Abstract
Rhodopsin is an extensively studied member of the G protein-coupled receptors (GPCRs). Although rhodopsin shares many features with the other GPCRs, it exhibits unique features as a photoreceptor molecule. A hallmark in the molecular structure of rhodopsin is the covalently bound chromophore that regulates the activity of the receptor acting as an agonist or inverse agonist. Here we show the pivotal role of the covalent bond between the retinal chromophore and the lysine residue at position 296 in the activation pathway of bovine rhodopsin, by use of a rhodopsin mutant K296G reconstituted with retinylidene Schiff bases. Our results show that photoreceptive functions of rhodopsin, such as regiospecific photoisomerization of the ligand, and its quantum yield were not affected by the absence of the covalent bond, whereas the activation mechanism triggered by photoisomerization of the retinal was severely affected. Furthermore, our results show that an active state similar to the Meta-II intermediate of wild-type rhodopsin did not form in the bleaching process of this mutant, although it exhibited relatively weak G protein activity after light irradiation because of an increased basal activity of the receptor. We propose that the covalent bond is required for transmitting structural changes from the photoisomerized agonist to the receptor and that the covalent bond forcibly keeps the low affinity agonist in the receptor, resulting in a more efficient G protein activation.
Keywords: G Proteins/Coupled Receptors (GPCR), Receptors/Photoreceptors, Signal Transduction/G proteins, Vision/Photoreceptors, Vision/Rhodopsin, Rhodopsin, Opsin, Retinal
Introduction
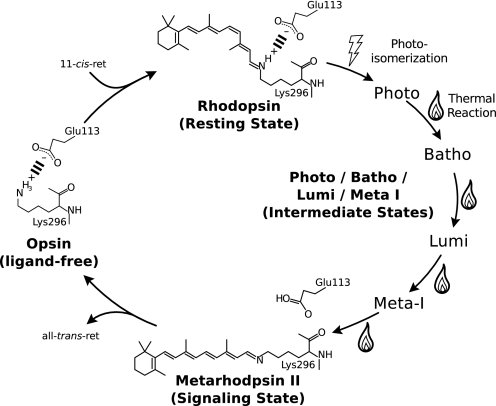
Rhodopsin is the photoreceptor molecule present in vertebrate eyes, which senses light stimuli and initiates a signaling cascade mediated by the G protein. Rhodopsin is a member of the family-A G protein-coupled receptors (GPCRs),3 and therefore it is presumed to have diverged from a diffusible ligand binding GPCR (1). However, in contrast with other GPCRs where diffusible ligands interact with their receptors strictly by noncovalent interactions, the binding of the retinal to opsin involves a covalent bond between the receptor and the ligand. Rhodopsin has the 11-cis-retinal chromophore covalently bound in its protein moiety acting as an inverse agonist in the dark, and light absorption causes the generation of the agonist all-trans-retinal through cis-trans photoisomerization of the chromophore (2).
The covalent bond is formed between the aldehyde group of the retinal and the ϵ-amino group of lysine 2964 located at the transmembrane helix VII of rhodopsin, through a protonated Schiff base linkage (3, 4). Formation of the protonated Schiff base is an important structural feature for photoreception, because the protonation of the Schiff base causes electron delocalization of the chromophore, resulting in a red shift of the absorption spectrum of rhodopsin, which allows it to absorb visible light (5). Moreover, the positive charge of Lys-296 and the negative charge of Glu-113 form an ionic pair, thus forming an intramolecular switch known as the opsin lock, which locks the receptor in an inactive state (6, 7). This ionic interaction is maintained in the 11-cis-retinal bound dark state by the protonated Schiff base and Glu-113; the activation of the receptor involves the unpairing of this salt bridge by the proton transfer from the Schiff base to Glu-113, which is observed as a big spectral shift to 380 nm because of the deprotonation of the Schiff base (Fig. 1) (8, 9). Although mutations at Lys-296 or Glu-113 that disrupt the opsin lock cause an increase of the intrinsic activation of the opsin, this constitutive activity is effectively suppressed by the binding of 11-cis-retinal (6, 10, 11).
FIGURE 1.
The photocycle of bovine rhodopsin. In the ligand-free opsin state, the rhodopsin basal activity is diminished by the ionic pairing, called the opsin lock, a salt bridge formed from the positive charge of Lys-296 and the negative charge of Glu-113 (6, 7). The 11-cis-retinal chromophore functions as an inverse agonist and further suppresses the activity of the receptor, until photon absorption causes stereospecific isomerization of the retinal to its all-trans form and initiates a chain of structural changes (thermal reaction) that form the signaling state. The signaling state is thermally unstable, and the retinal is eventually expelled from the receptor, which quickly reforms the opsin lock to shut off the receptor.
Retinal photoisomerization in rhodopsin is an extremely rapid event (200 fs), taking place in a time span so short that amino acid residues cannot immediately follow (12–14). Consequently, the photoisomerized retinal is in a highly twisted structure, and it is estimated that about 60% of the photon energy (∼30 kcal) is stored as potential energy in its twisted conformation (15, 16). Relaxation of the twisted retinal releases this potential energy, thus causing structural changes to the neighboring amino acid residues, which in turn leads to the formation of the signaling state of the photoreceptor molecule. Therefore, it is likely that the covalent bond plays a role in maintaining the twisted structure of the chromophore and efficiently transmitting structural changes. However, it was reported that the disruption of the covalent bond between the chromophore and the opsin had almost no effect on the G protein activation ability of rhodopsin (10, 11). Therefore, the detailed investigation of the photochemical and subsequent thermal reactions of rhodopsin, as well as the characterization of the signaling state are important for furthering our understanding of the molecular mechanism of light absorption and G protein activation by rhodopsin.
