman Coulter, Inc., Fullerton, CA).
Purification of 8-Cl-[3H]Ado by HPLC
The radiochemical purity of 8-Cl-[3H]Ado by HPLC decreased with storage time (greater than 10% loss/year). The primary impurity was putatively identified as adenosine by its retention time and UV absorbance ratios. To evaluate the metabolism of 8-Cl-Ado without adenosine interference, 8-Cl-[3H]Ado was purified just prior to use. Adenosine and 8-Cl-Ado were separated by HPLC using a C18 column (Xterra MS, 3.5 μm, 3.0 × 100 mm, Waters, Milford, MA) with a water/methanol linear gradient (5 to 30% methanol over 30 min, 0.3 ml/min). The methanol was removed from the 8-Cl-Ado fraction by evaporation.
Isolation of S-8-Cl-Ado by HPLC
To determine the chemical identity of S-8-Cl-Ado, JeKo cells (1 × 109 cells) were incubated with 8-Cl-Ado for 24 h and then extracted with perchloric acid (PCA) as previously described (11). S-8-Cl-Ado was isolated from the extract using two sequential HPLC methods with the same C18 column (described above) but different ion pairing agents, triethylammonium acetate (TEAA) and ammonium acetate. For the first separation with 10 mm TEAA (mobile phase A) and methanol (mobile phase B), the succinyl compounds were the most highly retained metabolites. Briefly, the analytes were eluted at 0.3 ml/min using the following gradient conditions: 0 min 0% B, 0–30 min linear increase to 50% B. The TEAA was removed during the second purification, an isocratic separation with 80 mm ammonium acetate. The S-8-Cl-Ado fraction was used directly for MS/MS analysis. The ammonium acetate and water were removed for NMR analysis by repeated lyophilization with D2O.
Intracellular Adenosine and 8-Cl-Ado Metabolite Quantification
The intracellular nucleotides were extracted using 0.4 n PCA or 60% methanol (1). Two separate HPLC methods using different pH buffers were required to quantify all the major 8-Cl-Ado metabolites and the adenosine nucleotides. For both methods, the compounds were separated on an analytical ion-exchange column (Partisil-10 SAX, 4.6 × 250 mm, Whatman, Maidstone, England) and quantified using external authentic standards at 265 nm. Radiochromatograms were generated using a Radiomatic Flow Scintillation Analyzer (PerkinElmer, Waltham, MA). To separate most analytes, a high pH method was used as previously described (12) with slight modifications. Briefly, the compositions of the mobile phases were 7.5 mm NH4H2PO4, pH 4.1 (mobile phase A) and 0.75 m NH4H2PO4, pH 4.9 (mobile phase B). The analytes were eluted at 1.7 ml/min using the following gradient conditions: 0 min 0.1% B, 0–10 min linear increase to 1% B, 10–15 min linear increase to 11% B, and 15–55 min linear increase to 60% B. To separate S-8-Cl-AMP from GDP and 8-Cl-IMP from 8-Cl-AMP, a low pH method was used. The compositions of the mobile phases were 7.5 mm NH4H2PO4, pH 4.1 (mobile phase A) and 0.75 m NH4H2PO4, pH 3.8 (mobile phase B). The analytes were eluted at 1.7 ml/min using the following gradient conditions: 0 min 0.1% B, 0–10 min linear increase to 1% B, 10–15 min linear increase to 11% B, 15–40 linear increase to 42%, and 40–55 min linear increase to 100% B. The intracellular concentrations of the analytes were estimated from the total cell count and the median cell volume.
Quantification of Fumarate Levels
The high pH HPLC method described above was the same used to quantify fumarate but at a wavelength of 210 nm with an approximate retention time of 20 min. To confirm specificity of the method, the fumarate concentrations as determined by this HPLC method were highly correlated to the relative proton NMR intensity of the fumarate authentic standard and the fumarate in JeKo cell extracts (data not shown).
NMR Spectroscopy
All NMR spectra were acquired at 500.13 MHz on a Bruker Avance DRX500 spectrometer equipped with a TXI (1H/13C/31P) probe. NMR samples for S-8-Cl-Ado identification were prepared by dissolving the dry powder in 550 μl of 99.9% D2O and experiments were performed at 15 °C to separate the peaks which were buried under the residual water peak at room temperature. The one-dimensional proton spectra were recorded with a 90 degree flip angle and a 5-s recycle delay. Data were zero-filled to 32K data points and multiplied by a 0.3 exponential function before Fourier transformation. All spectra were referenced to 3-(trimethylsilyl)propionic acid at 0.0 ppm. Two-dimensional COSY spectra were collected in absolute value mode with 128 t1 increments and 32 scans per fid. COSY data were zero-filled to 1024 points in the t1 dimension and multiplied by sine bell window function in both dimensions before Fourier transformation. Data acquisition and processing were completed using Bruker software TopSpin 2.0 (Billerica, MA).
Mass Spectrometry Analysis
The S-8-Cl-Ado dried powder was dissolved in 50:50 (0.2% formic acid in water/methanol) for electrospray positive mode and 50:50 water/methanol for electrospray negative mode. Solutions were directly infused to the Waters Quattro Premier or the Waters Quattro Premier XE (for accurate mass only) mass spectrometers (Milford, MA).
Statistical Analysis
Correlations, non-linear regressions, and t tests were performed using the GraphPad Prism 5 software (GraphPad Software, Inc. San Diego, CA). p values of < 0.05 were considered statistically significant.
