Abstract
The plasma membrane assembly of aquaporin-4 (AQP4) water channels into orthogonal arrays of particles (OAPs) involves interactions of AQP4 N-terminal domains. To study in live cells the site of OAP assembly, the size and dynamics of plasma membrane OAPs, and the heterotetrameric associations of AQP4, we constructed green fluorescent protein (GFP)-labeled AQP4 “long” (M1) and “short” (M23) isoforms in which GFP was inserted at the cytoplasm-facing N or C terminus or between Val-141 and Val-142 in the second extracellular loop of AQP4. The C-terminal and extracellular loop GFP insertions did not interfere with the rapid unrestricted membrane diffusion of GFP-labeled M1 or the restricted diffusion and OAP assembly of GFP-labeled M23. Photobleaching of brefeldin A-treated cells showed comparable and minimally restricted diffusion of M1 and M23, indicating that OAP assembly occurs post-endoplasmic reticulum. Single-molecule step photobleaching and intensity analysis of GFP-labeled M1 in the absence versus presence of excess unlabeled M1 or M23 with an OAP-disrupting mutation indicated heterotetrameric AQP4 association. Time-lapse total internal reflection fluorescence imaging of M23 in live cells at 37 °C indicated that OAPs diffuse slowly (D ∼ 10−12 cm2/s) and rearrange over tens of minutes. Our biophysical measurements in live cells thus reveal extensive AQP4 monomer-monomer and tetramer-tetramer interactions.
Keywords: Fluorescence, Membrane Biophysics, Membrane Proteins, Protein Assembly, Water Channel, Membrane Protein Assembly, Neuromyelitis Optica, Single-particle Tracking, Single-molecule Fluorescence
Introduction
Aquaporin-4 (AQP4)2 water channels are expressed in glial cells throughout the central nervous system and in skeletal muscle, lung, kidney, stomach, and exocrine glands. In brain, AQP4 is involved in water balance, neuroexcitation, and glial cell migration (reviewed in Ref. 1). AQP4 deletion in mice improves clinical outcome in models of ischemic stroke (2), bacterial meningitis (3), and compression spinal cord injury (4) and alters seizure dynamics (5). AQP4 autoantibodies are found in the sera of most patients with the multiple sclerosis variant neuromyelitis optica (6), in which AQP4 autoantibodies are involved in the pathogenesis of central nervous system neuroinflammation (7).
At the molecular level, AQP4 is expressed as two major isoforms, a short isoform (M23) with a translation initiation site at Met-23 and a longer isoform (M1) that initiates translation at Met-1. Additional AQP4 isoform(s) may be present at low levels, at least in rat brain (8). Like other aquaporins, AQP4 forms tetramers in membranes (9). AQP4 tetramers can form supramolecular assemblies called orthogonal arrays of particles (OAPs), which are regular square arrays of intramembrane particles seen by freeze-fracture electron microscopy in brain, skeletal muscle, and kidney (10). Our laboratory discovered that AQP4 is the OAP-forming protein by demonstrating OAPs in M23-transfected cells (11) and the absence of OAPs in brain and other tissues from AQP4 knock-out mice (12). Label-fracture electron microscopy subsequently confirmed AQP4 in OAPs (13). We found by quantum dot/single-particle tracking that M1, when expressed alone, diffuses freely in cell membranes, whereas M23 is highly confined because of its assembly in OAPs (14). Single-molecule measurements of the diffusion of various AQP4 mutants and chimeras in live cells showed that OAP formation by M23 involves hydrophobic intermolecular interactions of N-terminal AQP4 residues just downstream of Met-23 and that lack of OAP formation by M1 results from nonspecific blocking of N-terminal interactions by residues just upstream of Met-23 (15). More recently, we demonstrated rapid and reversible temperature-dependent assembly of certain weakly associated AQP4 mutants into OAPs (16) and found that the M1 and M23 isoforms of AQP4 can co-mingle in OAPs (17); however, it has not been possible to determine whether M1 and M23 co-associate at the individual tetramer level.
Here, we used green fluorescent protein (GFP)-labeled M1 and M23 isoforms of AQP4 to address questions about AQP4 associations and OAP dynamics than cannot be addressed by available freeze-fracture electron microscopy, biochemical (native gel electrophoresis), or biophysical (single-particle tracking) methods. For these studies, we generated GFP-labeled AQP4 isoforms in which the GFP additions do not interfere with OAP formation by M23. We previously used GFP chimeras of AQP1 and AQP2 in spot photobleaching studies of their plasma membrane and endoplasmic reticulum diffusion (18, 19). The GFP-AQP4 chimeras were used here to determine in live cells whether AQP4 assembles into OAPs at the endoplasmic reticulum, whether the M1 and M23 AQP4 isoforms co-assemble in heterotetramers, and whether AQP4-containing OAPs diffuse and reorganize.