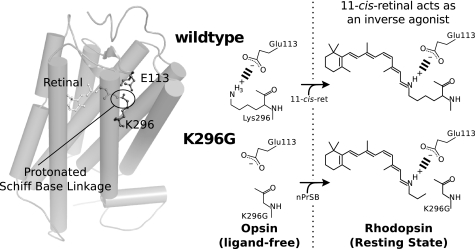
Following previous studies, we used the bovine rhodopsin mutant K296G with retinylidene Schiff bases and constructed artificial photoreceptor molecules (10). This system is ideal for the study of the covalent bond, because it preserves the Schiff base and its interaction with the counterion while disrupting the covalent bond (see Fig. 2). The resulting photoreceptor molecule contains a ligand bound by noncovalent interactions resembling a diffusible ligand binding GPCR. Our findings clearly show that the rhodopsin mutant K296G, lacking the covalent bond, exhibited absorption spectrum, photosensitivity, and low temperature photochemical reactions similar to those of the wild-type rhodopsin. In contrast, thermal reactions following the photoreaction were severely affected. Moreover, our detailed examination of the G protein activation by the K296G rhodopsin clearly showed that an active state similar to the Meta-II intermediate of wild-type rhodopsin did not form in the bleaching process of this mutant, although the mutant exhibited an apparent light-dependent G protein activity because of the constitutive activity caused by disrupting the opsin lock. The role of the covalent bond will be discussed in relation to the activation mechanism of the G protein by rhodopsin.
FIGURE 2.
Formation of a pigment from K296G without the covalent bond between retinal and opsin. The figure shows a schematic representation of retinal binding in K296G compared with wild-type rhodopsin. The figure shows how K296G opsin can be reconstituted with retinylidene-n-propylamine Schiff base (nPrSB). K296G can form similar visual pigments with retinylidene-ethylamine Schiff base (EtSB) and retinylidene-methylamine Schiff base (MeSB).
EXPERIMENTAL PROCEDURES
Preparation of the Alkyl-retinal Schiff Bases
Retinylidene Schiff bases with n-propylamine (nPrSB) and ethylamine (EtSB) were synthesized as previously described (10). MeSB was prepared in a similar way to EtSB, by reacting methyl-ammonium hydrochloride with 11-cis-retinal in ethanol in the presence of triethylamine. Although these retinylidene Schiff bases were relatively stable in ethanol, they were unstable in aqueous solutions because of hydrolysis of the Schiff base (17).
Heterologous Expression Rhodopsin Pigments
Bovine rhodopsin was heterologously expressed in HEK293 cells, and the expressed apoprotein was incubated with an appropriate chromophore as previously described (10, 18). Wild-type rhodopsin was incubated with 11-cis-retinal, and the mutant pigment K296G was incubated with retinylidene Schiff bases of varying lengths to form a rhodopsin pigment. The pigments were extracted by using 1% n-dodecyl-β-d-maltoside (DM) in buffer PM (50 mm HEPES, 140 mm NaCl, 3 mm MgCl2, pH adjusted to 7.0 at 273 K, unless otherwise indicated) and the DM extract was incubated with rho 1D4 antibody-agarose overnight. After washing with 0.02% DM/PM buffer, rhodopsin was eluted with the same buffer containing the C-terminal octadecapeptide of bovine rhodopsin. CHAPS/PC samples were purified likewise; however, instead of 0.02% DM 0.75% CHAPS mixed with 1 mg/ml of l-α-phosphatidylcholine from egg yolk (PC) was used for washing and elution of the pigments. CHAPS/PC-purified rhodopsin was used for the G protein α-subunit C-terminal high affinity peptide binding experiment, because this detergent condition favors the equilibrium between the active state and its predecessor. The rest of the experiments were conducted on pigments purified in DM, which favors the formation the signaling state over its precursor. For the G protein activity assays, we used membrane fractions obtained by 50% sucrose floatation; the membrane fractions in the supernatant were then isolated by diluting the sucrose to 25% and centrifuging, after which they were washed and suspended in buffer PM for G protein activity assays.
Photosensitivity
Photosensitivity of purified pigments was determined by UV-vis spectrophotometry using a Hitachi U-4100 spectrophotometer as previously described (19). Briefly, the sample was successively irradiated near the absorption maxima with an interference filter (500 nm; half-bandwidth, 5 nm; Optical Coatings Japan), in the presence of hydroxylamine (50 mm for the wild type and 20 mm for mutant pigments) at 273 K. The amount of remaining pigment after each irradiation was computed and plotted against the number of photons that were irradiated, then fitted with an exponential function to obtain the photosensitivity. The data were corrected for the differences in the absorbance maxima of the pigments and their absorbance at 500 nm, although these corrections were minor. We also estimated the extent of the correction for hydroxylamine bleach; however, under our experimental conditions, dark bleaching due to hydroxylamine was less than 1% of the total pigment and therefore negligible.
Chromophore Extraction and HPLC Analysis
Isomeric composition of the retinal was analyzed by high-performance liquid chromatography (HPLC) using a Shimadzu LC-10AT equipped with a silica column (150 × 6.0 mm, A-012–3; YMC) as previously described (19). Briefly, the retinaloximes of light-irradiated (Y52 cutoff filter; Toshiba) and non-irradiated purified samples were extracted, and HPLC analysis was performed (20, 21). Retinal composition was calculated from the area of the peaks and the absorption coefficients previously reported (22).
Absorption Coefficient
Acid denaturation of visual pigments was carried out to estimate the absorption coefficient of mutant pigments relative to the wild-type rhodopsin (19, 23, 24). After recording the dark state spectrum of purified rhodopsin, we added a small amount of 2 n HCl to achieve a final pH of <1.5 and corrected the denatured spectrum for dilution. Addition of the acid denatures the pigments and traps the chromophore as protonated Schiff base with a peak around 440 nm. All retinal-based pigments produce an identical acid denatured spectrum originating from protonated random Schiff bases, and therefore the absorption coefficient was estimated by comparing the ratio of the peaks before and after denaturation with that of the wild-type rhodopsin (17, 25).
Photoconversion
Low temperature spectroscopy was carried out as previously described (26). Rhodopsin samples were mixed with 66% glycerol (w/v) to avoid opalescence.
