Abstract
The neurotrophin receptor tyrosine kinase TrkB is critical to diverse biological processes. We investigated the interplay of Src family kinases (SFKs) and TrkB to better understand mechanisms of TrkB signaling in physiological and pathological conditions. We compared and contrasted the role of SFKs in TrkB signaling following activation of TrkB by two mechanisms, its transactivation by zinc, and its activation by its prototypic neurotrophin ligand, brain-derived neurotrophic factor (BDNF). Using biochemical, pharmacological, and chemical genetic studies of cultured rodent neurons, we found that zinc promotes preferential phosphorylation of Tyr-705/Tyr-706 of TrkB by a SFK-dependent but TrkB kinase-independent mechanism, a signaling event critical for transactivation of TrkB by zinc. By contrast, SFK activity is not essential for BDNF-mediated activation of TrkB, yet SFK activity is increased as a consequence of TrkB activation by BDNF. Moreover, BDNF-induced phosphorylation of Tyr-705/Tyr-706 of TrkB was inhibited by SFK inhibitors, implicating a regulatory role of SFKs in TrkB activation by BDNF. In sum, SFKs are activated by TrkB and, in turn, SFKs can promote TrkB activation. We propose models depicting the mutual regulation of SFKs and TrkB following activation of TrkB by zinc and BDNF.
Keywords: Neuron, Neurotrophin, Receptor Tyrosine Kinase, Src, Zinc, BDNF, TrkB
Introduction
The neurotrophin receptor tyrosine kinase TrkB is critical to diverse biological processes in the developing and mature nervous systems, including neuronal survival, proliferation, and differentiation as well as synaptic structure, function, and plasticity (1, 2). TrkB has been implicated in diverse neurological and psychiatric disorders, including Alzheimer disease, Huntington disease, schizophrenia, depression, addiction, neuropathic pain, and epilepsy (3–6). Accumulating evidence also implicates TrkB in diseases originating outside the nervous system, including cancer and metabolic disorders (7, 8). Understanding regulation of TrkB signaling will provide insight into its diverse functions in physiological and pathological conditions.
TrkB can undergo activation by its prototypic ligand, the neurotrophin BDNF,2 as well as transactivation by non-neurotrophin ligands. BDNF is a secreted 14-kDa polypeptide that binds to the ectodomain of TrkB, inducing receptor dimerization, resulting in increased intrinsic kinase activity and autophosphorylation of tyrosines, including Tyr-705/Tyr-706, Tyr-515, and Tyr-816 within the intracellular domain, in turn triggering activation of downstream signaling pathways, including Ras-Raf-MEK-MAPK, PI3K-Akt, and PLCγ1 (9, 10). TrkB can also undergo transactivation, a process in which the receptor is activated by a stimulus that does not interact directly with the receptor (11). We recently discovered that the divalent cation zinc can transactivate TrkB by a Src family kinase (SFK)-dependent but neurotrophin-independent mechanism (12).
SFKs are a family of non-receptor tyrosine kinases that have been implicated in regulation of diverse physiological and pathological processes in the nervous system as well as other organs. Among the SFK family, Src and Fyn in particular are highly expressed in the mammalian nervous system. Functional interactions of SFKs and diverse receptor tyrosine kinases have been established, notably involving the epidermal growth factor (EGF) and platelet-derived growth factor receptors (13). Co-immunoprecipitation of TrkB with Fyn suggested an association of TrkB with SFKs in cultured neurons (14). One potential biological consequence of this interaction is to promote the localization of TrkB in lipid rafts (15). That said, the functional interplay of SFKs and TrkB signaling is incompletely understood. Specifically, do SFKs regulate TrkB activation? Does TrkB activation regulate SFK activity?
Here we compared and contrasted the role of SFKs in TrkB signaling following activation of TrkB by two mechanisms, its transactivation by zinc and its activation by its prototypic neurotrophin ligand, BDNF. We found that SFKs are activated by TrkB and, in turn, SFKs can promote TrkB activation. We propose models depicting the mutual regulation of SFKs and TrkB following activation of TrkB by zinc and BDNF.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids
BDNF was purchased from Chemicon. AP5 and K252a were purchased from Tocris Crook. PP1, PP2, and PP3 were purchased from Calbiochem and Biomol. SMI11293 and CGP76030 were generously provided by Systems Medicine and Novartis, respectively. Other reagents were obtained from Sigma unless specified otherwise. 1NMPP1 was generously provided by Dr. David Ginty (Johns Hopkins University).
The DNA fragments encoding intracellular domain (ICD) of mouse TrkB (amino acids 454–821) or FLAG-tagged ICD were PCR-amplified and cloned into BamHI- and EcoRI-digested pGEXT-2T and pcDNA3 plasmids, respectively. pFLAG-TrkB was described previously (12). Y515F and Y705F/Y706F mutants of pFLAG-TrkB were generated by PCR-mediated site-directed mutagenesis. All the resultant plasmids were confirmed by DNA sequencing analyses.
Mice
TrkB conditional mutant mice in a C57BL/6 background were generated with Cre/loxP technology as described previously (26). In brief, crossing a TrkB loxP-floxed mouse to a KA1-Cre transgenic mouse (kindly provided by Dr. Susumu Tonegawa, Massachusetts Institute of Technology) expressing Cre driven by a KA1 promoter generated progeny in which expression of the floxed genes were selectively eliminated in principal neurons in CA3 and dentate gyrus (27). TrkBF616A mutant mice (kindly provided by Dr. David Ginty) in a C57BL/6 background carrying a point mutation of residue 616 (F → A) of TrkB was described previously (17, 12). The genotype of each animal was verified twice using PCR of genomic DNA isolated from tail before and after experiments. Animals were handled according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Duke University Animal Welfare Committee.
Cell Culture and Transfection
Cortical neuron/glia mixed cultures were prepared from pups of embryonic 18 (E18) pregnant Sprague-Dawley rat (Charles River) or P1 mouse pups. In brief, fetuses were removed under sterile conditions and kept in phosphate-buffered saline on ice. The cortices of rat embryos or mouse pups were dissected in Hanks' balanced salt solution buffer (Invitrogen) on ice. The tissue was minced with a scalpel and then dissociated by triturating with fire-polished glass Pasteur pipettes. The cortical cells were counted and then plated on tissue culture dishes that were pre-coated overnight with poly-d-lysine (0.1 mg/ml, Sigma). The neurons were cultured in Neurobasal with a B27 supplement and GlutaMAX (Invitrogen) for 12–24 days in vitro. HEK293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fatal bovine serum. For transfection experiments, cells were plated for 12 h at a density of 1 × 106 cells/10-cm culture dish and were transfected using a Lipofectamine method (Invitrogen). 24 h after transfection, cultured cells were lysed in modified radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, and proteinase inhibitors), and lysates were resolved on SDS-PAGE and subjected to immunoblotting.
GST Pulldown Assay
HEK293 cells stably expressing TrkB (12) were lysed in modified radioimmune precipitation assay buffer. Cell lysates were incubated with GST fusion proteins (2–5 μg) immobilized on glutathione-Sepharose beads at 4 °C overnight with constant rocking. The bound proteins were resolved on SDS-PAGE and subjected to immunoblotting analysis.
In Vitro Kinase Assay
HA epitope-tagged Src and FLAG epitope-tagged TrkB-ICD transiently expressed in HEK293 cells were immunoprecipitated with antibodies to HA and FLAG, respectively. The kinase assay was carried out at 30 °C for 20 min in kinase assay buffer (20 mm MOPS, pH 7.4, 15 mm MgCl2, 200 μm ATP) in the presence of vehicle or SMI11293 (100 nm). The phosphorylated proteins were resolved on SDS-PAGE. The phosphorylation states of Src and TrkB-ICD were monitored by immunoblotting with antibodies to pSrc (Tyr-416) and pTrk (Tyr-515). For measuring the phosphorylation of TrkB by Src, GST, or GST-TrkB-ICD expressed in SL21 bacteria were purified with glutathione-Sepharose beads and subsequently incubated with active Src protein (Upstate) in a 1:1 molar ratio. The phosphorylation states of TrkB-ICD were monitored by immunoblotting with pTrk (Tyr-705/Tyr-706) and pTrk (Tyr-515) antibodies.