EXPERIMENTAL PROCEDURES
AQP4 Constructs
DNA sequences encoding AQP4 fragments and GFP were generated by PCR using pCMV6.ratAQP4 and pEGFP-N1 (Clontech) as templates, respectively. For generation of AQP4 with GFP in the second extracellular loop (M1exGFP and M23exGFP), AQP4 and GFP fragments were sequentially ligated into the mammalian expression vector pcDNA3.1 at engineered HindIII and EcoRI restriction sites. To generate AQP4 labeled with GFP at its N or C terminus (M23NGFP, M1CGFP, or M23CGFP), sequences encoding AQP4 were ligated into vector pEGFP-C1 or pEGFP-N1. Other AQP4 constructs used in this study were described previously (15). All constructs were verified by sequencing.
Cell Culture and Transfection
LLC-PK1 renal epithelial cells (AS2459) were cultured at 37 °C in 5% CO2 and 95% air in Dulbecco's modified Eagle's/H21 medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Before transfection, cells were transferred to 12-well plates containing 18-mm diameter coverslips. Transfections were done at 90–95% confluence 24–48 h before experiments with Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. In some studies, cells were incubated with 5 μg/ml brefeldin A for 12 h. Experiments were done at 37 °C in phosphate-buffered saline containing 6 mm sucrose and 1 mm pyruvate using a custom-built perfusion chamber designed to fit a PDMI-2 micro-incubator controlled by a TC-201A temperature regulator (Harvard Apparatus).
Fluorescence Recovery after Photobleaching
Spot photobleaching was performed using a home-built apparatus as described (19). Excitation was done using a 488 nm argon-ion laser (Innova I-308C, Coherent, Palo Alto, CA) and an acousto-optic modulator. Light was directed onto the sample through a Z488RDC dichroic mirror (Chroma) and a 60× oil immersion objective (numerical aperture of 1.40; Nikon) mounted in a Nikon Eclipse TE2000U inverted epifluorescence microscope. GFP fluorescence was observed through an HQ525/50m emission filter (Chroma), detected by a photomultiplier, digitized, and collected using a custom program written in LabVIEW (National Instruments). In fluorescence recovery after photobleaching experiments, cells were bleached for 50 ms with an intense pulse (25 milliwatts), and fluorescence recovery after photobleaching was observed by intermittent illumination (20 ms of 1 s) at 10 microwatts. In continuous photobleaching experiments, fluorescence was measured during continuous illumination at 10 microwatts.
Single-particle Tracking
Single-particle tracking was performed on quantum dot-labeled AQP4 with labeling procedures as reported previously (17). AQP4exGFP was labeled with rabbit anti-GFP antibody (Santa Cruz Biotechnology) and quantum dot-conjugated goat anti-rabbit IgG (Invitrogen). AQP4 containing C-terminal GFP was labeled with a recombinant neuromyelitis optica monoclonal autoantibody (7, 17) and quantum dot-conjugated goat anti-human IgG (Invitrogen). Myc-tagged AQP4 was labeled with mouse anti-Myc antibody (Covance) and quantum dot-conjugated goat anti-mouse IgG (Invitrogen). Single-particle tracking measurements were done on a Nikon Eclipse TE2000S inverted epifluorescence microscope. Images were acquired continuously at 11 ms/frame (91 Hz) for 6 s using a deep-cooled charge-coupled device camera (Hamamatsu EM-CCD). Image processing, trajectory reconstruction, and data analysis were performed as described (20).
Total Internal Reflection Fluorescence Microscopy (TIRFM)
TIRFM was done using a Nikon Eclipse TE2000E microscope equipped with a through-objective TIRF attachment and a 100× oil immersion objective (numerical aperture of 1.49) mounted on a Perfect Focus module (Nikon). An argon-ion laser on a custom-built launch was coupled through a fiber optic to the TIRF module. GFP was excited using a Z488/10x excitation filter and a Z488RDC dichroic mirror and detected through an ET525/50m emission filter (Chroma). Images were acquired using a QuantEM 512SC deep-cooled charge-coupled device camera (Photometrics, Tucson, AZ).
Photobleaching of single fluorescent spots was done by continuous exposure to laser excitation and image acquisition at 10 Hz for 80 s or until all visible fluorescence was bleached to background levels. Images were processed, and background-subtracted area-integrated intensities were analyzed as described previously (21). Time-lapse images of AQP4 OAPs in live cells were acquired at three frames/min for up to 3 h, with the excitation laser shuttered between image acquisitions. Analysis of OAP diffusion was done as described for analysis of quantum dot diffusion.