The photoconversion of rhodopsin, bathorhodopsin, and isorhodopsin was described by Yoshizawa and Wald (27). Briefly, rhodopsin cooled to liquid nitrogen temperature is irradiated with 436-nm light (436-nm interference filter; half-bandwidth, 5.7 nm; Optical Coatings Japan) to create a photo-steady state mixture of rhodopsin, bathorhodopsin, and isorhodopsin, which we call PSS (photo-steady state) 1. The mixture is then irradiated with light <610 nm (R63 cutoff filter; Toshiba) to photoconvert bathorhodopsin to rhodopsin and create PSS 2. The sample is further irradiated with light <540 nm (O56 cutoff filter; Toshiba) to photoconvert rhodopsin to isorhodopsin via bathorhodopsin. Finally the sample is irradiated with 436 nm light again to create the initial mixture PSS 1.
Thermal Reaction
After the photoconversion, we gradually warmed the sample at a constant rate (1 K/5 min) and observed the different intermediate states of activation. Whereas rhodopsin and isorhodopsin remain in the same state, bathorhodopsin undergoes a thermal reaction marked by the formation of lumirhodopsin (Lumi), metarhodopsin-I (Meta-I), and the active state metarhodopsin-II (Meta-II). The transition temperatures at which one intermediate transforms into another can be clearly distinguished by their absorption maxima, and they mark the temperature stability ranges of the intermediates (27). We compared the transition temperatures for the wild type and mutant pigments.
We corrected our data to compare spectra taken at different temperatures due to baseline changes because of the temperature change and the phase changes. To correct for such baseline changes we fitted our data with a polynomial function of 4 terms (we used WaveMatrics Igor Pro Version 6 for all of our spectroscopic analyses). We took the fitted curve of the spectra at 273 K as a reference and fitted all other spectra with the polynomial function and then extracted the difference between the two curves. This procedure allowed us to compare spectra at different temperatures; however, the baseline changes were substantial, and the corrections were not 100% accurate; therefore, subtle changes in the spectra may not have been detected.
G Protein Binding
We used high affinity peptides derived from the C terminus of Gtα to assess the binding ability of the pigments by monitoring the formation of extra-Meta-II (28, 29). Time-resolved data were obtained as previously indicated (30). Measurements were recorded on a CCD spectrophotometer (Hamamatsu Photonics Co., Ltd), capable of continuously recording spectra (600–300 nm) with a wavelength resolution of 1.6 nm at time intervals of 9.7 ms at 253 K. Samples were prepared with 30% glycerol (w/v) to avoid freezing.
Measurement of Retinal Release
The intrinsic tryptophan fluorescence emission difference between the reconstituted pigment, and the opsin state was used to observe the release of the chromophore (31). We employed a photon-counting system (Hamamatsu Photonics Co., Ltd H7360 Photon Counting Head) to obtain high time resolution data. Samples were excited with the Xenon lamp of a Jasco J600 CD spectrometer, and fluorescence changes were recorded by monitoring the photon counts at a right angle with the excitation beam, with the photon counting head placed after a UV band pass filter (U360). A flash lamp was placed in front of the excitation beam, and samples were irradiated with a cutoff filter (Y52 cutoff filter; Toshiba), which activated about 50% of the rhodopsin in the sample. Measurements were taken at 288 K with 0.5 μm of rhodopsin purified in 0.02% DM at pH 6.5. Excitation light was kept low to avoid bleaching of the pigments.
G Protein Activation
The ability of visual pigments and of their opsins to activate Gt was assayed by radionucleotide filter binding assay, which measures the amount of GDP/GTPγS exchanged by the G protein as previously described (32). The buffer composition of the mixture in our experiments was 50 mm HEPES (pH 6.5), 3 mm MgCl2, 140 mm NaCl, 1 mm dithiothreitol, and 1 μm [35S]GTPγS. For the detergent-solubilized assays, an aliquot (15 μl) of DM-purified (final concentration 0.01% DM) rhodopsin (final concentration 12.5 nm) containing Gt (final concentration 600 nm) was incubated at 273 K in the dark or irradiated with orange light (Y52 cutoff filter for the wild type or Y51 for the mutant nPrSB; Toshiba), and subsequently mixed with 5 μl of GTPγS solution to initiate the GDP/GTPγS exchange. After 1 or 2 min of incubation at 288 K, 200 μl of stop solution (20 mm TrisCl, pH 7.4/100 mm NaCl/25 mm MgCl2/1 μm GTPγS/4 μm GDP) was added to the mixture. The mixture was then filtered through a nitrocellulose membrane, and it was washed three times with 200 μl of wash solution (20 mm Tris-Cl, pH 7.4/100 mm NaCl/25 mm MgCl2). The amount of GTPγS was quantitated by assaying the membrane filter with liquid scintillation counter. Cell membrane fractions obtained by 50% sucrose floatation of the transfected cells were likewise assayed to obtain data on membrane environment. After trying different rhodopsin concentrations of membrane fractions, we decided that the best results were obtained with 50 nm wild-type rhodopsin and 5 nm K296G.
To measure the opsin activity, we incubated rhodopsin with hydroxylamine (final concentrations: 22.2 mm for detergent-solubilized sample and 6.25 mm for the membrane fraction sample) and observed the effect of hydroxylamine on the light-dependent G protein activity. Hydroxylamine-containing samples were incubated after light irradiation, and GTPγS solution was added at a selected time. The GDP/GTPγS exchange reaction was terminated after a lapse of 2 min.
