Abstract
Active G protein-coupled receptors activate heterotrimeric Gαβγ proteins by catalyzing the exchange of GDP by GTP at the Gα subunit. A paradoxical attenuation of G protein-activated inwardly rectifying potassium channels (GIRK) upon stimulation of native cells with high concentrations of agonist is known. However, a deactivation of activated G proteins by active receptors has not been experimentally studied in intact cells. We monitored GIRK currents and Go protein activation by means of fluorescence resonance energy transfer (FRET) in parallel. The results suggested that GIRK currents were paradoxically attenuated due to an inactivation of Go proteins by active α2A-adrenergic receptors. To study the mechanisms, G protein activation and receptor-G protein interactions were analyzed as a function of nucleotide type and nucleotide concentrations by means of FRET, while controlling intracellular nucleotides upon permeabilization of the cell membrane. Results suggested a receptor-catalyzed dissociation of GTP from activated heterotrimeric Gαβγ. Consequently, nucleotide-free G proteins were sequestrated in heterotrimeric conformation at the active receptor, thus attenuating downstream signaling in an agonist-dependent manner.
Keywords: Biophysics, G Proteins/Heterotrimeric, G Proteins/Coupled Receptors (GPCR), Membrane/Fluorescence, Protein/Protein-Protein Interactions, Receptors/Membrane, Signal Transduction/G Proteins, FRET
Introduction
G protein-coupled receptors (GPCRs)2 represent the largest family of hormone- or neurotransmitter receptors (1) and account for roughly 30% of small molecule drug targets (2). The physiological role of GPCRs is directly linked to the activation of heterotrimeric Gαβγ proteins (3). Upon agonist binding, GPCRs become active and promote G protein activation by catalyzing GDP to GTP exchange at the Gα subunit (4). During interaction with active receptors, the affinity of GDP-bound G proteins toward GDP is lowered and a transient complex of agonist-bound receptor and nucleotide-free G protein may be formed (5). Efficient binding of GTP to the Gα subunit is based on the excess of intracellular GTP over GDP (6). Finally, active GTP-bound G proteins dissociate from active receptors, thus promoting G protein/effector interactions (7).
There are several models of receptor-G protein interaction. The model of collision coupling assumes conditions of free lateral diffusion in the cell membrane (8, 9), however some experimental evidence favors alternative models (10, 11). The model of collision coupling would allow a “reversed G protein activation”: interaction of heterotrimeric active G proteins with active receptors, receptor-catalyzed release of GTP from Gα, and consequent G protein deactivation. In computational G protein models, however, such a reaction was neglected (12). Hints for an agonist-induced release of GTP and a deactivation of G proteins have been described independently by several groups: An exchange of GTPγS and Gpp(NH)p to other nucleotides by active receptors was reported (13). Furthermore, G protein-activated inwardly rectifying potassium (GIRK) channels (14, 15) are paradoxically attenuated during strong stimulation of the adenosine A1 receptor (16, 17).
Recently established methods of fluorescent resonance energy transfer (FRET) allow for direct investigation of receptor-G protein interactions and G protein activation (18–21). Using these methods in conjunction with permeabilization of the cell membrane, we tested the hypothesis, that in intact cells activated G proteins interact with active receptors and become subsequently deactivated due to release of GTP.
EXPERIMENTAL PROCEDURES
Molecular Biology and Cell Culture
Measurement of G protein activation by means of FRET was performed by transient transfection of cDNA/5-cm dish (in micrograms): Gαo-(91)YFP (1.8), Gβ1 (0.5), and CFP-Gγ2 (0.2) as described (20) and illustrated in Fig. 1A in a HEK293 cell line stably expressing α2A-adrenergic receptor (α2A-AR) at 23 pmol/mg (clone I). GIRK currents were measured in clone I by transient transfection of cDNA/5-cm dish (in micrograms): GIRK1/4 (0.4), Gαo or Gαo-(91)YFP (1.8), and mGFP (0.2). Coexpression of Gαo was chosen for consistent experimental conditions and to minimize basal GIRK channel activation, thereby preventing death of transfected cells prior to analysis.
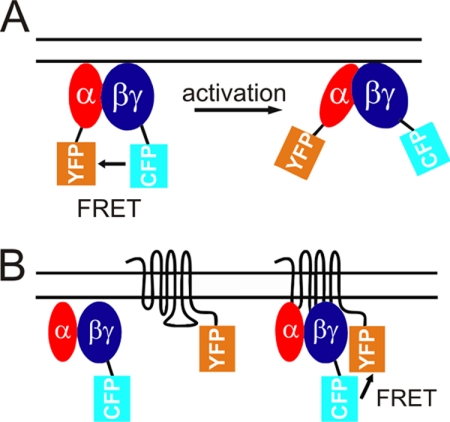
FIGURE 1.
Illustration of FRET-based monitoring of G protein activation and receptor-G protein interactions. A, schematic model for the FRET-based detection of G protein activation (20). Upon receptor-mediated activation, the distance between fluorescent moieties of YFP-tagged Gαo subunit and CFP-tagged Gγ subunit increased, leading to a loss of FRET. B, schematic model for the FRET-based detection of receptor-G protein interactions (21). Upon receptor activation, the distance between fluorescent moieties of YFP-tagged α2A-adrenergic receptor and CFP-tagged Gγ subunit decreased, leading to a gain of FRET.
FRET-based detection of receptor-G protein interactions was performed by transient transfection of cDNA/5-cm dish (in micrograms): Gαo (1.8), Gβ1 (0.5), CFP-Gγ2 (0.2), and α2A-AR-YFP (0.5) in a HEK293T cell as described before (21) and illustrated in Fig. 1B. All transfections were performed using the Effectene transfection kit according to the manufacturer's protocol (Qiagen).