SDS-PAGE and Immunoblot Analysis
For denaturing gel electrophoresis (SDS-PAGE), proteins from whole cell homogenates or vesicle fractions in lysis buffer containing a proteinase inhibitor mixture (Invitrogen) were denatured by NuPAGE lithium dodecyl sulfate sample buffer (Invitrogen). Samples were loaded onto NuPAGE 4–12% Bistris gels along with SeeBlue Plus2 markers (Invitrogen) and run with NuPAGE MES/SDS running buffer (Invitrogen) at 75 V. Proteins were transferred to Hybond ECL nitrocellulose membranes (Amersham Biosciences). Immunoblots were blocked with Tris-buffered saline/Tween 20 containing 5% nonfat milk and incubated with rabbit anti-AQP4 antibody (Santa Cruz Biotechnology) or rabbit anti-GFP polyclonal antibody (Abcam), followed by peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories), and signals were detected using an ECL Plus chemiluminescence kit (Amersham Biosciences).
RESULTS
Characterization of GFP-labeled AQP4
Cells expressing GFP-labeled AQP4 isoforms were characterized to ensure that the GFP insertions did not interfere with AQP4 plasma membrane targeting or OAP formation. Fig. 1A diagrams the useful sites of GFP insertion at the AQP4 C terminus and in its second extracellular loop. The latter insertion was made at the same site at which Myc and hemagglutinin epitopes were inserted without interference with AQP4 expression or function (14). Fig. 1 also shows the AQP4 Met-1 and Met-23 translation initiation sites.
FIGURE 1.
Expression GFP-labeled M1 and M23 AQP4 isoforms. A, membrane topography of AQP4 showing the sites of GFP insertion at the AQP4 C terminus and at an internal site in the second extracellular loop. Shown also are the M1 and M23 translation initiation sites. B, TIRFM of GFP-tagged M1 and M23 in transfected LLC-PK1 cells for the C-terminal (M23CGFP and M1CGFP) and extracellular loop (M23exGFP and M1exGFP) GFP insertions. C, immunoblot of total cell homogenates expressing GFP-tagged M1 and M23 isoforms or untagged AQP4. The blot was probed with antibody against the C terminus of AQP4. D, TIRFM of N-terminally GFP-tagged M23 (M23NGFP).
Fig. 1B shows efficient plasma membrane targeting of the GFP-tagged M1 and M23 isoforms of AQP4. By TIRFM, both GFP-tagged M23 isoforms showed punctate surface fluorescence characteristic of OAP-associated AQP4. In contrast, the M1-expressing cells showed quite uniform surface fluorescence characteristic of non-OAP-associated AQP4. Fig. 1C shows the expected molecular sizes of the various GFP-tagged and untagged M1 and M23 proteins by immunoblotting in which proteins were probed with an antibody against the AQP4 C terminus. Fig. 1D shows that M23 tagged with GFP at its N terminus had a smooth fluorescence pattern, indicating that the N-terminal GFP insertion interfered with M23 OAP formation, which is consistent with the involvement of N-terminal interactions in OAP formation (15).
For independent and quantitative verification that the C-terminal and extracellular loop insertions did not affect AQP4 supramolecular assembly, we measured AQP4 diffusion by quantum dot/single-particle tracking. Cells expressing the M1 and M23 isoforms of AQP4 were labeled with quantum dots using anti-Myc primary antibody (for AQP4 containing an extracellular Myc epitope), anti-GFP primary antibody (for GFP loop insertion chimeras M1exGFP and M23exGFP), or a recombinant neuromyelitis optica antibody against extracellular epitope(s) on native AQP4 (for C-terminal GFP chimeras M1CGFP and M23CGFP) (Fig. 2A, upper). Quantum dot trajectories of the GFP-labeled isoforms were compared with those of the M1 and M23 AQP4 isoforms containing a small extracellular Myc epitope as used previously (14).
FIGURE 2.
Quantum dot/single-particle tracking analysis of plasma membrane diffusion of GFP-labeled AQP4. A, upper, schematic of quantum dot (Qdot) labeling of Myc-tagged and GFP-labeled AQP4 isoforms showing extracellular AQP4 labeling with anti-Myc or anti-GFP antibodies or recombinant neuromyelitis optica monoclonal antibody (NMO-ab). Lower, representative single quantum dot trajectories (total duration of 6 s at 91 frames/s) for LLC-PK1 cells expressing the indicated constructs. B, analysis of single-particle trajectories showing mean squared displacement (MSD) analysis (left) and cumulative probability (range at 1 s) analysis (right). The data shown are for at least 250 trajectories for each condition.
