Abstract
There is a body of evidence suggesting that Ca2+ handling proteins assemble into signaling complexes required for a fine regulation of Ca2+ signals, events that regulate a variety of critical cellular processes. Canonical transient receptor potential (TRPC) and Orai proteins have both been proposed to form Ca2+-permeable channels mediating Ca2+ entry upon agonist stimulation. A number of studies have demonstrated that inositol 1,4,5-trisphosphate receptors (IP3Rs) interact with plasma membrane TRPC channels; however, at present there is no evidence supporting the interaction between Orai proteins and IP3Rs. Here we report that treatment with thapsigargin or cellular agonists results in association of Orai1 with types I and II IP3Rs. In addition, we have found that TRPC3, RACK1 (receptor for activated protein kinase C-1), and STIM1 (stromal interaction molecule 1) interact with Orai1 upon stimulation with agonists. TRPC3 expression silencing prevented both the interaction of Orai1 with TRPC3 and, more interestingly, the association of Orai1 with the type I IP3R, but not with the type II IP3R, thus suggesting that TRPC3 selectively mediates interaction between Orai1 and type I IP3R. In addition, TRPC3 expression silencing attenuated ATP- and CCh-stimulated interaction between RACK1 and the type I IP3R, as well as Ca2+ release and entry. In conclusion, our results indicate that agonist stimulation results in the formation of an Orai1-STIM1-TRPC3-RACK1-type I IP3R complex, where TRPC3 plays a central role. This Ca2+ signaling complex might be important for both agonist-induced Ca2+ release and entry.
Keywords: Calcium, Calcium/Cellular Regulation, Calcium/Channels, Calcium Intracellular Release, Ion Channels, IP3 Receptors, Orai1, TRPC3
Introduction
Cellular stimulation by agonists results in a rise in cytosolic free Ca2+ concentration ([Ca2+]i),4 an event that is essential for a large number of cellular processes. Agonist-evoked Ca2+ mobilization consists of two components: Ca2+ release from finite intracellular stores and Ca2+ entry. Often the increase in [Ca2+]i resulting from Ca2+ entry is of major magnitude than Ca2+ release, and is required for full activation of cellular functions (1, 2).
Agonist receptor activation results in the hydrolysis of membrane phosphoinositides by phospholipase C and the generation of Ca2+ mobilizing messenger inositol 1,4,5-trisphosphate (IP3), which, upon activation of different IP3 receptors (IP3Rs), releases Ca2+ from non-mitochondrial intracellular Ca2+ stores (3). In non-excitable cells, receptor occupation results in activation of two separate pathways for Ca2+ entry, named receptor-operated Ca2+ entry (ROCE) and capacitative or store-operated Ca2+ entry (SOCE). The latter is a major mechanism for Ca2+ influx regulated by the filling state of the intracellular Ca2+ stores (4), a mechanism where the stromal interaction molecule (STIM) 1 has been demonstrated to act as the transmembrane endoplasmic reticulum Ca2+ sensor (5–8). The nature of the plasma membrane Ca2+ permeable channels involved both in ROCE and SOCE are still under investigation but most studies have presented Orai1 as a putative SOC channel (9–14) and transient receptor potential (TRP) proteins as candidates to mediate both SOCE and ROCE (15–20). These channels have been shown to take part in signaling complexes, including the protein STIM1, which might be essential for the activation mode of the channel (19, 21–23).
In addition, a functional interaction between IP3Rs and human TRP channels has been demonstrated by different approaches in several cell types, including human platelets endogenously expressing TRPC1 and IP3Rs (24, 25), human embryonic kidney (HEK)-293 cells stably expressing hTRP3 (26) or TRPC1–6 proteins (27), and HEK293T transiently expressing different TRP proteins (28). IP3Rs have also been shown to be required for activation of TRPC1 in vascular smooth muscle cells (29) and for the IP3-dependent miniature Ca2+ channels (Imin), a Ca2+-selective channel activated by store depletion, in excised membrane patches from A431 human carcinoma cells (30).
A recent study has reported that TRPC3 regulates IP3R function by mediating interaction between IP3R and the scaffolding protein RACK1 (receptor for activated protein kinase C-1), a protein that plays a key role in transduction of plasma membrane signals to downstream effectors (31, 32), thus regulating agonist-induced Ca2+ release (33). Hence, in the present study we have investigated whether this complex is also important for agonist-induced Ca2+ entry with the participation of proteins involved in Ca2+ entry such as Orai1 and STIM1. We describe for the first time association of the Ca2+ permeable channel Orai1 with the type I and II IP3 receptors. The former appears to be mediated by TRPC3 proteins, which also mediates their interaction with RACK1. This protein complex might play a functional role in agonist-induced Ca2+ mobilization.
EXPERIMENTAL PROCEDURES
Materials
ATP, thapsigargin (TG), leupeptin, benzamidine, ionomycin, phenylmethylsulfonyl fluoride, dimethyl-BAPTA-AM, 4′,6- diamidino-2-phenylindole dihydrochloride (DAPI), carbachol (CCh), anti-G actin antibody, bovine serum albumin, and anti-Orai1 antibody (C-terminal) were from Sigma. Anti-TRPC3 (N-terminal) and anti-TRPC1 (C-terminal) antibodies were from Abcam (Cambridge, UK). Anti-type I IP3 receptor and anti-type II IP3 receptor antibodies, anti-RACK1 antibody and horseradish peroxidase-conjugated goat anti-rabbit IgG, and donkey anti-goat IgG antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-type III IP3 receptor antibody and anti-STIM1 antibody were from BD Biosciences. Horseradish peroxidase-conjugated ovine anti-mouse IgG antibody (NA931) and hyperfilm ECL were from Amersham Biosciences. Fura 2-AM, Alexa Fluor 488- and 568-conjugated secondary antibodies, and DAPI were from Invitrogen. Enhanced chemiluminescence detection reagents were from Pierce. All other reagents were of analytical grade.
Cell Culture and Transfection
Human HeLa and HEK293 cells were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium, and supplemented with 10% heat-inactivated fetal bovine serum in a 37 °C incubator with 5% CO2. The experiments were performed in HEPES-buffered saline containing (in mm): 145 NaCl, 10 HEPES, 10 d-glucose, 5 KCl, 1 MgSO4, pH 7.45. For dimethyl-BAPTA loading, cells were incubated for 30 min at 37 °C with 10 μm dimethyl-BAPTA-AM. Cells were then collected by centrifugation and resuspended in HEPES-buffered saline.
