Abstract
Previously we demonstrated that c-Jun N-terminal kinase (JNK) plays a central role in acetaminophen (APAP)-induced liver injury. In the current work, we examined other possible signaling pathways that may also contribute to APAP hepatotoxicity. APAP treatment to mice caused glycogen synthase kinase-3β (GSK-3β) activation and translocation to mitochondria during the initial phase of APAP-induced liver injury (∼1 h). The silencing of GSK-3β, but not Akt-2 (protein kinase B) or glycogen synthase kinase-3α (GSK-3α), using antisense significantly protected mice from APAP-induced liver injury. The silencing of GSK-3β affected several key pathways important in conferring protection against APAP-induced liver injury. APAP treatment was observed to promote the loss of glutamate cysteine ligase (GCL, rate-limiting enzyme in GSH synthesis) in liver. The silencing of GSK-3β decreased the loss of hepatic GCL, and promoted greater GSH recovery in liver following APAP treatment. Silencing JNK1 and -2 also prevented the loss of GCL. APAP treatment also resulted in GSK-3β translocation to mitochondria and the degradation of myeloid cell leukemia sequence 1 (Mcl-1) in mitochondrial membranes in liver. The silencing of GSK-3β reduced Mcl-1 degradation caused by APAP treatment. The silencing of GSK-3β also resulted in an inhibition of the early phase (0–2 h), and blunted the late phase (after 4 h) of JNK activation and translocation to mitochondria in liver following APAP treatment. Taken together our results suggest that activation of GSK-3β is a key mediator of the initial phase of APAP-induced liver injury through modulating GCL and Mcl-1 degradation, as well as JNK activation in liver.
Keywords: Metabolism/Drug, Phosphorylation/Kinases/Serine-Threonine, Signal Transduction/Protein Kinases/Serine/Threonine, Sulfhydryls/Glutathione, Tissue/Organ Systems/Liver, Toxins/Drugs, Toxins/Drugs/Xenobiotics/Drug Action, Glycogen Synthase Kinase 3, JNK, Mitochondria
Introduction
Acetaminophen (APAP)2 is the leading cause of drug-induced liver injury in the United States (1). APAP also remains an important experimental model for drug-induced liver injury, because it is one of the few drugs that cause liver injury in humans, which is reproducible in animals. Consequently, a great deal of our understanding of drug-induced liver injury has come from studying APAP-induced liver injury in animals (2). Although research on APAP hepatotoxicity spans nearly four decades, studies of the mechanisms underlying APAP hepatotoxicity continue to reveal many new and surprising findings, particularly regarding the importance of signaling pathways in mediating APAP-induced liver injury (3). Recent studies have shown that APAP-induced liver injury is not a passive process due to overwhelming injury caused by N-acetyl-p-benzo-quinoneimine (NAPQI; the reactive metabolite of APAP) as traditionally believed, but rather an active process involving the activation of mitogen-activated protein (MAP) kinases, particularly c-Jun N-terminal kinase (JNK) (4–6). Thus, APAP causes a type of programmed necrosis of hepatocytes. JNK is an important threonine/serine kinase that is activated by various stresses including oxidative stress (7, 8). When JNK activation is prolonged, it is believed to promote cell death (8, 9). The small molecule inhibitor of JNK (SP600125) or silencing expression of JNK1 and -2 markedly protected the liver against APAP-induced injury, despite extensive glutathione (GSH) depletion and covalent binding caused by the production of NAPQI (5, 6).
The JNK signaling pathway activated by APAP appears to target mitochondria to mediate APAP-induced liver injury (3). APAP hepatotoxicity involves two key processes centered on mitochondria: 1) GSH depletion and covalent binding by NAPQI in mitochondria and 2) the activation of JNK and translocation of JNK to mitochondria (5). APAP is metabolized by the CYP2E1 isoform of cytochrome P450 to NAPQI, which depletes GSH and covalently binds to protein thiols, which can inactivate proteins (2, 10). Traditionally, it had been believed that GSH depletion and covalent binding in mitochondria directly lead to inhibition of bioenergetics and trigger mitochondrial permeability transition (MPT; opening of the mitochondria mega-pore) resulting in hepatocyte necrosis and liver injury. However, recent studies by our lab suggest that mitochondrial GSH depletion and covalent binding act only as a first hit, which moderately impairs mitochondria function and leads to release of reactive oxygen species (ROS), but inhibition of mitochondrial bioenergetics remains below a critical threshold for hepatocyte viability. However, the first hit (mitochondrial GSH depletion and covalent binding) leads to ROS-mediated activation of MAP kinase and sensitizes mitochondria to a second hit, the translocation of activated JNK to mitochondria, which leads to MPT and marked inhibition of mitochondria function (3, 5, 6). JNK translocates to mitochondria and promotes MPT, cytochrome c release, and causes loss of mitochondrial bioenergetics in various model systems (7, 8, 11). Inhibition of JNK protects against mitochondrial dysfunction and MPT caused by APAP treatment, which prevents hepatocyte death and liver injury (5). Similarly, deletion of apoptosis signaling-regulated kinase 1 (ASK-1), an important upstream MAP kinase involved in JNK activation has also been shown to prevent APAP-induced liver injury (12). The exact mechanism by which JNK impairs mitochondria function and promotes MPT has not been completely elucidated.
The importance of the ROS-activated MAP kinase pathway in APAP hepatotoxicity suggests the possibility that other ROS-activated signaling pathways, both related and unrelated to JNK, may also modulate APAP liver injury. Recently, glycogen synthase kinase-3β (GSK-3β) has received a great deal of attention in various pathologies including ischemia reperfusion injury in the heart (13, 14). GSK-3β is an important kinase that was first identified as the major regulator of glycogen synthase, the key enzyme involved in glycogen synthesis. However, like many other kinases, GSK-3β was found to regulate many other processes, including those involving cell death (14, 15). In myocardial ischemia-reperfusion injury, GSK-3β, like JNK, has been shown to translocate to mitochondria and promote MPT, through interactions with voltage-dependent anion channels or through degradation of Mcl-1, an antiapoptotic Bcl-2 protein in mitochondrial membranes (16–18). Therefore, the present studies were designed to determine whether GSK-3β plays a role in APAP hepatotoxicity and to understand its mechanism of action.
EXPERIMENTAL PROCEDURES
Materials
Antisense oligonucleotides (ASO) targeting mouse GSK-3α, GSK-3β, Akt2, JNK1, JNK2, and a control oligonucleotide were synthesized as 20 nucleotides, uniform phosphorothioate chimeric oligonucleotides, and purified as previously described (5). The oligonucleotides used in these studies were chimeric oligonucleotides containing five nuclease-resistant 2′-O-methoxyethylribose-modified phosphorothioate residues on the 5′ and 3′ ends, flanking a 2′-deoxyribonucleotide/phosphorothioate region that supports RNase H-based cleavage of the targeted mRNA. The sequences of the mouse GSK-3β ASO and GSK-3α ASO are 5′-TGGCTTGATATACCACACCA-3′ and 5′-CACACATCGATGGACGAGGT-3′, respectively. The sequence for Akt2 ASO was 5′-ACCTCATTCATGGTGGCAGC-3′ and the control oligonucleotide nonsense sequence was 5′-CCTTCCCTGAAGGTTCCTCC-3′. Anti-phospho-GSK-3β (Tyr-216, Ser-9), GSK-3β, phospho-Akt (Thr-308), Akt, phospho-PKA, PKA, phospho-JNK, JNK, glycogen synthase, phosphoglycogen synthase, actin, and cytochrome oxidase IV (COX IV) antisera were purchased from Cell Signaling (Beverly, MA). Anti-GCL was purchased from Novus (Littleton, CO). Anti-Bax and cytochrome c antisera were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-myeloid cell leukemia sequence 1 (Mcl-1) antibody was purchased from either Santa Cruz Biotechnology or Rockland (Gilbertsville, PA).
Animals
Male mice (C57BL/6; 6–8 weeks of age) were obtained from Harlan Bioproducts for Science Inc. (Indianapolis, IN). The animals were housed in a temperature-controlled room and allowed to acclimatize for a minimum of 3 days prior to use in experiments. They were maintained on a commercial pellet diet ad libitum. The animals were fasted overnight (no food, but water was available) prior to experiments. All the treatments were administered intraperitoneally. APAP (Sigma) was dissolved in warm PBS (55 °C) and cooled to 37 °C before injection into mice (5). For ASO experiments, the animals were given 50 mg/kg in sterile saline (intraperitoneally, 7 injections total, one every other day, an ∼0.3 ml volume injected) prior to APAP administration (6). The last dose of ASO was given 1 day prior to APAP administration. In experiments involving JNK inhibitor (SP600125), JNK inhibitor was dissolved in DMSO (8.3%, v/v) in PBS (1 mg in 125 μl of DMSO diluted with 1375 μl of PBS). JNK inhibitor (10 mg/kg) was injected (intraperitoneally, ∼0.3 ml volume injected) into mice 1 h prior to APAP injection. Blood was obtained after mice were anesthetized at the indicated time periods and serum alanine transaminase (ALT) was measured at the University of Southern California Pathology Reference Laboratory. All animals received care according to methods approved under institutional guidelines for the care and use of laboratory animals in research.