Fig. 2A (lower) shows representative single-particle trajectories for quantum dot-labeled cells expressing the Myc- and GFP-containing M1 and M23 AQP4 isoforms. Substantial diffusion (>500 nm in 6 s) was found for the Myc- and GFP-containing M1 isoforms, whereas little diffusion was seen for the M23 isoforms, supporting the conclusion that M23CGFP and M23exGFP assemble into OAPs. Fig. 2B shows quantitative analyses of the single-particle trajectory data as mean squared displacement (left) and cumulative probability range (right) plots. The GFP-containing M1 isoforms diffused rapidly, similar to the diffusion of M1-Myc, whereas the GFP-containing M23 isoforms diffused very slowly, as seen for M23-Myc. The slightly (∼15%) reduced diffusion of M1exGFP and M1CGFP compared with M1-Myc may be related to the increased molecular size of M1 containing the GFP tag. These measurements validate the utility of the GFP-labeled AQP4 isoforms for use in the biophysical studies to follow.
Photobleaching Reveals that M23 Does Not Assemble into OAPs at the Endoplasmic Reticulum (ER)
Although membrane protein oligomerization generally occurs at the ER (22), it is not known whether supramolecular protein assembly, such as OAP formation by M23, occurs at the ER versus downstream in the Golgi or the plasma membrane. To determine whether AQP4 OAPs form at the ER, spot photobleaching measurements were done in cells expressing GFP-labeled M1 and M23 isoforms. Fluorescence microscopy showed a predominant plasma membrane pattern of GFP fluorescence after transfections in control (non-brefeldin A (BFA)-treated) LLC-PK1 cells (Fig. 3A, −BFA panels). To study ER diffusion of AQP4, cells were treated with BFA, which produced a characteristic ER pattern of GFP fluorescence (Fig. 3A, +BFA panels).
FIGURE 3.
Photobleaching shows that M23 OAP formation occurs post-endoplasmic reticulum. A, wide-field fluorescence micrographs of M1CGFP- and M23CGFP-transfected LLC-PK1 cells without and with BFA treatment. B, upper, representative fluorescence recovery after photobleaching curves of the GFP-labeled M1 and M23 AQP4 isoforms without and with BFA treatment. Spot photobleaching was done using a brief laser pulse (50 ms) with ∼1.4-μm diameter to bleach initial fluorescence by ∼40%. GFP fluorescence recovery was measured in the bleached spot. Bleaching of plasma membrane GFP-labeled AQP4 (non-BFA-treated) was done at the edge of confluent cells; bleaching of ER-localized GFP-labeled AQP4 (BFA-treated) was done in a perinuclear region of the ER. Lower, summary of the percentage mobile fraction (from exponential regression of fluorescence recovery curves) for GFP-labeled AQP4 at the plasma membrane (PM) and ER. Error bars indicate means ± S.E. (n > 10 cells). *, p < 0.05. C, continuous photobleaching in which GFP fluorescence was measured in an ∼1.4-μm spot during continuous light exposure. Upper, representative fluorescence curve shown for the indicated GFP-tagged AQP4 isoforms at the plasma membrane (upper set of curves; non-BFA-treated) and ER (lower set of curves; BFA-treated). Lower, summary of percentage fluorescence loss at 3 min. Error bars indicate means ± S.E. (n > 10 cells). *, p < 0.05.
Spot photobleaching was done using a 60× objective lens producing an ∼1.4-μm diameter spot on the cell membrane. First, photobleaching measurements were done in cells expressing GFP-tagged M1 and M23 at the plasma membrane in which the well demarcated edges of closely apposed cells were targeted by the laser spot; under this condition, fluorescence recovery is well described by one-dimensional diffusion as reported previously (19). Representative fluorescence recovery after photobleaching curves in Fig. 3B (upper set of curves) show substantial recovery of GFP-labeled M1, with percentage recovery generally of ∼80%, with 50% recovery in 10–20 s. Relatively little recovery (generally <40%) was seen for GFP-labeled M23, consistent with the quantum dot/single-particle tracking. Fig. 3B (lower) summarizes recovery fractions for a series of measurements. Spot photobleaching of AQP4 in the ER was done in the BFA-treated cells in which a region near the cell nucleus was targeted by the laser spot. Remarkably, unlike the findings for AQP4 diffusion at the plasma membrane, the ER diffusion of the M1 and M23 AQP4 isoforms was quite similar, with substantial recovery over 30 s (Fig. 3B, lower set of curves). These results suggest that M23 does not assemble into relatively immobile OAPs at the ER.
Continuous photobleaching measurements were done to substantiate the spot photobleaching data. In continuous photobleaching, the fluorescence from a continuously illuminated spot is measured. The kinetics of fluorescence decline depends on illumination intensity and diffusion, with greater diffusion manifest as a slower decline in fluorescence. The laser beam intensity was chosen to produce ∼20% decline in fluorescence in 3 min for M1-GFP. Representative continuous photobleaching curves in Fig. 3C (upper set of curves) show much greater decline in fluorescence for M23 versus M1 at the plasma membrane but comparable decline in fluorescence at the ER in BFA-treated cells. The comparable decline in fluorescence for M23 and M1 in the ER (Fig. 3C, lower set of curves) supports the fluorescence recovery after photobleaching data showing comparable ER diffusion of the M1 and M23 AQP4 isoforms.