Cell transfection with shTRPC3, shTRPC1, and shOrai1 was performed as described previously (33, 34) using shTRPC3, shTRPC1, and shOrai1 kindly provided by Dr. Ambudkar. For shTRPC3, the sense sequence was 5′-CACCGTGATGTGGTCTGAATGTAACGAATTACATTCAGACCACATCAC-3′, and the antisense sequence was 5′- AAAAGTGATGTGGTCTGAATGTAATTCGTTACATTCAGACCACATCAC-3′. For the shRNA targeting human TRPC1, the sense sequence was 5′-CACCGGGTGACTTTATATGGTTCGAAAACCATATAATAGTCACCC-3′, and the antisense sequence was 5′-AAAAGGGTGACTATTATATGGTTTTCGAACCATATAATAGTCACCC-3′. For the shOrai1, the sense sequence was 5′-CACCTCACTGGTTAGCCATAAGACGAATCTTATGGCTAACCAGTGA-3′, and the antisense sequence was 5′-AAAACCTTTACACGCTAGATGGTttcgTCTTATGGCTAACCAGTGA-3′. These sequences were synthesized and hybridized as previously described (33, 34). Plasmids were used for silencing experiments at 1 μg/μl.
Immunoprecipitation
Cell suspension aliquots (500 μl) were treated as described and lysed with an equal volume of lysis buffer, pH 7.2, containing 316 mm NaCl, 20 mm Tris, 2 mm EGTA, 0.2% SDS, 2% sodium deoxycholate, 2% Triton X-100, 2 mm Na3VO4, 2 mm phenylmethylsulfonyl fluoride, 100 μg/ml of leupeptin, and 10 mm benzamidine. Protein immunoprecipitation was achieved by incubating samples with 2 μg of the specific antibody and protein A-agarose overnight at 4 °C.
Western Blotting
Western blotting was performed as described previously (35). Briefly, proteins were separated by 10% SDS-PAGE and electrophoretically transferred for 2 h at 0.8 mA/cm2, in a semi-dry blotter (Hoefer Scientific, Newcastle, Staffshire, UK) onto nitrocellulose membranes for subsequent probing. Blots were incubated overnight with 10% (w/v) bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBST) to block residual protein binding sites. Immunodetection of Orai1, TRPC3, RACK1, G actin, or types I, II, or III IP3 receptors was achieved using the anti-Orai1, anti-TRPC3, anti-RACK1, anti-STIM1, anti-G actin, anti-type I IP3 receptor, anti-type II IP3 receptor, or anti-type III IP3 receptor antibodies diluted 1:1000 in TBST for 2 h or the anti-TRPC1 antibody diluted 1:200 in TBST for 2 h. To detect the primary antibody, blots were incubated for 1 h with the appropriate horseradish peroxidase-conjugated anti-IgG antibody diluted 1:10,000 in TBST and then exposed to enhanced chemiluminescence reagents for 4 min. Blots were then exposed to photographic films. The density of bands on the film was measured using a scanning densitometry.
Immunofluorescence
Cultured cells were fixed using 3% paraformaldehyde (in phosphate-buffered saline) for 10 min at room temperature. The cells were then permeabilized in phosphate-buffered saline containing 0.025% (v/v) Nonidet P-40 detergent for 10 min at 4 °C. Samples were incubated with rabbit anti-Orai1 or goat anti-type I IP3 receptor antibodies overnight at room temperature in phosphate-buffered saline containing 0.5% bovine serum albumin as blocking agent, followed by incubation with Alexa Fluor 488- and 568-conjugated secondary antibodies and DAPI for 1 h. The samples were examined using a Zeiss LSM 510 confocal microscope. For quantitative colocalization analysis the overlap coefficient according to Manders et al. (36) was calculated using Image J software.
Measurement of Intracellular Free Calcium Concentration ([Ca2+]i)
Cells were loaded with fura-2 by incubation with 2 μm fura-2/AM for 45 min at 37 °C. Fluorescence was recorded from 2-ml aliquots of magnetically stirred cellular suspension (106 cells/ml) at 37 °C using a Cary Eclipse Spectrophotometer (Varian Ltd., Madrid, Spain) with excitation wavelengths of 340 and 380 nm and emission at 505 nm. Changes in [Ca2+]i were monitored using the fura-2 340/380 fluorescence ratio and calibrated according to an established method (37).
Ca2+ release by ATP or CCh was estimated using the integral of the rise in [Ca2+]i for 3 min after the addition of the agonist (38). Ca2+ entry was estimated using the integral of the rise in [Ca2+]i for 2.5 min after addition of CaCl2 (39). Ca2+ entry was corrected by subtraction of the [Ca2+]i elevation due to leakage of the indicator.
Statistical Analysis
Analysis of statistical significance was performed using Student's t test. The difference was considered statistically significant when at least p < 0.05.
RESULTS
Thapsigargin Enhances the Association of Orai1 with Type I IP3 Receptor
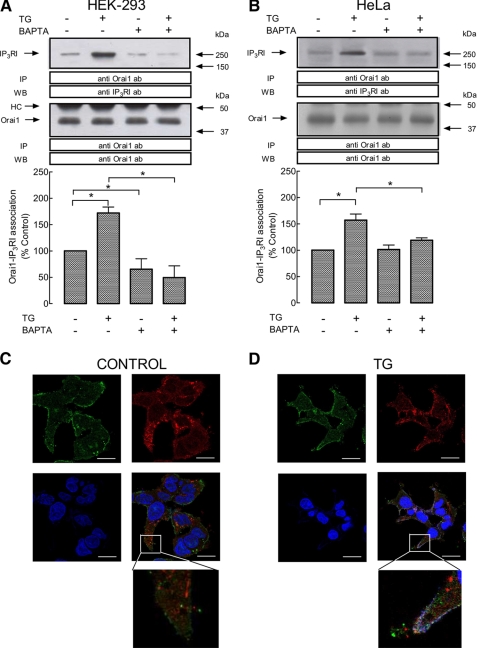
We have investigated the possible association between the plasma membrane protein Orai1 and the type I IP3R in two unrelated human cell lines, HEK293 and HeLa cells, by looking for co-immunoprecipitation from cell lysates. Immunoprecipitation and subsequent SDS-PAGE and Western blotting were conducted using resting cells and cells treated with TG, a specific inhibitor of SERCA (40) that promotes passive Ca2+ efflux from the endoplasmic reticulum and, subsequently, rises in [Ca2+]i. As depicted in Fig. 1, A and B, upper panels, our results show detectable association between Orai1 and type I IP3R in resting HEK293 and HeLa cells. Interestingly, we have found that treatment with TG in a medium containing 1 mm Ca2+ enhanced the co-immunoprecipitation between Orai1 and the type I IP3R in HEK293 and HeLa cells by 72 and 57%, respectively (Fig. 1, A and B, upper panels; p < 0.05; n = 6). Western blotting with anti-Orai1 antibody confirmed a similar content of this protein in all lanes (Fig. 1, A and B, lower panels).
FIGURE 1.