Cell Isolation and Culture
Primary cultured hepatocytes were isolated as previously described (19). The liver was perfused with collagenase and isolated hepatocytes were suspended in Dulbecco's modified Eagle's medium/F-12 containing 10% heat-inactive fetal bovine serum, 1 nm bovine insulin, 100 units/ml of penicillin, and 0.1 mg/ml of streptomycin, 50 nm hydrocortisone, and 0.15 mg/ml of methionine. 1.2 × 106 cells in 4 ml were plated in individual 60-mm diameter LUX culture dishes coated with 0.03% (w/v) rat tail collagen and cultured in a 5% CO2 atmosphere at 37 °C. The viability of the isolated hepatocytes was greater than 90% as judged by trypan blue exclusion. After 3 h, the culture medium was changed to serum-free medium containing 100 units/ml of penicillin and 0.1 mg/ml of streptomycin. At this time, APAP and buthionine sulfoximine (BSO) were added to cultured hepatocytes. APAP was dissolved in culture medium and sterile filtered before treatment to cells. BSO, dissolved in PBS, was immediately added after APAP treatment. The GSK-3β inhibitor (SB216763) was dissolved in DMSO and added 2 h before APAP treatment. After 20–24 h of treatment, cells were double-stained with 8 μg/ml of Hoechst 33258 and 1 μm Sytox Green (20). After staining, the culture dishes were observed under an OLYMPUS fluorescent microscope. Quantitation of total and apoptotic cells were performed by counting a minimum of 1000 cells in 10 different fields. Necrotic cells (Sytox Green positive) were determined by counting the same field. APAP, in the presence or absence of BSO, primarily caused necrosis with very little apoptosis being observed (<1%).
Isolation of Liver Mitochondria and Cytoplasm
Mitochondria were isolated from liver of mice by differential centrifugation as previously described (21). Livers were excised, washed with 0.25 m sucrose, and homogenized in H-medium (210 mm mannitol, 70 mm sucrose, 2 mm HEPES, 0.05% bovine serum albumin (w/v), plus protease and phosphatase inhibitors). The homogenate was centrifuged at 800 × g for 10 min, the pellet removed, and the centrifugation process repeated. The resulting supernatant was centrifuged at 8,500 × g for 15 min. The supernatant (“cytoplasmic fraction,” postmitochondrial S9 fraction) was collected and saved at −80 °C for future analysis. The pellet, which represents the mitochondria fraction, was washed with H-medium and the centrifugation repeated. The mitochondria were resuspended in H-medium before oxygen electrode and Western blot analysis.
Measurements of Respiration in Isolated Mitochondria
Respiration was measured in freshly isolated mitochondria by monitoring oxygen consumption polarographically with a Clark-type electrode (Hanstech, UK) in respiration buffer containing 230 mm mannitol, 70 mm sucrose, 30 mm Tris-HCl, 5 mm KH2PO4, 1 mm EDTA, pH 7.4 (21). For isolated mitochondria, 0.75 mg of mitochondria protein was added to 1 ml of respiration buffer and oxygen consumption was monitored in the presence of mitochondrial substrates (21).
Immunoblotting
Aliquots of cytoplasmic or mitochondria extracts were fractionated by electrophoresis on a 12% SDS-polyacrylamide gel (Bio-Rad). Subsequently, proteins were transferred to nitrocellulose membrane and blots were blocked with 5% (w/v) nonfat milk dissolved in Tris-buffered saline with Tween 20. The blots were then incubated with the desired primary and secondary antibodies. Finally, the proteins were detected by Luminol ECL reagent (Santa Cruz Biotechnology, Santa Cruz, CA). Multiple bands in immunoblots of GS and GSK-3β often observed are likely to be the result of multiple phosphorylation sites on these proteins. All gels shown are representative samples from three experiments. Densitometry was performed using Image J program from NIH.
GSH Measurements
GSH was detected using HPLC electrochemical detection as previously described (19). Total liver homogenate or mitochondria were mixed with metaphosphoric acid to obtain a 5% metaphosphoric acid solution to prevent GSH autoxidation. Samples were centrifuged (12,000 × g for 5 min) and the supernatant injected into the HPLC.
Glutamate Cysteine Ligase (GCL) Measurements
GCL activity was measured spectrophotometrically as previously described (22). Cytoplasmic liver fraction was run twice, once without and once with BSO, a specific irreversible inhibitor of GCL (23). The specific GCL activity was calculated from the NADH consumption in samples without BSO minus the samples with BSO.
Quantitative Reverse Transcription-PCR for GCL mRNA Levels
Total RNA was extracted using the RNeasy Plus Mini kit according to the manufacturer's instructions (Qiagen). First strand cDNA was produced using the Omniscript reverse transcription kit (Qiagen) with 10 μm random primers (Applied Biosystems). Two micrograms of total RNA were used for each reverse transcription reaction mixture (20 μl). Real time PCR was carried out using an ABI Prism 7900HT (Applied Biosystems). 10-μl reactions were carried out in a 384-well PCR plate using the following final concentrations: 1 μmol each of forward and reverse primers, 1× SYBR Green PCR master mix (Quanti Tect SYBR Green PCR kit, Qiagen), and 5 ng of cDNA. For each condition triplicates were used to minimize the variation. Cycling conditions were as follows: initial step (50 °C for 2 min), hot activation (95 °C for 10 min), amplification (95 °C for 15 s and 60 °C for 1 min), repeated 40 times, and quantification with a single fluorescence measurement at the dissociation stage. Data were analyzed using ABI Prism SDS 2.1 software. We used the standard curve method provided by the system software (SDS2.1) for relative quantification following the manufacturer's instructions (Applied Biosystems). Briefly, the resultant mRNA was normalized to its own glyceraldehyde-3-phosphate dehydrogenase. Final results were expressed as n-fold difference in gene expression relative to glyceraldehyde-3-phosphate dehydrogenase mRNA. For C57BL/6 mouse, the primer pair for Gcl-c was 5′-GGGGTGACGAGGTGGAGTA-3′ and 5′-GTTGGGGTTTGTCCTCTCCC-3′), and the primer pair for glyceraldehyde-3-phosphate dehydrogenase was 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Statistical Analysis
Statistical analyses were performed using the Student's t test for unpaired data or analysis of variance for comparison of multiple groups. p < 0.05 was defined as statistically significant.
RESULTS
Signaling Pathways Involved in APAP-induced Liver Injury
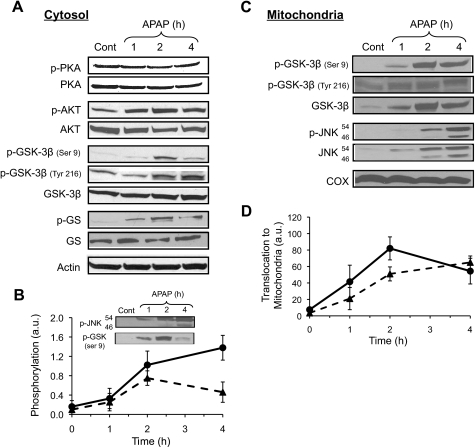
To determine whether other signaling pathways besides JNK play an important role in APAP hepatotoxicity, the activation of various signaling pathways following APAP treatment was investigated in liver. Fig. 1A shows that APAP treatment results in increased phosphorylation of both GSK-3β and Akt, but does not affect the protein kinase A (PKA) phosphorylation status. Phosphorylation of Akt at the site of threonine 308 is generally associated with activation and once activated, Akt can phosphorylate many proteins including GSK-3β on the site of serine 9 (14). GSK-3β is constitutively active in the cytoplasm, but its activity can be enhanced by phosphorylation at tyrosine 216 or inhibited by phosphorylation at the site of serine 9 (15, 24). Although APAP treatment increased levels of both the active and inhibited forms of GSK-3β, APAP caused an increase in glycogen synthase phosphorylation, an important downstream target of GSK-3β, suggesting APAP treatment leads to an overall increase in GSK-3β activity. Fig. 1B shows the time course of GSK-3β (serine 9) and JNK phosphorylation. Both GSK-3β (serine 9) and JNK phosphorylation occurred within 1 h of APAP treatment; however, GSK-3β phosphorylation appears to peak at 2 h, and begins to decline at 4 h, unlike JNK phosphorylation, which peaked at 4 h. Previous studies have also suggested that GSK-3β, like JNK, once further activated, translocates to mitochondria. APAP treatment resulted in translocation of GSK-3β (both phosphorylated forms and total GSK-3β) to mitochondria, similar to JNK (Fig. 1C). Again, both GSK-3β and JNK translocation to mitochondria started at 1 h. GSK-3β translocation to mitochondria peaked at 2 h, whereas JNK translocation peaked at 4 h. The signaling changes to Akt, JNK, GSK-3β, and glycogen synthase as well as GSK-3β and JNK translocation to mitochondria, all preceded liver injury (ALT increased at ∼6 h) caused by APAP. GSK-3β translocation to mitochondria only occurred at APAP doses that caused liver injury (i.e. >200 mg/kg) and not at doses that do not cause liver injury (i.e. <200 mg/kg).