Immunoprecipitation and Immunoblotting
Cells were lysed in modified radioimmune precipitation assay buffer and centrifuged at 16,000 × g for 10 min; the supernatant was defined as cell lysate. Acutely isolated mouse hippocampal slices were homogenized in buffer (10 mm Tris·HCl, pH 7.2, and 0.32 mm sucrose) and centrifuged at 1,500 × g for 10 min. The supernatant was saved for further analysis. Immunoprecipitations were performed as described previously (12). In brief, cell lysates (500 μg) were incubated with indicated antibodies (1 μg) and protein A/G beads at 4 °C overnight. Cell lysates (10–20 μg) or immunoprecipitates were resolved by SDS-PAGE. The blots were incubated overnight with primary antibodies and subsequently with secondary antibodies (1:5,000) for 1 h at room temperature. The antibodies and dilution used in this study are as follow: pTrk (pY515), pTrk (pY705/706), pSrc (Tyr-416), pAkt, pErk, Erk (1:1,000, Cell Signaling); TrkB, Fyn (1:500, BD Transduction Laboratories); pan-Trk (Santa Cruz Biotechnology); Src (GD11), phosphotyrosine (4G10, 1:1,000, Upstate); and β-actin (1:10,000, Sigma). pTrkB (pY816, 1:1,000) was kindly provided by Dr. Moses Chao (New York University). The immunoblots were developed with enhanced chemiluminescence (ECL, Amersham Biosciences). An equivalent amount of protein loaded in each lane was verified with immunoblotting with antibodies to TrkB, Erk, or actin. Shown are representative results of immunoblotting from at least three independent experiments.
Acute Hippocampal Slice Preparation
Acute hippocampal slices were prepared as described previously (12). In brief, mice (P28–P42) were anesthetized with halothane and decapitated. The brain was quickly removed and placed in ice-cold sucrose slicing artificial cerebrospinal fluid containing (in mm): sucrose 110, NaCl 60, KCl 3, NaH2PO4 1.25, NaHCO3 28, CaCl2 0.5, MgCl2 7.0, and Dextrose 5, saturated with 95% O2 plus 5% CO2. Transverse hippocampal slices (400 μm in thickness) were prepared using a tissue chopper and incubated in oxygenated normal artificial cerebrospinal fluid containing (in mm): NaCl 124, KCl 1.75, KH2PO4 1.25, NaHCO3 26, CaCl2 2.4, MgCl2 1.3, and Dextrose 10 for at least 1 h at room temperature before treatments.
RESULTS
Zinc Induces Phosphorylation of Tyr-705/Tyr-706 of TrkB by a TrkB Kinase-independent Mechanism
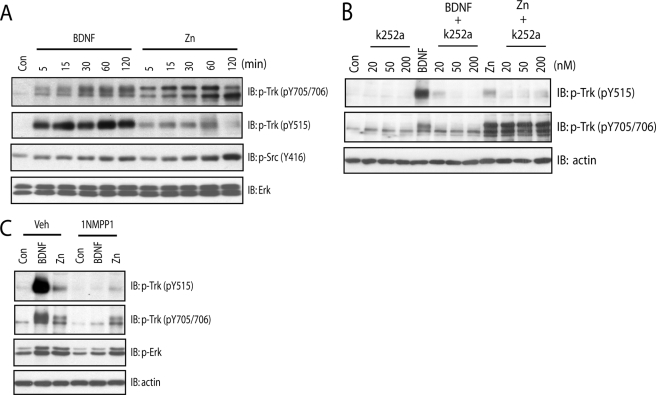
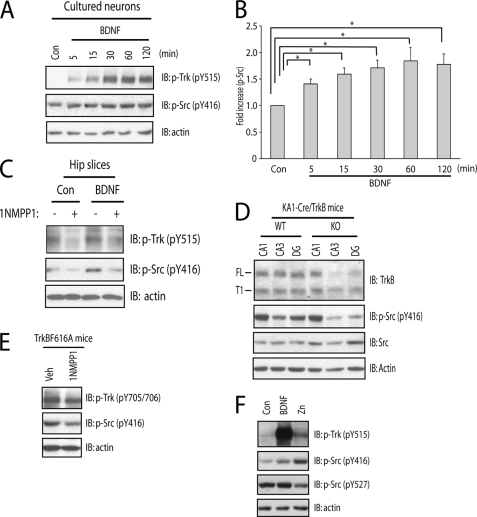
We have previously shown that zinc transactivates TrkB, but the detail of phosphorylation events by which TrkB is activated is incompletely understood. The model for TrkB activation by neurotrophin ligands, including BDNF, has been proposed. The binding of BDNF homodimers to the ectodomain of TrkB induces receptor dimerization and a conformational change in the intracellular domain, permitting binding of ATP to lysine and resulting in autophosphorylation of Tyr-705/Tyr-706 within the activation loop of TrkB kinase domain, a critical signaling event required for enhanced intrinsic kinase activity. This is followed by phosphorylation of Tyr-515 and Tyr-816 and subsequent activation of downstream signaling cascades, including Shc-Ras-MAPK, PI3K/Akt, and PLCγ1 signaling pathways (9, 10). Therefore, the phosphorylation state of tyrosines of TrkB is considered as a surrogate measure of its activation (16, 17). Consistent with this model, incubation of BDNF in cultured neurons resulted in marked increases of phosphorylation of Tyr-705/Tyr-706 and Tyr-515 (Fig. 1A) and Tyr-816 (data not shown) in a time-dependent manner. We asked whether zinc-mediated transactivation of TrkB induces a pattern of increased tyrosine phosphorylation similar to that of BDNF. Likewise, zinc induced dramatic increases of phosphorylation of Tyr-705/Tyr-706 (Fig. 1A) yet unexpectedly induced only modest increases of phosphorylation of Tyr-515 in cultured neurons (Fig. 1A; also see Ref. 12). This finding was confirmed by quantitative analysis. The molecular mechanism underlying differential phosphorylation of tyrosine residues of TrkB induced by zinc compared with BDNF is unclear.
FIGURE 1.
Zinc increases phosphorylation of Tyr-705/Tyr-706 in a TrkB kinase-independent mechanism. In all of the experiments below, E18 rat (A and B) or P0-P1 mouse (C) cortical neurons were maintained in vitro for 12–14 days. Cell lysates were subjected to immunoblotting with the indicated antibodies after the treatments described. A, exogenous zinc induced preferential phosphorylation of Tyr-705/Tyr-706 in a time-dependent manner. Cortical neurons were incubated with vehicle, BDNF (10 ng/ml), or zinc (100 μm) for the indicated periods of time. B, the small molecule Trk inhibitor K252a reduced zinc-induced phosphorylation of Tyr-515 but not Tyr-705/Tyr-706. Cortical neurons were preincubated with vehicle or indicated concentrations of K252a for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm). C, the small molecule Trk inhibitor 1NMPP reduced zinc-induced phosphorylation of Tyr-515 but not Tyr-705/Tyr-706. Cortical neurons cultured from TrkBF616A mice were preincubated with vehicle or 1NMPP1 (1 μm) for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm).