Fluorescence Measurements and Electrophysiology
Transfected cells growing on poly-l-lysine-coated coverslips were mounted on a dual-emission photometric microscope, and FRET was measured as described (19–21). Illumination time was 50 ms at a frequency of 10 Hz for experiments shown in Fig. 3 and at 1 Hz for all other experiments. FRET was calculated ratiometrically by dividing spillover-corrected YFP emission by CFP emission (19–21). To permeabilize cells attached to poly-l-lysine-coated coverslips, incubation with internal buffer containing 0.05% saponine for 1 min, was performed, and cells were subsequently washed with internal buffer in experiments shown in Fig. 3–6. Confocal images were taken using a Leica TCS SP5 system, similar to those of a previous study (21).
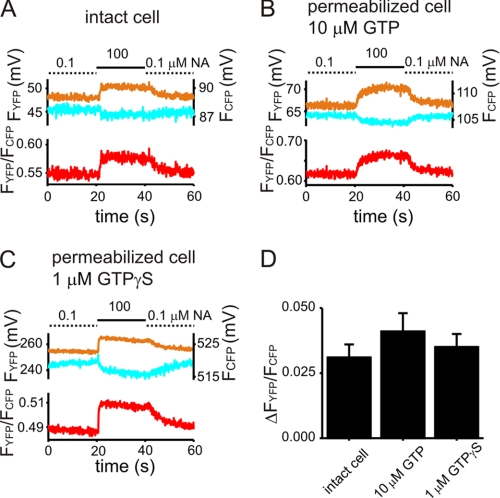
FIGURE 3.
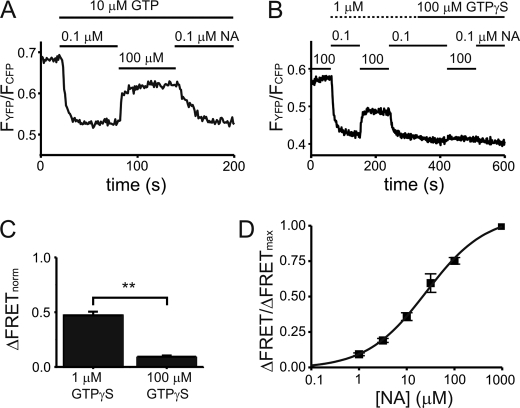
Noradrenaline-induced interactions of GTP- and GTPγS-preactivated G proteins with α2A-ARs in permeabilized cells. NA-induced receptor-G protein interactions were monitored in single HEK293 cells expressing YFP-tagged α2A-adrenergic receptors (α2A-AR-YFP), Gαo, Gβ1, and CFP-Gγ2 by means of FRET as illustrated in Fig. 1B. The upper panels of A–C show bleed-through and bleaching-corrected intensities of the YFP and CFP channels as measured by the photodiodes in millivolts. The corresponding FRET, calculated ratiometrically as described, is shown in the lower panel. Cells were superfused with NA as indicated. A representative FRET recording for NA-induced receptor-G protein interactions in a single intact cell is given in A. B and C show similarly conducted representative recordings of single permeabilized cells, either in the presence of 10 μm GTP (B) or in the presence of 1 μm GTPγS (C). D, summarized data are given for the mean increase in FRET induced by switching superfusion from 0.1 μm NA to 100 μm NA for all three conditions (n = 6–9, not significant).
Whole cell, patch clamp recordings for experiments shown in Fig. 2 were performed in the presence of 20 mm extracellular K+ to measure GIRK currents at −90 mV membrane potential in an inward direction as described before (22). All solutions were applied using a rapid superfusion device (20) that allowed for solution exchange times within 10 ms.
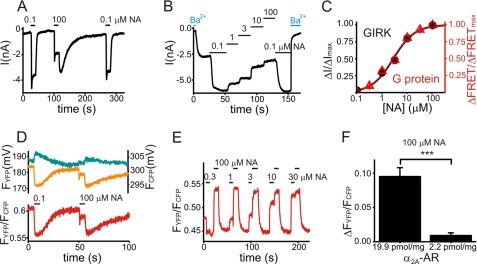
FIGURE 2.