Interaction of Orai1 with type I IP3R in HEK293 and HeLa cells. A and B, HEK (A) and HeLa cells (B) were either loaded with dimethyl-BAPTA or left treated, as indicated, and then suspended in a medium containing 1 mm Ca2+ or a Ca2+-free medium (BAPTA-loaded cells; 100 μm EGTA was added). Cells were then stimulated with TG (1 μm) for 3 min or left untreated and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-Orai1 antibody and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting with a specific anti-IP3RI antibody. Membranes were reprobed with the anti-Orai1 antibody for protein loading control. The panel shows results from one experiment representative of five. Molecular masses indicated on the right were determined using molecular mass markers run in the same gel. Histograms represent the quantification of Orai1-IP3RI association under different experimental conditions. Results are expressed as mean ± S.E. and presented as percentage of control (non-stimulated cells not loaded with dimethyl-BAPTA). *, p < 0.05. HC, heavy chain of the immunoglobulin used for immunoprecipitation. C and D, confocal images of resting (C) and TG-treated (D) HEK293 cells immunostained with anti-Orai1 and anti-IP3RI antibodies followed by Alexa Fluor 488- and 568-conjugated secondary antibodies, respectively, or DAPI. An overlay of the three images is depicted on the bottom right-hand image. Cells were stimulated in the absence (control) or presence of 1 μm TG, as indicated, in a medium containing 1 mm Ca2+. Scale bar, 10 μm. Insets depict a zoom of two representative areas. Error bars, S.E.
We have further investigated whether the interaction between these two Ca2+-handling proteins requires rises in [Ca2+]i by using the intracellular Ca2+ chelator dimethyl- BAPTA, a procedure that abolishes the rise in [Ca2+]i induced by TG (data not shown, but see Ref. 35). As shown in Fig. 1, A and B, in a Ca2+-free medium (100 μm EGTA was added) dimethyl-BAPTA loading reduced the interaction between Orai1 and the type I IP3R in resting HEK293 cells and prevented the TG-stimulated increase in co-immunoprecipitation of both proteins in HEK293 and HeLa cells. These findings suggest that TG enhances Ca2+-dependent association between Orai1 and type I IP3R.
Next, we explored co-localization between Orai1 and type I IP3R by immunofluorescence using specific anti-Orai1 and anti-type I IP3R antibodies followed by Alexa Fluor 488- or 568-conjugated secondary antibodies both in control cells and cells stimulated with TG. As shown in Fig. 1, C and D, and previously described (35), Orai1 was distributed in the cell periphery (plasma membrane) but there is also detectable staining in the cytoplasmic area, whereas type I IP3R shows a cytoplasmic distribution in resting HEK293 cells. Stimulation with TG for 3 min resulted in re-distribution of type I IP3R in the cell periphery and enhanced co-localization (Fig. 1, C and D; the value of overlap coefficient according to Manders et al. (36) increased following stimulation with TG from 0.484 in resting cells to 0.723 in TG-treated cells).
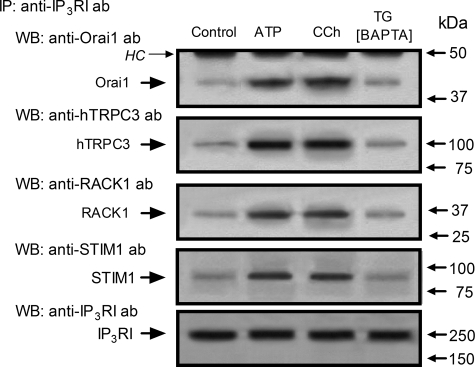
Association of Type I IP3 Receptor with Orai1, TRPC3, RACK1, and STIM1
Studies in HEK293 cells have reported that IP3Rs, RACK1, and TRPC3 form a dynamically regulated complex that is important for agonist-induced Ca2+ mobilization (33, 41). Hence we have investigated the association of the type I IP3R with TRPC3 and RACK1, as well as with STIM1, which associates with Orai1 during SOCE (10, 42, 43). As shown in Fig. 2, Orai1, TRPC3, RACK1, and STIM1 were detected in type I IP3R immunoprecipitates from resting cells. Association between these proteins with the type I IP3R was enhanced by treatment with ATP and CCh by 69 ± 6 and 67 ± 9% for Orai1, 89 ± 4 and 95 ± 10% for TRPC3, 82 ± 7 and 89 ± 6% for RACK1, and 63 ± 4 and 59 ± 3% for STIM1, respectively (p < 0.05; n = 6). We have further investigated whether association of these proteins is related with the capacitative pathway for Ca2+ entry by inducing store depletion with TG in dimethyl-BAPTA-loaded cells. Dimethyl-BAPTA loading did not significantly modify the association between Orai1, TRPC3, RACK1, and STIM1 and slightly reduced the association of Orai1 with type I IP3R in non-stimulated cells (data not shown). As shown in Fig. 2, in BAPTA-loaded cells TG was unable to induce association between type I IP3R and Orai1, TRPC3, RACK1, or STIM1.
FIGURE 2.
Interaction of type I IP3R with Orai1, STIM1, TRPC3, and RACK1 in HEK293. HEK293 cells were suspended in a medium containing 1 mm Ca2+ and then stimulated with ATP (10 μm) or CCh (100 μm) for 3 min and lysed. Alternatively, cells were loaded with dimethyl-BAPTA, suspended in Ca2+-free medium (100 μm EGTA was added), and then stimulated with TG (1 μm) for 3 min and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-type I IP3R antibody and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with specific anti-Orai1, anti-TRPC3, anti-RACK1, or anti-STIM1 antibodies. Membranes were reprobed with the anti-type I IP3R antibody for protein loading control. The panels show results from one experiment representative of five. Molecular masses indicated on the right were determined using molecular mass markers run in the same gel. HC, heavy chain of the immunoglobulin used for immunoprecipitation.
Role of TRPC3 in the Association between the Type I IP3 Receptor and Orai1 and RACK1 Proteins
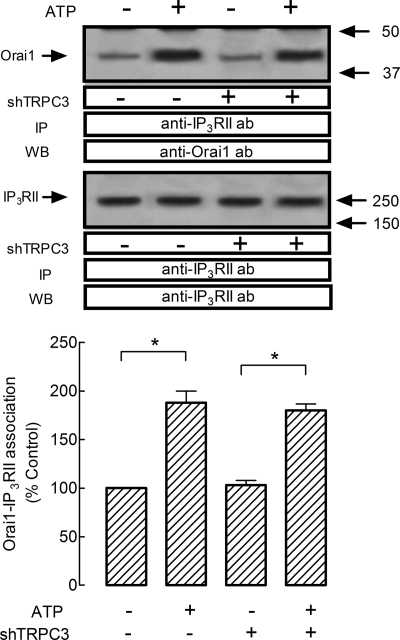
HEK293 cells have been reported to endogenously express several TRPC proteins, including TRPC3 (43, 44). In these cells, Orai1 has been demonstrated to physically interact with the N and C termini of the TRPC3 proteins (43). Recently, TRPC3 has been reported to regulate agonist-stimulated Ca2+ release by mediating interaction between IP3R and RACK1 (33). Hence, we investigated the possibility that TRPC3 mediates interaction between Orai1 and type I IP3R by using TRPC3 expression silencing.