The model by which BDNF activates TrkB also predicts that limiting access of ATP to its binding site in the kinase domain of TrkB should abolish autophosphorylation of Tyr-705/Tyr-706 and other tyrosine residues, including Tyr-515 and Tyr-816. If correct, a small molecule that selectively inhibits ATP binding will prevent BDNF-mediated activation of TrkB. We tested this idea in two ways, namely pharmacology and chemical genetics. As predicted, BDNF induced robust increases of phosphorylation of Tyr-705/Tyr-706 as well as Tyr-515 in cultured neurons (Figs. 1B and 2E). Prevention of ATP binding to TrkB by K252a, a selective Trk inhibitor, abolished BDNF-induced phosphorylation of Tyr-705/Tyr-706 and Tyr-515 in cultured rat cortical neurons in a concentration-dependent fashion (Fig. 1B). The requirement of ATP binding to TrkB for receptor autophosphorylation was also examined by a chemical-genetic approach. Introduction of a phenylalanine to alanine substitution within kinase subdomain V (TrkBF616A) of TrkB exon 14 in the region of the TrkB kinase ATP binding pocket (17) renders TrkBF616A sensitive to specific inhibition by a cell membrane-permeable, small-molecule PP1 derivative, 1NMPP1 (17, 12). Consistent with this prediction, addition of 1NMPP1 prevented BDNF-induced phosphorylation of Tyr-705/Tyr-706 and Tyr-515 in cortical neurons cultured from TrkBF616A mice (Figs. 1C and 2E). Furthermore, activation of the downstream signaling proteins such as pErk was also impaired in the presence of 1NMPP1 (Fig. 1C). Importantly, 1NMPP1 did not affect BDNF-induced phosphorylation of TrkB in cortical neurons cultured from wild-type (WT) mice (data not shown), thereby demonstrating the specificity of inhibition by 1NMPP1.
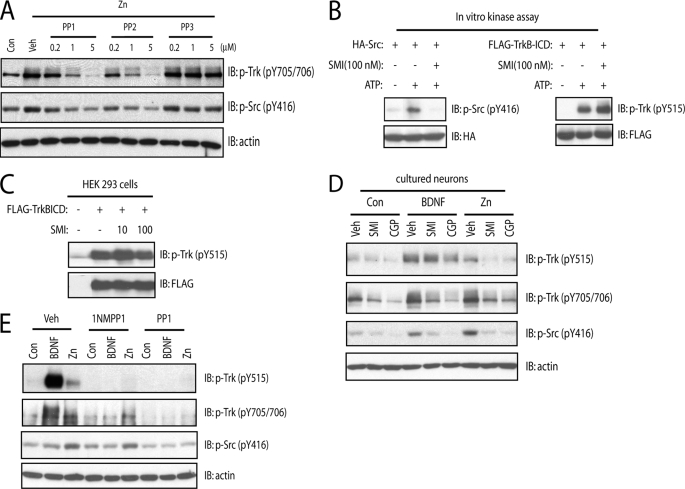
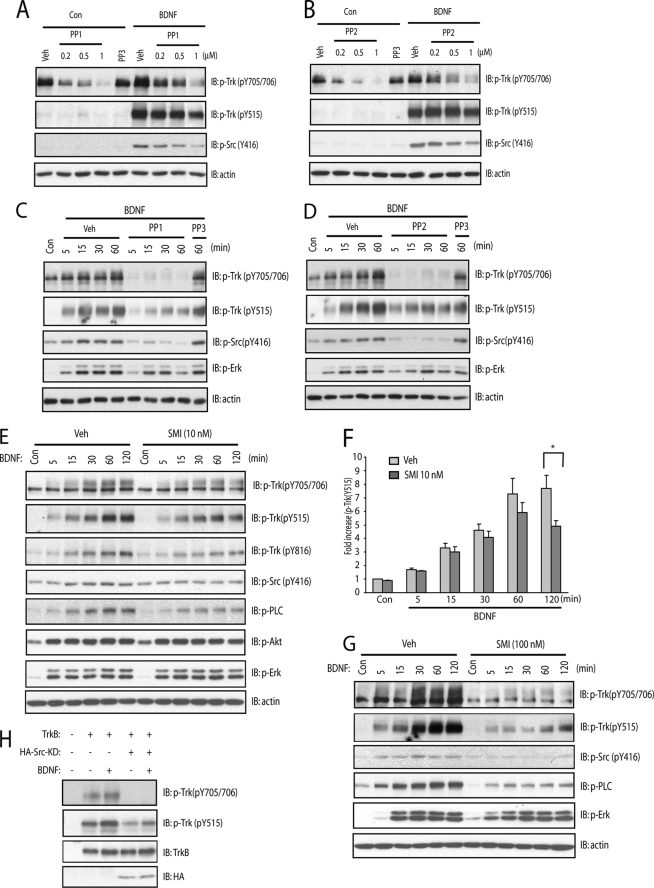
FIGURE 2.
SFKs are required for zinc-induced phosphorylation of Tyr-705/Tyr-706. In all of the experiments below, E18 rat (A–D) or P0–P1 mouse (E) cortical neurons were maintained in vitro for 12–14 days. Unless specified otherwise, cell lysates were subjected to immunoblotting with the indicated antibodies after the treatments described. A, the small molecule inhibitors of SFKs reduced exogenous zinc-mediated phosphorylation of Tyr-705/Tyr-706 in a concentration-dependent manner. Cortical neurons were preincubated with varying concentrations of SFK inhibitors, PP1 or PP2, or the inactive analog, PP3, for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle or zinc (100 μm). B and C, the specificity of a novel small molecule SFK inhibitor, SMI11293 compound. Src or TrkB-ICD proteins transiently expressed in HEK293 cells were purified by immunoprecipitation with anti-HA and FLAG antibodies, respectively. The immunoprecipitates were incubated in the kinase assay buffer in the presence of vehicle or SMI11293 (100 nm). In C, HEK293 cells expressing TrkB-ICD were incubated with vehicle or indicated concentrations of SMI11293 compound for 30 min. The phosphorylation of Src or TrkB was evident with immunoblotting with indicated antibodies. D, the structurally distinct small molecule SFK inhibitors prevented exogenous zinc-induced phosphorylation of Tyr-705/Tyr-706. Cortical neurons were preincubated with SFK inhibitors, SMI11293 (10 nm), or CGP76030 (100 nm) for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm). E, the SFK inhibitor PP1 reduced exogenous zinc-induced phosphorylation of Tyr-705/Tyr-706 in mouse TrkBF616A neurons. Cortical neurons were preincubated with vehicle, 1NMPP1 (1 μm), or PP1 (1 μm) for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle, BDNF (10 ng/ml), or zinc (100 μm).
The divergent pattern of increased pTrkB induced by zinc compared with BDNF led us to ask whether TrkB kinase is also required for transactivation by zinc. Unexpectedly, prevention of ATP binding to TrkB by K252a did not inhibit zinc-induced phosphorylation of Tyr-705/Tyr-706 but reduced phosphorylation of Tyr-515 (Fig. 1B). Likewise, prevention of ATP binding to TrkB by 1NMPP1 resulted in minimal inhibition of zinc-mediated phosphorylation of Tyr-705/Tyr-706 but reduced phosphorylation of Tyr-515 (Figs. 1C and 2E), thereby demonstrating that the kinase activity intrinsic to TrkB is required for zinc-induced phosphorylation of Tyr-515 but not Tyr-705/Tyr-706. The differences in phosphorylation of Tyr-705/Tyr-706 compared with Tyr-515 together with differences in inhibition mediated by small molecules preventing binding of ATP to TrkB reveal fundamental differences in the mechanisms by which TrkB is activated by BDNF compared with zinc. Specifically, these results suggest that zinc-mediated phosphorylation of Tyr-705/Tyr-706 is independent of TrkB kinase activity.