Attenuation of GIRK currents upon strong receptor stimulation is paralleled by an attenuation of Go protein activation. A and B, GIRK currents were recorded in single HEK293 cells stably expressing α2A-adrenergic receptors (α2A-AR, clone I) and transiently expressing GIRK1 and GIRK4, along with Gαo to keep basal GIRK currents as low as possible. GIRK currents were activated by reversible stimulation of receptors with noradrenaline (NA) as indicated. By whole cell patch clamp, membrane potential was set to −90 mV, and GIRK currents were measured as inward currents. The slow recovery of GIRK currents after stimulation with 100 μm NA most likely reflects the limits of the used superfusion system in context of very high expressing and therefore very NA-sensitive α2A-AR cells. C, data for the concentration-response curve for the NA-induced attenuation of both GIRK currents (●, n = 7) and G protein deactivation detected by FRET (Δ, n = 9, see D and E for more details) were based on experiments similar to those depicted in B for GIRK currents and E for FRET between G protein subunits. Steady-state attenuation of GIRK currents (ΔI) for each tested concentration of NA was normalized to the maximal attenuation in the presence of 100 μm NA (ΔImax). Steady-state increase in FRET for each tested concentration of NA (ΔFRET) was normalized to the maximal increase of FRET in the presence of 100 μm NA (ΔFRETmax). D and E, recordings of FRET between fluorescence-tagged G protein subunits were performed in single HEK293 cells stably expressing α2A-AR (clone I) and transiently expressing Gαo-YFP, Gβ1, and CFP-Gγ2. G proteins were activated by reversible stimulation of receptors with NA as indicated. An agonist-induced loss of FRET is compatible with Go protein activation (23), as illustrated in Fig. 1A. In D: the upper panel shows bleed-through and bleaching-corrected intensities of the YFP-channel (FYFP) and CFP-channel (FCFP) as measured by the photodiodes in millivolts. The lower panel shows the corresponding FRET, calculated ratiometrically by division of the corrected YFP intensity by the corrected CFP intensity (FYFP/FCFP). Note that both GIRK currents and loss of FRET between G protein subunits were maximal during stimulation with 0.1 μm NA, attenuated during stimulation with 100 μm NA, and became maximally stimulated again during washout of 100 μm NA. E, FRET between tagged G protein subunits upon superfusion with NA as indicated is plotted. F, the mean increase in FRET (ΔFYFP/FCFP) during stimulation with 100 μm NA was significantly larger for cells from clone I (expressing α2A-AR at 19.9 pmol/mg) compared with cells expressing α2A-AR at 2.2 pmol/mg (0.095 ± 0.013, n = 14 versus 0.009 ± 0.004, n = 14, p < 0.001).
Data Normalization for Permeabilized Cell Conditions
G protein activation in Figs. 5 and 6 was normalized to the maximum level of G protein activation (defined as steady-state FRET in the presence of internal buffer containing 0.1 μm noradrenaline (NA) and 1 μm GTPγS) and normalized to the minimum level of G protein activation (defined as steady-state FRET during superfusion with internal buffer containing 100 μm NA without nucleotides).
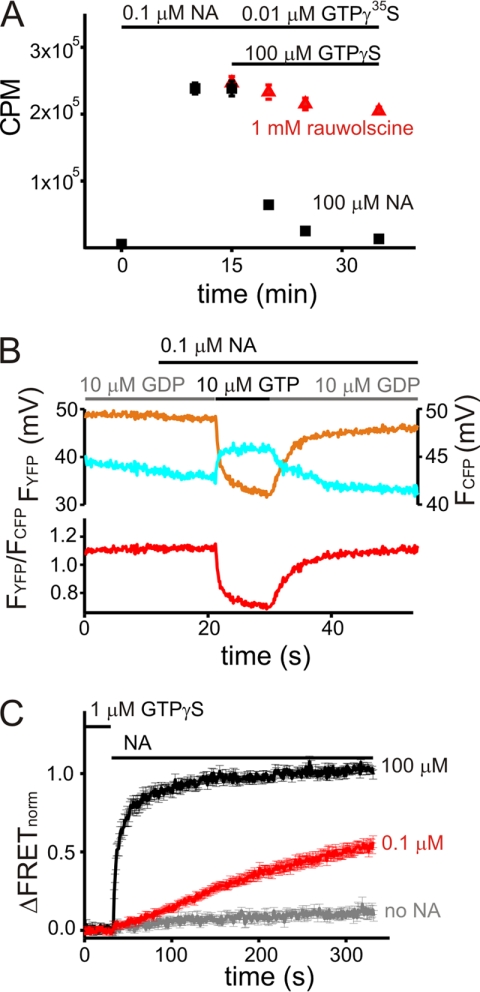
FIGURE 5.
α2A-ARs induce GTPγS release from GTPγS-preactivated Go proteins in an agonist-dependent manner. A, cell membranes from an α2A-AR-expressing cell line (clone I) were incubated with GTPγ35S and NA as indicated. Upon equilibration, the reaction batch was split into two groups. The first group was incubated with an antagonist for the α2A-AR (rauwolscine, to antagonize the presence of NA) and an excess of non-radioactive GTPγS. The second group was incubated with an excess of NA and GTPγS. Counts per minute (CPM; mean ± S.E.) were taken from samples of the two different groups at the indicated time points for three independent experiments. B, FRET between G protein subunits was recorded in single permeabilized cells, transiently expressing Gαo-YFP, Gβ1, and CFP-Gγ2 and stably expressing α2A-AR (clone I). Cells were superfused with nucleotides and NA as indicated. The upper panel presents bleed-through and bleaching corrected intensities of the YFP and CFP channels. The lower panel shows the corresponding FRET, calculated ratiometrically as described. A decrease in FRET is compatible with Go protein activation. C, FRET between GTPγS-preactivated G protein subunits was recorded for 5 min in single permeabilized cells upon withdrawal of nucleotides and superfusion with NA as indicated. Averaged data plotted were normalized as explained under “Experimental Procedures” prior to averaging. To preactivate G proteins, cells were superfused with GTPγS and NA until a steady state of FRET was observed (not shown). GTPγS and NA were subsequently withdrawn. As indicated, superfusion was switched to nucleotide-free internal buffer containing no NA (gray), 0.1 μm NA (red), or 100 μm NA (black). The observed increase in FRET is compatible with inactivation of Go proteins.
FIGURE 6.