RESULTS
Intracellular Metabolites of 8-Cl-Ado
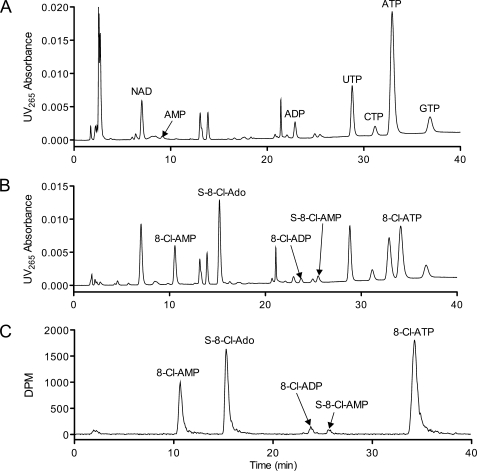
To determine the intracellular metabolic profile of 8-Cl-Ado, JeKo cells were incubated with radiolabeled 8-Cl-Ado, and the cell extracts were separated by HPLC (Fig. 1). Consistent with previous results (1), the ATP concentration after 8-Cl-Ado treatment was depleted, and NAD concentration was increased as compared with the untreated control (Fig. 1, A and B). Using radiodetection (Fig. 1C), five major metabolites were observed including the three known phosphorylated metabolites and two previously unreported compounds, S-8-Cl-Ado and S-8-Cl-AMP (Fig. 2). 8-Cl-Ado equivalent metabolites were not observed at retention times close to other potential adenosine conjugated or derived molecules, such as NAD, with the exception of 8-Cl-IMP. For this particular HPLC method, 8-Cl-IMP had a similar retention time to 8-Cl-AMP. However, using a low pH HPLC method to evaluate 8-Cl-IMP and 8-Cl-AMP separately, the peak at 11 min (Fig. 1B) was composed almost exclusively of 8-Cl-AMP (>97%, data not shown).
FIGURE 1.
8-Cl-Ado accumulates as 5 major intracellular metabolites. A, HPLC/UV chromatogram of untreated JeKo cell extracts. B–C, HPLC/UV chromatogram and radiochromatogram of 10 μm 8-Cl-[3H]Ado-treated cells. JeKo cells were incubated with and without 10 μm 8-Cl-[3H]Ado (50 μCi) for 24 h. 8-Cl-[3H]Ado was purified by HPLC immediately before cell treatment (see “Experimental Procedures”). Cells were extracted with PCA and neutralized prior to HPLC analysis. For each chromatogram, extracts from an equal number of cells were injected on the column.
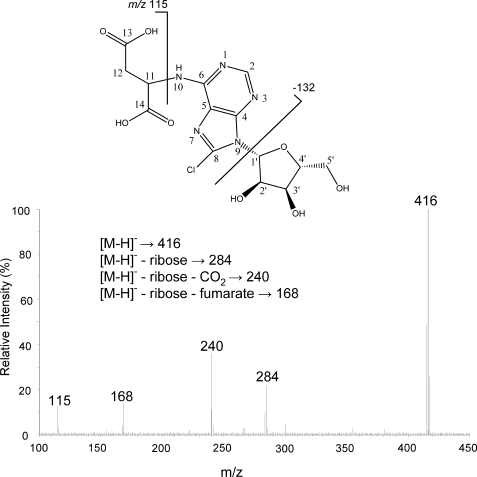
FIGURE 2.
MS/MS spectrum and proposed fragmentation pattern of succinyl-8-chloro-adenosine in negative ion mode. Succinyl-8-Cl-Ado was isolated and purified from 8-Cl-Ado-treated JeKo cells by PCA extraction and two HPLC separations (see “Experimental Procedures”). The eluate was dissolved in MeOH/water (1:1) for direct infusion to the qTOF.
The UV absorbance of S-8-Cl-Ado was different from that of the 8-Cl-Ado nucleotides. Thus, to quantify the molar formation of S-8-Cl-Ado by UV absorbance without an authentic standard, the relative extinction coefficient for S-8-Cl-Ado was calculated using the UV area/DPM ratios after treatment with 8-Cl-[3H]Ado (Fig. 1, B and C). The relative extinction coefficient of S-8-Cl-Ado was 1.25 ± 0.05 (S.D.) when normalized to 8-Cl-ATP. The maximum UV absorbance of S-8-Cl-Ado (269 nm) was 8 nm higher than that of 8-Cl-Ado (261 nm). This increase was consistent with the addition of an acetyl group to a primary aromatic amine, a theoretical increase of 7 nm (13). The maximum UV absorbance of S-AMP (267 nm) was also 8 nm higher than that of AMP (259 nm) (14).
Characterization of S-8-Cl-Ado and S-8-Cl-AMP
The chemical identity of S-8-Cl-Ado was determined using 1D-1H and 2D-COSY NMR (Table 1) and by electrospray ionization mass spectrometry (Fig. 2). For NMR analysis, S-8-Cl-Ado in D2O was compared with commercially available authentic standards, 8-Cl-Ado and S-AMP. Consistent with the proposed structure (Fig. 2), S-8-Cl-Ado had nearly identical chemical shifts to 8-Cl-Ado for position 2 and the ribose protons (1′ to 5′). Likewise, S-8-Cl-Ado proton chemical shifts and the peak splitting patterns for the succinyl group (11 and 12) almost exactly matched those of S-AMP.
TABLE 1.