Src Family Kinases Are Required for Zinc-induced Phosphorylation of Tyr-705/Tyr-706 of TrkB
Insight into the kinase(s) required for zinc-induced phosphorylation of TrkB is critical for understanding the signaling mechanisms by which TrkB is transactivated by zinc. Two facts led us to suspect that a SFK was required: (a) exogenous zinc activates SFK in cultured cortical neurons (12) and (b) Src phosphorylates another receptor tyrosine kinase, namely the EGF receptor, on the tyrosine residue analogous to Tyr-705/Tyr-706 of TrkB. If our suspicion was correct, inhibition of SFKs will reduce zinc-mediated phosphorylation of Tyr-705/Tyr-706. We tested this idea with four structurally distinct small molecule inhibitors of SFK activation. In accord with our previous findings, addition of zinc (100 μm) to cultured neurons induced increased phosphorylation of both Tyr-705/Tyr-706 and Tyr-416 of SFKs (a surrogate measure of SFK activation) (Fig. 2A; also see Ref. 12). The SFK inhibitors, PP1 and PP2, but not their inactive structural analog, PP3, inhibited zinc-induced phosphorylation of Tyr-705/Tyr-706 and pSrc with similar concentration dependence (Fig. 2A). To further test this idea, we examined the effect of an additional structurally distinct SFK inhibitor, namely SMI11293. Pretreatment of SMI11293 (10 nm) reduced exogenous zinc-mediated phosphorylation of Tyr-705/Tyr-706 as well as Tyr-515 in cultured neurons (Fig. 2D). Because treatment with zinc activated both SFKs and TrkB, the question arose as to whether SMI11293 (10 nm) directly inhibited either or both SFKs and TrkB kinase. To address this question, we examined the effects of SMI11293 on SFKs and TrkB in cell-free systems in vitro. Epitope-tagged Src and the ICD of TrkB were expressed in HEK293 cells and purified by immunoprecipitation, and kinase assay was measured in the presence of ATP. Addition of SMI11293 (100 nm) abolished phosphorylation of Src yet did not inhibit phosphorylation of ICD (Fig. 2B), demonstrating that SMI11293 at concentrations up to 100 nm did not directly inhibit TrkB kinase activity in vitro. That SMI11293 had little effect on autophosphorylation of ICD present in HEK293 cells (Fig. 2C) further supported the specificity of inhibition of SMI11293 of SFKs but not TrkB expressed in mammalian cells. Finally, a fourth structurally distinct SFK inhibitor CGP76030 (100 nm) inhibited zinc-induced phosphorylation of Tyr-705/Tyr-706 and Tyr-515 in cultured neurons (Fig. 2D). In sum, the inhibition of zinc-mediated activation of SFKs and TrkB by four structurally distinct small molecules supports the assertion that SFKs are required for zinc-mediated transactivation of TrkB.
The evidence above led us to query whether SFKs were required for the zinc-mediated phosphorylation of TrkB that persisted in the presence of TrkB kinase inhibition effected by 1NMPP1 treatment of TrkBF616A neurons. Indeed, the zinc-induced phosphorylation of Tyr-705/Tyr-706 in the presence of 1NMPP1 in TrkBF616A neurons was abolished by inclusion of PP1 (Fig. 2E) but not PP3 (data not shown). This demonstrates that the TrkB kinase-independent phosphorylation of Tyr-705/Tyr-706 induced by zinc requires SFK activity.
SFK Directly Phosphorylates Tyr-705/Tyr-706 of TrkB
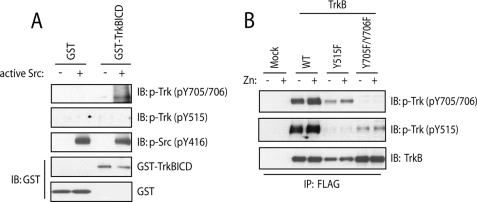
The requirement of SFKs for zinc-mediated activation of TrkB raises the possibility that SFKs can directly phosphorylate TrkB. Moreover, the fact that SFKs were required for TrkB kinase-independent phosphorylation of Tyr-705/Tyr-706 suggested that SFKs may directly phosphorylate TrkB on residues Tyr-705/Tyr-706 in particular. To test this idea, we asked whether purified active Src could phosphorylate the purified recombinant intracellular domain of TrkB (ICD) in a cell-free system. Consistent with our prediction, addition of Src resulted in increased phosphorylation of Tyr-705/Tyr-706 as evidenced by immunoblotting with antibodies to pTrk (Fig. 3A) or phosphotyrosines (data not shown). Despite directly phosphorylating TrkB Tyr-705/Tyr-706, Src did not phosphorylate Tyr-515 of TrkB. In sum, these findings demonstrate that SFKs can directly phosphorylate TrkB at Tyr-705 and Tyr-706 in vitro.
FIGURE 3.
Phosphorylation of Tyr-705/Tyr-706 promotes zinc-induced transactivation of TrkB. A, Src directly phosphorylated TrkB in a cell-free system. Purified GST-TrkB-ICD protein was incubated with active Src at 30 °C for 20 min in kinase assay buffer. B, Tyr-705/Tyr-706 facilitated exogenous zinc-induced phosphorylation of TrkB. HEK 293 cells stably expressing WT or indicated tyrosine mutants of TrkB were incubated with vehicle or exogenous zinc (500 μm) for 20 min. TrkB was immunoprecipitated with FLAG antibody. The immunoprecipitates or kinase reaction mixtures were resolved by SDS-PAGE and subjected to immunoblotting with indicated antibodies.
Phosphorylation of Tyr-705/Tyr-706 Is Essential for Zinc-induced Transactivation of TrkB
The fact that zinc induces phosphorylation of Tyr-705/Tyr-706 in an SFK requiring but TrkB kinase-independent mechanism raises the question as to the role of phosphorylation of Tyr-705/Tyr-706 in Trk activation. Studies of Trks and other receptor tyrosine kinases suggest a model whereby phosphorylation of tyrosine residues within the activation loop analogous to Tyr-705/Tyr-706 facilitates activation of intrinsic kinase activity and subsequent phosphorylation of other tyrosine residues of the intracellular domain. We therefore hypothesized a signaling event whereby zinc initially activates SFKs, which phosphorylate Tyr-705/Tyr-706, which in turn promotes activation of intrinsic kinase activity of TrkB and phosphorylation of other tyrosine residues such as Tyr-515. If correct, mutation of Tyr-705 and Tyr-706 should prevent zinc-induced phosphorylation of Tyr-515. To test this idea, a series of FLAG-tagged tyrosine point mutants of TrkB were generated and stably expressed in HEK293 cells. Addition of zinc to HEK cells expressing WT TrkB induced phosphorylation of both Tyr-705/Tyr-706 and Tyr-515 (Fig. 3B). As predicted, addition of zinc to HEK cells expressing Y705F/Y706F mutant reduced zinc-mediated increase of phosphorylation of Tyr-515. By contrast, addition of zinc to HEK cells expressing Y515F mutant was still capable of inducing increased phosphorylation of Tyr-705/Tyr-706. Note that Y705F/Y706F mutant was still able to be phosphorylated at Tyr-515, probably due to overexpression-induced forced phosphorylation and consistent with previous findings (16, 18). Collectively, these findings support the assertion that phosphorylation of Tyr-705/Tyr-706 is the initial event in zinc-mediated transactivation of TrkB.