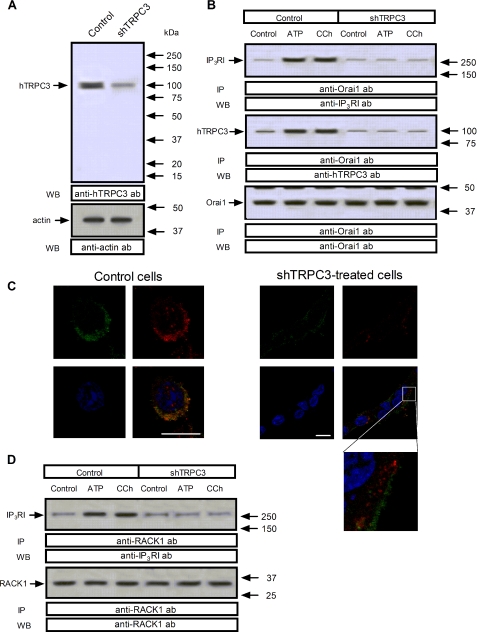
As shown in Fig. 3A, top panel, shTRPC3 reduced significantly the amount of TRPC3 detected in HEK293 cells as compared with controls. Western blotting with anti-actin antibody revealed a similar amount of protein in all lanes (Fig. 3A, bottom panel). As reported above, treatment with ATP (10 μm) or CCh (100 μm) significantly increased the association between Orai1 and type I IP3R by 70 ± 5 and 68 ± 6%, respectively, and also between Orai1 and TRPC3 by 64 ± 5 and 68 ± 4%, respectively (Fig. 3B, lanes 1–3; p < 0.05; n = 5). We found that shTRPC3 abolishes ATP- and CCh-mediated association between Orai1 and the type I IP3R or TRPC3 (Fig. 3B, top and middle panel; p < 0.05; n = 6). Western blotting with anti-Orai1 antibody confirmed a similar content of this protein in all lanes (Fig. 3B, lower panel).
FIGURE 3.
Role of TRPC3 in the association between IP3RI and Orai1 or RACK1 in HEK cells. HEK293 cells were transfected with shTRPC3 or control vector, as indicated, and used after 48 h for protein detection. A, Western blotting (WB) showing the expression levels of TRPC3, which was significantly reduced in lysates from cells transfected with shTRPC3. Bottom panel, Western blotting with anti-G actin antibody for protein loading control. B and D, HEK293 cells were transfected with shTRPC3 or control vector, as indicated, and then stimulated for 3 min with 10 μm ATP or 100 μm CCh or left untreated and lysed. Samples were immunoprecipitated (IP) with anti-Orai1 antibody (B) or anti-RACK1 antibody (D) and immunoprecipitates were analyzed by Western blotting (WB) using anti-type I IP3R (B and D, upper panels) or anti-TRPC3 antibodies (B, middle panel) and reprobed with either anti-Orai1 antibody (B, lower panel) or anti-RACK1 antibody (D, lower panel) as described under “Experimental Procedures.” The panels show results from one experiment representative of three to five. C, confocal images of TG-treated HEK293 cells (left panels) or TG-treated HEK-293 cells transfected with shTRPC3 (right panels) immunostained with anti-Orai1 and anti-IP3RI antibodies followed by Alexa Fluor 488- and 568-conjugated secondary antibodies, respectively, or DAPI. An overlay of the three images is depicted on the bottom right-hand image. Cells were stimulated with 1 μm TG in medium containing 1 mm Ca2+. Scale bar, 10 μm. Inset depicts a zoom of a representative area.
We have analyzed co-localization between Orai1 and type I IP3R in shTRPC3-treated cells by immunofluorescence in cells stimulated with CCh. As shown in Fig. 3C, stimulation with CCh for 3 min resulted in redistribution of the type I IP3R in the cell periphery and enhanced co-localization, which was impaired in shTRPC3-treated cells (the value of overlap coefficient according to Manders et al. (36) decreased in shTRPC3-treated cells stimulated with CCh from 0.795 in control cells to 0.184 in shTRPC3-treated cells).
We further explored the effect of shTRPC3 on interaction between type I IP3R and RACK1. As shown in Figs. 2 and 3D and described above, treatment with ATP or CCh enhanced the association between the type I IP3R and RACK1. Our results indicate that shTRPC3 abolishes ATP- and CCh-stimulated association between these proteins (Fig. 3D, top panel; p < 0.05; n = 4). Western blotting with anti-RACK1 antibody confirmed a similar content of this protein in all lanes (Fig. 3D, lower panel).
TRPC3 Modulates Agonist-evoked Intracellular Ca2+ Release
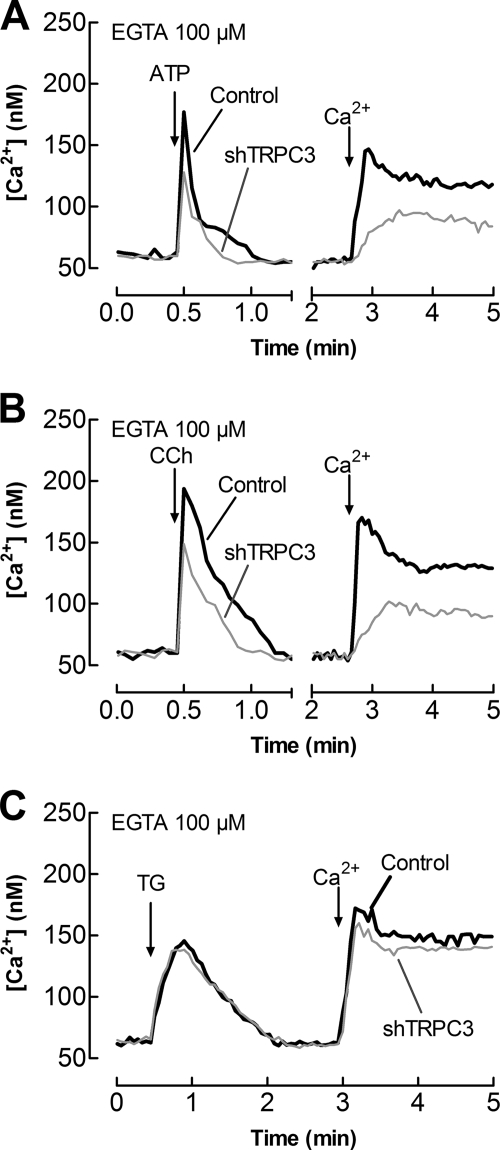
Because silencing TRPC3 expression was effective preventing the association between Orai1 and type I IP3R in HEK293 cells, we investigated the functional relevance of this interaction in agonist-evoked Ca2+ mobilization. Treatment of HEK293 cells with 10 μm ATP or 100 μm CCh induced a rapid and transient increase in [Ca2+]i due to release of Ca2+ from the intracellular stores (Fig. 4). As shown in Fig. 4, A and B, shTRPC3 significantly decreased ATP- and CCh-induced intracellular Ca2+ release by 47 ± 9 and 39 ± 7% as compared with cells transfected with control vector (p < 0.05; n = 5). The effect observed is unlikely due to a different amount of Ca2+ accumulated in the intracellular stores because Ca2+ release evoked by TG was unaffected in cells treated with shTRPC3 (Fig. 4C). In addition, we found that TRPC3 expression silencing significantly attenuated ATP- and CCh-induced Ca2+ entry by 49 ± 9 and 51 ± 7%, respectively; however, TRPC3 silencing did not significantly modify TG-evoked Ca2+ entry (Fig. 4, A–C; p < 0.05; n = 10).