Proton chemical shift assignments of S-8-Cl-Ado, 8-Cl-Ado, and S-AMP
Proton assignments were determined or confirmed by 2D-COSY. All experiments were performed at 15 °C in D2O. Chemical shifts were referenced to 3-(Trimethylsilyl)propionic acid.
| Position | δH multiplicity (J in Hz) |
||
|---|---|---|---|
| S-8-Cl-Ado | 8-Cl-Ado | S-AMP | |
| 2 | 8.09 sa | 8.09 s | 8.11 s |
| 8 | N/Ab | N/A | 8.38 s |
| 11 | 4.62 mc | N/A | 4.62 m |
| 12a | 2.73 ddd (15.7, 3.6) | N/A | 2.73 dd (15.7, 3.6) |
| 12b | 2.56 dd (15.7, 9.9) | N/A | 2.56 dd (15.7, 9.9) |
| 1′ | 6.00 de (6.0) | 6.08 d (7.2) | 5.98 d(6.0) |
| 2′ | 4.89 m | 4.95 m | 4.61 m |
| 3′ | 4.34 dd (5.3, 1.2) | 4.46 dd (5.3, 1.8) | 4.33 dd (5.2, 1.5) |
| 4′ | 4.19 m | 4.32 m | 4.20 m |
| 5a′ | 3.80 dd (13, 2.5) | 3.95 dd (13, 2.5) | 3.85 m |
| 5b′ | 3.71 dd (13, 2.5) | 3.85 dd (13, 2.5) | 3.85 m |
a s, singlet.
b N/A, not applicable.
c m, multiplet.
d dd, double doublet.
e d, doublet.
The chemical composition of S-8-Cl-Ado was also confirmed using mass spectrometry. S-8-Cl-Ado displayed a typical chlorine isotope signature (not shown) proving that S-8-Cl-Ado was not an endogenous molecule but derived from 8-Cl-Ado. Unlike other adenosine conjugates such as NAD or SAM, S-8-Cl-Ado had an intact ribose ring without phosphorylation, supported by the loss of 132 from the parent anion (Fig. 2). The fragmentation pattern as described in Fig. 2 was consistent with succinyl conjugation on the adenine base. The accurate mass values (m/z 418.077 [M+H]+, 416.062 [M-H]−) were also consistent with the elemental formula of S-8-Cl-Ado (C14H17N5O8Cl).
Given that the S-8-Cl-AMP concentration was much lower than that of S-8-Cl-Ado, S-8-Cl-AMP was not isolated for mass spectrometry or NMR characterization. Instead, using a low pH HPLC method (see “Experimental Procedures”), S-8-Cl-AMP was putatively identified by HPLC using the UV265/280 ratio of S-8-Cl-Ado. The retention time of S-8-Cl-AMP compared with that of 8-Cl-ADP was consistent with the radiochromatogram peaks (Fig. 1C). In addition, a related authentic standard, S-AMP, was analyzed by HPLC and had a retention time nearly identical to that of S-8-Cl-AMP (data not shown).
Relationship between S-8-Cl-Ado Metabolites and 8-Cl-Ado Nucleotides
After demonstrating that succinylated 8-Cl-Ado analogs were accumulating in the cells (Fig. 2), we hypothesized that S-8-Cl-AMP may be the conjugated product of 8-Cl-AMP and fumarate, synthesized by adenylosuccinase (ASase). This metabolic pathway is consistent with the relatively high concentration of 8-Cl-AMP (Fig. 1C) and the reversible nature of ASase in the de novo purine pathway (14). S-8-Cl-AMP may then be dephosphorylated by cytosolic nucleotidase and/or phosphatase to S-8-Cl-Ado, an analogous metabolic pathway previously reported for adenosine in patients with ASase deficiency (15, 16). Similarly, succinylpurines including S-Ado accumulate in the cerebrospinal fluid of patients with fumarase deficiency, a condition that elevates intracellular fumarate concentrations (17). Based on these relationships, 8-Cl-AMP is the primary metabolite, a precursor for all other metabolites; 8-Cl-ATP and S-8-Cl-Ado are the terminal products.
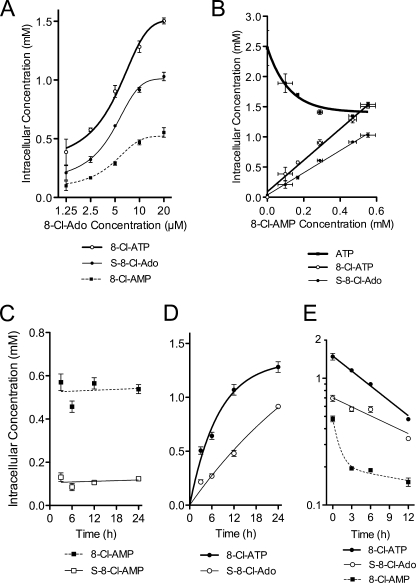
Consistent with this proposed pathway, 8-Cl-AMP, 8-Cl-ATP, and S-8-Cl-Ado concentrations after 24 h of treatment increased with 8-Cl-Ado dose (Fig. 3A) and were correlated to each other (r2 > 0.98, p < 0.01, Fig. 3B). The EC50 values for metabolite accumulation were estimated by non-linear regression using a four-parameter dose-response curve (5.1 to 5.9 μm, Fig. 3A) and were not different from each other (p > 0.1). The relationship between 8-Cl-AMP concentrations and ATP levels was more complex (Fig. 3B); the ATP concentrations were depleted but reached a plateau at 0.3 mm 8-Cl-AMP.
FIGURE 3.
Intracellular metabolism of 8-Cl-Ado as a function of dose and time. A, dose-dependent accumulation of 8-Cl-AMP, S-8-Cl-Ado, and 8-Cl-ATP. B, 8-Cl-AMP association with accumulation of its terminal metabolites (8-Cl-ATP and S-8-Cl-Ado) and depletion of ATP. For A and B, JeKo cells were incubated with 0, 1.25, 2.5, 5, 10, and 20 μm 8-Cl-Ado for 24 h. C and D, time-dependent accumulation of 8-Cl-Ado metabolites: 8-Cl-AMP, S-8-Cl-AMP, S-8-Cl-Ado, and 8-Cl-ATP. JeKo cells were incubated with 10 μm 8-Cl-Ado for up to 24 h. E, elimination curves for 8-Cl-ATP, S-8-Cl-Ado, and 8-Cl-AMP. After 24 h of preincubation with 10 μm 8-Cl-Ado, the medium was replaced with drug-free medium, and the metabolite concentrations were quantified at various time points up to 12 h. For all experiments, cells were extracted with PCA or 60% methanol prior to HPLC analysis. Data points are averages ± S.D. of three independent experiments.