Association of SFKs with TrkB
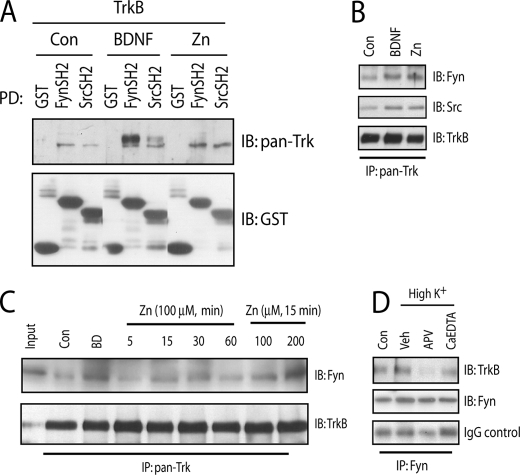
The requirement of SFK activation for zinc-induced transactivation of TrkB together with the fact that SFKs can directly phosphorylate TrkB raises the possibility that SFKs are physically associated with TrkB and that the physical association can be regulated by zinc. The binding of SFKs to receptor tyrosine kinases is mediated by interaction of the SH2 domain of SFKs with phosphotyrosines of receptor (13). We investigated the interactions of SFKs and TrkB with two approaches, namely SH2 protein pulldown and co-immunoprecipitation assays. We first asked whether SH2 domains of Fyn and Src are able to pull down TrkB stably expressed in heterologous cells. Under control conditions, the SH2 domains of both Fyn and Src pulled down TrkB (Fig. 4A). Incubation of the HEK cells with zinc (500 μm for 20 min) increased the TrkB pulled down by both Fyn and Src (Fig. 4A). Next, we addressed whether zinc can induce the association of TrkB and SFKs in cultured neurons. These data revealed that addition of exogenous zinc enhanced the amounts of both Src and Fyn in the immunoprecipitates of Trk receptors (Fig. 4B), consistent with the idea that zinc promotes the association of Src and Fyn with TrkB in cultured neurons. Furthermore, the effect of zinc on co-immunoprecipitation of TrkB and Fyn was both concentration- and time-dependent Fig. 4C). Together these findings support the idea that the SH2 domains of both Fyn and Src can interact with TrkB and that these interactions can be enhanced by zinc.
FIGURE 4.
SFKs are associated with TrkB. In the experiments below (B–D), E18 rat cortical neurons were maintained in vitro for 12–14 days. Unless specified otherwise, cell lysates or proteins were subjected to immunoblotting with the indicated antibodies after the treatments described. A, association of TrkB and SH2 domains of Src and Fyn. HEK 293 cells stably expressing TrkB were incubated with vehicle, BDNF (10 ng/ml), or exogenous zinc (500 μm) for 20 min. Cell lysates were incubated with GST alone or GST-SH2 domain fusions of Src and Fyn conjugated with glutathione-Sepharose beads. B, BDNF and exogenous zinc promoted the association of Src and Fyn with Trk receptors in cultured neurons. Cortical neurons were incubated with vehicle, BDNF (10 ng/ml), or zinc (100 μm) for 30 min. C, BDNF and exogenous zinc promoted the association of Fyn and Trk receptors in cultured neurons in a time- and concentration-dependent manner. Cortical neurons were incubated with vehicle, BDNF (10 ng/ml), or zinc (100 μm) for the indicated concentration or periods of time. D, neuronal activity facilitated the association of Fyn and TrkB in cultured neurons. Cortical neurons were preincubated with APV (50 μm) or CaEDTA (1 mm) for 15 min; each of these treatments was continued for an additional 30 min in the presence of a high (50 mm) K+ buffer.
Because we previously showed that KCl facilitates zinc-mediated transactivation of TrkB in cultured neurons (12), we inquired whether the interaction of Fyn and TrkB is enhanced by KCl. Brief addition of 50 mm KCl (20 min) to cultured neurons enhanced the amount of TrkB co-immunoprecipitated with Fyn (Fig. 4D), suggesting that enhanced neuronal activity facilitates the association of Fyn and TrkB. Interestingly, inclusion of the N-methyl-d-aspartic acid receptor antagonist, AP5, reduced the KCl-induced increase of binding of Fyn and TrkB. Likewise, inclusion of cell membrane-impermeable-specific zinc chelator CaEDTA reduced the amount of TrkB in the immunoprecipitates of Fyn (Fig. 4D). Collectively, our results demonstrate that zinc promotes the association of Fyn and TrkB in cultured neurons. Our results are consistent with the idea that neuronal activity facilitates the association of Fyn and TrkB in part through endogenous zinc.
SFKs Are Downstream of TrkB
The above findings demonstrate that zinc-mediated transactivation of TrkB requires SFKs, implying that SFKs are upstream of TrkB in this setting. The fact that exogenous zinc facilitated recruitment of SFKs to TrkB in turn raised the possibility that SFKs could be activated as a consequence of TrkB activation. The fact that SFKs are upstream of TrkB when transactivated by zinc complicates addressing this question with zinc. We therefore asked whether a neurotrophin that activates TrkB directly by binding to its ectodomain in turn results in activation of SFKs.
To address this question, we first asked whether SFKs are recruited to TrkB when activated by BDNF. Brief incubation of TrkB-expressing HEK cells with BDNF enhanced the amounts of TrkB pulled down by the SH2 domains of both Src and Fyn (Fig. 4A). Addition of BDNF to cultured neurons also increased the amount of TrkB co-immunoprecipitated with both Src and Fyn (Fig. 4, B and C), thereby confirming and extending previous findings (14). Moreover, incubation of cultured neurons with BDNF increased phosphorylation of Tyr-416 (pY416) of SFKs, a surrogate measure of SFK activity, in a time-dependent manner (Fig. 5, A and B). The activation of SFKs by BDNF requires TrkB kinase activity, because inclusion of 1NMPP1 abolished BDNF-induced increases of pY416 in both cultured cortical neurons and acute hippocampal slices isolated from TrkBF616A mice (Figs. 2E and 5C, respectively).
FIGURE 5.
SFK activity is enhanced by the activation of TrkB. In the experiments below (A and F), E18 rat cortical neurons were maintained in vitro for 12–14 days. Unless specified otherwise, cell lysates were subjected to immunoblotting with the indicated antibodies after the treatments described. A, BDNF induced modest increases of phosphorylation of Tyr-416 of SFKs (pY416) in vitro in a time-dependent manner. Cortical neurons were incubated with vehicle or BDNF (10 ng/ml) for the indicated periods of time. B, quantitative analysis of BDNF-induced increased pSrc (Y416) in A. Statistical analyses were performed by one-way analysis of variance with post hoc test. *, indicates p < 0.05. C, 1NMPP1 reduced BDNF-mediated increased pSrc in ex vivo. Hippocampal slices acutely isolated from TrkBF616A mice were preincubated with vehicle or 1NMPP1 (1 μm) for 30 min followed by an additional incubation of vehicle or BDNF (20 ng/ml) for 20 min. D, conditional deletion of TrkB postnatally reduced phosphorylation of TrkB protein in hippocampal membranes ex vivo. Hippocampal CA1, CA3, and dentate gyrus regions were isolated by microdissection from WT and TrkB knock-out mice. E, 1NMPP1 reduced pSrc in vivo. TrkF616A mice were treated with vehicle or 1NMPP1 (25 μm) in drinking water for 7 days. F, the effect of BDNF on the phosphorylation of Y527 of SFKs. Cortical neurons were incubated with vehicle, BDNF (10 ng/ml), or zinc (100 μm) for 30 min.
We also asked whether SFK activation is dependent upon TrkB in vivo. To address this issue, two different approaches were employed, namely conditional deletion of TrkB from subsets of CNS neurons and chemical-genetic inhibition of TrkB kinase activity. Conditional deletion of TrkB in KA1-cre/TrkBf/f mice resulted in striking reductions of TrkB protein in dentate gyrus and CA3 but not CA1 of hippocampus (Fig. 5D); interestingly, the reductions of TrkB correlated with striking reductions of pY416 of SFKs. In separate experiments, TrkB kinase activity was selectively inhibited by treatment of adult TrkBF616A mice with 1NMPP1 in drinking water for 7 days (Fig. 5E); the modest reduction of pTrkB was paralleled by a modest reduction of pY416 of SFKs (Fig. 5E), suggesting that persistence of basal activity of SFKs is regulated by TrkB in vivo. Collectively, these results demonstrate that TrkB, in particular TrkB kinase activity, promotes SFK activation in vivo.