RESULTS AND DISCUSSION
Effect of K296G on the Quantum Yield
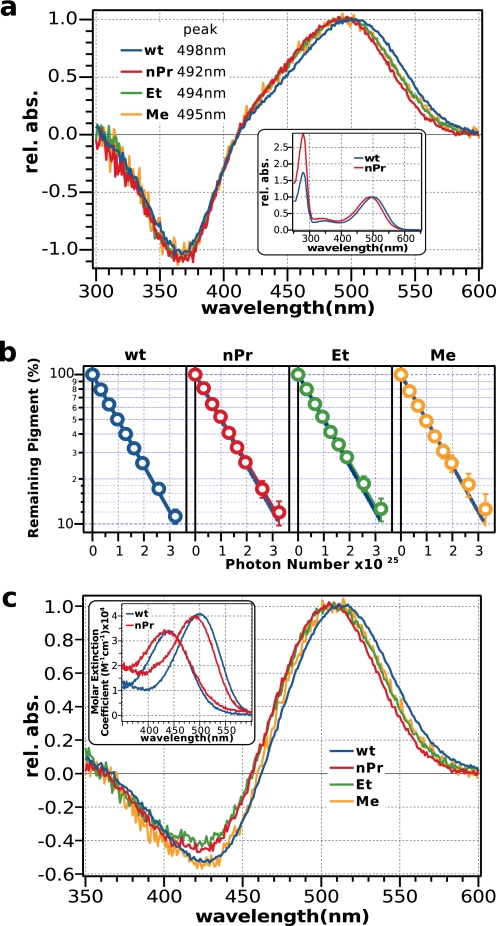
Fig. 4a shows that the difference spectra of K296G pigments before and after irradiation are similar with the wild-type rhodopsin, albeit with characteristic absorbance maxima for each pigment. The inset shows the absolute spectra of K296G/nPrSB in comparison to the wild type as an example. A difference in the peak at 280 nm is evident because of the impurities in the mutant sample that cannot be eliminated because of its low expression level and reconstitution rate. Photosensitivity measurements of these pigments indicate that they have identical photosensitivities (Fig. 4b). Also, HPLC analysis of the retinal conformation of K296G/nPrSB confirms that 11-cis-retinal is photoisomerized to all-trans-retinal (Fig. 3).
FIGURE 4.
Spectroscopic analyses of K296G pigments. a, difference spectra of K296G pigments purified in DM; nPrSB (red), EtSB (green), and MeSB (yellow) relative to the wild type (blue). The inset shows the absolute spectra of K296G/nPrSB (red) purified in CHAPS/PC. b, photosensitivities of K296G pigments relative to the wild-type rhodopsin. The error bars represent the deviation from two independent measurements, and the blue dotted line represents the photosensitivity of the wild type. c, difference spectra of acid denaturation of K296G pigments relative to the wild type. The inset shows the absolute spectra for K296G/nPrSB (red). The addition of acid produced a denatured pigment with a peak at 437 nm. The pH after adding the acid was <1.5. Denaturation of the wild type (blue) is shown for comparison.
FIGURE 3.
Isomeric composition of retinal before and after light irradiation. Retinal composition before and after light stimulation shows that the main reaction in K296G/nPrSB is cis-trans isomerization of the 11-cis double bond similar to the wild-type rhodopsin. The mutant shows some all-trans isomers before light irradiation, presumably due to the excess chromophore added to reconstitute the pigment. It also shows some formation of 13-cis isomer after light irradiation; however, its contribution is small.
To calculate the quantum yield of the cis-trans photoisomerization, we estimated the absorption coefficients of K296G pigments by acid denaturation. Although the K296G mutant lacks the lysine residue that forms the protonated random Schiff base, it is possible to estimate the absorption coefficient of K296G pigments by acid denaturation, because the retinylidene Schiff base compensates for the lysine residue. Moreover, K296G pigments have similar spectral properties to the wild type in the dark, and therefore we can estimate the absorption coefficient of K296G pigments from the difference spectra before and after acid denaturation. Fig. 4c shows that the difference spectra of acid denaturation of K296G pigments are almost identical to the wild type. Therefore, we assumed that the absorption coefficient is the same as the wild type. Assuming that the absorption coefficients are not affected by the disruption of the covalent bond, the measured photosensitivity becomes directly proportional to the quantum yield, revealing that K296G pigments and the wild type have identical isomerization efficiencies (Table 1).
TABLE 1.
Summary of the photosensitivities and quantum yields of the pigments
| Rhodopsin | K296G/nPrSB | K296G/EtSB | K296G/MeSB | |
|---|---|---|---|---|
| Photosensitivity | 1.00 ± 0.02 | 0.97 ± 0.03 | 0.97 ± 0.03 | 1.00 ± 0.04 |
| Quantum yielda | 0.65 ± 0.01 | 0.63 ± 0.02 | 0.63 ± 0.02 | 0.65 ± 0.03 |
a The quantum yield was computed from the photosensitivity and the molar extinction coefficient, which was assumed to be the same for all pigments.