FIGURE 1.
Signaling pathways in liver activated by APAP treatment in vivo. A, effect of APAP treatment on PKA, AKT, JNK, GSK-3β, and glycogen synthase (GS) phosphorylation in liver cytoplasm. B, time course of JNK (●) and GSK-3β (▴; Ser-9) phosphorylation following APAP treatment in the liver. C, translocation of GSK-3β and JNK to mitochondria. D, time course of total JNK (●) and GSK-3β (▴) translocation to mitochondria. Mice were treated with APAP (300 mg/kg, intraperitoneally). At the times indicated, mice were sacrificed, the liver was removed, and the cytoplasm and mitochondria fractions were separated using differential centrifugation. Immunoblotting was performed on the cytoplasm and mitochondria. Immunoblotting of actin was used to show equal loading in cytoplasm samples, whereas COX was utilized to show equal loading in mitochondrial fractions. Densitometry was performed using the Image J program from NIH. Gels are representative of three samples and B and D show mean ± S.D. (n = 3–5).
Protective Effects of Silencing GSK-3β against APAP Hepatotoxicity in Vivo and in Cultured Primary Hepatocytes
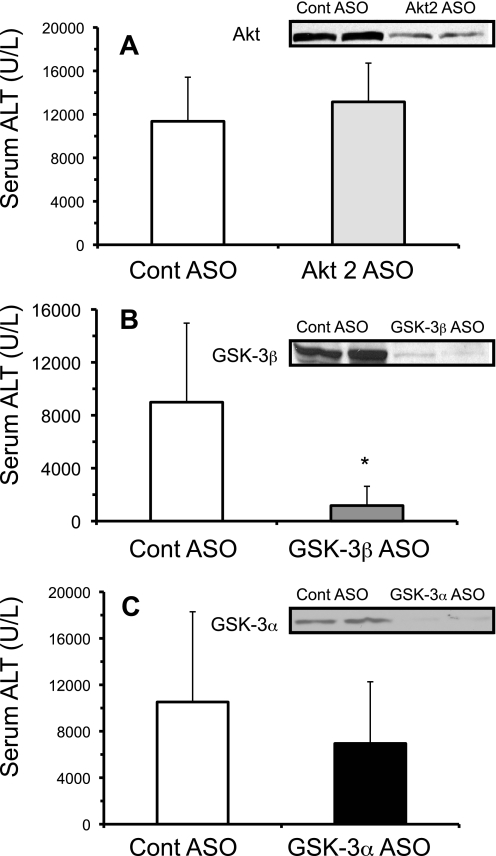
We next examined whether the signaling pathways activated (Akt, GSK-3β) by APAP treatment in vivo were important in mediating hepatotoxicity. In liver, there are three isoforms of Akt (Akt1, Akt2, and Akt3), with Akt2 being an important regulator of insulin signaling (25). To determine whether Akt2 may be important in APAP-induced liver injury, Akt2 was silenced using antisense. Although Akt levels were significantly reduced in liver, no differences in serum ALT levels occurred with the silencing of Akt2 in vivo following APAP treatment (300 mg/kg) (Fig. 2A). On the other hand, silencing of GSK-3β significantly reduced serum ALT levels compared with control, suggesting that GSK-3β activation is important in mediating liver injury caused by APAP (Fig. 2B). The protection against APAP-induced liver injury was not observed when GSK-3α, the other GSK isoform, was silenced using antisense (Fig. 2C). The lack of protection by silencing GSK-3α confirms that GSK-3β specifically mediates APAP hepatotoxicity.
FIGURE 2.
Silencing GSK-3β, but not Akt2 or GSK-3α, protects against APAP-induced liver injury in vivo. Effect of Akt2 antisense (A), GSK-3β antisense (B), and GSK-3α (C) antisense treatment on APAP-induced liver injury. Mice were treated with Akt2, GSK-3β, GSK-3α, or control scrambled antisense for 2 weeks. The day following the last antisense injection, APAP (300 mg/kg, intraperitoneally) was injected into mice. Serum was taken 20–24 h following APAP injection for ALT measurements. Western blots (insets) were performed on liver samples from mice not treated with APAP to confirm the silencing of the proteins by antisense injection. Results are mean ± S.D. from experiments using 4–9 mice. *, p < 0.05 versus control antisense-treated mice.
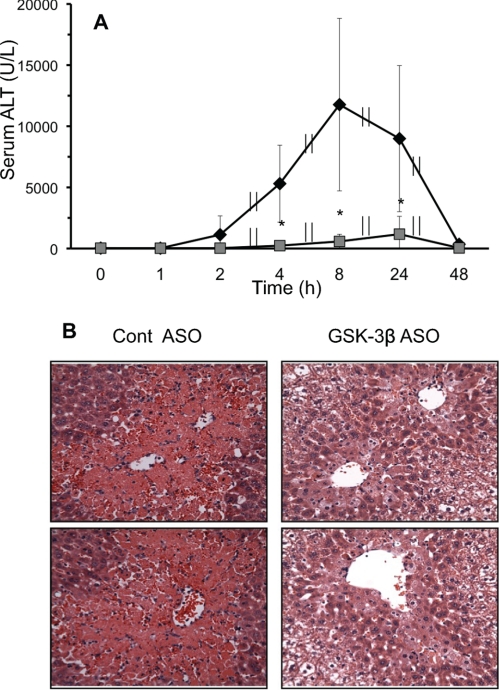
A detailed time course revealed that silencing GSK-3β significantly lowered ALT levels compared with control at all time points examined, up to 48 h (Fig. 3A). This demonstrates that silencing GSK-3β actually prevents, and does not simply delay, APAP-induced liver injury. Liver histological sections showed a significant decrease in necrosis following APAP treatment when GSK-3β was silenced (Fig. 3B), confirming the ALT findings. Several chemical GSK-3β inhibitors (SB216763 and SB415286) were also tested in vivo to determine their protective effects against APAP-induced liver injury. However, problems with solubility requiring large amounts of DMSO, which greatly modulates APAP injury, and ineffectiveness of inhibiting GSK-3β activity precluded the use of these chemical GSK-3β inhibitors in vivo (data not shown).
FIGURE 3.
Silencing GSK-3β protects mice from APAP-induced liver injury. A, ALT time course for control (♦) and GSK-3β-silenced (■) mice. B, H&E histology of APAP-induced liver injury in control and GSK-3β-silenced mice at 24 h (top and bottom panels show different mice). Control and GSK-3β-silenced mice, using antisense (ASO), were treated with APAP (300 mg/kg, intraperitoneally). At various times, mice were sacrificed, the liver removed for histology, and serum was collected for ALT measurements. Results are mean ± S.D. for three to nine experiments. *, p < 0.05 versus control ASO-treated mice.
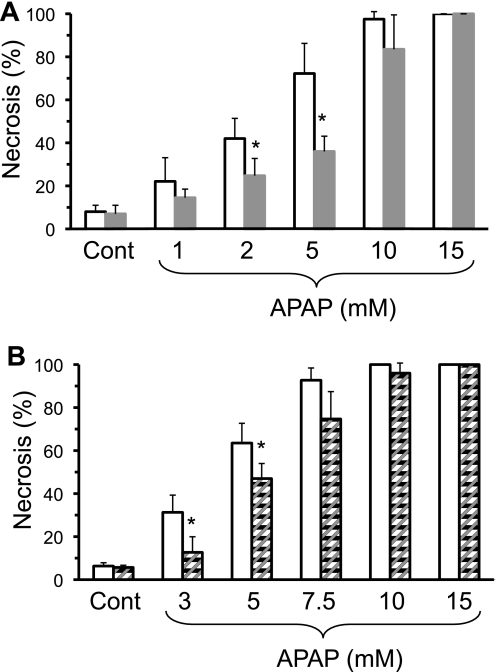
Cultured primary hepatocytes isolated from GSK-3β-silenced mice were also significantly protected from necrosis caused by various doses of APAP (Fig. 4A), supporting the role of GSK-3β in hepatocytes as opposed to nonparenchymal cells. Similarly, pretreatment (2 h) of hepatocytes (both β and α isoforms) with 20 μm SB216763 (an inhibitor specific for GSK-3) (26), also protected hepatocytes from APAP-induced necrosis (Fig. 4B). Because the GSK-3 inhibitor was dissolved in DMSO, which alters APAP hepatotoxicity (27), slightly higher levels of APAP were used in experiments involving GSK-3 inhibitor. At very high doses of APAP (>10 mm), GSK-3β silencing or inhibition no longer conferred protection against APAP, suggesting that there is a threshold of protection. The protective effects of silencing or inhibiting GSK-3β in primary cultured hepatocytes was not as effective in preventing APAP hepatotoxicity as was observed when GSK-3β was silenced in vivo. However, this is very similar to effects observed with JNK inhibition, in which the inhibition of JNK only partially protected against APAP hepatotoxicity in cultured hepatocytes (∼35%), but JNK inhibition almost completely protected against APAP hepatotoxicity in vivo (6, 28), suggesting significant differences exist between the two models. Silencing GSK-3β did not protect hepatocytes against tumor necrosis factor and actinomycin-induced apoptosis. Hepatocytes with GSK-3β silenced had equal death (∼99%) as control hepatocytes when treated with tumor necrosis factor (5 ng) plus actinomycin D (10 μm). These findings suggest that GSK-3β in hepatocytes plays a key role in APAP-induced liver injury, but does not play a role in all forms of liver injury.