We have proposed that zinc activates SFKs by inhibiting C-terminal Src kinase (Csk), thereby resulting in reduced phosphorylation of Tyr-527 of SFK (12). Here we asked whether BDNF induces increased SFK activity by a mechanism similar to zinc. Addition of exogenous zinc resulted in an increase of pY416 paralleled by a reduction of pY527 (Fig. 5F), thereby confirming our prior finding (12). In contrast to zinc, addition of BDNF induced a modest increase of pY416 while the level of pY527 remained unchanged (Fig. 5F). These results support the conclusion that BDNF enhances SFK activation by a Csk-independent mechanism.
SFKs Facilitate BDNF-induced Autophosphorylation of TrkB
The fact that SFKs are upstream of TrkB when activated by zinc and that SFKs are downstream of TrkB when activated by BDNF raised the following question: might SFKs activated by TrkB in turn feed back and promote phosphorylation of TrkB itself? The fact that Src phosphorylates Tyr-705/Tyr-706 in particular (Fig. 3A) predicted that inhibition of SFK activity would limit the extent of pTyr-705/Tyr-706 induced by BDNF. We used both pharmacological and dominant negative approaches to test this idea.
Four structurally distinct inhibitors were employed to inhibit SFK activity when activated by BDNF. First, BDNF-induced phosphorylation of Tyr-705/Tyr-706 in cultured neurons was reduced by the SFK inhibitor, PP1, in a concentration- and time-dependent manner, whereas an inactive structural analog, PP3, was ineffective (Fig. 6, A and C). Similar results were obtained with another SFK inhibitor, PP2 (Fig. 6, B and D). A third SFK inhibitor, SMI11293, reduced BDNF-induced phosphorylation of Tyr-705/Tyr-706 in a time- and concentration-dependent manner (Fig. 6, E and G). A fourth SFK inhibitor, CGP76030 (0.1 μm), also reduced BDNF-induced phosphorylation of Tyr-705/Tyr-706 (Fig. 2D). We also demonstrated that overexpression of a dominant negative mutant of Src inhibited BDNF-induced phosphorylation of Tyr-705/Tyr-706 of TrkB in HEK293 cells (Fig. 6H), thereby reinforcing the pharmacological findings.
FIGURE 6.
SFKs is involved in BDNF-induced phosphorylation of Tyr-705/Tyr-706. In the experiments below, E18 rat cortical neurons were maintained in vitro for 12–14 days. Unless specified otherwise, cell lysates were subjected to immunoblotting with the indicated antibodies after the treatments described. A, the small molecule SFK inhibitor, PP1, inhibited BDNF-induced phosphorylation of TrkB in a concentration-dependent manner. Cortical neurons were preincubated with indicated concentrations of PP1 for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle or BDNF (10 ng/ml). B, the small molecule SFK inhibitor, PP2, inhibited BDNF-induced phosphorylation of TrkB in a concentration-dependent manner. Cortical neurons were preincubated with indicated concentrations of PP2 for 15 min; each of these treatments was continued for an additional 15 min in the presence of vehicle or BDNF (10 ng/ml). C, PP1 inhibited BDNF-induced phosphorylation of TrkB in a time-dependent manner. Cortical neurons were preincubated with PP1 (1 μm) or PP3 (1 μm) for 15 min; each of these treatments was continued for the indicated periods of time in the presence of vehicle or BDNF (10 ng/ml). D, PP2 inhibited BDNF-induced phosphorylation of TrkB in a time-dependent manner. Cortical neurons were preincubated with PP2 (1 μm) or PP3 (1 μm) for 15 min; each of these treatments was continued for the indicated periods of time in the presence of vehicle or BDNF (10 ng/ml). E and G, SMI11293 reduced phosphorylation of TrkB in a time-dependent manner. Cortical neurons were preincubated with SMI11293 (10 nm in E; 100 nm in G) for 15 min; each of these treatments was continued for the indicated periods of time in the presence of vehicle or BDNF (10 ng/ml). F, quantitative analysis of SMI11293-mediated reduction of phosphorylation of Tyr-515 induced by BDNF in E. H, overexpression of the dominant negative form of Src protein reduced phosphorylation of TrkB in heterologous cells. HEK293 cells were transiently transfected with plasmids expressing mock, TrkB alone, or TrkB together with HA-tagged dominant negative Src (Src-KD). Transfected cells were incubated with vehicle or BDNF (10 nm) for 15 min. Statistical analyses were performed by one-way analysis of variance with post hoc test. *, p < 0.05.
One striking feature of each of the experiments described in the preceding paragraph was that inhibition of SFKs resulted in smaller reduction of BDNF-induced phosphorylation of Tyr-515 than of Tyr-705/Tyr-706 (Fig. 6, E and F). This finding is consistent with earlier findings (Fig. 3A) that SFKs can directly phosphorylate Tyr-705/Tyr-706. Collectively, these findings support the assertion that SFKs activated by TrkB in turn feed back and promote phosphorylation of TrkB itself.
We also examined the role of SFKs on signaling pathways downstream of TrkB. Interestingly, TrkB-mediated PLCγ1 activation was inhibited by SMI11293 in a concentration-dependent manner as evident by probing immunoblots with antibodies to pY816 of Trk receptors and pPLCγ1 (Fig. 6, E and G), surrogate measures of activation of TrkB-PLCγ1 signaling pathway. By contrast, only a minimal reduction of pErk and pAkt was detected in the presence of SMI11293 (Fig. 6, E and G). These results suggest that SFKs function differently on distinct signaling pathways downstream of TrkB.
SFKs Are Involved in BDNF-induced Regulation of K+-Cl− Cotransporter (KCC2) Expression
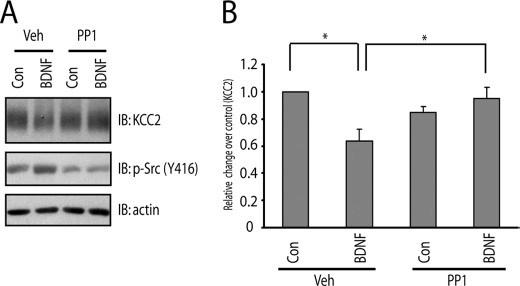
Our results reveal a role of SFKs in TrkB signaling and raise the question as to whether SFKs are required for some molecular consequences of TrkB activation. One such molecular consequence is the reduced expression of the K+-Cl− cotransporter, KCC2, induced by BDNF. Reduced expression of KCC2 is of particular interest, because the change in chloride gradient resulting from its reduced expression in the mature brain can transform GABA-mediated neuronal responses from hyperpolarizing to depolarizing, a cellular mechanism identified in disorders of neuropathic pain and epilepsy (19). Consistent with previous findings in organotypic hippocampal slices, addition of BDNF to cultured cortical neurons for 60 min reduced KCC2 protein (Fig. 7A). Pretreatment with the SFK inhibitor, PP1 (1 μm), prevented the BDNF-mediated reduction of KCC2 protein (Fig. 7, A and B). These results were confirmed with other SFK inhibitors, including SMI11293 and PP2 (data not shown), reinforcing the role of SFK activity in TrkB-mediated regulation of KCC2 protein content.
FIGURE 7.
SFKs are required for BDNF-induced down-regulation of Cl−, K+ co-transportor (KCC2) expression. E18 rat cortical neurons were maintained in vitro for 21 days. A, PP1 prevented BDNF-mediated reduction of KCC2 protein content. Cortical neurons were preincubated with vehicle or PP1 (1 μm) for 15 min; each of these treatments was continued for 60 min in the presence of vehicle or BDNF (10 ng/ml). B, quantitative analysis of the effect of PP1 on BDNF-induced reduction of KCC2 in A. Statistical analyses were performed by student's t test. *, p < 0.05.