Inactivation of G proteins preactivated by GTP or GTPγS upon receptor stimulation. FRET between G protein subunits was recorded in single permeabilized cells transiently expressing Gαo-YFP, Gβ1, and CFP-Gγ2 and stably expressing α2A-AR (clone I). A and B, show representative FRET recordings as a function of NA superfusion in the presence of GTP (A) or in the presence of low and high concentrations of GTPγS (B). As previously reported, agonist-induced loss of FRET is indicative for Go protein activation. Note that, during stimulation with 100 μm NA, FRET was reversibly increased in the presence of 10 μm GTP and 1 μm GTPγS. C, summarized data (mean ± S.E., n = 8) of NA-induced increase in FRET (normalized as explained under “Experimental Procedures”) is illustrated for the presence of 1 μm GTPγS and 100 μm GTPγS (p < 0.01). D, a concentration-response curve for the NA-induced increase in FRET in the presence of 1 μm GTPγS was determined. Data were normalized to the increase in FRET observed during stimulation with 1000 μm NA in each individual experiment.
Receptor-G protein interactions in Fig. 4 were normalized to the maximum levels of receptor-G protein interactions (defined as steady-state FRET in the presence of 100 μm NA without nucleotides) and minimum levels of receptor-G protein interactions (defined as steady-state FRET in the presence of internal buffer containing nucleotide but no NA). Only experiments free of non-linear shifts were analyzed.
FIGURE 4.
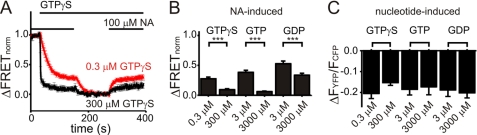
Noradrenaline-induced interactions of Go proteins with α2A-ARs in permeabilized cells as a function of nucleotide type and concentration. A, FRET was recorded in permeabilized cells expressing α2A-AR-YFP, Gαo, Gβ1, and CFP-Gγ2 and normalized as explained under “Experimental Procedures” to the maximum and minimum levels of receptor-G protein interactions (ΔFRETnorm). As a pretreatment (not shown), cells were superfused with 100 μm NA without nucleotide until a steady state of FRET was observed. Subsequently, superfusion was switched to contain 0.3 μm (red, n = 11) or 300 μm (black, n = 7) GTPγS and NA as indicated. Upon reaching a steady state, NA was withdrawn and reapplied as indicated. B and C, summarized data (mean ± S.E.) are given for the NA-induced increase in normalized FRET in the presence of nucleotide as indicated (p < 0.001) (B) and for the nucleotide-induced decrease in FRET (C) (n = 8–12, not significant).
Buffers
Intact cells (in both FRET and patch clamp experiments) were continuously superfused with external buffer containing (in mm): NaCl 124, KCl 20, CaCl2 2, MgCl2 1, HEPES 10 at pH 7.35, and NA as indicated. Permeabilized cells were continuously superfused with internal buffer containing (in mm): K+-aspartate 100, KCl 30, NaCl 10, MgCl2 1, EGTA 5, HEPES 10 at pH 7.35, NA, and nucleotides as indicated. Patch clamp pipettes were filled with a buffer containing (in mm): K+-aspartate 100, KCl 40, MgCl2 1, EGTA 5, Na+-ATP 2.5, Na+-GTP 0.03, HEPES 10 at pH 7.35. All buffers were calibrated to 330 mosm using KCl. All chemicals were from Sigma or AppliChem.
GTPγ35S Release Assay
GTPγ35S binding was performed as described previously (23). Briefly, 10 μg of cell membrane of HEK293 cells stably expressing α2A-AR (clone I) were incubated with 3 × 105 cpm GTPγ35S in a binding buffer containing (in mm) 20 HEPES, 1 MgCl2, 100 NaCl, 1 EDTA, and 1 dithiothreitol at pH 7.4 with 0.1 μm NA at room temperature in a total volume of 4 ml. Upon steady-state binding of GTPγ35S (determined to occur within 10 min of incubation), the reaction batch was split into two groups. Group 1 was additionally incubated with 1 mm rauwolscine and 100 μm GTPγS, group 2 with 100 μm NA and 100 μm GTPγS. GTPγ35S binding at each indicated time point was assessed by filtration of 2 × 200 μl of the reaction batch on GF/F filters after washing four times with 5 ml of binding buffer and resuspension in 8 ml of Lumasafe Plus (PELAS) buffer. Radioactivity was determined using a Beckman LS-1801 counter.
Membrane Preparation and Western Blots
HEK293 cells were disrupted with an Ultra-Turrax cell disrupter, and crude cell membrane preparations were obtained by centrifugation of the lysate at 1,200 × g followed by centrifugation of the supernatant at 100,000 × g. Pellets were resuspended in resuspension buffer (5 mm Tris (pH 7.4), 2 mm EDTA). Proteins were solubilized in SDS-sample buffer and boiled for 5 min at 95 °C. Equal amounts of total protein were separated by SDS-PAGE and were electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore). Bound proteins were probed with polyclonal anti-Gαo/Gαi3 antibodies (Calbiochem), followed by peroxidase-coupled secondary antibodies (Dianova). Signal detection was performed with an ECL Plus Western Blotting Detection Reagent (Amersham Biosciences) in accordance with the manufacturer's instructions, and signals were recorded on a LAS-1000 imaging system and quantified with Multi Gauge software (Fuji).
Statistical Analysis
If not indicated otherwise, all data are given as mean and standard error of the mean. Statistical analysis was performed using Student's t test and Origin 7.5b (OriginLab). Ordinary concentration-response curves were fitted using PRISM 4.0 (GraphPad Software Inc.).
RESULTS
Attenuation of GIRK Currents and G Protein Activation upon Strong Receptor Stimulation
Strong stimulation of adenosine A1 receptors coexpressed in atrial myocytes (16) or HEK293 cells (17) was reported not only to activate but also to paradoxically attenuate preactivated GIRK currents.