Photoconversion
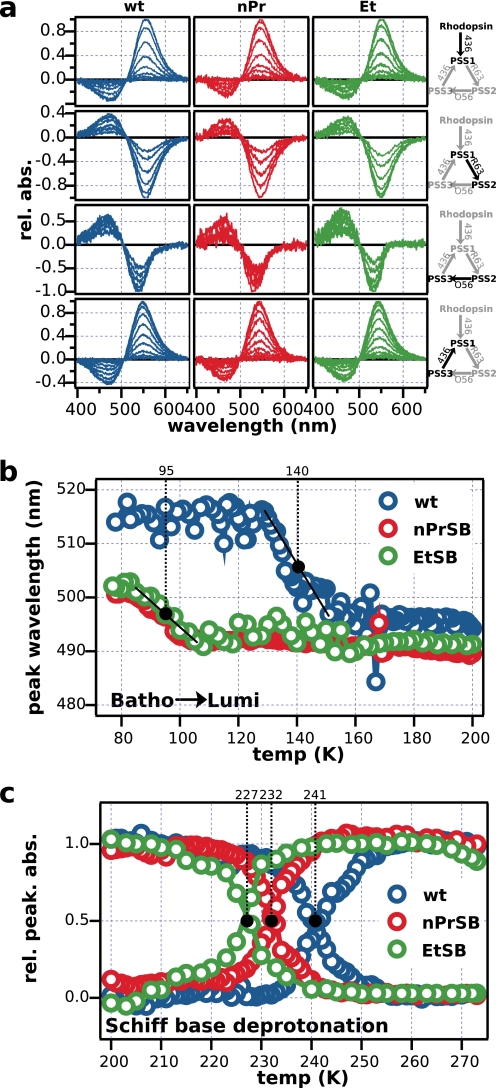
At liquid nitrogen temperature, light irradiation of rhodopsin only results in the geometric isomerization of the chromophore, leaving the apoprotein relatively unaltered (27). We monitored these changes by photoconverting retinal isomers to characterize the photochemistry of K296G. Fig. 5a shows the normalized difference spectra of the photoconversion. Each figure shows the spectral change of a mutant pigment as it moves toward a PSS, and the respective change for the wild-type rhodopsin is shown for comparison. Overall, the photoconversion seems to be homologous and the disruption of the covalent bond seems to be irrelevant in these reactions. All spectra are very similar to the wild type, varying mostly in the position of the peaks, because the pigments have slightly different absorption maxima. These results corroborate photosensitivity measurements and reveal that the behaviors of photoproducts bathorhodopsin and isorhodopsin of K296G/nPrSB and K296G/EtSB are very similar to the wild-type rhodopsin, manifesting that even without the covalent bond rhodopsin, bathorhodopsin, and isorhodopsin are freely interconvertible just like in the wild type (27).
FIGURE 5.
Low temperature spectroscopy of K296G. a, kinetics of the interconversion of rhodopsin, bathorhodopsin, and isorhodopsin at 77 K. The scheme on the right shows the filters used to create different photosteady states. Light was initially irradiated for 5 s, then the irradiation time was incremented by a factor of two for every irradiation (e.g. 5 s, 10 s, 20 s, 40 s, etc.) until pigments reached a photo-steady state. b, thermal reaction subsequent to photoconversion. The position of the absorbance maximum was monitored as the photoexcited pigments were gradually heated up. Black dots indicate the midpoint temperature in the transition from bathorhodopsin to lumirhodopsin, as the temperature was increased at a fixed rate. c, absorbance value of the peaks of protonated and deprotonated photoproducts was monitored likewise. As the protonated photoproduct decays, a deprotonated photoproduct is formed. Black dots indicate the temperatures at which peaks were at their half-values. In the wild type, this indicates the transition from Meta-I to Meta-II. Similar changes were observed in K296G/nPrSB and EtSB, with earlier transitions observed in K296G.
Thermal Reaction
After the photoconversion at 77 K, we gradually warmed up the sample, and observed the spectral changes that reflected the formation of intermediate states as the photoexcited pigment thermally relaxed and underwent structural changes. Fig. 5b shows the position of the peaks, and Fig. 5c shows the relative absorbances as samples were warmed up at a constant rate from 77 to 273 K. Our wild-type control is in good agreement with previous experiments (27). On the other hand, K296G pigments show a much faster transition from bathorhodopsin to lumirhodopsin, with a difference in transition temperature of ∼40 degrees. Differences in the peak wavelength between the wild type and the mutant pigments reflect the differences of the peak wavelengths in the dark state.
Although subsequent thermal reactions from lumirhodopsin to meta-I were not clearly detected, Fig. 5c shows the transition from the protonated state to the deprotonated state. Deprotonation of the Schiff base, which in the wild type corresponds to the transition from Meta-I to Meta-II, was clearly observed as a decay of the peak in the visible region and formation of a peak at 380 nm. The same deprotonation event was observed for the mutant with faster transitions; curiously, these transitions were affected by the length of the retinylidene Schiff base, showing lower thermal stability as the retinylidene Schiff base alkyl tail is made shorter. Presumably, potential energy stored in the twisted structure of the chromophore is released much faster in the absence of the covalent bond. These results suggest that in contrast with the unaltered photochemical properties of the pigments lacking the covalent bond, the thermal relaxation is severely affected, destabilizing the intermediates that lack the covalent bond.
Extra Meta-II Formation
The severely affected thermal reaction revealed by low temperature experiments suggested the possibility that the formation and thermal stability of the signaling state Meta-II are affected by the disruption of the covalent bond. To test this hypothesis, we assumed that the deprotonated spectral species observed in the mutant pigments is Meta-II and analyzed the kinetics of photobleaching in the presence and absence of a high affinity C-terminal peptide of Gtα to monitor the change in the formation of Meta-II (28, 29). The experiment was carried out at different temperatures, and 253 K was determined to be the optimum temperature to monitor the changes in K296G/nPrSB. All samples were adjusted to pH 7.5 ± 0.05 at 273 K, a pH that favors the formations of Meta-I over Meta-II in our detergent conditions (0.75% CHAPS mixed with 1 mg/ml of PC).
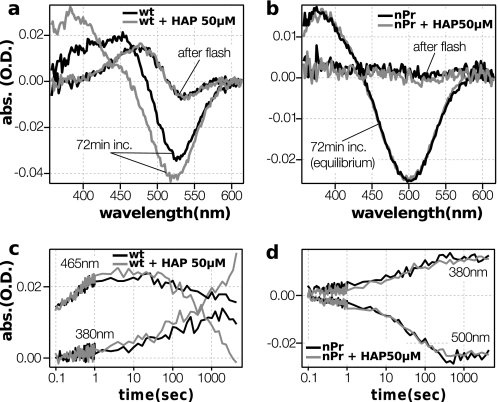
Fig. 6 shows the difference spectra of pigments before and after the flash irradiation, and the time course of the reaction at key wavelengths. The wild-type rhodopsin manifests Meta-I formation immediately after the flash, and a subsequent transition to Meta-II. The presence of the high affinity peptide enhances the transition from Meta-I to Meta-II by binding and stabilizing the deprotonated species (29). The reaction of K296G/nPrSB is in sharp contrast with the wild type. Spectra immediately after the flash show almost no change, indicating that this photoproduct has similar absorption to the dark state. This intermediate then decays and forms a product that absorbs at around 380 nm. Had the high affinity peptides bound to and stabilized a meta-II like conformation, a change in the kinetics or the final equilibrium would have been expected; however the high affinity peptides had no effect in the reaction kinetics or in the spectrum of the final product, suggesting that the peptide does not interact with these spectral species. The difference spectra after 72 min resembles the simple difference spectra of the dark subtracted from the spectra with a peak at around 380 nm, which can be derived either from Meta-II or from the mixture of free retinylidene Schiff base and hydrolyzed free retinal. This led us to consider the possibility that the 380-nm product was in fact the spectrum of the mixture of retinylidene Schiff base and its hydrolyzed retinal detached from the Schiff base, rather than the signaling state Meta-II.