FIGURE 4.
Silencing or inhibiting GSK-3β protects primary cultured hepatocytes from APAP hepatotoxicity. A, effect of APAP treatment on primary cultured hepatocytes from control and GSK-3β-silenced mice; B, effect of APAP treatment on primary cultured hepatocytes pretreated with GSK-3β inhibitor (SB216763; 20 μm pretreatment for 2 h). Open bar, control hepatocytes; solid bar, GSK-3β silenced hepatocytes; and striped bars, GSK-3β inhibitor-pretreated hepatocytes. Primary cultured hepatocytes were isolated from mice treated with control ASO, GSK-3β ASO, or untreated. Necrosis was determined 20–24 h following APAP treatment using Sytox green as described under ”Experimental Procedures.“ Results are mean ± S.D. for four to five experiments. *, p < 0.05 versus control.
Effect of GSK-3β on GSH Levels in Liver following APAP Treatment
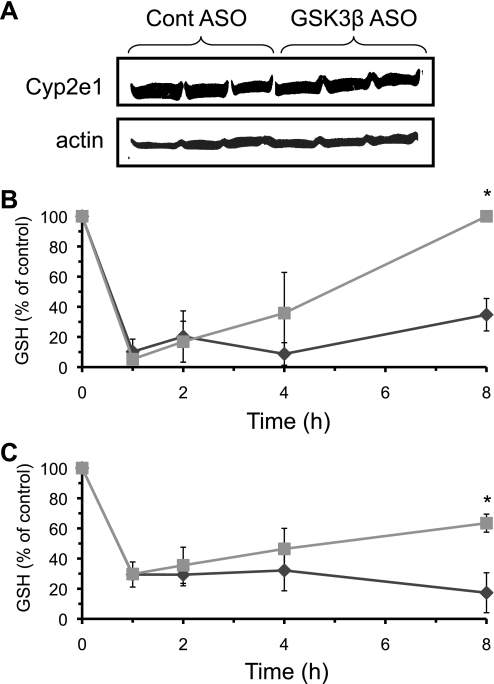
Many potential mechanisms may underlie the protective effects of silencing GSK-3β against APAP-induced liver injury. First, we investigated the possibility that silencing GSK-3β could be modulating Cyp2e1 levels, the major protein involved in generating NAPQI responsible for GSH depletion (2). Fig. 5A shows that silencing GSK-3β did not affect expression of Cyp2e1, suggesting NAPQI formation was unaffected by GSK-3β. GSH depletion is a major component of APAP hepatotoxicity and modulation of GSH synthesis can greatly influence APAP-induced injury. To determine whether GSK-3β modulates GSH in liver following APAP treatment, we next examined the time course of GSH levels in the liver of GSK-3β-silenced and control mice following APAP treatment (Fig. 5, B and C). Baseline GSH levels were not altered by silencing GSK-3β (control ASO = 46.3 + 9.5, GSK-3β ASO = 49.6 + 17.5 nmol/mg of protein). APAP treatment (300 mg/kg) caused an identical sharp decline in GSH levels in whole liver and mitochondria (1–2 h following APAP treatment) in both control and GSK-3β-silenced mice. This suggests that the initial NAPQI production was not altered, consistent with the lack of effect of silencing GSK-3β on Cyp2e1 expression. On the other hand, the recovery of GSH beginning 2 h following APAP treatment was accelerated in mice lacking GSK-3β compared with control. Because GSH depletion is a key component in APAP-induced liver injury, the inhibition of GSH recovery when mice were treated with APAP alone may be an important component in APAP-induced liver injury.
FIGURE 5.
GSH recovery in liver is enhanced in GSK-3β-silenced mice following APAP treatment. A, Cyp2e1 protein levels; B, GSH levels in total liver homogenate; C, GSH levels in isolated liver mitochondria. Control ASO (♦) and GSK-3β ASO treated (■) mice were given APAP (300 mg/kg, intraperitoneally). At the indicated times, mice were sacrificed, the liver removed and homogenized, and the cytoplasm and mitochondrial fractions were separated using differential centrifugation. Liver homogenate and isolated mitochondria were immediately placed in 5% metaphosphoric acid to prevent GSH oxidation. GSH levels were analyzed using HPLC with electrochemical detection as described under ”Experimental Procedures.“ Results are mean ± S.D. for three to five experiments. *, p < 0.05 versus control ASO-treated mice.
Effect of APAP on GCL mRNA and Protein Levels
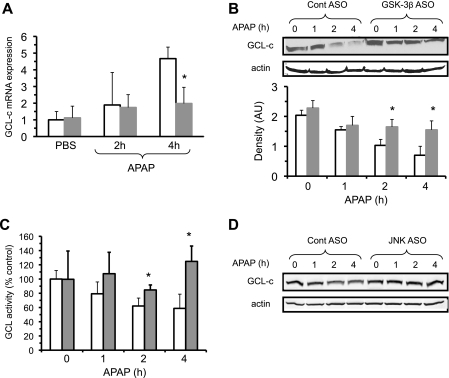
There are several possible mechanisms by which GSK-3β could be suppressing GSH recovery following APAP treatment. Therefore, we examined the possibility that GSK-3β or a downstream effect may regulate GSH synthesis by modulating synthesis or degradation of GCL (the rate-limiting enzyme in GSH biosynthesis) (23, 29). GCL is a heterodimeric protein composed of two subunits, a catalytic heavy subunit (GCL-c) and a modulator light subunit (GCL-m) (30, 31). The GCL-c subunit is up-regulated by various stresses including oxidative stress. GCL-c mRNA levels have also been shown to be up-regulated by APAP treatment as a defense to counter GSH depletion caused by NAPQI (32). The transcription of GCL-c is regulated by Nrf-2, a transcription factor that translocates to the nucleus following activation by oxidative stress or covalent binding of NAPQI (32, 33). In certain contexts GSK-3β has been shown to phosphorylate Nrf-2, which causes Nrf-2 to move out of the nucleus, thus preventing Nrf-2 transcriptional activity (i.e. synthesis of GCL-c mRNA) (34). The effect of silencing GSK-3β on GCL-c mRNA levels were measured by quantitative reverse transcription-PCR. Fig. 6A shows that GCL-c mRNA levels increased following APAP treatment, in agreement with previous results that APAP treatment induces Nrf-2-dependent transcription of GCL mRNA levels (32). However, GCL-c mRNA levels were lower in mice having GSK-3β silenced compared with control ASO-treated mice, suggesting that silencing GSK-3β did not enhance but rather reduced GCL transcription, presumably by protecting against APAP hepatotoxicity (e.g. decreased mitochondrial reactive oxygen species generation). However, mRNA levels are not always a direct measure of transcription and other factors besides changes in transcription (i.e. mRNA stability) may account for differences in GCL-c mRNA levels when GSK-3β is silenced. Similar results were seen in GCL-m mRNA levels (data not shown).
FIGURE 6.
Silencing GSK-3β prevents loss of GCL-c induced by APAP, but does not increase GCL-c mRNA. GCL-c mRNA levels (A), GCL-c protein levels (B), and GCL-c activity (C) following APAP treatment (300 mg/kg, intraperitoneally) in control and GSK-3β-silenced mice. D, GCL-c protein levels in JNK1 and -2-silenced mice. Open bar, control ASO; solid bar, GSK-3β-silenced mice. GCL-c protein levels were analyzed using immunoblotting with antisera to GCL-c. Densitometry was performed using the Image J program from NIH (AU, arbitrary units). A portion of liver was sectioned off, RNA extracted, and qPCR was performed to assess GCL-c mRNA levels as described under ”Experimental Procedures.“ GCL activity was analyzed using a spectophotometric assay utilizing BSO as described under ”Experimental Procedures.“ Results are mean ± S.D. for four to five experiments. *, p < 0.05 versus control antisense untreated (no APAP) mice.
We next examined whether GCL-c protein levels were modulated by GSK-3β and APAP in liver. Surprisingly, GCL-c levels in liver dramatically declined following APAP treatment (Fig. 6B), an effect that was significantly abrogated in mice treated with GSK-3β ASO. To confirm that APAP treatment causes a decline in GCL-c protein levels, GCL-c activity was measured. In agreement with the Western blot data, GCL-c activity significantly declined at 2 and 4 h following APAP treatment in the liver of control mice, but not in the liver of GSK-3β-silenced mice (Fig. 6C). No changes in GCL-m protein levels were observed after APAP treatment (data not shown). This data suggests that silencing GSK-3β preserves GCL-c protein levels and GCL activity, which helps explain the greater recovery of GSH following APAP treatment.