We hypothesized that this attenuation reflects an inactivation of G proteins by high levels of active GPCRs. Therefore, GIRK currents and G protein activation were assessed in an HEK293 cell line, stably expressing α2A-adrenergic receptors (α2A-AR) at 19.9 pmol/mg (clone I). Based on comparative confocal fluorescence measurements, radioligand binding, and immunoblotting, we estimated the expression of α2A-AR and Gαo to be 3:1 for these conditions (supplemental Fig. S1). The Ki of NA binding to the α2A-AR is in the order of 5 μm (22).
GIRK currents were maximal upon stimulation of transfected cells with 0.1 μm NA. Upon stimulation with 100 μm NA, GIRK currents rapidly decreased to an attenuated steady state. When NA was washed out, GIRK currents were quickly reactivated to a level as elicited by stimulation with 0.1 μm NA (Fig. 2A). A concentration-response curve with a −log EC50 of 5.5 ± 0.04 (Fig. 2C, black trace, n = 7) was obtained, when the GIRK current attenuation at 1 μm, 3 μm, and 10 μm NA was normalized to the attenuation observed at 100 μm NA (Fig. 2B). This result corresponded with the binding affinity of NA toward the α2A-AR (22).
We next tested whether a corresponding NA-induced attenuation could also be detected on the level of G protein activation. G protein activation was monitored by means of FRET between YFP-tagged Gαo and CFP-tagged Gγ2-subunits (19). As illustrated in Fig. 1A, FRET between tagged Gαo and Gγ2 becomes less efficient upon receptor-mediated activation of G proteins (20), consistent with either dissociation or conformational rearrangement of G protein subunits.
G protein activation paralleled the activation of GIRK currents: FRET decreased maximally upon stimulation of cells with 0.1 μm NA, suggesting maximal activation of Go proteins (Fig. 2D). Upon stimulation with 100 μm NA, FRET increased markedly, suggesting only a partial activation of Go proteins (Fig. 2D). When NA was washed out, FRET decreased until reaching equivalent levels as observed upon stimulation with 0.1 μm NA (Fig. 2D), indicative of maximum Go protein reactivation. The kinetics of the initiation and relief of the attenuation of GIRK currents induced by 100 μm NA were not significantly different from those measured for G protein activity (supplemental Fig. S2).
A concentration-response curve for the attenuation of G protein activation with a −log EC50 of 5.6 ± 0.04 (n = 8) (Fig. 2C, red trace) was obtained, when the increase in FRET at 0.3 μm, 1 μm, 3 μm, 10 μm, and 30 μm NA (Fig. 2E) was normalized to the increase at 100 μm NA (representing maximum attenuation of Go protein activation). This result again corresponded to the published NA binding at α2A-AR (22). The mean increase in FRET upon stimulation with 100 μm NA (Fig. 2F) was significantly larger for clone I (0.095 ± 0.013, n = 14) compared with a second cell line, expressing α2A-AR at 2.2 pmol/mg (0.009 ± 0.004, n = 14).
In summary, these results suggested that the amount of active receptors primarily determines the extent of the paradoxical attenuation of GIRK currents and G protein activation. To explore the underlying mechanisms, we attempted to monitor receptor-G protein interactions and G protein activation in single permeabilized cells while controlling cytoplasmic guanine nucleotides. First, we tested, whether preactivated Go proteins interact with active α2A-AR and second, whether interactions depend on the cytoplasmic guanine nucleotide levels.
Active α2A-Adrenergic Receptors Physically Interact with Preactivated Go Proteins
Receptor-G protein interactions were directly studied by means of FRET in transiently transfected HEK293T cells (21) as illustrated in Fig. 1B. Upon interaction of CFP-tagged Gγ2 and YFP-tagged α2A-AR, the distance between CFP and YFP moieties decreases and thereby gives rise to a more efficient FRET.
An increase in FRET was observed in single intact cells (Fig. 3A) as well as in single permeabilized cells in the presence of either a low physiological (24) 10 μm GTP (Fig. 3B) or 1 μm GTPγS (Fig. 3C) upon reversibly switching superfusion of cells from 0.1 μm NA to 100 μm NA. The mean increase in FRET was not significantly different between these conditions (Fig. 3D). Reversibly switching the superfusion between 0.1 μm NA and NA-free buffer showed no increase in FRET, in line with results by Hein et al. (21).
For GDP, the affinity of Gα toward GDP is lowered, when Gα interacts with active receptors, promoting formation of a stable complex of nucleotide-free G proteins and active receptors (5). This process may be enhanced by low GDP concentrations, resulting in increased receptor-G protein interactions. The same principle may also apply to the interactions of active receptors with GTP- or GTPγS-bound G proteins. Therefore, we tested whether NA-induced receptor-G protein interactions measured by FRET increase in the presence of low nucleotide concentrations. A significantly larger NA-induced gain of FRET, normalized to maximum and minimum levels of receptor-G protein interactions, as explained under “Experimental Procedure” was observed upon decreasing GTPγS, GTP, and GDP concentrations (Fig. 4, A and B). The nucleotide-induced decrease in FRET was not significantly different among all conditions tested (Fig. 4C).
These results imply transient interactions of receptors with GTP- or GTPγS-bound G proteins. Additionally, they suggest a fast receptor-mediated release of GTPγS and GTP from Gαo resulting in complexes of nucleotide-free heterotrimeric G proteins and agonist-bound receptors. Therefore, a receptor-mediated release of radioactively labeled GTPγ35S is feasible, corresponding to reports of a GTPγ35S exchange in native, atrial cell membranes upon muscarinic acetylcholine receptor stimulation (13).