Single-molecule Analysis Shows AQP4 Heterotetramers
Single-molecule analysis of GFP intensity and photobleaching was done to investigate whether the M1 and M23 isoforms of AQP4 can associate in heterotetramers. The idea in these studies is that a homotetramer containing four GFP-labeled AQP4 molecules would undergo multistep photobleaching as each of the four GFPs are sequentially bleached and have four times the intensity of a GFP monomer. If, however, a tetramer consists of one GFP-labeled AQP4 molecule and three unlabeled AQP4 molecules, then photobleaching would occur in a single step, and the spot intensity would be the same as that of a GFP monomer. We compared the photobleaching kinetics and spot intensity distributions of purified recombinant GFP monomers with those of LLC-PK1 cells expressing GFP-labeled M1 alone and GFP-labeled M1 coexpressed with an excess of unlabeled M1 or an M23 mutant, M23-G28P, which has an OAP-disrupting proline downstream of Met-23 (15). For these studies, it was necessary to use an M23 variant that cannot form OAPs because single-spot photobleaching and intensity analysis can be applied only to physically distinct AQP4 tetramers (and not to AQP4 in OAPs). Also, AQP4 expression at very low levels was required to avoid AQP4 clustering due to crowding effects, and cells were lightly fixed prior to measurements to freeze tetramers in situ, which gave improved the signal-to-noise ratio compared with unfixed cells, in which the tetramers diffused rapidly. Imaging was done using TIRFM to reduce background signal for single-molecule imaging.
Fig. 4A shows examples of serial GFP images of purified recombinant GFP monomers (upper) and cells expressing GFP-labeled M1 (lower). Single GFP spots were readily visualized. Spots containing purified GFP monomers had similar fluorescence and disappeared in an all-or-none manner with continuous illumination. Spots in the cells expressing GFP-labeled M1 were brighter than those of purified GFP and appeared to undergo multistep photobleaching, showing reduced intensity in serial image frames.
FIGURE 4.
Single-molecule step photobleaching and intensity analysis shows AQP4 heterotetrameric association. A, serial TIRFM images of recombinant monomeric GFP on a cover glass (upper) showing single-step loss of fluorescence. Fluorescent GFP spots are indicated by colored circles. Serial TIRFM images of M1exGFP in fixed LLC-PK1 cells show examples (red and blue circles) of multistep loss of fluorescence. B, left, single-spot (background-subtracted) integrated fluorescence intensity histograms obtained during initial illumination for recombinant GFP and for cells expressing M1exGFP alone or with excess non-GFP-labeled M1 or M23-G28P (non-OAP-forming M23 mutant). 1× (vertical dashed red line) indicates the average intensity of recombinant GFP. Right, representative single-spot intensities as a function of time during continuous illumination, showing single-step versus multistep photobleaching.
Fig. 4B (left) summarizes histogram distributions of single-spot fluorescence (background-subtracted area-integrated) of recombinant GFP monomers and cells expressing GFP-labeled M1 without versus with excess unlabeled M1 or M23-G28P. The mean fluorescence intensity of recombinant GFP monomers was defined as 1×. The intensity histogram was broad and shifted to ∼4× for cells expressing GFP-labeled M1 alone, where each M1 tetramer is expected to contain four GFP molecules. The broadness of the histograms is related to experimental noise in these challenging single-molecule GFP measurements. Unimodal intensity distributions similar to that for recombinant GFP were seen for cells expressing GFP-labeled M1 and a 10-fold excess of unlabeled M1 or M23-G28P. The relative mean intensities of these distributions were slightly greater than 1× because some AQP4 tetramers contain two GFPs under our experimental conditions, in which a 10-fold (rather than infinite-fold) excess of unlabeled AQP4 was used. Fig. 4B (right) shows representative single-spot photobleaching kinetics. Except for cells expressing GFP-labeled M1 alone, single-step photobleaching was seen in >90% of spots, with >25 spots analyzed per condition. For cells expressing GFP-labeled M1 alone, multistep bleaching was seen, often with four distinct steps. These results provide evidence that the M1 and M23 AQP4 isoforms are able to form heterotetramers.