To determine whether GCL-c loss was a direct or specific effect of GSK-3β, or was downstream of JNK activation, we examined whether GCl-c degradation was also inhibited in JNK-silenced (both 1 and 2) mice. Silencing JNK1 and -2 dramatically reduces APAP-induced liver injury, and was also associated with prevention of GCL-c degradation (Fig. 6D). This suggests that GCL-c degradation may be not be directly mediated by GSK-3β.
Inhibiting or Silencing GSK-3β Protects Primary Cultured Hepatocytes from APAP Despite Inhibition of GSH Synthesis by Buthionine Sulfoximine
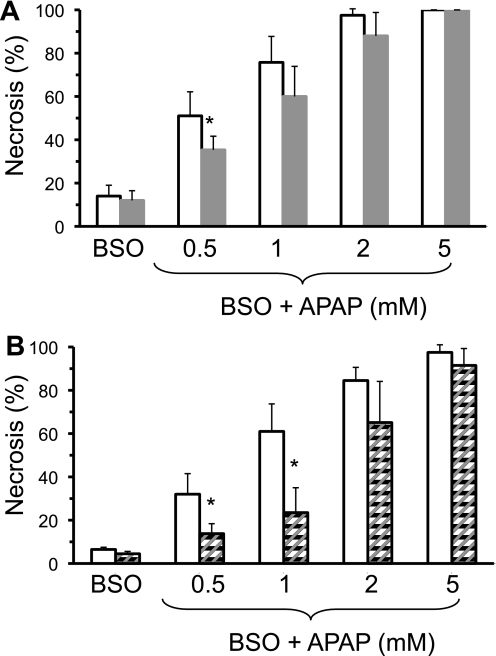
To determine whether GSK-3β mediates APAP hepatotoxicity solely through GSH modulation, primary cultured hepatocytes were simultaneously treated with APAP and BSO, an irreversible inhibitor of GCL (23). BSO treatment completely inhibits GSH synthesis and nullifies any effect GSK-3β exerts on GSH levels. Fig. 7A demonstrates that hepatocytes taken from mice treated with GSK-3β ASO exhibited resistance to necrosis induced by APAP and BSO. Because BSO inhibits GSH synthesis, the co-treatment of hepatocytes with BSO shifts the toxic dose curve of APAP, requiring significantly lower levels of APAP to induce necrosis in hepatocytes. Similarly, GSK-3β inhibitor pretreatment significantly protected against hepatotoxicity induced by APAP and BSO in primary cultured hepatocytes (Fig. 7B). Thus, inhibiting or silencing GSK-3β protected against APAP hepatotoxicity even when GSH synthesis was inhibited by BSO, suggesting that there is also a mechanism of action by GSK-3β in APAP hepatotoxicity independent of its effect on GSH recovery.
FIGURE 7.
Silencing or inhibiting GSK-3β protects primary cultured hepatocytes from APAP hepatotoxicity despite inhibition of GSH synthesis with buthionine sulfoximine. A, effect of APAP plus BSO (1 mm; GSH synthesis inhibitor) treatment of control and GSK-3β-silenced hepatocytes; B, effect of APAP plus BSO treatment on primary hepatocytes pretreated with GSK-3β inhibitor (SB216763; 20 μm pretreatment for 2 h). Open bar, control; solid bar, GSK-3β-silenced hepatocytes; and striped bars, GSK-3β inhibitor-treated hepatocytes. Necrosis was determined 20–24 h following APAP treatment using Sytox green as described under ”Experimental Procedures.“ Results are mean ± S.D. for four to five experiments. *, p < 0.05 versus control.
Effect of GSK-3β on Mitochondrial Function
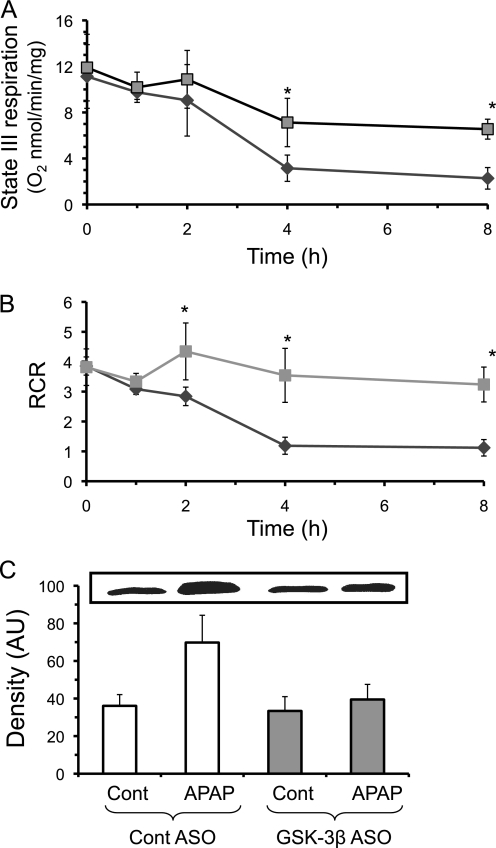
We next investigated possible GSH independent pathways involved in protecting liver from APAP when GSK-3β is silenced. Loss of mitochondrial bioenergetics and MPT play a central role in APAP-induced liver injury (5). Mitochondrial respiration was measured to determine whether GSK-3β plays a role in mitochondrial dysfunction during APAP-induced liver injury. Fig. 8, A and B, shows that the decline in state III respiration (glutamate/malate plus ADP) and respiratory control ratio (state III/state IV (glutamate/malate but no ADP)) caused by APAP treatment was significantly reduced in mitochondria of mice lacking GSK-3β. Similarly, the release of cytochrome c into the cytoplasm, which indicates mitochondrial damage, was reduced following APAP treatment (4 h) in mice when GSK-3β was silenced (Fig. 8C). Overall, silencing GSK-3β was associated with a protection against loss of mitochondrial function caused by APAP treatment.
FIGURE 8.
Silencing GSK-3β protects against mitochondrial dysfunction and cytochrome c release caused by APAP treatment in vivo. A, state III respiration (using glutamate/malate plus ADP); B, RCR (respiratory control ratio, state III/state IV); and C, cytochrome c release from mitochondria. Control (♦) and GSK-3β-silenced (■) mice were treated with APAP (300 mg/kg, intraperitoneally) and at the times indicated, the liver was removed and cytoplasm and mitochondria fractions were separated using differential centrifugation. State III and respiratory control ratio measurements were performed by monitoring oxygen consumption using a Clarke-type electrode as described under ”Experimental Procedures.“ Immunoblotting for cytochrome c was performed in the cytoplasmic fraction. Actin showed equal loading of cytoplasmic proteins (data not shown). Results are mean ± S.D. for three to five experiments. *, p < 0.05 versus control antisense-treated mice. AU, arbitrary unit.
Effect of GSK-3β on the Activation of JNK during APAP Hepatotoxicity
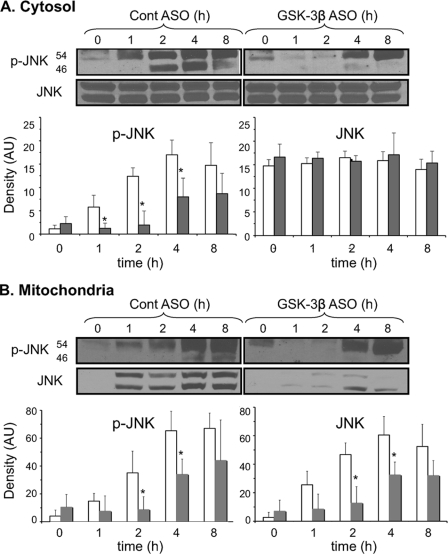
Previously, we showed that activation and translocation of JNK to mitochondria was an important step in causing a decline in mitochondrial bioenergetics during APAP-induced liver injury (5). Several studies have suggested that GSK-3β is involved in JNK activation through interaction with upstream kinases such as mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) and mixed lineage kinase (MLK) (35, 36). To determine whether GSK-3β is upstream of JNK activation after APAP treatment, JNK activity and translocation to mitochondria were measured in control ASO and GSK-3β ASO-treated mice. Fig. 9 demonstrates that the silencing of GSK-3β both significantly delayed and reduced JNK activation induced by APAP treatment. The phosphorylation of JNK, which occurs within an hour of APAP treatment in control mice, did not occur in GSK-3β-silenced mice until 4 h following APAP treatment and remained reduced up to 8 h following APAP treatment (Fig. 9A). Similarly, translocation of P-JNK and total JNK to mitochondria was delayed and at a reduced level (Fig. 9B). The silencing of GSK-3β did not affect JNK expression, although there was a trend of increased basal JNK activation (P-JNK in cytoplasm and mitochondria) when GSK-3β was silenced. These data suggest that GSK-3β plays a role in the early activation of JNK by APAP treatment and suggests that the protective effects of silencing GSK-3β may include suppression of JNK activation and translocation to mitochondria.