Active α2A-ARs Induce GTPγS Release from G Proteins in an Agonist-dependent Manner
We tested for displacement of radioactively labeled GTPγ35S by an excess of non-labeled GTPγS in an agonist-dependent manner using membranes of the α2A-AR-expressing HEK293 cell line (clone I). After steady-state incubation with GTPγ35S and 0.1 μm NA, the reaction batch was split into two groups. The first group was incubated with the α2A-AR antagonist rauwolscine (1 mm to antagonize the presence of NA) and an excess of non-labeled GTPγS, showing a small decrease in GTPγ35S binding after 15 min by 16.9% ± 1.6% (n = 3). The second group was incubated with 100 μm NA and an excess of non-labeled GTPγS, resulting in a loss of GTPγ35S binding by 94.4% ± 0.7% (n = 3) after 15 min (Fig. 5A).
An agonist-induced GTPγS exchange of preactivated G proteins may also be detectable as an agonist-induced deactivation of GTPγS-preactivated G proteins upon withdrawal of GTPγS. We tested whether an activation of G proteins corresponding to physiological activation of G proteins can be observed in a single permeabilized cell. α2A-AR-expressing cells (clone I) transfected with fluorescent Go protein subunits showed a decrease in FRET upon switching the superfusion of cells from 10 μm GDP to 10 μm GTP in the presence of 0.1 μm NA (Fig. 5B), indicative of Go protein activation with kinetics expected for physiological Go protein or Go protein effector activation (20, 22, 25).
To assess deactivation of GTPγS-preactivated Go proteins, permeabilized cells were superfused with internal buffer containing 0.1 μm NA and 1 μm GTPγS until saturated binding of GTPγS to Gαo, as reflected by a steady-state decrease in FRET. Subsequently, NA and GTPγS were withdrawn from the buffer. Continuing superfusion of cells with internal buffer lead at most to an increase in FRET of 10% over 5 min (normalized as explained under “Experimental Procedures”). In contrast, addition of NA to superfused internal buffer resulted in a substantially faster increase in FRET in the presence of 100 μm NA compared with 0.1 μm NA (Fig. 5C, n = 7 each), indicating Go protein deactivation. Experiments conducted for 10 μm NA and 1 μm NA showed intermediate kinetics (data not shown, n = 7 each). These data corroborated that active α2A-ARs induce GTPγS release of GTPγS-bound Gαo proteins leading to nucleotide-free, inactive G proteins.
Attenuation of G Protein Activation upon Strong Receptor Stimulation in the Continuous Presence of GTPγS or GTP
Collectively, the results of the study suggest that during strong receptor stimulation a transient deactivation of GTP- or GTPγS-preactivated Go proteins may be observable in the continuous presence of GTP or GTPγS. A decrease in FRET upon receptor stimulation with 0.1 μm NA was observed with single α2A-AR-expressing cells (clone I), transfected with fluorescent Go protein subunits, in the presence of either 10 μm GTP (Fig. 6A) or 1 μm GTPγS (Fig. 6B, beginning of the trace). FRET increased markedly (Fig. 6A and beginning of trace in Fig. 6B) upon switching superfusion from 0.1 μm NA to 100 μm NA, indicating a substantial deactivation of preactivated Go proteins.
The deactivation of Go proteins was significantly larger in the presence of 1 μm GTPγS compared with the presence of 100 μm GTPγS (Fig. 6C, 47.1% ± 9.7% versus 9.3% ± 3.7%, n = 8 each, data normalized as explained under “Experimental Procedures”). Moreover, the deactivation depended on the concentration of NA applied and exhibited a −log EC50 of 4.6 ± 0.06 (n = 7) (Fig. 6D) in the presence of 1 μm GTPγS. Compared with intact cells, the EC50 was shifted by approximately one order of magnitude. A similar shift of the EC50 was observed for the activation of the FRET-based α2A-adrenergic receptor sensor α2A-cam (26) in permeabilized cells in the presence of 1 μm GTPγS compared with intact cells (permeabilized: −log EC50 3.8 ± 0.05, n = 5; intact: −log EC50 5.4 ± 0.02, n = 6).
Taken together, a coherent explanation of these results would be a receptor-induced loss of nucleotides of preactivated Go proteins upon interaction with active α2A-AR and subsequent formation of a complex of nucleotide-free Go proteins and active α2A-receptors.
DISCUSSION
The focus of the present study is an under-appreciated aspect of the G protein cycle: interactions of active G proteins with active receptors. These interactions will lead to recognizable effects only at high expression levels of one or several GPCRs competing for the same subtype of G protein. In such a case, increasing stimulation of receptors may not only lead to an increase in G protein effector activity but may also paradoxically deactivate G protein effectors at very strong stimulation.
Several potential mechanisms may account for this paradoxical effect: mechanism I, transient receptor desensitization or GIRK channel desensitization; mechanism II, “scavenging” of Gβγ (which activates the GIRK channel) by GPCR kinases during agonist stimulation; mechanism III, scavenging of active, GTP-bound G proteins by active receptors; or mechanism IV, scavenging of deactivated G proteins by active receptors, either due to forced GTP hydrolysis and subsequent release of GDP or by a direct GTP release.
Several results argue against a transient receptor or GIRK channel desensitization (mechanism I) as well as scavenging of Gβγ by GPCR kinases (mechanism II): GIRK currents and G protein activation become quickly reactivated during washout of strong agonist stimulation (Fig. 1 and supplemental Fig. S2) to an extent, as elicited by weak agonist stimulation. The beginning and end of the investigated attenuation correspond to kinetics published for receptor activation and receptor-G protein interactions (21, 26). Moreover, a strong receptor reserve and attenuation were observed in patch clamp experiments over 15 min.