DISCUSSION
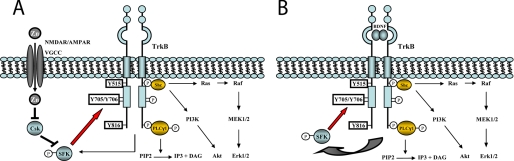
Our objective was to elucidate the interplay of SFKs and the neurotrophin receptor TrkB. Toward that end, we compared and contrasted the role of SFKs in activation of TrkB by two mechanisms, namely its transactivation by zinc and its activation by its prototypic neurotrophin ligand, BDNF. We used biochemical, pharmacological, and chemical-genetic studies of cultured cortical neurons and recombinant proteins in heterologous cells in vitro and acute hippocampal slices and hippocampal membranes ex vivo. Several principal findings emerged. With respect to zinc-mediated transactivation of TrkB, exogenous zinc promoted the preferential phosphorylation of Tyr-705/Tyr-706 by a SFK-dependent but TrkB kinase-independent mechanism. This signaling event is critical to zinc-mediated transactivation of TrkB. With respect to BDNF, its activation of TrkB resulted in increased SFK activity, demonstrating SFK to be downstream of TrkB. SFK activity is required for the TrkB-mediated reduction of KCC2 protein content in cultured neurons. Interestingly, inhibition of SFKs preferentially inhibited BDNF-induced phosphorylation of Tyr-705/Tyr-706, revealing a regulatory role of SFKs in the activation of TrkB by BDNF. Collectively, these findings support a model in which SFKs and TrkB are mutually regulated (Fig. 8).
FIGURE 8.
Model of mutual regulation of SFKs and TrkB signaling. A, SFKs are essential for zinc-induced transactivation of TrkB. Synaptically released zinc permeates neurons through N-methyl-d-aspartic acid receptors (NMDAR), calcium-permeable AMPA receptors (AMPAR), and voltage-gated calcium channels (VGCC). Increased intracellular zinc results in activation of SFKs through inhibiting C-terminal Src kinase (Csk) activity. Activated SFKs in turn promote phosphorylation of Tyr-705/Tyr-706 of TrkB and subsequent phosphorylation of other tyrosines, including Tyr-515, triggering activation of downstream signaling cascades. It seems likely that transactivated TrkB subsequently promotes SFK activity. B, SFKs are both downstream and upstream of TrkB signaling activated by BDNF. TrkB activation by BDNF promotes the association of SFKs and TrkB and SFK activity through an as yet unidentified mechanism. Activated SFKs in turn feedback and promote full activation of TrkB through facilitating phosphorylation of Tyr-705/Tyr-706.
Specificity of Small Molecule Inhibitors of SFKs
While the various compounds used here are known to inhibit SFKs, demonstrating that these compounds inhibit SFKs but not TrkB kinase directly is critical to meaningful interpretation of several experiments. The following evidence supports the specificity of these compounds for SFK inhibition. 1) Obtaining similar results with each of four structurally distinct small molecule inhibitors of SFKs renders it unlikely that each compound has the same off-target effect, namely direct inhibition of TrkB kinase. 2) Concentrations of a novel SFK inhibitor, SMI11293, that inhibited SFKs were ineffective in inhibition of TrkB kinase activity in a cell-free system. 3) Whereas selective inhibition of TrkB kinase by 1NMPP1 (Fig. 1C) eliminated phosphorylation of both Tyr-705/Tyr-706 and Tyr-515 induced by BDNF, SFK inhibitors preferentially reduced BDNF-mediated phosphorylation of Tyr-705/Tyr-706 compared with Tyr-515 (Fig. 6, E and F). The distinct pattern of inhibition implies a distinct mechanism. 4) The effects of SFK inhibitors were mimicked by a dominant negative mutant of Src, which inhibited BDNF-induced phosphorylation of TrkB in heterologous cells. Taken together, this findings strongly support the assertion that these small molecules are inhibiting SFK but not TrkB kinase directly under the conditions of these experiments.
Significance of Phosphorylation of Tyr-705/Tyr-706 of TrkB by SFKs
Among the various tyrosines in the intracellular domain of TrkB, exogenous zinc induces preferential phosphorylation of Tyr-705/Tyr-706 of TrkB by a SFK-dependent mechanism. This raises the question as to the functional significance of phosphorylation of Tyr-705/Tyr-706 in TrkB activation. X-ray crystallographic studies of the kinase domains of other receptor tyrosine kinases, unphosphorylated insulin receptor β subunit (20) and fibroblast growth factor receptor (21), suggest a model in which the tyrosines (e.g. Tyr-1162/Tyr-1163 in the insulin receptor) homologous to Tyr-705/Tyr-706 of TrkB play an important role in ligand-mediated full activation of receptor tyrosine kinases. The crystal structures reveal that three tyrosine residues in the subdomain VII lie within a flexible activation loop that prevents binding of ATP to the receptor. Phosphorylation of Tyr-1162 of the insulin receptor results in preferred conformation of the activation loop, thereby facilitating accessibility of ATP and substrate binding and resulting in full activation of insulin receptor (20). It seems plausible that phosphorylation of Tyr-705/Tyr-706 of TrkB serves a similar function in activation of this receptor. Consistent with this idea, work of multiple investigators (16, 22–24) implicates an important role for Tyr-705/Tyr-706 in the BDNF-induced activation of Trk receptors and their biological responses. Interestingly, Segal et al. (16) showed that phosphorylation of Tyr-705/Tyr-706 precedes and facilitates the phosphorylation of other tyrosines, including Tyr-515. Importantly, the extent of phosphorylation of Tyr-705/Tyr-706 is correlated with the level of tyrosine kinase activity of Trk receptor (16). In this context, SFK-dependent phosphorylation of Tyr-705/Tyr-706 of TrkB following activation by either zinc- or BDNF likely facilitates full activation of TrkB signaling, thereby revealing a role for SFKs in TrkB signaling.
SFKs Are Necessary for Zinc-mediated Transactivation of TrkB
Our prior studies suggest a role of SFKs in zinc-induced transactivation of TrkB. Yet the sequence of molecular events by which zinc transactivates TrkB is incompletely understood. In this study, we further elucidate the molecular events by which zinc transactivates TrkB. First, zinc preferentially promotes phosphorylation of Tyr-705/Tyr-706 of TrkB in cultured neurons. Second, zinc-induced phosphorylation of Tyr-705/Tyr-706 requires SFKs. Third, zinc promotes the formation of a protein complex that includes TrkB and SFKs, Src and Fyn. Fourth, active Src is capable of directly phosphorylating Tyr-705/Tyr-706 of TrkB in a cell-free system. Fifth, point mutations of Y705F/Y706F reduced the zinc-induced phosphorylation of TrkB at Tyr-515, implying an important role of phosphorylation of Tyr-705/Tyr-706 in transactivation of TrkB by zinc. The role of SFKs in zinc-mediated transactivation of TrkB is reminiscent of zinc-induced transactivation of another receptor tyrosine kinase, the EGF receptor (EGFR), in that Src-dependent phosphorylation of Tyr-845 of EGFR (homologous to Tyr-705/Tyr-706 of TrkB) (25) is critical for EGFR-mediated Ras activation by zinc in fibroblasts. Collectively, it seems plausible that SFK-dependent phosphorylation of tyrosines homologous to Tyr-705/Tyr-706 is a mechanism common to transactivation of receptor tyrosine kinases by zinc. Together with our prior findings (12), we propose the following model in which zinc transactivates TrkB (Fig. 8A): zinc activates SFKs through dephosphorylation of C-terminal inhibitory tyrosine residue Tyr-527 by inhibiting Csk activity. Activated SFKs in turn result in phosphorylation of Tyr-705/Tyr-706, either directly or indirectly, resulting in full activation of TrkB and subsequent phosphorylation of other tyrosine residues of TrkB, including Tyr-515, triggering activation of downstream signaling cascades, including Ras/MEK/MAPK, PI3K/Akt, and PLCγ1 signaling pathways.