FIGURE 9.
Silencing GSK-3β inhibits JNK activation and translocation to mitochondria following APAP treatment. JNK activation in the cytoplasm (A) and translocation to mitochondria (B). Open bar, control ASO; solid bar, GSK-3β-silenced mice for total p-JNK and JNK (JNK 1 + 2). Control ASO and GSK-3β ASO-treated mice were given APAP (300 mg/kg, intraperitoneally). At the indicated times, the liver was excised, and cytoplasm and mitochondria fractions were separated using differential centrifugation. Immunoblotting was performed using antisera against p-JNK, JNK, actin (cytoplasmic loading control), and COX (mitochondria loading control). Densitometry of total p-JNK and JNK levels was performed using Image J (AU, arbitrary units). Cytoplasmic p-JNK and JNK density were normalized to actin (not shown), and p-JNK and JNK in mitochondria were normalized to COX (data not shown). Results are mean ± S.D. for three to seven experiments. *, p < 0.05 versus control antisense-treated mice.
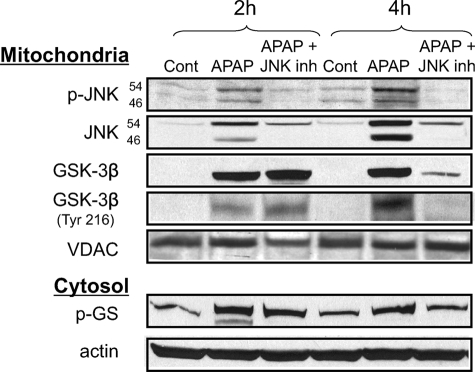
Next, we examined the converse, the effect of inhibition of JNK on GSK-3β activation during APAP-induced liver injury (Fig. 10). The pretreatment of mice with the JNK inhibitor (SP600125) decreased JNK activation (phosphorylation) and JNK translocation to mitochondria caused by APAP, confirming our previous studies (5, 6). However, JNK inhibitor treatment did not affect the initial GSK-3β activation (tyrosine 216 phosphorylation), GSK-3β activity (glycogen synthase phosphorylation), or GSK-3β translocation to mitochondria at 2 h following APAP treatments. These findings further support the conclusion that GSK-3β acts upstream of JNK in APAP hepatotoxicity. However, at 4 h GSK-3β phosphorylation, activity, and mitochondrial translocation declined when JNK was inhibited, suggesting that JNK is important in maintaining sustained GSK-3β activity possibly through a feed-forward mechanism.
FIGURE 10.
JNK inhibition does not inhibit initial GSK-3β activation following APAP treatment. Mice were given APAP (300 mg/kg, intraperitoneally) with or without JNK inhibitor (inh) (SP600125, 10 mg/kg intraperitoneally; 1 h prior to APAP injection). JNK inhibitor was dissolved in 8.3% DMSO/PBS solution (v/v) and the control received the same amount of DMSO. At the indicated times, the liver was excised, and mitochondria were separated from cytoplasm using differential centrifugation. Immunoblot analysis was performed in the cytoplasm and isolated mitochondria. Actin served as loading control in the cytoplasm, whereas voltage-dependent anion channels (VDAC) served as loading control for mitochondria. Gels are representative of three samples.
Effect of GSK-3β on Mcl-1 and Bax Levels in Mitochondria
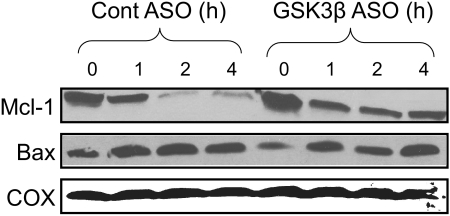
GSK-3β is known to regulate both pro-apoptotic (Bax) and anti-apoptotic (Mcl-1) members of the Bcl-2 family that modulate mitochondrial death pathways (14, 24). GSK-3β has been shown to phosphorylate Bax and induce its translocation to mitochondria in neurons (37). Similarly, GSK-3β has been shown to phosphorylate mitochondrial Mcl-1, which promotes its degradation (38). We observed that APAP treatment resulted in both Bax translocation to mitochondria and loss of Mcl-1 from mitochondria (Fig. 11). Silencing GSK-3β reduced Mcl-1 loss, but did not affect Bax translocation to mitochondria. This suggests that GSK-3β promotes Mcl-1 degradation following APAP treatment, but does not regulate Bax activation induced by APAP. The stabilization of Mcl-1, which protects against MPT in mitochondria, may also be an important GSH independent mechanism by which silencing GSK-3β protects against mitochondrial dysfunction and hepatocyte death caused by APAP.
FIGURE 11.
Silencing GSK-3β prevents loss of Mcl-1 but has no effect on bax translocation. Control and GSK-3β-silenced mice were treated with APAP (300 mg/kg, intraperitoneally). At the times indicated, the liver was excised and mitochondria were isolated using differential centrifugation. Immunoblot analysis was performed with mitochondria using antisera against Mcl-1, bax, and COX. Gels are representative of three samples.
DISCUSSION
APAP Treatment Induces GSK-3β Activation and Translocation to Mitochondria
In this study, we have identified an important role for GSK-3β in APAP-induced liver injury in mice. The death promoting properties of GSK-3β have been observed previously in ischemia-reperfusion injury in the heart, in brain injury, and various degenerative diseases (13, 14, 24). Following APAP treatment, we observed an increase in both the activated (p-tyrosine 216) and inactivated (p-serine 9) forms of GSK-3β in liver (15, 24). The fact that GSK-3β translocated to mitochondria and increased glycogen synthase phosphorylation, a major downstream target of GSK-3β, suggests that overall GSK-3β activity is increased during APAP-induced liver injury. However, it remains to be further assessed if only the kinase activated form of GSK-3β (p-tyrosine) is important in mediating APAP hepatotoxicity, or whether the increase in inactive GSK-3β (p-serine 9) plays some role in mediating liver injury caused by APAP, possibly by interacting with mitochondrial proteins (13). Why both a protective and detrimental form of GSK-3β is activated by APAP remains unclear; however, this is not completely surprising because APAP hepatotoxicity is associated with activation of both detrimental (e.g. JNK and Bax) and protective (e.g. Bcl-2 and Bcl-xl) signaling pathways in liver (3). Furthermore, the mechanism of GSK-3β activation following APAP treatment is uncertain at the present. However, it is known that oxidative stress can activate GSK-3β by Src- and calcium-dependent mechanisms (39).
Primary cultured hepatocytes from GSK-3β-silenced mice or treated with GSK-3β inhibitor were also significantly protected from APAP, suggesting that GSK-3β activation in hepatocytes, and not other cells such as Kupffer cells, is an important event in APAP-induced liver injury. Some studies have suggested that Kupffer cells may play a role in APAP-induced liver injury, although this is somewhat controversial (40–42). Even if GSK-3β silencing was affecting Kupffer cell activation, Kupffer cells only modestly affect the extent of APAP-induced liver injury, and therefore the extensive protective effects of silencing GSK-3β in mice cannot be attributed to effects on Kupffer cells. Interestingly, the silencing of GSK-3β did not have any observable pathophysiologic baseline effects in cultured hepatocytes or in vivo, whereas GSK-3β knock-out has been observed to be embryonic lethal. Thus, although GSK-3β is critical in development, its loss is well tolerated in adult mice.
Effect of GSK-3β on GCL Levels during APAP-induced Liver Injury
The mechanism by which GSK-3β modulates APAP-induced liver injury is complex. Based on work with cultured primary hepatocytes and GSH measurements in vivo, it appears that GSK-3β affects several pathways to promote APAP hepatotoxicity. GSK-3β appears to indirectly modulate loss of GCL-c degradation in liver following APAP treatment. Thus, silencing JNK also prevented loss of GCL-c. This observation of a rapid decline in GCL-c protein levels and activity is a novel finding in APAP hepatotoxicity, which has not been previously described, and may play an important role in allowing APAP hepatotoxicity to progress by interfering with compensatory up-regulation of GSH synthesis, despite activation of Nrf-2-dependent transcription of GCL. The ∼40–70% decrease of GCL-c caused by APAP is likely to significantly inhibit GSH synthesis and recovery during APAP-induced liver injury. However, the decline in GCL-c levels during APAP hepatotoxicity is not complete and if cysteine supplementation were to occur (i.e. NAC treatment), GSH levels in liver should still improve. At present, we do not know if the loss of GCL-c is due to enhanced degradation or impaired translation. In cultured hepatocytes, GCL has been reported to undergo cleavage by caspases during apoptosis caused by transforming growth factor-β1 (43) and toxins such as diphenylarsinic acid (44). However, caspase activity does not play a role in APAP-induced liver injury, probably due to the highly oxidized environment caused by NAPQI, GSH depletion, and oxidative stress. Whether the loss of GCL-c is due to degradation by proteasomes initiated by GSK-3β phosphorylation, as observed with β-catenin, or through the participation of proteases (e.g. calpains or cathepsins) that might degrade GCL-c is currently under investigation.