Monitoring GIRK currents and G protein activation in intact cells did not allow us to distinguish whether scavenging of GTP-bound G proteins (mechanism III) or inactivation of GTP-bound G proteins (mechanism IV) by active receptors is primarily responsible for the paradoxical GIRK current attenuation. Performance of single cell FRET assays subsequent to permeabilization of the plasma membrane allowed analysis of receptor-G protein interactions and G protein activation while controlling intracellular nucleotides.
Data suggest that GTP-bound heterotrimeric G proteins interact with active receptors rather transiently. However, a subsequent release of nucleotide upon interaction may lead to a stable receptor-G protein complex, as proposed by the ternary complex model (5), thus resulting in the paradoxical G protein deactivation. The study provides evidence to support this model: (i) Agonist-induced receptor-G protein interactions were increased in the presence of low concentrations of nucleotides (Fig. 4B). Moreover, receptor-G protein interactions were maximal in the absence of nucleotides, as recently reported using fluorescence recovery after photobleaching experiments (27). (ii) A receptor-mediated inactivation of GTPγS-preactivated Go proteins was observed (Fig. 5C). (iii) During strong receptor stimulation, a transient attenuation of GTPγS-preactivated Go proteins was found only in the presence of a low GTPγS concentration (Fig. 6), corresponding to the increased receptor-G protein interactions in the presence of low GTPγS concentrations (Fig. 4). Moreover, this result excludes a rapid hydrolysis of GTP upon interaction of GTP-bound G proteins with active receptors.
Active G proteins interact with active receptors in heterotrimeric G protein conformation (Fig. 7): changes in FRET for the used CFP/YFP donor/acceptor pair can only be detected if CFP and YFP are within a range of <10 nm (28). The FRET-based assay for the G protein activation used an YFP-tagged Gαo and a CFP-tagged Gγ subunit. Upon strong receptor activation, an increase in FRET was observed, reflecting not only G protein deactivation but also requiring both YFP and CFP tags of G protein subunits to come within 10 nm distance, as feasible in a heterotrimeric G protein conformation. The fast kinetics of the attenuation may also suggest that activated Go proteins (similarly to Gi (19)) do not fully dissociate into Gα and Gβγ subunits upon their activation. Consequently, data would support models of the G protein cycle, in which activated G proteins remain in equilibrium between heterotrimeric conformation and subunit dissociation.
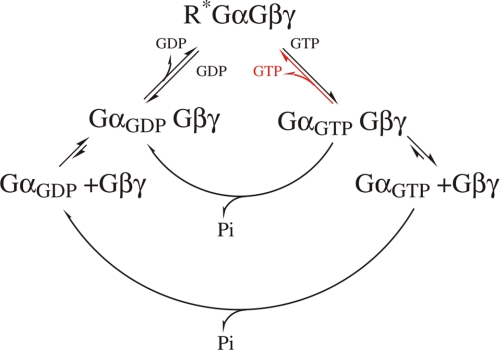
FIGURE 7.
Schematic model of the G protein cycle. Active receptors interact with heterotrimeric GDP- or GTP-bound G proteins, as investigated in this study and indicated in red in the model. Upon interaction, nucleotide is released. Subsequently, complexes of nucleotide-free G proteins and active receptors are transiently formed. Upon binding GTP, G proteins become active, facilitating dissociation from active receptors. Both GDP- and GTP-bound G proteins are in equilibrium between heterotrimeric conformation and dissociation of subunits. GGTP, GGDP, and G indicate GTP-bound, GDP-bound, or nucleotide-free G proteins, respectively; R*, indicates active receptors.
The physiological role of the “reversed activation” remains presently unresolved. It may well account for bell-shaped concentration-response curves, in which a paradoxical decrease in effect is observed at high agonist concentrations. Tissues with a high receptor expression or low GTP concentrations, which are exposed to high agonist concentrations, would be strong candidates. The level of endogenous receptor expression could so far only be determined by means of radioligand binding in membranes derived from whole tissue. In the case of α2-adrenergic receptors the average expression level of the cortex is about 1/70th (29) of our HEK cells. Given the low abundance of noradrenergic neurons in the cortex, it does not seem unlikely that local receptor concentrations such as in synaptic terminals could reach levels close to the range of those of heterologous expression systems. In the present study we estimated that α2A-AR and Go were expressed at a ratio about 3:1 (supplemental Fig. S1). Exogenous expression of Gαo-YFP in individual HEK cells lead to an estimated increase of 5- to 6-fold in total Gαo content (supplemental Fig. S1, B and C). Physiological expression levels for Go proteins vary over a wide range for different tissues, some expressing rather low levels such as human atrial myocytes (1 pmol/mg (30)). In rod cells, the concentration of rhodopsin is up to 3 mm, but the GTP concentration is also up to 1000 μm GTP (31, 32). In context with the almost complete dissociation of transducin upon its activation (33), interactions of activated transducin with rhodopsin during light stimulation are very unlikely.
Taken together, data of this study are in line with the present model of G protein cycling. Data result from transient interactions of nucleotide-bound G proteins with active receptors and stable interactions of nucleotide-free G proteins with active receptors. The equilibrium between these states results from the interplay of nucleotide concentrations and active-receptor concentrations.
Supplementary Material
Acknowledgments
We thank Dr. B. Hille for the critical reading of the manuscript and helpful advice. For sharing equipment and productive discussion, we are grateful to M. J. Lohse.