TIRFM Analysis of OAP Dynamics
The ability of GFP-labeled M23 to form OAPs allows direct examination of OAP size and dynamics in live cells by time-lapse TIRFM imaging. Fig. 5A (left) shows a TIRFM image of LLC-PK1 cells expressing GFP-labeled M1. There were distinct well demarcated spots corresponding to individual OAPs. Because the actual sizes of OAPs are generally less than the x,y spatial resolution of TIRFM, most OAPs appear as diffraction-limited fluorescent spots whose intensity is proportional to the number of GFP-AQP4 molecules in the OAP. Fig. 5A (right) shows individual OAP trajectories from time-lapse TIRFM imaging done over 3 h in which cells were maintained at 37 °C. Most trajectories appear to show a random Brownian pattern of motion. Examination of time-lapse images (see the supplemental movie) shows the slow diffusive motion of OAPs, as well as various reorganization events such OAP fusion and fission. An example of OAP fission is shown in Fig. 5A (lower), where a single fluorescent OAP spot appears to separate over tens of minutes into at least four distinct fluorescent spots.
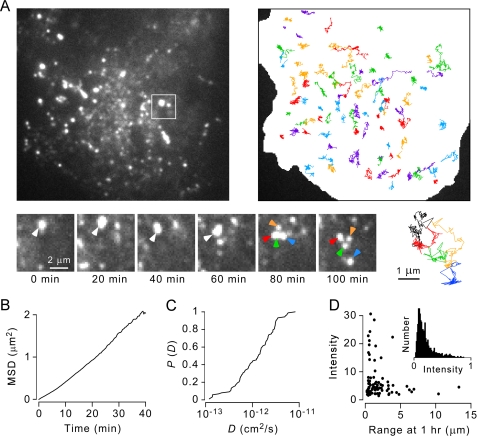
FIGURE 5.
TIRFM analysis of plasma membrane OAP dynamics in live cells. Measurements were made in LLC-PK1 cells expressing M23CGFP. A, TIRFM image (left) showing distinct fluorescent spots corresponding to OAPs, with deduced single-OAP trajectories over 3 h shown at the right. Lower, high magnification of the boxed region showing a spontaneous OAP disruption event. Trajectories of original OAP (black) and daughter OAPs (red, green, yellow, and blue) are shown at the right. B, averaged mean squared displacement (MSD) plot of OAP diffusion with a deduced diffusion coefficient of ∼10−12 cm2/s. C, cumulative probably distribution of OAP diffusion coefficients. D, correlation between single-spot intensity (proportional to OAP size) and displacement (range) at 1 h. The inset shows an intensity histogram of many individual OAPs.
The time-lapse image data were analyzed to characterize OAP diffusion. Fig. 5B shows an averaged mean squared displacement plot deduced from trajectories of many fluorescent spots from many cells. The plot shows slight upward curvature, suggesting primarily Brownian diffusion with mild convection (see “Discussion”), with a median diffusion coefficient of ∼10−12 cm2/s. Fig. 5C shows a cumulative probability plot of the OAP diffusion coefficient. To determine whether apparent OAP size (quantified by integrated spot intensity) correlated with OAP diffusion, spot intensity was plotted against spot displacement (range) at 1 h (Fig. 5D). A wide range of diffusive rates was seen. Although the largest OAPs diffused relatively little (<2 μm in 1 h), there was little correlation between OAP size and diffusion for most OAPs. The inset in Fig. 5D shows a skewed unimodal distribution of spot intensities (OAP sizes) for measurements made on many cells.
DISCUSSION
We labeled the M1 and M23 isoforms of AQP4 with GFP at three sites: at their N and C termini and at residues 141–142 in their second extracellular loops. The extracellular loop site was selected from previous exploratory studies showing that labeling of AQP4 at the equivalent site with various epitopes, including hemagglutinin and Myc, did not affect its expression, membrane targeting, or water permeability (14, 17). We found here that GFP labeling of AQP4 isoforms at their C termini or in their extracellular loops did not impair their plasma membrane targeting or the ability of M23 to form OAPs. There are several other examples of successful insertions of GFP in internal loops in membrane proteins, including CFTR (cystic fibrosis transmembrane conductance regulator) (21) and the vesicular glutamate transporter VGLUT1 (23). It is thought that the proximity of the N and C termini of GFP and its tight β-barrel structure allow its insertion in internal loops with minimal perturbation of protein folding or structure. We found that AQP4 labeling at its N terminus interfered with OAP assembly, which is not unexpected because interactions of the AQP4 N terminus are responsible for OAP formation and disruption (15).
The GFP-labeled AQP4 isoforms were used to address questions about AQP4 assembly and dynamics in live cells at 37 °C that cannot be addressed easily by other approaches. We showed recently that, under some conditions, reduced temperature greatly alters OAP assembly (16), indicating the importance of studying OAPs at physiological temperature. Freeze-fracture electron microscopy and native gel studies of AQP4 cannot provide dynamic information about AQP4 interactions and do not provide reliable information about the AQP4 association state at 37 °C. Native gel analysis of AQP4 structure involves incubations with solubilizing detergents that are generally done on ice (17); freeze-fracture electron microscopy requires membrane fixation and extensive processing (10, 24). Strategies to label epitope-tagged AQP4 with small fluorophores generally label a small and unknown fraction of AQP4 molecules, precluding the types of analyses needed for the questions addressed here.