Regulation of JNK by GSK-3β
The silencing or inhibition of GSK-3β in primary cultured hepatocytes also protected against cell death induced by APAP, even when GSH synthesis was inhibited by BSO. This suggests that GSK-3β mediates APAP-induced injury by an additional mechanism independent of impairing GSH synthesis, which likely involves inhibition of both JNK activation and Mcl-1 degradation. Silencing GSK-3β in mice delayed and suppressed JNK activation and translocation to mitochondria, which corresponded to significantly improved mitochondrial functionality and reduced cytochrome c loss caused by APAP treatment. Although some JNK activation and translocation to mitochondria occurred in GSK-3β-silenced mice, the levels were lower and occurred when mitochondrial GSH levels were recovering (starting at 4 h). Because JNK appears to only inhibit redox-modified mitochondria, the low amounts of JNK that translocate to mitochondria in GSK-3β-silenced mice may not be effective in inhibiting mitochondria bioenergetics because GSH levels are recovering in mitochondria at these later time points. Thus, the partial inhibition of JNK activation, as well as delaying JNK translocation to mitochondria to a time when GSH levels are recovering, may be an important mechanism by which silencing GSK-3β preserves mitochondrial functionality to sustain viability of hepatocytes following APAP administration.
Because of our previous findings on the pivotal role of JNK in APAP-induced necrosis, it is important to understand how GSK-3β relates to JNK, particularly in the sequence of events leading to loss of viability. Our findings suggest that GSK-3β activation is upstream of JNK activation. Several previous studies have suggested that GSK-3β mediates JNK activation through activation of MEKK1 or MLK in neuronal and kidney cell lines (35, 36). MEKK1 and MLK, like ASK-1, are MAP kinase kinase kinases (MAPKKK), which phosphorylate and activate MKK4/7, which in turn activates JNK. As previously mentioned, ASK-1 plays a significant role in JNK activation and APAP-induced liver injury; mice lacking ASK-1 were more resistant to APAP-induced liver injury (12). However, ASK-1 may not be the only MAPKKK involved in JNK activation following APAP treatment. ASK-1 knock-out mice exhibited an initial ASK-1 independent activation of JNK (starting ∼1.5 h) following APAP treatment, which was followed by a late ASK-1-dependent activation of JNK (>3 h) (12). Our data suggest that silencing GSK-3β inhibited the initial phase of JNK activation, whereas the later phase of JNK activation, possibly mediated by ASK-1, was less affected. Inhibition of the initial phase of JNK activation may be a key factor in determining how silencing GSK-3β protects against APAP-induced liver injury. The role of GSK-3β-induced activation of MEKK1 or MLK in early JNK activation after APAP will require additional studies. No link between GSK-3β and ASK-1 has yet been demonstrated. Although GSK-3β appears to be upstream of JNK, our data suggests that JNK may still be necessary to sustain GSK-3β activation by APAP. Treatment with JNK inhibitor did not prevent the initial GSK-3β activation and translocation to mitochondria caused by APAP (2 h), but did result in decreased GSK-3β activity and translocation at 4 h. It is conceivable that JNK directly interacts with GSK-3β. In human embryonic kidney cells, JNK has been shown to form a complex with GSK-3β and increase its activity (45). Alternatively, a feed-forward mechanism may exist between GSK-3β and JNK, whereby GSK-3β plays an important role in activating JNK, which then may be necessary to sustain GSK-3β activation by promoting the stimulus for GSK-3β activation (e.g. mitochondrial ROS production).
Effect of GSK-3β on Mcl-1 Levels
Other effects involving GSK-3β, besides JNK activation, that may also contribute to the decline in mitochondrial function following APAP treatment involve the translocation of GSK-3β to mitochondria, important in Mcl-1 degradation and possibly MPT. Mcl-1 is an anti-apoptotic Bcl-2 family member that is known to be a target for GSK-3β phosphorylation, which leads to its rapid proteosomal degradation (38, 46). Rapid degradation of Mcl-1 following APAP may render mitochondria more susceptible to cytochrome c release, MPT, and other mitochondria changes. Mcl-1 has been shown to play a protective role in other types of liver injury (47, 48), and thus its degradation may play an important role in APAP-induced liver injury. The translocation of GSK-3β to mitochondria, independent of Mcl-1 regulation, has also been shown to promote MPT in heart (17, 18). Thus, GSK-3β translocation to mitochondria during APAP hepatotoxicity may also play an important role in mediating mitochondria dysfunction. Consequently, JNK translocation to mitochondria, GSK-3β translocation to mitochondria, and Mcl-1 degradation may all contribute to mitochondrial decline and MPT during APAP-induced liver injury. Whether all these factors work in concert and are essential for mitochondrial dysfunction during APAP-induced liver injury needs to be further investigated. Another Bcl-2 family member of interest is Bax, a pro-apoptotic protein, which translocates to mitochondria to promote MPT and cytochrome c release. Interestingly, Bax translocation was unaltered when GSK-3β was silenced. Although this finding does not rule out some role of Bax (when GSK-3β and JNK also translocate to mitochondria), Bax knock-out mice are not protected against APAP (49).
Summary
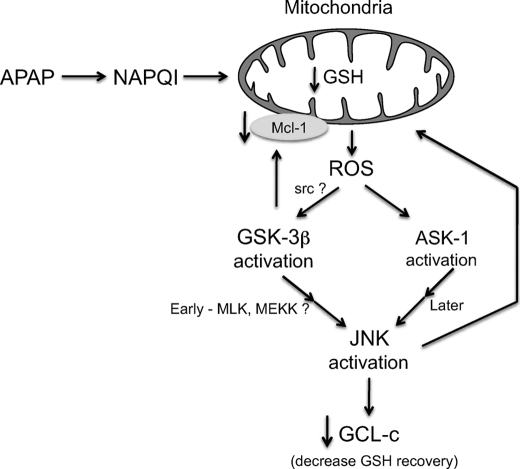
In conclusion, we provide evidence that GSK-3β participates in APAP hepatotoxicity through modulating several pathways (Fig. 12). We present the novel finding that APAP induced a rapid loss of GCL-c and Mcl-1 degradation. The early degradation of these two important proteins in cell survival may be a key factor in APAP-induced liver injury. GSK-3β also controls the downstream activation of JNK and its translocation to mitochondria, a critical event in APAP-induced necrosis. Overall, our findings on the role of GSK-3β, coupled with our previous work on the role of JNK, provide a striking example of the importance of signal transduction pathways in mediating the necrotic response to a toxin (3).
FIGURE 12.
Hypothesis for the role of signal transduction in APAP-induced liver injury. Production and targeting of NAPQI (first hit) to mitochondria leads to ROS production, which then activates extramitochondrial MAPKK kinase upstream of GSK-3β and JNK. Activation of GSK-3β leads to its translocation to mitochondria where it targets Mcl-1 for degradation and activates MAPKK kinase (i.e. MLP and MEKK1) leading to rapid activation of JNK. Subsequently, ROS release activates ASK-1 leading to sustained JNK activation. JNK then leads to loss of GCL-c and JNK translocates to mitochondria in the early and late phases of the evolution of toxicity providing a sustained second hit to mitochondria. Ultimately, the interplay of these effects of GSK-3β and JNK may promote mitochondrial membrane permeability transition pore opening, full collapse of mitochondrial function, and necrotic death.
Acknowledgments
The support of the Cell & Tissue Imaging, Analytical, Metabolic, Instrumentation, and Cell Culture Cores of the University of Southern California Research Center for Liver Diseases (Grant DK48522, to N. K.) is gratefully acknowledged.
This work was supported, in whole or in part, by National Institutes of Health Grants DK067215 (to N. K.) and AA016911 (to D. H.). This work was also supported by a Zumberge award (Office of the Provost at the University of Southern California; to D. H.).
- APAP
- acetaminophen
- Akt
- protein kinase B
- ASK-1
- apoptosis signaling-regulated kinase 1
- ASO
- antisense oligonucleotides
- BSO
- buthionine sulfoximine
- COX
- cytochrome oxidase
- GCL
- glutamate cysteine ligase
- GCL-c
- glutamate cysteine ligase heavy catalytic subunit
- GS
- glycogen synthase
- GSH
- glutathione
- GSK-3β
- glycogen synthase kinase 3β
- JNK
- c-Jun N-terminal kinase
- MAP kinase
- mitogen-activated protein kinase
- Mcl-1
- myeloid cell leukemia sequence 1
- MEKK1
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1
- MLK
- mixed lineage kinase
- MPT
- mitochondrial permeability transition
- NAPQI
- N-acetyl-p-benzo-quinoneimine
- PKA
- protein kinase A
- ROS
- reactive oxygen species
- MAPKKK
- MAP kinase kinase kinase
- DMSO
- dimethyl sulfoxide
- PBS
- phosphate-buffered saline
- ALT
- alanine transaminase
- HPLC
- high pressure liquid chromatography.