This work was supported by Deutsch Forschungsgemeinschaft Grant SFB 487 TP A10 (to M. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- GPCR
- G protein-coupled receptor
- GIRK
- G protein-activated inwardly rectifying potassium
- AR
- adrenergic receptor
- CFP
- cyan fluorescent protein
- YFP
- yellow fluorescent protein
- FRET
- fluorescence resonance energy transfer
- GTPγS
- guanosine 5′3′-O-(thio)triphosphate
- Gpp(NH)p
- guanosine 5′-(β,γ-imido)triphosphate
- HEK
- human embryonic kidney
- NA
- noradrenaline
- PTX
- pertussis toxin
- GPCR kinase
- G protein-coupled receptor kinase.
REFERENCES
- 1.Bjarnadóttir T. K., Gloriam D. E., Hellstrand S. H., Kristiansson H., Frederiksson R., Schiöth H. B. (2006) Genomics 88, 263–273 [DOI] [PubMed] [Google Scholar]
- 2.Hopkins A. L., Groom C. R. (2002) Nat. Rev. Drug. Discov. 1, 727–730 [DOI] [PubMed] [Google Scholar]
- 3.Bourne H. R. (1997) Curr. Opin. Cell Biol. 9, 134–142 [DOI] [PubMed] [Google Scholar]
- 4.Gilman A. G. (1987) Annu. Rev. Biochem. 56, 615–649 [DOI] [PubMed] [Google Scholar]
- 5.Kagawa H., Asai H. (1980) J. Biol. Chem. 255, 7106–7117 [PubMed] [Google Scholar]
- 6.Heck M., Hofmann K. P. (2001) J. Biol. Chem. 276, 10000–10009 [DOI] [PubMed] [Google Scholar]
- 7.Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 8.Lefkowitz R. J., De Lean A., Hoffman B. B., Stadel J. M., Kent R., Michel T., Limbird L. (1981) Adv. Cyclic Nucleotide Res. 14, 145–161 [PubMed] [Google Scholar]
- 9.Levitzki A. (1988) Science 241, 800–806 [DOI] [PubMed] [Google Scholar]
- 10.Neubig R. R. (1994) FASEB J. 8, 939–946 [DOI] [PubMed] [Google Scholar]
- 11.Chidiac P. (1998) Biochem. Pharmacol. 55, 549–556 [DOI] [PubMed] [Google Scholar]
- 12.Bornheimer S. J., Maurya M. R., Farquhar M. G., Subramaniam S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15899–15904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilf G., Kupprion C., Wieland T., Jakobs K. H. (1992) Eur. J. Biochem. 204, 725–731 [DOI] [PubMed] [Google Scholar]
- 14.Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. (1987) Nature 325, 321–326 [DOI] [PubMed] [Google Scholar]
- 15.Dascal N. (2001) Trends Endocrinol. Metab. 12, 391–398 [DOI] [PubMed] [Google Scholar]
- 16.Bösche L. I., Wellner-Kienitz M. C., Bender K., Pott L. (2003) J. Physiol. 550, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leaney J. L., Benians A., Brown S., Nobles M., Kelly D., Tinker A. (2004) Am. J. Physiol. Cell Physiol. 287, C182–C191 [DOI] [PubMed] [Google Scholar]
- 18.Janetopoulos C., Jin T., Devreotes P. (2001) Science 291, 2408–2411 [DOI] [PubMed] [Google Scholar]
- 19.Bünemann M., Frank M., Lohse M. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank M., Thümer L., Lohse M. J., Bünemann M. (2005) J. Biol. Chem. 280, 24584–24590 [DOI] [PubMed] [Google Scholar]
- 21.Hein P., Frank M., Hoffmann C., Lohse M. J., Bünemann M. (2005) EMBO J. 24, 4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bünemann M., Bücheler M. M., Philipp M., Lohse M. J., Hein L. (2001) J. Biol. Chem. 276, 47512–47517 [DOI] [PubMed] [Google Scholar]
- 23.Sternweis P. C., Robishaw J. D. (1984) J. Biol. Chem. 259, 13806–13813 [PubMed] [Google Scholar]
- 24.Breitwieser G. E., Szabo G. (1988) J. Gen. Physiol. 91, 469–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doupnik C. A., Davidson N., Lester H. A., Kofuji P. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10461–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilardaga J. P., Bünemann M., Krasel C., Castro M., Lohse M. J. (2003) Nat. Biotechnol. 21, 807–812 [DOI] [PubMed] [Google Scholar]
- 27.Qin K., Sethi P. R., Lambert N. A. (2008) FASEB J. 22, 2920–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsien R. Y., Bacskai B. J., Adams S. R. (1993) Trends Cell Biol. 3, 242–245 [DOI] [PubMed] [Google Scholar]
- 29.MacKinnon A. C., Brown C. M., Spedding M., Kilpatrick A. T. (1989) Br. J. Pharmacol. 98, 1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano T., Shinohara H., Morishita R., Norota I., Kato K., Endoh M. (1995) J. Biochem. 117, 183–189 [DOI] [PubMed] [Google Scholar]
- 31.Biernbaum M. S., Bownds M. D. (1979) J. Gen. Physiol. 74, 649–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hárosi F. I. (1975) J. Gen. Physiol. 66, 357–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenzweig D. H., Nair K. S., Wei J., Wang Q., Garwin G., Saari J. C., Chen C. K., Smrcka A. V., Swaroop A., Lem J., Hurley J. B., Slepak V. Z. (2007) J. Neurosci. 27, 5484–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.