FIGURE 4.
TRPC3 modulates Ca2+ mobilization. HEK293 cells were transfected with shTRPC3 (gray traces) or control vector (black traces) and used after 48 h for Ca2+ measurements. Cells were loaded with fura-2 and stimulated with 10 μm ATP (A), 100 μm CCh (B), or 1 μm TG (C), in a Ca2+-free medium (100 μm EGTA was added), followed by addition of CaCl2 (final concentration 1 mm) to initiate Ca2+ entry. Traces are representative of five to 10 independent experiments.
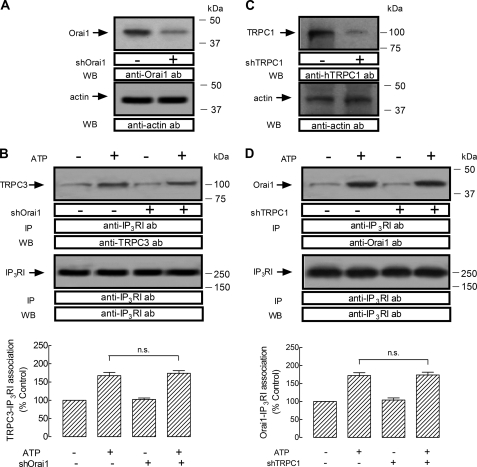
We further investigated whether Orai1 is essential for the interaction between TRPC3 and type I IP3R by Orai1 expression silencing. As shown in Fig. 5A, top panel, shOrai1 significantly reduced the amount of Orai1 detected in HEK293 cells. Western blotting with anti-actin antibody revealed a similar amount of protein in all lanes (Fig. 5A, bottom panel). Our results indicate that shOrai1 did not affect resting or the ATP-evoked increase in TRPC3-type I IP3R association (Fig. 5B).
FIGURE 5.
Role of Orai1 in the interaction TRPC3-type I IP3R and lack of involvement of TRPC1 on the Orai1-type I IP3R association. A and B, HEK293 cells were transfected with shOrai1 or control vector, as indicated, and used after 48 h for protein detection. A, Western blot (WB) showing the expression levels of Orai1, which was significantly reduced in lysates from cells transfected with shOrai1. Bottom panel, Western blotting with anti-G actin antibody for protein loading control. B, HEK293 cells were transfected with shOrai1 or control vector, as indicated, and then stimulated for 3 min with 10 μm ATP or left untreated and lysed. Samples were immunoprecipitated (IP) with anti-type I IP3R antibody and immunoprecipitates were analyzed by Western blotting using anti-TRPC3 antibody (upper panel) and reprobed with anti-type I IP3R antibody (lower panel) as described under “Experimental Procedures.” The panels show results from one experiment representative of five. C and D, HEK293 cells were transfected with shTRPC1 or control vector, as indicated, and used after 48 h for protein detection. C, Western blot showing the expression levels of TRPC1, which was significantly reduced in lysates from cells transfected with shTRPC1. Bottom panel, Western blot with anti-G actin antibody for protein loading control. D, HEK293 cells were transfected with shTRPC1 or control vector, as indicated, and then stimulated for 3 min with 10 μm ATP or left untreated and lysed. Samples were immunoprecipitated with anti-type I IP3R antibody and immunoprecipitates were analyzed by Western blotting using anti-Orai1 antibody (upper panel) and reprobed with anti-type I IP3R antibody (lower panel) as described under “Experimental Procedures.” The panels show results from one experiment representative of five. n.s., not significant; error bars, S.E.
Because TRPC3 plays a crucial role in interaction between Orai1 and type I IP3R, we investigated whether other TRPCs, such as TRPC1, would play such a role in this interaction. Our results indicate that TRPC1 expression silencing by using shTRPC1, which reduces TRPC1 expression as compared with control cells (Fig. 5C), was without effect on resting or ATP-induced association between Orai1 and type I IP3R (Fig. 5D). This finding indicates that TRPC1 is not involved in association between Orai1 and type I IP3R, and suggest a specific role for TRPC3 in mediation of this complex.
Interaction of Orai1 with Types II and III IP3 Receptors
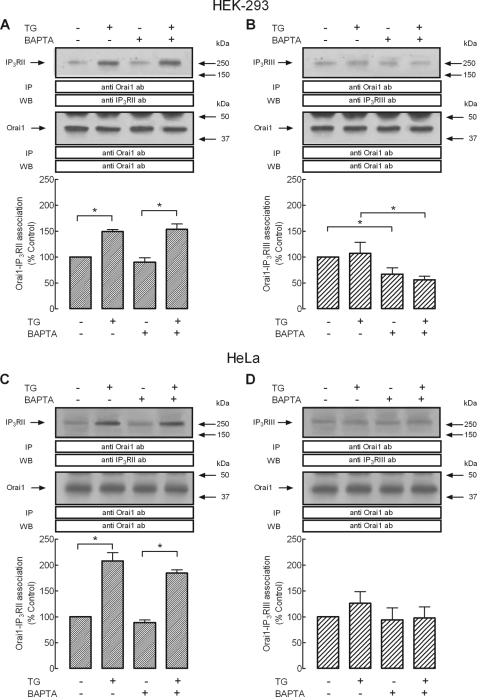
To investigate whether the role of TRPC3 is specific for interaction between Orai1 and type I IP3R and does not involve other IP3R isoforms, we performed a series of experiments to assess the possible association of Orai1 with types II and III IP3R in HEK293 cells. After immunoprecipitation with anti-Orai1 antibody, Western blotting revealed the presence of type II IP3R in samples from TG-treated cells and also a faint immunoreactive band over 250 kDa was detected in resting cells (Fig. 6A, upper panel, n = 6). Similar results were observed in HeLa cells (Fig. 6C, upper panel), thus suggesting that this might be a general event in human cells. TG-induced coupling between Orai1 and type II IP3R was independent of elevations in [Ca2+]i because it was also observed, without significant decrement, in dimethyl-BAPTA-loaded cells. Western blotting with anti-Orai1 antibody confirmed a similar content of this protein in all lanes (Fig. 6, A and C, lower panels). By contrast, although we observed detectable association between Orai1 and the type III IP3R at resting conditions both in HEK293 (Fig. 6B, upper panel) and HeLa (Fig. 6D, upper panel), we were unable to detect a significant increase in interaction between Orai1 and type III IP3R upon stimulation with TG in these cells (Fig. 6, B and D), even though we used the same experimental procedures followed to detect coupling between Orai1 and types I or II IP3R. It is noteworthy to mention that dimethyl- BAPTA loading reduced basal association between Orai1 and type III IP3R in HEK293 cells (Fig. 6B, upper panel), as reported above for the type I IP3R (Fig. 1A, upper panel). These findings indicate that although there is a small interaction between Orai1 and the three IP3R isoforms, agonists only enhanced the interaction between Orai1 and types I and II IP3R.