FIGURE 6.
Kinetics of the bleaching process in K296G/nPrSB and wild-type rhodopsin. a and b, effect of the high affinity C-terminal peptide of Gtα on the formation of Meta-II (CHAPS/PC purified pigments, 273 K, pH 7.6, 50 μm high affinity peptides). The difference spectra of pigments immediately after and 72 min after light excitation, without (black lines) and with (gray lines) high affinity peptides. c and d, time course of the absorbances at 380, 465, and 500 nm. The wild type exhibits a classic behavior, producing more Meta-II in the presence of high affinity peptides. On the other hand, the spectra and the kinetics of K296G/nPrSB are completely unaffected by the high affinity peptides.
Retinal Release
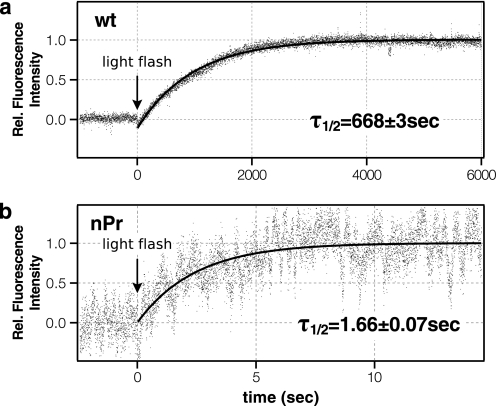
Fig. 7 shows the time course of retinal release as monitored by the fluorescence emission change after flash light irradiation in the wild-type rhodopsin and K296G/nPrSB. The decay rate of the wild-type rhodopsin is slightly faster than the accepted lifetime of meta-II in ROS samples but reasonable for a detergent (DM)-solubilized pigment. On the other hand, the mutant K296G shows an immediate increase in fluorescence after light bleaching with a rate constant 400 times faster than the wild type, suggesting that the retinal is immediately expelled from the receptor. The elevated release rate of the photoisomerized retinal indicates that the covalent bond is required to forcibly keep the low affinity agonist in the receptor.
FIGURE 7.
Change of intrinsic fluorescence after flash light irradiation at 288 K of the wild type (a) and K296G/nPrSB (b). The data were fitted with a single exponential function, and the lifetime of retinal release was estimated. It is of note that the noise is much more prominent in K296G/nPrSB because of the difference in time resolution (1 s for the wild-type rhodopsin and 2 ms for K296G/nPrSB).
G Protein Activation
Fluorescent measurements and high affinity peptides experiments suggest that the photoisomerized retinal is immediately expelled, and K296G does not form a signaling state similar to Meta-II. However, past studies have reported the light-induced G protein activity of this mutant (10, 11). We therefore measured the G protein activity of the K296G mutant opsin to assess the formation of a signaling state.
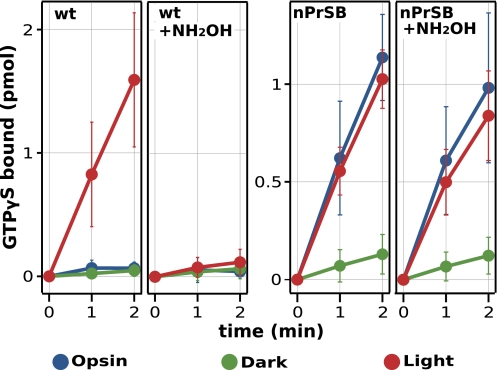
To obtain measurements of the opsin activity as well as the activity of the reconstituted pigment, we measured the activity in a membrane environment. Fig. 8 shows that K296G has a significant basal (opsin) activity compared with the wild type, whereas dark activity is largely suppressed by the binding of retinal. It is of note that the basal opsin activity and the light-dependent activity of K296G are very similar. The figure also shows the effect of hydroxylamine on activation. The active state is more accessible to hydroxylamine, whereas the dark state or the opsin state is not significantly affected (33). It is clear that K296G/nPrSB has a significant component in the presence of hydroxylamine, whereas wild-type activity is suppressed by it.
FIGURE 8.
Time course of G protein activity of membrane fraction expressing wild-type rhodopsin and K296G/nPrSB. The G protein activation ability of the opsin (blue), the dark state pigment (green), and the light-irradiated pigment (red) were assayed. The assay was carried out at 288 K in the presence of 600 nm Gt. Receptor concentrations were 50 nm for the wild type and 5 nm for the mutant K296G. The same measurements were repeated in the presence of 6.25 mm hydroxylamine. Error bars represent the S.D. of five independent measurements.