To demonstrate that 8-Cl-AMP and S-8-Cl-AMP were precursors to 8-Cl-ATP and S-8-Cl-Ado, respectively, the kinetics of metabolite formation were evaluated at 10 μm 8-Cl-Ado for up to 24 h. Consistent with precursor compounds, 8-Cl-AMP and S-8-Cl-AMP levels reached steady-state levels in less than 3 h (Fig. 3C). In contrast, S-8-Cl-Ado and 8-Cl-ATP continued to accumulate over the incubation period of 24 h (Fig. 3D). Interestingly, the rates at which 8-Cl-ATP and S-8-Cl-Ado accumulated in the cells indicated that 8-Cl-ATP had a shorter half-life than S-8-Cl-Ado (Fig. 3D). Given that 8-Cl-AMP levels were relatively constant, the half-life of 8-Cl-ATP was estimated by non-linear regression assuming first-order elimination (5.4 ± 0.5 h, estimate ± S.D.). Although the exact value could not be estimated for S-8-Cl-Ado in this experiment, its half-life was clearly greater than that of 8-Cl-ATP. To estimate a more accurate half-life, a separate elimination experiment was performed after 10 μm 8-Cl-Ado treatment for 12 h (Fig. 3E). Assuming first-order elimination, the estimated half-life of 8-Cl-ATP was similar to its estimated value from the previous accumulation experiment (7.7 ± 0.5 h, estimate ± S.D.) but less than that of S-8-Cl-Ado (12.8 ± 2.4 h, estimate ± S.D.).
For the elimination experiment, 8-Cl-AMP depletion was also evaluated to confirm the order of metabolite formation (Fig. 3E). 8-Cl-AMP concentrations did not follow first-order elimination kinetics. Instead, 8-Cl-AMP elimination was second order with the first elimination phase prior to 3 h, consistent with the rapid steady-state observed previously during 8-Cl-Ado treatment (Fig. 3C). For the second elimination phase, 8-Cl-AMP levels were maintained presumably by 8-Cl-ATP and S-8-Cl-Ado catabolism. Elimination of S-8-Cl-AMP was also second-order (data not shown). Thus, like 8-Cl-AMP, S-8-Cl-AMP was a short half-life metabolite possibly maintained by catabolism of terminal metabolites.
Succinyl-8-Cl-Ado Accumulated in Multiple Cell Lines
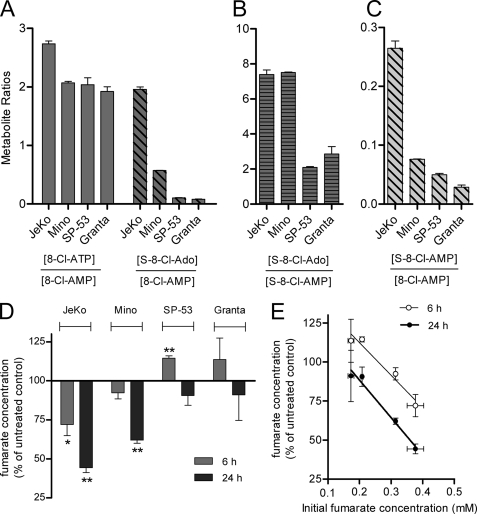
To understand the variability in the formation of succinyl and phosphorylated metabolites, we compared the metabolism of 8-Cl-Ado in four MCL cell lines previously evaluated for efficacy (1). The relationship between the metabolites was determined after 24 h of continuous 10 μm 8-Cl-Ado treatment; this concentration is a therapeutically achievable plasma concentration using data from previous clinical trials with 8-Cl-cAMP (18). At these conditions, the ratios of 8-Cl-ATP/8-Cl-AMP were predicted to be at a steady-state given that the half-life of 8-Cl-ATP was ∼6 h (Fig. 3E). Indeed, the 8-Cl-ATP/8-Cl-AMP ratios were similar for all four cell lines (1.9 to 2.7). Although succinyl 8-Cl-Ado metabolites were detected in all cell lines, the S-8-Cl-Ado/8-Cl-AMP ratios were variable (24-fold, Fig. 4A). For JeKo cells with the highest ratio, 8-Cl-ATP and S-8-Cl-Ado concentrations were almost equal. Mino cells also accumulated substantial S-8-Cl-Ado (22% of 8-Cl-ATP), enough to surpass that of 8-Cl-ADP in the cell (13% of 8-Cl-ATP). In contrast, two cell lines, SP-53 and Granta, did not accumulate significant quantities of S-8-Cl-Ado (4% of 8-Cl-ATP levels).
FIGURE 4.
Succinyl-8-Cl-adenylate metabolism of multiple cell lines was associated with fumarate loss and initial fumarate concentration. A, accumulation of 8-Cl-ATP and S-8-Cl-Ado normalized to 8-Cl-AMP levels. B, accumulation of S-8-Cl-Ado normalized to S-8-Cl-AMP levels. C, accumulation of S-8-Cl-AMP normalized to 8-Cl-AMP. D, fumarate concentrations after 8-Cl-Ado treatment compared with the untreated controls. E, association between initial fumarate concentrations and fumarate change after 8-Cl-Ado treatment. Four MCL cell lines were continuously incubated with 10 μm 8-Cl-Ado for 6 h (D and E) or 24 h (A–E). Cells were extracted with 60% methanol and analyzed by HPLC. Data points are averages ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01.