FIGURE 6.
Interaction of Orai1 with types II and III IP3R in HEK293 and HeLa cells. HEK and HeLa cells were either loaded with dimethyl-BAPTA or left treated, as indicated, and then suspended in medium containing 1 mm Ca2+ or Ca2+-free medium (BAPTA-loaded cells, 100 μm EGTA was added). Cells were then stimulated with TG (1 μm) for 3 min or left untreated and lysed. Whole cell lysates were immunoprecipitated (IP) with anti-Orai1 antibody and immunoprecipitates were subjected to 10% SDS-PAGE and subsequent Western blotting (WB) with specific anti-IP3RII (A and C) or anti-IP3RIII (B and D) antibodies. Membranes were reprobed with the anti-Orai1 antibody for protein loading control. The panel shows results from one experiment representative of five. Molecular masses indicated on the right were determined using molecular mass markers run in the same gel. Histograms represent the quantification of Orai1-IP3RII or Orai1-IP3RIII association under different experimental conditions. Results are expressed as mean ± S.E. and presented as percentage of control (non-stimulated cells not loaded with dimethyl-BAPTA). *, p < 0.05. Error bars, S.E.
Subsequently, we investigated whether TRPC3 expression silencing impairs the association between Orai1 and type II IP3R. We observed that treatment with 10 μm ATP resulted in a significant increase in association between Orai1 and type II IP3R (Fig. 7, upper panel, lanes 1 and 2; p < 0.05). TRPC3 expression silencing had a negligible effect, if any, on resting or a ATP-evoked increase in Orai1-type II IP3R association (Fig. 7, upper panel, lanes 3 and 4; p < 0.05). Western blotting with anti-type II IP3R confirmed a similar content of this protein in all lanes (Fig. 7, lower panels). Therefore, our results indicate that the effect of TRPC3 silencing on agonist-induced Ca2+ release is specific for interaction between Orai1 and type I IP3R.
FIGURE 7.
TRPC3 silencing does not alter the interaction between Orai1 and IP3RII in HEK cells. HEK293 cells were transfected with shTRPC3 or control vector, as indicated, and used after 48 h for protein detection. Cells were stimulated for 3 min with 10 μm ATP or left untreated and lysed. Samples were immunoprecipitated (IP) with anti-type II IP3R antibody and immunoprecipitates were analyzed by Western blotting (WB) using anti-Orai1 antibody (upper panel) and reprobed with anti-type II IP3R antibody (lower panel) as described under “Experimental Procedures.” The panels show results from one experiment representative of five. Histograms represent the quantification of Orai1-IP3RII association under different experimental conditions. Results are expressed as mean ± S.E. of six independent experiments and presented as percentage of control (cells transfected with control vector not treated with ATP). *, p < 0.05 compared to resting cells. Error bars, S.E.
DISCUSSION
TRPC3 proteins are among the first mammalian homologs of Drosophila TRP identified. The ability of TRPC3 to associate with a number of partner proteins has been reported to enable TRPC3 to participate in a variety of signals and transduction mechanisms (45). For instance, TRPC3 forming channels have been shown to be activated by diacylglycerol, although there is evidence suggesting that these channels can also be gated by IP3Rs through a conformational coupling mechanism (46, 47). Moreover, evidence suggests that IP3Rs are involved in translocation of TRPC3 proteins to the cell surface in response to agonist stimulation (48, 49). Reciprocally, recent studies have reported that TRPC3 is involved in modulation of Ca2+ release from the intracellular stores in different cell types, such as skeletal muscle cells (50) or HEK293 cells (33). The latter has been shown to be mediated by formation of a ternary complex, TRPC3-RACK1-IP3R, although the nature of the IP3R isoform involved has not yet been identified (33).
Our results report interaction between the type I IP3R and Ca2+-handling proteins Orai1, STIM1, TRPC3, and RACK1. This protein complex is dynamically regulated by agonists, such as ATP and CCh, and plays a relevant role in Ca2+ release from intracellular stores and also Ca2+ entry. These findings are based on the effect of TRPC3 expression silencing, which impairs association between type I IP3R and the proteins Orai1 and RACK1, the latter is in agreement with previous studies (33). In addition, TRPC3 expression silencing inhibits agonist-evoked Ca2+ mobilization.
IP3Rs have been reported to form macro signal complexes and function as a point of convergence for signaling cascades. A number of studies have reported the association between IP3Rs and the scaffold protein RACK1 (41, 51, 52), which plays a crucial role in agonist-evoked Ca2+ release from intracellular stores, thus suggesting that RACK1 is a physiologic regulator of agonist-induced Ca2+ release (41).
We have found that cell treatment with shTRPC3 has no detectable effect on TG-induced Ca2+ release and entry; this finding, together with the observation that TG was unable to induce association between type I IP3R and proteins Orai1, STIM1, TRPC3, and RACK1 in BAPTA-loaded cells, suggests that TRPC3 plays a crucial role in agonist-induced Ca2+ entry but not in SOCE in these cells. Our results indicate that functional Orai1 is not required for the TRPC3-type I IP3R association, which further supports that TRPC3 plays a central role in the Orai1-TRPC3-type I IP3R complex.