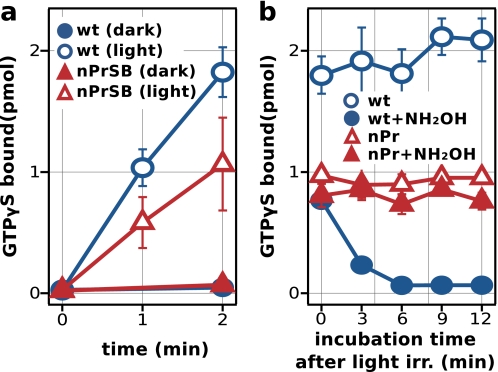
Measurements of the wild type and K296G in membrane fractions cannot be directly compared, because the receptor to membrane ratio differs greatly between the samples due to the much lower expression and reconstitution rate of the mutant protein. Therefore, to compare the activation efficiency between the wild type and K296G, we measured the G protein activity of detergent-solubilized and purified samples, ensuring that the receptor activities were assayed under the same conditions (Fig. 9). As with the membrane fractions, K296G/nPrSB showed a suppression of its activity in the dark and a clear signal of activation upon light irradiation, which was not affected by hydroxylamine. Whereas the wild type quickly lost the ability to activate the G protein because of the bleaching of the signaling state Meta-II by the hydroxylamine, K296G/nPrSB retained most of its activity after a long period of hydroxylamine incubation, indicating that this component is hydroxylamine insensitive. Furthermore, comparison between the wild type and K296G revealed that the activity of K296G/nPrSB was considerably smaller than the wild type, indicating that K296G is not fully active.
FIGURE 9.
G protein activity of detergent-solubilized and purified pigments. a, light-dependent G protein activity of the wild type and K296G/nPrSB. Error bars represent the S.D. of five independent measurements. b, time course of G protein activity in the presence of hydroxylamine (22 mm). The G protein activity was measured similar to a, but samples were incubated with hydroxylamine after light irradiation, and GDP/GTPγS exchange was initiated at selected times. The assay was carried out at 288 K in the presence of 600 nm Gt. Receptor concentrations were 12.5 nm for both wild type and the mutant K296G. Error bars represent the S.D. of three independent measurements.
These results, together with fluorescent measurements of retinal release and time-resolved measurements of the interaction with the G protein high affinity peptide, suggest that the partial activation observed upon light irradiation of K296G pigment is simply its basal activity. Previous works have concluded that the covalent bond is not required for the activation of rhodopsin (10, 11). However, our results suggest that this is only a basal activation, and thus the covalent bond is required for the formation of an efficient and stable active state.
Implications on the Activation Mechanism of Rhodopsin
Our results indicate that photoreceptive functions of rhodopsin are not affected by the disruption of the covalent bond; rather it seems that high affinity for the 11-cis inverse agonist, high quantum yield, and regio-specific isomerization of the retinal are achieved as a result of the evolutionary process that shaped the chemical environment surrounding the chromophore. Therefore, the importance of the covalent bond cannot lay in the rapid and efficient photoconversion of an inverse agonist to an agonist. Instead, our results show that the activation mechanism triggered by the photoisomerization of the retinal to an agonist was severely affected by the absence of this covalent bond.
Based on our findings, we propose a model where the covalent bond has two functions upon receptor activation. First, the covalent bond is required to efficiently transmit the structural changes caused by the isomerization of the retinal to the surrounding helices. Thus the disruption of the covalent bond results in destabilization of intermediate states. Second, we think the covalent bond is required to maintain the active conformation of the receptor. Although K296G pigments were reasonably stable in the dark, indicating that the covalent bond is not necessary to keep the retinal in the binding pocket in the resting state, the signaling state is predicted to have a more open structure, and thus the covalent bond may be required to trap the retinal inside (34, 35). We propose that the isomerized all-trans-retinal acts as a jamming device that locks the receptor in an open conformation, and the covalent bond is required to forcibly keep the low affinity agonist from leaving the receptor. Once the retinal leaves the receptor in the wild type, the salt bridge (Lys-296—Glu-113) is swiftly reformed, and the activation is shut off (6, 9, 11). On the other hand, K296G lacks the salt bridge in the absence of a Schiff base, and thus the receptor fluctuates between an open (active) and a closed (inactive) conformation, in the absence of the retinal, resulting in a partial activation of the receptor.
Vertebrate visual pigments, such as bovine rhodopsin, are characterized by their extremely efficient activation compared with other opsins, and we think vertebrate visual pigments underwent an evolutionary adaptation to increase sensitivity (36). In fact, vertebrate visual opsins cannot bind exogenous all-trans-retinal, ensuring that they are activated exclusively by light, although allosteric effects of all-trans-retinal and partial and intrinsic activation by some retinoids have been reported (37, 38). Vertebrate visual opsins also have a deprotonated active state in contrast with invertebrate type protonated bistable visual pigments (39, 40). Furthermore, it was recently reported that the magnitude of the light-induced conformational change correlates with the ability to activate the G protein (41). Thus, a more open conformation of the active state is more capable of activating the G protein. Our findings suggest that the function of the covalent bond of forcibly keeping the low affinity agonist in the receptor is a newly evolved trait that vertebrate visual pigments acquired, resulting in a more open active state and therefore more efficient G protein activation. Even though rhodopsin is a member of the GPCR superfamily, our results show that one of the defining features of opsins, its covalent bond with the ligand, has functional implications on its activation mechanism, which distinguish it from diffusible ligand binding GPCRs.
Acknowledgments
We thank Prof. A Wada at Kobe Pharmaceutical University for helpful advice in the synthesis of different retinylidene Schiff bases. We also thank Prof. Y. Imamoto and I. Seki of Kyoto University for the setting up of the photon counting system for fluorescence measurements.
This work was supported in part by a grant-in-aid for scientific research and the Global Center of Excellence Program (A06) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to Y. S.).
The numbers of all amino acid residues in this report are based on the bovine rhodopsin numbering system.
- GPCR
- G protein-coupled receptor
- nPrSB
- retinylidene-n-propylamine Schiff base
- EtSB
- retinylidene-ethylamine Schiff base
- MeSB
- retinylidene-methylamine Schiff base
- Gtα
- transducin α-subunit
- PSS
- photo-steady state
- ROS
- rod outer segment
- Meta
- metarhodopsin
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- DM
- n-dodecyl-β-d-maltoside.