To determine what enzymes were likely contributing to the variable metabolism of 8-Cl-Ado, the conversion of S-8-Cl-AMP to S-8-Cl-Ado and the conversion of 8-Cl-AMP to S-8-Cl-AMP were evaluated separately (Fig. 4, B and C). Interestingly, the ratios of S-8-Cl-Ado to S-8-Cl-AMP were almost identical for JeKo and Mino, the cell lines with the most succinyl metabolism; the nucleotidase activity was 3-fold higher than that of the other two cell lines (Fig. 4B). However, the nucleotidase activity did not completely account for the variability in succinyl metabolism. As compared with the other cell lines, JeKo cells had a much higher ratio of S-8-Cl-AMP to 8-Cl-AMP, a measure of the ASase activity (Fig. 4C).
8-Cl-Ado Treatment Reduced Fumarate Concentrations
Given that ASase utilizes fumarate as a substrate to generate succinyl metabolites, we hypothesized that fumarate levels may be reduced after 8-Cl-Ado treatment. Indeed, after 6 and 24 h of 10 μm 8-Cl-Ado treatment, fumarate concentrations were reduced in JeKo and Mino cell lines (Fig. 4D), the cell lines with the highest relative accumulation of succinyl metabolites (Fig. 4A). In contrast, for SP-53 cells, the fumarate levels increased after 6 h of 8-Cl-Ado treatment. The reduction in fumarate was highly associated with succinyl metabolism but not loss of mitochondrial potential; JeKo, Mino, and SP-53 at these conditions had similar cell death responses (1). In addition, the equivalent loss of ATP by alanosine, an inhibitor of adenylosuccinate synthase (ADSS), reduced the fumarate concentrations of JeKo cells much less than that of 8-Cl-Ado in a paired experiment after 24 h of continuous treatment (18% versus 39% loss, p < 0.01). Interestingly, the percent reduction of fumarate by 8-Cl-Ado was highly associated with the pre-treated fumarate concentrations in the cells (r2 > 0.83, p < 0.01, Fig. 4E). These results suggest that intracellular fumarate concentrations may have been rate-limiting in the formation of S-8-Cl-AMP from 8-Cl-AMP. Thus, normal fumarate levels (<0.4 mm) did not saturate ASase, a finding consistent with the in vitro Km of fumarate (0.5 mm) (14).
Combination of 8-Cl-Ado with Metabolic Inhibitors
To further characterize 8-Cl-Ado metabolite formation, we decided to evaluate 8-Cl-Ado metabolism in combination with pharmacologic inhibitors of ATP synthase (oligomycin), adenosine/AMP deaminase (deoxycoformycin), and electron transfer chain complex I (ETC1) (metformin) (19). Metformin is a commonly prescribed anti-diabetic drug used in the treatment of type 2 diabetes (20) that has shown some anticancer activity in breast cancer (21). The JeKo cell line was chosen as a model system because JeKo cells accumulated the greatest quantities of succinyl metabolites compared with the other MCL cell lines. After treatment with different metabolic agents, we hypothesized that the relationship between the 8-Cl-Ado metabolites would be consistent with changes in adenosine metabolism and the fumarate pool.
8-Cl-AMP Was Not a Major Substrate of AMP Deaminase and/or Adenosine Deaminase
Co-treatment of JeKo cells with 1 μm dCF or 1 μm CF did not change the distribution of adenosine or the 8-Cl-Ado products (data not shown). Also, 8-Cl-IMP, a potential product of 8-Cl-AMP catalyzed by AMP deaminase, was not a major intracellular metabolite (≤1% of the 8-Cl-adenylate pool). These results were not surprising given that 8-Cl-Ado is a poor substrate for adenosine deaminase (efficiency 0.06% that of adenosine) (22). However, adenosine deaminase may still be important in the systemic clearance of 8-Cl-Ado; 8-Cl-inosine was identified as a major metabolite of 8-Cl-Ado in plasma during rodent pharmacokinetic studies (3).
Acute Inhibition of ATP Synthase Increased 8-Cl-AMP and S-8-Cl-AMP Levels
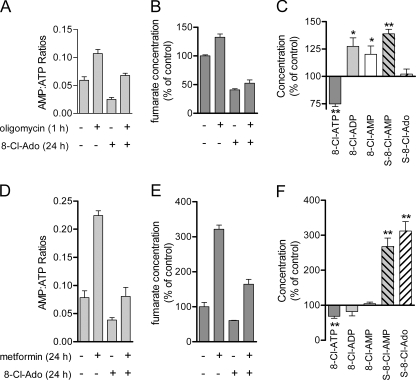
Oligomycin was used to determine the impact of ATP synthase inhibition on 8-Cl-Ado metabolism (Fig. 5, A–C). First, we evaluated the combination effect on the adenosine nucleotides and the fumarate pool. After oligomycin treatment for 1 h, the AMP:ATP ratios increased both for untreated and 8-Cl-Ado-treated cells (Fig. 5A), a combination of ATP loss and AMP gain. In contrast, although ATP levels were reduced 54% by 8-Cl-Ado treatment, the AMP:ATP ratios were also reduced because of the 80% reduction in AMP levels. As shown earlier (Fig. 4D), 8-Cl-Ado treatment of JeKo cells reduced fumarate levels, presumably as a consequence of succinyl metabolite formation (Fig. 5B). The fumarate levels were slightly increased with oligomycin treatment regardless of 8-Cl-Ado treatment (Fig. 5B).
FIGURE 5.
8-Cl-Ado metabolite distribution after oligomycin and metformin co-treatment. A–C, JeKo cells were preincubated with 10 μm 8-Cl-Ado for 24 h and/or then exposed to oligomycin (2 μg/ml) for 1 h. Cell extracts were quantified for (A) AMP:ATP ratios, (B) fumarate concentrations, and (C) 8-Cl-adenylate metabolite levels, oligomycin versus no oligomycin controls. D–F, JeKo cells were co-incubated with 10 μm 8-Cl-Ado and/or 5 mm metformin for 24 h. Cell extracts were quantified for (D) AMP:ATP ratios, (E) fumarate concentrations, and (F) 8-Cl-adenylate metabolite levels, metformin versus no metformin controls. Cells contents were extracted using 60% methanol and analyzed by HPLC. Data points are averages ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01.