Role of SFKs in BDNF-mediated TrkB Activation
Results of our studies of SFKs in zinc-mediated transactivation of TrkB led us to reexamine the role of SFKs in BDNF-mediated activation of TrkB. The following evidence supports the idea that TrkB activation by BDNF is capable of promoting SFK activity. First, BDNF promotes formation of a protein complex that includes TrkB and the SFKs, Fyn and Src, in cultured neurons. Second, BDNF induces increased pY416 of SFKs, a surrogate measure of SFK activity, in both cultured neurons and hippocampal slices. This effect of BDNF requires TrkB kinase activity, because 1NMPP1 reduced this activation in slices from TrkBF616A but not WT mice, demonstrating that SFK activation is downstream of TrkB. Third, reduction of SFK activity was found in Western blots of hippocampi isolated from TrkB conditional knock-out mice. Additional evidence supports the idea that SFKs participate in BDNF-mediated activation of TrkB. That is, pharmacological inhibition of SFKs reduced BDNF-mediated phosphorylation of Tyr-705/Tyr-706 to a greater extent than Tyr-515 in cultured neurons. Likewise, a dominant negative mutant of Src reduced BDNF-induced phosphorylation of TrkB in heterologous cells, implicating SFKs in BDNF-induced activation of TrkB. Collectively, these findings support a model in which SFK are both downstream and upstream of BDNF-mediated TrkB activation and that SFK can provide positive feedback regulation of TrkB signaling (Fig. 8B). This model is consistent with and extends prior studies of SFKs in modulation of ligand-mediated receptor tyrosine kinase signaling, including the EGF receptor (13).
CONCLUSIONS
In sum, our findings support a model in which SFKs are both downstream and upstream of BDNF-mediated TrkB activation and further that SFKs can provide positive feedback regulation of TrkB signaling induced by BDNF (Fig. 8B). This model is consistent with and extends prior studies of SFKs in modulation of ligand-mediated receptor tyrosine kinase signaling, including the EGF receptor (13). SFKs also play an important role in zinc-mediated transactivation of TrkB. In contrast to the regulatory role of SFKs in BDNF-mediated activation of TrkB, SFKs are essential for the transactivation of TrkB by zinc (Fig. 8A). It also seems likely that TrkB transactivated by zinc promotes SFK activity.
This work was supported, in whole or in part, by National Institutes of Health Grant NS056217 (to J. O. M.) from NINDS. This work was also supported by an Epilepsy Foundation postdoctoral fellowship (to Y. Z. H.).
- BDNF
- brain-derived neurotrophic factor
- MAPK
- mitogen-activated protein kinase
- Erk
- extracellular signal-regulated kinase
- MEK
- MAPK/Erk kinase
- PI3K
- phosphatidylinositol 3-kinase
- EGF
- epidermal growth factor
- PLC
- phospholipase C
- SFK
- Src family kinase
- PDGF
- platelet-derived growth factor
- ICD
- intracellular domain
- E
- embryonic day
- GST
- glutathione S-transferase
- HA
- hemagglutinin
- MOPS
- 4-morpholinepropanesulfonic acid
- WT
- wild type
- Csk
- C-terminal Src kinase.
REFERENCES
- 1.McAllister A. K., Katz L. C., Lo D. C. (1999) Annu. Rev. Neurosci. 22, 295–318 [DOI] [PubMed] [Google Scholar]
- 2.Poo M. M. (2001) Nat. Rev. Neurosci. 2, 24–32 [DOI] [PubMed] [Google Scholar]
- 3.Chao M. V., Rajagopal R., Lee F. S. (2006) Clin. Sci. (Lond) 110, 167–173 [DOI] [PubMed] [Google Scholar]
- 4.Coull J. A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M. W., De Koninck Y. (2005) Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 5.Dietz D. M., Dietz K. C., Nestler E. J., Russo S. J. (2009) Pharmacopsychiatry 42, Suppl. 1, S69–S78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara J. O., Huang Y. Z., Leonard A. S. (2006) Sci. STKE 2006, re12. [DOI] [PubMed] [Google Scholar]
- 7.Desmet C. J., Peeper D. S. (2006) Cell Mol. Life Sci. 63, 755–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo G. S., Connie Hung C. C., Rochford J., Keogh J., Gray J., Sivaramakrishnan S., O'Rahilly S., Farooqi I. S. (2004) Nat. Neurosci. 7, 1187–1189 [DOI] [PubMed] [Google Scholar]
- 9.Huang E. J., Reichardt L. F. (2003) Annu. Rev. Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- 10.Reichardt L. F. (2006) Philos. Trans. R. Soc. Lond. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee F. S., Rajagopal R., Chao M. V. (2002) Cytokine Growth Factor Rev. 13, 11–17 [DOI] [PubMed] [Google Scholar]
- 12.Huang Y. Z., Pan E., Xiong Z. Q., McNamara J. O. (2008) Neuron 57, 546–558 [DOI] [PubMed] [Google Scholar]
- 13.Bromann P. A., Korkaya H., Courtneidge S. A. (2004) Oncogene 23, 7957–7968 [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki Y., Gay B., Wada K., Koizumi S. (1998) J. Neurochem. 71, 106–111 [DOI] [PubMed] [Google Scholar]
- 15.Pereira D. B., Chao M. V. (2007) J. Neurosci. 27, 4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal R. A., Bhattacharyya A., Rua L. A., Alberta J. A., Stephens R. M., Kaplan D. R., Stiles C. D. (1996) J. Biol. Chem. 271, 20175–20181 [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Ye H., Kuruvilla R., Ramanan N., Scangos K. W., Zhang C., Johnson N. M., England P. M., Shokat K. M., Ginty D. D. (2005) Neuron 46, 13–21 [DOI] [PubMed] [Google Scholar]
- 18.McCarty J. H., Feinstein S. C. (1998) Oncogene 16, 1691–1700 [DOI] [PubMed] [Google Scholar]
- 19.De Koninck Y. (2007) Curr. Opin. Pharmacol. 7, 93–99 [DOI] [PubMed] [Google Scholar]
- 20.Hubbard S. R., Wei L., Ellis L., Hendrickson W. A. (1994) Nature 372, 746–754 [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi M., Schlessinger J., Hubbard S. R. (1996) Cell 86, 577–587 [DOI] [PubMed] [Google Scholar]
- 22.Guiton M., Gunn-Moore F. J., Stitt T. N., Yancopoulos G. D., Tavaré J. M. (1994) J. Biol. Chem. 269, 30370–30377 [PubMed] [Google Scholar]
- 23.Guiton M., Gunn-Moore F. J., Tavare J. M. (1995) Biochem. Soc. Trans. 23, 176S. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham M. E., Stephens R. M., Kaplan D. R., Greene L. A. (1997) J. Biol. Chem. 272, 10957–10967 [DOI] [PubMed] [Google Scholar]
- 25.Wu W., Graves L. M., Gill G. N., Parsons S. J., Samet J. M. (2002) J. Biol. Chem. 277, 24252–24257 [DOI] [PubMed] [Google Scholar]
- 26.He X. P., Kotloski R., Nef S., Luikart B. W., Parada L. F., McNamara J. O. (2004) Neuron 43, 31–42 [DOI] [PubMed] [Google Scholar]
- 27.McHugh T. J., Jones M. W., Quinn J. J., Balthasar N., Coppari R., Elmquist J. K., Lowell B. B., Fanselow M. S., Wilson M. A., Tonegawa S. (2007) Science 317, 94–99 [DOI] [PubMed] [Google Scholar]