The GFP-labeled AQP4 isoforms were used to test the hypothesis that AQP4 OAPs are formed in the ER. Although membrane protein oligomerization generally occurs at the ER (22), there is no information to our knowledge about when and where supramolecular assemblies such as OAPs are formed. Photobleaching showed rapid diffusion of M1, faster at the ER than at the plasma membrane, with largely complete recovery of fluorescence, supporting the conclusion that most M1 molecules are mobile. Similarly increased membrane protein diffusion in the ER versus the plasma membrane has been seen for other proteins such as AQP2 (19), which is probably related to the low content of cholesterol in the ER membrane and to differences in membrane protein composition and density. Photobleaching showed that M23 is largely immobile at the plasma membrane, as anticipated from previous single-particle tracking studies in which it was concluded that slow diffusion of M23 is due primarily to its assembly in OAP-like rafts rather than to tethering effects (14). However, unexpectedly, the diffusion of M23 was rapid and comparable with that of M1 at the ER, providing biophysical evidence that OAPs do not assemble at the ER. These measurements do not formally distinguish whether AQP4 at the ER is monomeric versus tetrameric versus in small OAPs whose diffusion is not slowed. We speculate that differences in ER lipid composition and physical properties, such as absence of lipid rafts, may be responsible for the inability of M23 to form OAPs at the ER. Alternative possibilities include the strong curvature of the ER membrane, which might inhibit OAP formation, or differential interactions between AQP4 and non-AQP4 membrane proteins in the ER versus the plasma membrane.
Previous biochemical studies utilizing velocity sedimentation, cross-linking, and immunoblot analysis of rat brain lysates provided indirect evidence that the M1 and M23 isoforms are able to co-mingle (25). Whether AQP4 heterotetramers form in intact cell membranes had not been studied. The approach used here was single-molecule intensity and step photobleaching analysis, which provides information, by two separate measures, on the number of GFP molecules in diffraction-limited distinct fluorescent spots visualized by TIRFM. We used this strategy recently to establish that CFTR is in monomeric form in live cell membranes (21), and others have used single-step versus multistep photobleaching to determine the oligomeric state of postsynaptic proteins (26) and N-methyl-d-aspartate receptors (27). We found that spots containing GFP-labeled M1 alone had severalfold greater brightness than recombinant monomeric GFP and underwent multistep photobleaching, consistent with the assembly of GFP-labeled AQP4 molecules as tetramers. In contrast, one GFP molecule/fluorescent spot was found in membranes containing GFP-labeled M1 together with an excess of unlabeled M1 or an M23 variant that does not form OAPs. These findings indicate the ability of M1 and M23 to associate in heterotetramers in live cell membranes. The association of M1 and M23 at the single tetramer level would account for the extensive co-mingling of M1 and M23 in OAPs as deduced from two-color single-particle tracking and native gel studies (17).
GFP-labeled M23 allowed time-lapse TIRFM imaging of OAPs to study their size and dynamics. OAPs containing M23 diffused slowly and remained largely isolated, although some OAPs underwent spontaneous fission and fusion events. Prior quantum dot/single-particle tracking measurements over many minutes showed slow diffusion of M23 in arrays with slight upward curvature by mean squared displacement analysis (14). The direct visualization of OAPs containing GFP-labeled M23 is consistent with the interpretation of the prior quantum dot tracking results in terms of ascribing the slow phase of M23 diffusion to the raft-like diffusion of individual OAPs. Interestingly, the OAP tracking data here also showed slight upward curvature by mean squared displacement analysis, which may arise from subtle convective effects in the plasma membrane of live cells. Such subtle convective effects may not have been appreciated previously because they require measurements of the diffusion of large raft-like intramembrane domains that are free from tethering to other cellular components.
Measurement of spot intensities showed a broad distribution of OAP sizes. Comparison of apparent OAP size with diffusion did not yield any distinct relationship. Only a few of the smaller OAPs covered a range >5 μm over 1 h, whereas the largest OAPs moved substantially less. We should point out, however, that real glial cell membranes contain both M1 and M23 isoforms and so contain smaller OAPs than those of pure M23 imaged here. Super-resolution imaging methods may overcome some of the limitations of conventional TIRFM in terms of visualizing very small OAPs.
In conclusion, the biophysical studies of GFP-labeled AQP4 chimeras here have provided new information about AQP4 monomer-monomer associations and the dynamics and site of OAP assembly. Labeling of AQP4 with specialized GFP mutants should allow super-resolution imaging of AQP4 OAPs and dynamic sensing of the AQP4 environment during its cellular processing and regulation.