Consistent with an increase in AMP:ATP ratios (Fig. 5A), ATP synthase inhibition by oligomycin after 8-Cl-Ado pretreatment shifted the distribution of 8-Cl-adenylate metabolites from 8-Cl-ATP to 8-Cl-ADP and 8-Cl-AMP (Fig. 5C). Because 8-Cl-AMP is proposed to be in rapid equilibrium with S-8-Cl-AMP (Fig. 3C), the increase in 8-Cl-AMP levels and slight increase in fumarate levels were consistent with increased levels of S-8-Cl-AMP. Although the S-8-Cl-AMP levels increased, the S-8-Cl-Ado levels did not increase because the cells were treated with oligomycin for only 1 h. Extended incubation times with oligomycin did increase the S-8-Cl-Ado concentrations (data not shown) as expected given the relatively slow rate of accumulation for S-8-Cl-Ado (Fig. 3D).
Metformin Increased Fumarate Levels and Succinyl Metabolism of 8-Cl-Ado
Next, we wanted to increase the concentration of fumarate and evaluate the metabolic profile of 8-Cl-Ado. Because fumarate is a dicarboxylic acid and cannot readily cross the plasma membrane, we used metformin to increase the fumarate concentrations (3-fold increase, Fig. 5E). Although its mechanism for increased fumarate levels is currently unknown, metformin increased the AMP:ATP ratios independently of 8-Cl-Ado treatment (Fig. 5D) presumably by inhibiting ETC1 (19). Without a change in 8-Cl-AMP concentrations, metformin and 8-Cl-Ado co-treatment increased fumarate levels (3-fold) and correspondingly increased the formation of both succinyl 8-Cl-Ado metabolites (3-fold, Fig. 5F). Unlike the oligomycin experiment, the S-8-Cl-AMP and S-8-Cl-Ado levels with metformin treatment increased proportionately as compared with the 8-Cl-Ado-treated cells without metformin. Equal accumulation of the succinyl metabolites was expected because metformin and 8-Cl-Ado were added simultaneously to allow enough time for metformin translocation across the membrane. Although the accumulation of 8-Cl-ATP was reduced slightly as expected by inhibition of ETC1 (Fig. 5D), the depletion of ATP and cell death were unaffected by metformin co-treatment (data not shown). We also tried to increase fumarate levels by addition of fumarate mono or diethyl ester as described previously (23, 24). However, in our system, both the mono ester fumarate (5 mm) and diethyl ester fumarate (0.5 mm) were highly cytotoxic after 24 h of exposure; shorter exposure times and non-cytotoxic concentrations either did not change or reduced the intracellular fumarate levels (data not shown).
DISCUSSION
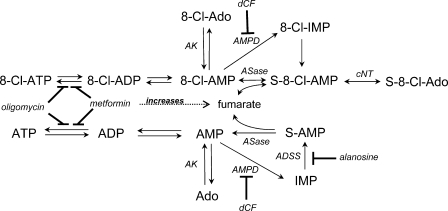
Previous reports on the metabolism of 8-Cl-Ado have focused exclusively on the formation of 8-Cl-ATP, an ATP analog that inhibits RNA transcription (1, 2, 5). The objective of the current study was to determine whether 8-Cl-Ado is a precursor to other adenosine-derived analogs that may contribute to its mechanism of action. While we hypothesized the formation of downstream adenosine-conjugated products like NAD, this study discovered the formation of two adenosine precursor metabolites, S-8-Cl-AMP and S-8-Cl-Ado (Fig. 2). The synthesis of these succinylated metabolites was tentatively attributed to ASase (Fig. 6), a reversible enzyme in the purine de novo and salvage pathways (13). Although we did not directly evaluate the metabolism of 8-Cl-Ado using purified enzymes, ASase is the only known enzyme that is capable of fumarate conjugation with AMP (13). Succinylation of 8-Cl-Ado may have clinically relevant consequences related to loss of fumarate and the intracellular pharmacokinetics of 8-Cl-Ado.
FIGURE 6.
Proposed 8-Cl-Ado and Ado metabolic pathways. AK, adenosine kinase; AMPD, AMP deaminase; ASase, adenylosuccinase; ADSS, adenylosuccinate synthase; cNT, cytosolic nucleotidase; dCF, 2′-deoxycoformycin.
One major consequence of 8-Cl-Ado succinylation was the utilization and subsequent depletion of fumarate, an intermediate of the citric acid cycle (Figs. 4 and 5). Loss of fumarate may inhibit the normal function of the citric acid cycle and thus reduce the rate of ATP formation by oxidative phosphorylation. Indeed, ATP depletion after 8-Cl-Ado treatment has been observed in a variety of cell lines (1, 2, 5, 6, 25) and primary leukemia cells (4). Furthermore, decrease in intracellular ATP has been previously associated with cell death (1, 4, 5). Specifically for these MCL cell lines, ATP loss was more pronounced for those cells with greater accumulation of succinyl metabolites and fumarate loss (JeKo and Mino) as compared with SP-53 cells (Fig. 4A) (1).
Perhaps more important than its effect on oxidative phosphorylation, loss of fumarate by 8-Cl-Ado may reduce the direct, tumorigenic actions of fumarate in cancer cells. Fumarate itself inhibits HIF prolyl 4-hydroxylases and consequently stabilizes hypoxia-inducible factor (HIF)-1α, a key regulator of tumor metabolism (23, 24, 26, 27). Specifically, germline mutations of fumarase, an enzyme in the Krebs cycle that converts fumarate to malate, increase fumarate concentrations, and predispose individuals to multiple leiomyomas, testicular tumors, and hereditary leiomyomatosis renal cell cancer (HLRCC) (23, 24, 28–30). Consequently, fumarase is one of only two mitochondrial proteins that is classified as a bona-fide tumor suppressor protein (31).