A major finding of this study is that TRPC3 mediates the interaction between Orai1 and type I IP3R, whereas we suggest that it does not mediate the association between Orai1 and type II IP3R. We show that disrupting TRPC3-Orai1 association by TRPC3 expression silencing impairs agonist-induced association between Orai1 and the type I IP3R and results in significant inhibition of agonist-induced Ca2+ release and entry. By contrast, we have found that other TRPC proteins, such as TRPC1, are not required for the Orai1-type I IP3R interaction. Our findings are in agreement with previous observations suggesting that TRPC3 mediates the interaction between IP3R and RACK1, which has been reported to enhance IP3-binding affinity and augment Ca2+ release (41). Recent studies have reported that impairment of TRPC3-RACK1 association by expressing mut-C3 or knocking down endogenous TRPC3 leads to attenuation of IP3-mediated Ca2+ release (33), which, together with our results, indicates that TRPC3 might be a point of convergence of distinct signals regulating the IP3R function.
Our results are consistent with previous studies reporting that TRPC proteins are involved in a dynamic interaction with IP3Rs and Ca2+ signaling protein complexes (53–55). In addition, TRPC3 has been reported to regulate type I ryanodine receptor in primary mouse skeletal muscle myoblasts/myotubes (56).
Cell stimulation by agonists has been reported to lead to dynamic changes in Ca2+ signaling proteins involving trafficking and activation of cation permeable channels, such as TRPC3 and Orai1 (35, 57, 58). There is a body of evidence suggesting that TRPC3 proteins and Orai1 are recruited into the plasma membrane in signaling complexes containing IP3Rs and other Ca2+-handling proteins (33, 48, 54, 57, 58). Consistent with this, we found the association between type I IP3R and Orai1, STIM1, TRPC3, and RACK1. In addition, impairment of the association of Orai1 with TRPC3 and the type I IP3R results in attenuation of agonist-evoked Ca2+ entry. Our results provide evidence supporting that the association between the type I IP3R and TRPC3, RACK1, Orai1, and STIM1 is not capacitative in nature because TG was unable to induce formation of this complex in the absence of rises in [Ca2+]i. In addition, impairment of this complex by shTRPC3 was without effect on TG-evoked SOCE. The role of STIM1 in non-capacitative receptor-regulated Ca2+ entry pathways, such as Ca2+ entry via arachidonate-regulated calcium (ARC) channels (59, 60) has previously been demonstrated. Our results reporting the association of STIM1 with type I IP3R upon stimulation with ATP or CCh also shows the involvement of STIM1 in ROCE, further suggesting that STIM1 might be a universal regulator of Ca2+ entry.
In conclusion, our results indicate that agonist stimulation leads to the formation of a protein complex involving Orai1, STIM1, TRPC3, RACK1, and type I IP3R, as well as Ca2+ release from the intracellular stores and Ca2+ entry from the extracellular medium. Here we report for the first time that disruption of association between Orai1 and TRPC3 prevents agonist-induced association of Orai1 with the type I IP3R, leading to attenuated agonist-induced Ca2+ release and Ca2+ entry. Together these findings suggest that TRPC3 mediates the functional coupling between Orai1 and the type I IP3R and, therefore, modulates agonist-stimulated Ca2+ mobilization.
Acknowledgment
We are extremely grateful to Dr. Indu Ambudkar for generously providing plasmids.
This work was supported by Spanish Ministry of Science and Innovation Grant BFI2007-60104. This work was authored, in whole or in part, by National Institutes of Health staff.
- [Ca2+]i
- intracellular free calcium concentration
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- inositol 1,4,5-trisphosphate receptor
- ROCE
- receptor-operated calcium entry
- SOCE
- store-operated calcium entry
- STIM1
- stromal interaction molecule 1
- TG
- thapsigargin
- TRP
- transient receptor potential
- DAPI
- 4′,6-diamidino-2-phenylindole
- CCh
- carbachol
- HEK
- human embryonic kidney
- shRNA
- short hairpin RNA
- BAPTA
- 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 2.Putney J. W., Bird G. S. (2008) J. Physiol. 586, 3055–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berridge M. J., Lipp P., Bootman M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 4.Putney J. W., Jr. (1986) Cell Calcium 7, 1–12 [DOI] [PubMed] [Google Scholar]
- 5.Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López J. J., Salido G. M., Pariente J. A., Rosado J. A. (2006) J. Biol. Chem. 281, 28254–28264 [DOI] [PubMed] [Google Scholar]
- 8.Spassova M. A., Soboloff J., He L. P., Xu W., Dziadek M. A., Gill D. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 4040–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 10.Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. (2006) Nat. Cell Biol. 8, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 11.Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr. (2006) J. Biol. Chem. 281, 24979–24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. (2006) J. Biol. Chem. 281, 20661–20665 [DOI] [PubMed] [Google Scholar]
- 13.Vig M., Beck A., Billingsley J. M., Lis A., Parvez S., Peinelt C., Koomoa D. L., Soboloff J., Gill D. L., Fleig A., Kinet J. P., Penner R. (2006) Curr. Biol. 16, 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruyama Y., Ogura T., Mio K., Kato K., Kaneko T., Kiyonaka S., Mori Y., Sato C. (2009) J. Biol. Chem. 284, 13676–13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Singh B. B., Ambudkar I. S. (2003) J. Biol. Chem. 278, 11337–11343 [DOI] [PubMed] [Google Scholar]
- 16.Soboloff J., Spassova M., Xu W., He L. P., Cuesta N., Gill D. L. (2005) J. Biol. Chem. 280, 39786–39794 [DOI] [PubMed] [Google Scholar]
- 17.Thebault S., Zholos A., Enfissi A., Slomianny C., Dewailly E., Roudbaraki M., Parys J., Prevarskaya N. (2005) J. Cell. Physiol. 204, 320–328 [DOI] [PubMed] [Google Scholar]
- 18.Ambudkar I. S., Ong H. L., Liu X., Bandyopadhyay B. C., Cheng K. T. (2007) Cell Calcium 42, 213–223 [DOI] [PubMed] [Google Scholar]