Supplementary Material
This work was supported by National Institutes of Health Grants EB00415, HL73856, DK35124, EY13574, and DK72517 and by a grant from the Guthy-Jackson Charitable Foundation.

The on-line version of this article (available at http://www.jbc.org) contains a supplemental video.
- AQP4
- aquaporin-4
- OAP
- orthogonal arrays of particles
- GFP
- green fluorescent protein
- TIRFM
- total internal reflection fluorescence microscopy
- Bistris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MES
- 4-morpholineethanesulfonic acid
- ER
- endoplasmic reticulum
- BFA
- brefeldin A.
REFERENCES
- 1.Verkman A. S., Binder D. K., Bloch O., Auguste K., Papadopoulos M. C. (2006) Biochim. Biophys. Acta 1758, 1085–1093 [DOI] [PubMed] [Google Scholar]
- 2.Manley G. T., Fujimura M., Ma T., Noshita N., Filiz F., Bollen A. W., Chan P., Verkman A. S. (2000) Nat. Med. 6, 159–163 [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos M. C., Verkman A. S. (2005) J. Biol. Chem. 280, 13906–13912 [DOI] [PubMed] [Google Scholar]
- 4.Saadoun S., Bell B. A., Verkman A. S., Papadopoulos M. C. (2008) Brain 131, 1087–1098 [DOI] [PubMed] [Google Scholar]
- 5.Binder D. K., Yao X., Zador Z., Sick T. J., Verkman A. S., Manley G. T. (2006) Glia 53, 631–636 [DOI] [PubMed] [Google Scholar]
- 6.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R. (2005) J. Exp. Med. 202, 473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett J. L., Lam C., Kalluri S. R., Saikali P., Bautista K., Dupree C., Glogowska M., Case D., Antel J. P., Owens G. P., Gilden D., Nessler S., Stadelmann C., Hemmer B. (2009) Ann. Neurol. 66, 617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moe S. E., Sorbo J. G., Sogaard R., Zeuthen T., Petter Ottersen O., Holen T. (2008) Genomics 91, 367–377 [DOI] [PubMed] [Google Scholar]
- 9.Ho J. D., Yeh R., Sandstrom A., Chorny I., Harries W. E., Robbins R. A., Miercke L. J., Stroud R. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7437–7442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolburg H. (1995) J. Hirnforsch. 36, 239–258 [PubMed] [Google Scholar]
- 11.Yang B., Brown D., Verkman A. S. (1996) J. Biol. Chem. 271, 4577–4580 [PubMed] [Google Scholar]
- 12.Verbavatz J. M., Ma T., Gobin R., Verkman A. S. (1997) J. Cell Sci. 110, 2855–2860 [DOI] [PubMed] [Google Scholar]
- 13.Rash J. E., Yasumura T., Hudson C. S., Agre P., Nielsen S. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 11981–11986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane J. M., Van Hoek A. N., Skach W. R., Verkman A. S. (2008) Mol. Biol. Cell 19, 3369–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane J. M., Verkman A. S. (2009) J. Cell Sci. 122, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crane J. M., Verkman A. S. (2009) Biophys. J. 97, 3010–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane J. M., Bennett J. L., Verkman A. S. (2009) J. Biol. Chem. 284, 35850–35860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umenishi F., Verbavatz J. M., Verkman A. S. (2000) Biophys. J. 78, 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin M. H., Haggie P. M., Vetrivel L., Verkman A. S. (2001) J. Biol. Chem. 276, 21331–21336 [DOI] [PubMed] [Google Scholar]
- 20.Crane J. M., Verkman A. S. (2008) Biophys. J. 94, 702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haggie P. M., Verkman A. S. (2008) J. Biol. Chem. 283, 23510–23513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurtley S. M., Helenius A. (1989) Annu. Rev. Cell Biol. 5, 277–307 [DOI] [PubMed] [Google Scholar]
- 23.Voglmaier S. M., Kam K., Yang H., Fortin D. L., Hua Z., Nicoll R. A., Edwards R. H. (2006) Neuron 51, 71–84 [DOI] [PubMed] [Google Scholar]
- 24.Furman C. S., Gorelick-Feldman D. A., Davidson K. G., Yasumura T., Neely J. D., Agre P., Rash J. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13609–13614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neely J. D., Christensen B. M., Nielsen S., Agre P. (1999) Biochemistry 38, 11156–11163 [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama Y., Kawabata I., Sobue K., Okabe S. (2005) Nat. Methods 2, 677–684 [DOI] [PubMed] [Google Scholar]
- 27.Ulbrich M. H., Isacoff E. Y. (2007) Nat. Methods 4, 319–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.