Whereas previous therapeutic strategies have targeted downstream effectors of HIF-1α such as lactate dehydrogenase A in HLRCC (32), we propose that fumarate is a more attractive target given that fumarate accumulation initiates HIF-1α stabilization. Of note, 2-fold changes in fumarate levels have been reported to directly activate hypoxia and angiogenesis pathways in HLRCC models (24); using Mino and JeKo cells, we observed similar 2 to 3-fold differences in fumarate levels after 8-Cl-Ado treatment (Figs. 4 and 5).
Results from our study not only indicate that fumarate levels were reduced by 8-Cl-Ado, but also show that succinylation and subsequent fumarate loss were selective for cells with high fumarate concentrations. S-8-Cl-Ado and S-8-Cl-AMP formation was directly associated with the initial concentration of fumarate (Fig. 4E), and increased fumarate concentrations resulted in increased rates of 8-Cl-AMP succinylation (Fig. 5, E and F). Based on these results, we expect 8-Cl-Ado succinylation to occur primarily in cells with relatively high concentrations of fumarate, as might be expected for cells with reduced fumarase activity.
Selective accumulation of 8-Cl-Ado succinyl metabolites may also allow 8-Cl-Ado a pharmacokinetic advantage by sustaining its therapeutic effects. For example, the succinyl metabolites may accumulate selectively in fumarase-deficient cells, prolonging the therapeutic actions of 8-Cl-Ado in the tumor. Interestingly, S-8-Cl-Ado itself had a much longer half-life than that of 8-Cl-ATP, the putative active metabolite (12 h versus 6 h, Fig. 3E). Thus, S-8-Cl-Ado may maintain intracellular 8-Cl-AMP levels as 8-Cl-Ado concentrations fall in the plasma and consequently prolong 8-Cl-Ado therapeutic effects.
Finally, one unexpected finding of this study was the change in 8-Cl-Ado metabolism after co-treatment with metformin, a commonly prescribed type 2 diabetes drug and potential anti-cancer therapeutic (20, 21). Because it is an ETC1 inhibitor (19), metformin was expected to decrease ATP levels and consequently increase the AMP/ATP ratios (Fig. 5D). However, we were surprised that metformin increased fumarate levels and drastically changed the 8-Cl-Ado metabolite distribution to favor succinylation (Fig. 5, E and F). One consequence of this drug-drug interaction was the increased intracellular half-life of 8-Cl-ATP; the 8-Cl-ATP half-life was increased with metformin co-treatment of JeKo cells (45% increase, p < 0.05). However, the accumulation of 8-Cl-ATP, the putative active metabolite, was reduced by metformin, presumably caused by inhibition of ETC1 (Fig. 5E). Thus, the therapeutic consequences of this interaction could be deleterious or advantageous and require further study.
In conclusion, S-8-Cl-AMP and S-8-Cl-Ado were identified as major 8-Cl-Ado metabolites in MCL cell lines, and their formation was accompanied by loss of fumarate. This connection to the citric acid cycle supports further investigation of therapeutic strategies for individualized therapy with 8-Cl-Ado, specifically in the treatment of fumarase deficient tumors. In addition, the monitoring of all metabolites will be important during clinical trials because the formation of succinyl metabolites may influence the intracellular pharmacokinetics of phosphorylated 8-Cl-Ado.
Acknowledgments
The Characterized Cell Line, Pharmacology and Analytical, and NMR Core Facilities at M. D. Anderson are supported in part by a Cancer Center Support Grant, NCI CA16672. We thank Robert J. Barbuch at Eli Lilly for helpful suggestions and review of the mass spectrometry data.
This work was supported, in whole or in part, by National Institutes of Health Grants CA 85915 from the NCI and Lymphoma SPORE CA136411.
- 8-Cl-Ado
- 8-chloroadenosine
- AK
- adenosine kinase
- AMPD
- AMP deaminase
- ASase
- adenylosuccinase
- ADSS
- adenylosuccinate synthase
- CLL
- chronic lymphocytic leukemia
- cNT
- cytosolic nucleotidase
- dCF
- 2′-deoxycoformycin
- ETC1
- electron transport chain 1
- HLRCC
- hereditary leiomyomatosis renal cell cancer
- MCL
- mantle cell lymphoma
- PCA
- perchloric acid
- S-8-Cl-Ado
- succinyl-8-chloroadenosine.