- 19.Jardin I., Lopez J. J., Salido G. M., Rosado J. A. (2008) J. Biol. Chem. 283, 25296–25304 [DOI] [PubMed] [Google Scholar]
- 20.Salido G. M., Sage S. O., Rosado J. A. (2009) Biochim. Biophys. Acta 1793, 223–230 [DOI] [PubMed] [Google Scholar]
- 21.Jardin I., Gómez L. J., Salido G. M., Rosado J. A. (2009) Biochem. J. 420, 267–276 [DOI] [PubMed] [Google Scholar]
- 22.Liao Y., Erxleben C., Abramowitz J., Flockerzi V., Zhu M. X., Armstrong D. L., Birnbaumer L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2895–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y., Plummer N. W., George M. D., Abramowitz J., Zhu M. X., Birnbaumer L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3202–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosado J. A., Sage S. O. (2001) Biochem. J. 356, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardín I., López J. J., Salido G. M., Rosado J. A. (2008) Cell. Signal. 20, 737–747 [DOI] [PubMed] [Google Scholar]
- 26.Kiselyov K., Xu X., Mozhayeva G., Kuo T., Pessah I., Mignery G., Zhu X., Birnbaumer L., Muallem S. (1998) Nature 396, 478–482 [DOI] [PubMed] [Google Scholar]
- 27.Yuan J. P., Kiselyov K., Shin D. M., Chen J., Shcheynikov N., Kang S. H., Dehoff M. H., Schwarz M. K., Seeburg P. H., Muallem S., Worley P. F. (2003) Cell 114, 777–789 [DOI] [PubMed] [Google Scholar]
- 28.Boulay G., Brown D. M., Qin N., Jiang M., Dietrich A., Zhu M. X., Chen Z., Birnbaumer M., Mikoshiba K., Birnbaumer L. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14955–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai K., Hamaide M. C., Debaix H., Gailly P., Wibo M., Morel N. (2008) Eur. J. Pharmacol. 583, 135–147 [DOI] [PubMed] [Google Scholar]
- 30.Zubov A. I., Kaznacheeva E. V., Nikolaev A. V., Alexeenko V. A., Kiselyov K., Muallem S., Mozhayeva G. N. (1999) J. Biol. Chem. 274, 25983–25985 [DOI] [PubMed] [Google Scholar]
- 31.Chang B. Y., Chiang M., Cartwright C. A. (2001) J. Biol. Chem. 276, 20346–20356 [DOI] [PubMed] [Google Scholar]
- 32.Miller L. D., Lee K. C., Mochly-Rosen D., Cartwright C. A. (2004) Oncogene 23, 5682–5686 [DOI] [PubMed] [Google Scholar]
- 33.Bandyopadhyay B. C., Ong H. L., Lockwich T. P., Liu X., Paria B. C., Singh B. B., Ambudkar I. S. (2008) J. Biol. Chem. 283, 32821–32830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong H. L., Cheng K. T., Liu X., Bandyopadhyay B. C., Paria B. C., Soboloff J., Pani B., Gwack Y., Srikanth S., Singh B. B., Gill D. L., Ambudkar I. S. (2007) J. Biol. Chem. 282, 9105–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodard G. E., Salido G. M., Rosado J. A. (2008) Am. J. Physiol. Cell Physiol. 294, C1323–C1331 [DOI] [PubMed] [Google Scholar]
- 36.Manders E. M., Stap J., Brakenhoff G. J., van Driel R., Aten J. A. (1992) J. Cell Sci. 103, 857–862 [DOI] [PubMed] [Google Scholar]
- 37.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 38.López J. J., Redondo P. C., Salido G. M., Pariente J. A., Rosado J. A. (2006) Cell. Signal. 18, 373–381 [DOI] [PubMed] [Google Scholar]
- 39.Rosado J. A., Lopez J. J., Gomez-Arteta E., Redondo P. C., Salido G. M., Pariente J. A. (2006) J. Cell. Physiol. 209, 142–152 [DOI] [PubMed] [Google Scholar]
- 40.Thastrup O., Foder B., Scharff O. (1987) Biochem. Biophys. Res. Commun. 142, 654–660 [DOI] [PubMed] [Google Scholar]
- 41.Patterson R. L., van Rossum D. B., Barrow R. K., Snyder S. H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2328–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derler I., Fahrner M., Muik M., Lackner B., Schindl R., Groschner K., Romanin C. (2009) J. Biol. Chem. 284, 24933–24938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Y., Erxleben C., Yildirim E., Abramowitz J., Armstrong D. L., Birnbaumer L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4682–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zagranichnaya T. K., Wu X., Villereal M. L. (2005) J. Biol. Chem. 280, 29559–29569 [DOI] [PubMed] [Google Scholar]
- 45.Groschner K., Rosker C. (2005) Naunyn-Schmiedebergs Arch. Pharmacol. 371, 251–256 [DOI] [PubMed] [Google Scholar]
- 46.Putney J. W., Jr., Trebak M., Vazquez G., Wedel B., Bird G. S. (2004) Novartis Found. Symp. 258, 123–133 [PubMed] [Google Scholar]
- 47.Xi Q., Adebiyi A., Zhao G., Chapman K. E., Waters C. M., Hassid A., Jaggar J. H. (2008) Circ. Res. 102, 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J. Y., Zeng W., Kiselyov K., Yuan J. P., Dehoff M. H., Mikoshiba K., Worley P. F., Muallem S. (2006) J. Biol. Chem. 281, 32540–32549 [DOI] [PubMed] [Google Scholar]
- 49.Wedel B. J., Vazquez G., McKay R. R., St. J. Bird G., Putney J. W., Jr. (2003) J. Biol. Chem. 278, 25758–25765 [DOI] [PubMed] [Google Scholar]
- 50.Woo J. S., Kim do H., Allen P. D., Lee E. H. (2008) Biochem. J. 411, 399–405 [DOI] [PubMed] [Google Scholar]
- 51.Bosanac I., Yamazaki H., Matsu-Ura T., Michikawa T., Mikoshiba K., Ikura M. (2005) Mol. Cell 17, 193–203 [DOI] [PubMed] [Google Scholar]
- 52.Sklan E. H., Podoly E., Soreq H. (2006) Prog. Neurobiol. 78, 117–134 [DOI] [PubMed] [Google Scholar]
- 53.Kim J. Y., Saffen D. (2005) J. Biol. Chem. 280, 32035–32047 [DOI] [PubMed] [Google Scholar]
- 54.Redondo P. C., Jardin I., Lopez J. J., Salido G. M., Rosado J. A. (2008) Biochim. Biophys. Acta 1783, 1163–1176 [DOI] [PubMed] [Google Scholar]
- 55.Rosado J. A., Sage S. O. (2000) Biochem. J. 350, 631–635 [PMC free article] [PubMed] [Google Scholar]
- 56.Lee E. H., Cherednichenko G., Pessah I. N., Allen P. D. (2006) J. Biol. Chem. 281, 10042–10048 [DOI] [PubMed] [Google Scholar]
- 57.Singh B. B., Lockwich T. P., Bandyopadhyay B. C., Liu X., Bollimuntha S., Brazer S. C., Combs C., Das S., Leenders A. G., Sheng Z. H., Knepper M. A., Ambudkar S. V., Ambudkar I. S. (2004) Mol. Cell 15, 635–646 [DOI] [PubMed] [Google Scholar]
- 58.Soboloff J., Spassova M., Hewavitharana T., He L. P., Luncsford P., Xu W., Venkatachalam K., van Rossum D., Patterson R. L., Gill D. L. (2007) Handb. Exp. Pharmacol. 179, 575–591 [DOI] [PubMed] [Google Scholar]
- 59.Mignen O., Thompson J. L., Shuttleworth T. J. (2007) J. Physiol. 579, 703–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shuttleworth T. J., Thompson J. L., Mignen O. (2007) Cell Calcium 42, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]