REFERENCES
- 1.Shichida Y., Matsuyama T. (2009) Philos. Trans. R. Soc. Lond., B., Biol. Sci. 364, 2881–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald G. (1968) Science 162, 230–239 [DOI] [PubMed] [Google Scholar]
- 3.Bownds D. (1967) Nature 216, 1178–1181 [DOI] [PubMed] [Google Scholar]
- 4.Wang J. K., McDowell J. H., Hargrave P. A. (1980) Biochemistry. 19, 5111–5117 [DOI] [PubMed] [Google Scholar]
- 5.Honig B., Greenberg A. D., Dinur U., Ebrey T. G. (1976) Biochemistry. 15, 4593–4599 [DOI] [PubMed] [Google Scholar]
- 6.Cohen G. B., Oprian D. D., Robinson P. R. (1992) Biochemistry. 31, 12592–12601 [DOI] [PubMed] [Google Scholar]
- 7.Cohen G. B., Yang T., Robinson P. R., Oprian D. D. (1993) Biochemistry. 32, 6111–6115 [DOI] [PubMed] [Google Scholar]
- 8.Jäger F., Fahmy K., Sakmar T. P., Siebert F. (1994) Biochemistry. 33, 10878–10882 [DOI] [PubMed] [Google Scholar]
- 9.Kim J. M., Altenbach C., Kono M., Oprian D. D., Hubbell W. L., Khorana H. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12508–12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhukovsky E. A., Robinson P. R., Oprian D. D. (1991) Science. 251, 558–560 [DOI] [PubMed] [Google Scholar]
- 11.Robinson P. R., Cohen G. B., Zhukovsky E. A., Oprian D. D. (1992) Neuron. 9, 719–725 [DOI] [PubMed] [Google Scholar]
- 12.Matuoka S., Shichida Y., Yoshizawa T. (1984) Biochim. Biophys. Acta. 765, 38–42 [DOI] [PubMed] [Google Scholar]
- 13.Schoenlein R. W., Peteanu L. A., Mathies R. A., Shank C. V. (1991) Science. 254, 412–415 [DOI] [PubMed] [Google Scholar]
- 14.Kandori H., Shichida Y., Yoshizawa T. (2001) Biochemistry Mosc. 66, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 15.Nakamichi H., Okada T. (2006) Angew. Chem. Int. Ed. Engl. 45, 4270–4273 [DOI] [PubMed] [Google Scholar]
- 16.Cooper A. (1979) Nature 282, 531–533 [DOI] [PubMed] [Google Scholar]
- 17.Pitt G. A., Collins F. D., Morton R. A., Stok P. (1955) Biochem. J. 59, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai H., Terakita A., Shichida Y. (2000) Methods Enzymol. 315, 293–312 [DOI] [PubMed] [Google Scholar]
- 19.Tsutsui K., Imai H., Shichida Y. (2007) Biochemistry 46, 6437–6445 [DOI] [PubMed] [Google Scholar]
- 20.Shichida Y., Nakamura K., Yoshizawa T., Trehan A., Denny M., Liu R. S. (1988) Biochemistry 27, 6495–6499 [DOI] [PubMed] [Google Scholar]
- 21.Imamoto Y., Yoshizawa T., Shichida Y. (1996) Biochemistry 35, 14599–14607 [DOI] [PubMed] [Google Scholar]
- 22.Trehan A., Liu R. S., Shichida Y., Imamoto Y., Nakamura K., Yoshizawa T. (1990) Bioorg. Chem. 18, 30–40 [Google Scholar]
- 23.Kito Y., Suzuki T., Azuma M., Sekoguti Y. (1968) Nature 218, 955–957 [DOI] [PubMed] [Google Scholar]
- 24.Sakmar T. P., Franke R. R., Khorana H. G. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8309–8313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H., Komatsu T., Kato T. (1973) J. Phys. Soc. Jpn. 34, 156–165 [Google Scholar]
- 26.Imai H., Mizukami T., Imamoto Y., Shichida Y. (1994) Biochemistry 33, 14351–14358 [DOI] [PubMed] [Google Scholar]
- 27.Yoshizawa T., Wald G. (1963) Nature 197, 1279–1286 [DOI] [PubMed] [Google Scholar]
- 28.Emeis D., Kühn H., Reichert J., Hofmann K. P. (1982) FEBS Lett. 143, 29–34 [DOI] [PubMed] [Google Scholar]
- 29.Martin E. L., Rens-Domiano S., Schatz P. J., Hamm H. E. (1996) J. Biol. Chem. 271, 361–366 [DOI] [PubMed] [Google Scholar]
- 30.Morizumi T., Imai H., Shichida Y. (2005) Biochemistry 44, 9936–9943 [DOI] [PubMed] [Google Scholar]
- 31.Farrens D. L., Khorana H. G. (1995) J. Biol. Chem. 270, 5073–5076 [DOI] [PubMed] [Google Scholar]
- 32.Terakita A., Yamashita T., Tachibanaki S., Shichida Y. (1998) FEBS Lett. 439, 110–114 [DOI] [PubMed] [Google Scholar]
- 33.Wald G., Brown P. K. (1953) J. Gen. Physiol. 37, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 35.Altenbach C., Kusnetzow A. K., Ernst O. P., Hofmann K. P., Hubbell W. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7439–7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terakita A., Koyanagi M., Tsukamoto H., Yamashita T., Miyata T., Shichida Y. (2004) Nat. Struct. Mol. Biol. 11, 284–289 [DOI] [PubMed] [Google Scholar]
- 37.Surya A., Knox B. E. (1998) Exp. Eye Res. 66, 599–603 [DOI] [PubMed] [Google Scholar]
- 38.Kefalov V. J., Crouch R. K., Cornwall M. C. (2001) Neuron 29, 749–755 [DOI] [PubMed] [Google Scholar]
- 39.Tsukamoto H., Terakita A., Shichida Y. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyanagi M., Kawano E., Kinugawa Y., Oishi T., Shichida Y., Tamotsu S., Terakita A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsukamoto H., Farrens D. L., Koyanagi M., Terakita A. (2009) J. Biol. Chem. 284, 20676–20683 [DOI] [PMC free article] [PubMed] [Google Scholar]