REFERENCES
- 1.Dennison J. B., Balakrishnan K., Gandhi V. (2009) Br. J. Haematol. 147, 297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stellrecht C. M., Rodriguez C. O., Jr., Ayres M., Gandhi V. (2003) Cancer Res. 63, 7968–7974 [PubMed] [Google Scholar]
- 3.Gandhi V., Chen W., Ayres M., Rhie J. K., Madden T. L., Newman R. A. (2002) Cancer Chemother. Pharmacol. 50, 85–94 [DOI] [PubMed] [Google Scholar]
- 4.Balakrishnan K., Stellrecht C. M., Genini D., Ayres M., Wierda W. G., Keating M. J., Leoni L. M., Gandhi V. (2005) Blood 105, 4455–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi V., Ayres M., Halgren R. G., Krett N. L., Newman R. A., Rosen S. T. (2001) Cancer Res. 61, 5474–5479 [PubMed] [Google Scholar]
- 6.Stellrecht C. M., Phillip C. J., Cervantes-Gomez F., Gandhi V. (2007) Cancer Res. 67, 9913–9920 [DOI] [PubMed] [Google Scholar]
- 7.Ghias K., Ma C., Gandhi V., Platanias L. C., Krett N. L., Rosen S. T. (2005) Mol. Cancer Ther. 4, 569–577 [DOI] [PubMed] [Google Scholar]
- 8.Krett N. L., Davies K. M., Ayres M., Ma C., Nabhan C., Gandhi V., Rosen S. T. (2004) Mol. Cancer Ther. 3, 1411–1420 [PubMed] [Google Scholar]
- 9.Chen L. S., Sheppard T. L. (2004) J. Biol. Chem. 279, 40405–40411 [DOI] [PubMed] [Google Scholar]
- 10.Chen L. S., Nowak B. J., Ayres M. L., Krett N. L., Rosen S. T., Zhang S., Gandhi V. (2009) Biochem. Pharmacol. 78, 583–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez C. O., Jr., Plunkett W., Paff M. T., Du M., Nowak B., Ramakrishna P., Keating M. J., Gandhi V. (2000) J. Chromatogr. B. Biomed. Sci. App. 745, 421–430 [DOI] [PubMed] [Google Scholar]
- 12.de Korte D., Haverkort W. A., Roos D., van Gennip A. H. (1985) Clin. Chim. Acta 148, 185–196 [DOI] [PubMed] [Google Scholar]
- 13.Silverstein R. M., Bassler G., Morrill T. (2005) Spectrometric Identification of Organic Compounds, 7th Ed., John Wiley and Sons, New York [Google Scholar]
- 14.Carter C. E., Cohen L. H. (1956) J. Biol. Chem. 222, 17–30 [PubMed] [Google Scholar]
- 15.Van den Bergh F., Vincent M. F., Jaeken J., Van den Berghe G. (1991) Anal. Biochem. 193, 287–291 [DOI] [PubMed] [Google Scholar]
- 16.Van den Berghe G., Vincent M. F., Jaeken J. (1997) J. Inherit. Metab. Dis. 20, 193–202 [DOI] [PubMed] [Google Scholar]
- 17.Zeman J., Krijt J., Stratilová L., Hansíková H., Wenchich L., Kmoch S., Chrastina P., Houstek J. (2000) J. Inherit. Metab. Dis. 23, 371–374 [DOI] [PubMed] [Google Scholar]
- 18.Tortora G., Ciardiello F., Pepe S., Tagliaferri P., Ruggiero A., Bianco C., Guarrasi R., Miki K., Bianco A. R. (1995) Clin. Cancer Res. 1, 377–384 [PubMed] [Google Scholar]
- 19.El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) J. Biol. Chem. 275, 223–228 [DOI] [PubMed] [Google Scholar]
- 20.Bosi E. (2009) Diabetes, Obesity, Metabol. 11, 3–8 [DOI] [PubMed] [Google Scholar]
- 21.Jiralerspong S., Palla S. L., Giordano S. H., Meric-Bernstam F., Liedtke C., Barnett C. M., Hsu L., Hung M. C., Hortobagyi G. N., Gonzalez-Angulo A. M. (2009) J. Clin. Oncol. 27, 3297–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett L. L., Chang C. H., Allan P. W., Adamson D. J., Rose L. M., Brockman R. W., Secrist J. A., Shortnacy A., Montgomery J. A. (1985) Nucleosides Nucleotides Nucleic Acids 4, 107–116 [Google Scholar]
- 23.Isaacs J. S., Jung Y. J., Mole D. R., Lee S., Torres-Cabala C., Chung Y. L., Merino M., Trepel J., Zbar B., Toro J., Ratcliffe P. J., Linehan W. M., Neckers L. (2005) Cancer Cell 8, 143–153 [DOI] [PubMed] [Google Scholar]
- 24.Koivunen P., Hirsilä M., Remes A. M., Hassinen I. E., Kivirikko K. I., Myllyharju J. (2007) J. Biol. Chem. 282, 4524–4532 [DOI] [PubMed] [Google Scholar]
- 25.Stellrecht C., Ayres M., Arya R., Gandhi V. (2009) Breast Cancer Res. Treat, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudarshan S., Sourbier C., Kong H. S., Block K., Romero V. A., Yang Y., Galindo C., Mollapour M., Scroggins B., Goode N., Lee M. J., Gourlay C. W., Trepel J., Linehan W. M., Neckers L. (2009) Mol. Cell Biol. 29, 4080–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R., Li Z., Bai S., Zhang H., Tang M., Lei Y., Chen L., Liang S., Zhao Y. L., Wei Y., Huang C. (2009) Mol. Cell Proteomics 8, 70–85 [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson I. P., Alam N. A., Rowan A. J., Barclay E., Jaeger E. E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., Roylance R. R., Olpin S., Bevan S., Barker K., Hearle N., Houlston R. S., Kiuru M., Lehtonen R., Karhu A., Vilkki S., Laiho P., Eklund C., Vierimaa O., Aittomäki K., Hietala M., Sistonen P., Paetau A., Salovaara R., Herva R., Launonen V., Aaltonen L. A. (2002) Nat. Genet. 30, 406–410 [DOI] [PubMed] [Google Scholar]
- 29.Carvajal-Carmona L. G., Alam N. A., Pollard P. J., Jones A. M., Barclay E., Wortham N., Pignatelli M., Freeman A., Pomplun S., Ellis I., Poulsom R., El-Bahrawy M. A., Berney D. M., Tomlinson I. P. M. (2006) J. Clin. Endocrinol. Metab. 91, 3071–3075 [DOI] [PubMed] [Google Scholar]
- 30.King A., Selak M. A., Gottlieb E. (2006) Oncogene 25, 4675–4682 [DOI] [PubMed] [Google Scholar]
- 31.Vander Heiden M. G., Cantley L. C., Thompson C. B. (2009) Science 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie H., Valera V. A., Merino M. J., Amato A. M., Signoretti S., Linehan W. M., Sukhatme V. P., Seth P. (2009) Mol. Cancer Ther. 8, 626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]