REFERENCES
- 1.Ostapowicz G., Fontana R. J., Schiødt F. V., Larson A., Davern T. J., Han S. H., McCashland T. M., Shakil A. O., Hay J. E., Hynan L., Crippin J. S., Blei A. T., Samuel G., Reisch J., Lee W. M. (2002) Ann. Intern. Med. 137, 947–954 [DOI] [PubMed] [Google Scholar]
- 2.Kaplowitz N. (2005) Nat. Rev. Drug Discov. 4, 489–499 [DOI] [PubMed] [Google Scholar]
- 3.Han D., Shinohara M., Ybanez M. D., Saberi B., Kaplowitz N. (2009) Handb. Exp. Pharmacol. 196, 267–310 [DOI] [PubMed] [Google Scholar]
- 4.Kaplowitz N., Shinohara M., Liu Z. X., Han D. (2008) Gastroenterology 135, 1047–1051 [DOI] [PubMed] [Google Scholar]
- 5.Hanawa N., Shinohara M., Saberi B., Gaarde W. A., Han D., Kaplowitz N. (2008) J. Biol. Chem. 283, 13565–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunawan B. K., Liu Z. X., Han D., Hanawa N., Gaarde W. A., Kaplowitz N. (2006) Gastroenterology 131, 165–178 [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q., Lam P. Y., Han D., Cadenas E. (2008) J. Neurochem. 104, 325–335 [DOI] [PubMed] [Google Scholar]
- 8.Han D., Ybanez M. D., Ahmadi S., Yeh K., Kaplowitz N. (2009) Antioxid. Redox. Signal. 9, 2245–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czaja M. J. (2007) Semin. Liver Dis. 27, 378–389 [DOI] [PubMed] [Google Scholar]
- 10.Hinson J. A., Reid A. B., McCullough S. S., James L. P. (2004) Drug Metab. Rev. 36, 805–822 [DOI] [PubMed] [Google Scholar]
- 11.Aoki H., Kang P. M., Hampe J., Yoshimura K., Noma T., Matsuzaki M., Izumo S. (2002) J. Biol. Chem. 277, 10244–10250 [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa H., Maeda S., Hikiba Y., Ohmae T., Shibata W., Yanai A., Sakamoto K., Ogura K., Noguchi T., Karin M., Ichijo H., Omata M. (2008) Gastroenterology 135, 1311–1321 [DOI] [PubMed] [Google Scholar]
- 13.Miura T., Nishihara M., Miki T. (2009) J. Pharmacol. Sci. 109, 162–167 [DOI] [PubMed] [Google Scholar]
- 14.Jope R. S., Johnson G. V. (2004) Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 15.Frame S., Cohen P. (2001) Biochem. J. 359, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastorino J. G., Hoek J. B., Shulga N. (2005) Cancer Res. 65, 10545–10554 [DOI] [PubMed] [Google Scholar]
- 17.Juhaszova M., Zorov D. B., Kim S. H., Pepe S., Fu Q., Fishbein K. W., Ziman B. D., Wang S., Ytrehus K., Antos C. L., Olson E. N., Sollott S. J. (2004) J. Clin. Invest. 113, 1535–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishihara M., Miura T., Miki T., Tanno M., Yano T., Naitoh K., Ohori K., Hotta H., Terashima Y., Shimamoto K. (2007) J. Mol. Cell Cardiol. 43, 564–570 [DOI] [PubMed] [Google Scholar]
- 19.Han D., Hanawa N., Saberi B., Kaplowitz N. (2006) Free Radic. Biol. Med. 41, 627–639 [DOI] [PubMed] [Google Scholar]
- 20.Saberi B., Shinohara M., Ybanez M. D., Hanawa N., Gaarde W. A., Kaplowitz N., Han D. (2008) Am. J. Physiol. Cell Physiol. 295, C50–C63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han D., Williams E., Cadenas E. (2001) Biochem. J. 353, 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seelig G. F., Meister A. (1985) Methods Enzymol. 113, 379–390 [DOI] [PubMed] [Google Scholar]
- 23.Meister A. (1995) Methods Enzymol. 251, 3–7 [DOI] [PubMed] [Google Scholar]
- 24.Eldar-Finkelman H. (2002) Trends Mol. Med. 8, 126–132 [DOI] [PubMed] [Google Scholar]
- 25.Whiteman E. L., Cho H., Birnbaum M. J. (2002) Trends Endocrinol. Metab. 13, 444–451 [DOI] [PubMed] [Google Scholar]
- 26.Coghlan M. P., Culbert A. A., Cross D. A., Corcoran S. L., Yates J. W., Pearce N. J., Rausch O. L., Murphy G. J., Carter P. S., Roxbee Cox L., Mills D., Brown M. J., Haigh D., Ward R. W., Smith D. G., Murray K. J., Reith A. D., Holder J. C. (2000) Chem. Biol. 7, 793–803 [DOI] [PubMed] [Google Scholar]
- 27.Park Y., Smith R. D., Combs A. B., Kehrer J. P. (1988) Toxicology 52, 165–175 [DOI] [PubMed] [Google Scholar]
- 28.Matsumaru K., Ji C., Kaplowitz N. (2003) Hepatology 37, 1425–1434 [DOI] [PubMed] [Google Scholar]
- 29.Kaplowitz N., Aw T. Y., Ookhtens M. (1985) Annu. Rev. Pharmacol. Toxicol. 25, 715–744 [DOI] [PubMed] [Google Scholar]
- 30.Iles K. E., Liu R. M. (2005) Free Radic. Biol. Med. 38, 547–556 [DOI] [PubMed] [Google Scholar]
- 31.Lu S. C. (2000) Curr. Top. Cell Regul. 36, 95–116 [DOI] [PubMed] [Google Scholar]
- 32.Goldring C. E., Kitteringham N. R., Elsby R., Randle L. E., Clement Y. N., Williams D. P., McMahon M., Hayes J. D., Itoh K., Yamamoto M., Park B. K. (2004) Hepatology 39, 1267–1276 [DOI] [PubMed] [Google Scholar]
- 33.Copple I. M., Goldring C. E., Jenkins R. E., Chia A. J., Randle L. E., Hayes J. D., Kitteringham N. R., Park B. K. (2008) Hepatology 48, 1292–1301 [DOI] [PubMed] [Google Scholar]
- 34.Salazar M., Rojo A. I., Velasco D., de Sagarra R. M., Cuadrado A. (2006) J. Biol. Chem. 281, 14841–14851 [DOI] [PubMed] [Google Scholar]
- 35.Mishra R., Barthwal M. K., Sondarva G., Rana B., Wong L., Chatterjee M., Woodgett J. R., Rana A. (2007) J. Biol. Chem. 282, 30393–30405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J. W., Lee J. E., Kim M. J., Cho E. G., Cho S. G., Choi E. J. (2003) J. Biol. Chem. 278, 13995–14001 [DOI] [PubMed] [Google Scholar]
- 37.Linseman D. A., Butts B. D., Precht T. A., Phelps R. A., Le S. S., Laessig T. A., Bouchard R. J., Florez-McClure M. L., Heidenreich K. A. (2004) J. Neurosci. 24, 9993–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maurer U., Charvet C., Wagman A. S., Dejardin E., Green D. R. (2006) Mol. Cell 21, 749–760 [DOI] [PubMed] [Google Scholar]
- 39.Grimes C. A., Jope R. S. (2001) Prog. Neurobiol. 65, 391–426 [DOI] [PubMed] [Google Scholar]
- 40.Ju C., Reilly T. P., Bourdi M., Radonovich M. F., Brady J. N., George J. W., Pohl L. R. (2002) Chem. Res. Toxicol. 15, 1504–1513 [DOI] [PubMed] [Google Scholar]
- 41.Laskin D. L., Gardner C. R., Price V. F., Jollow D. J. (1995) Hepatology 21, 1045–1050 [PubMed] [Google Scholar]
- 42.Michael S. L., Pumford N. R., Mayeux P. R., Niesman M. R., Hinson J. A. (1999) Hepatology 30, 186–195 [DOI] [PubMed] [Google Scholar]
- 43.Franklin C. C., Rosenfeld-Franklin M. E., White C., Kavanagh T. J., Fausto N. (2003) FASEB J. 17, 1535–1537 [DOI] [PubMed] [Google Scholar]
- 44.Sumi D., Manji A., Shinkai Y., Toyama T., Kumagai Y. (2007) Toxicol. Appl. Pharmacol. 223, 218–224 [DOI] [PubMed] [Google Scholar]
- 45.Hu D., Fang W., Han A., Gallagher L., Davis R. J., Xiong B., Yang W. (2008) Carcinogenesis 29, 2317–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warr M. R., Shore G. C. (2008) Curr. Mol. Med. 8, 138–147 [DOI] [PubMed] [Google Scholar]
- 47.Vick B., Weber A., Urbanik T., Maass T., Teufel A., Krammer P. H., Opferman J. T., Schuchmann M., Galle P. R., Schulze-Bergkamen H. (2009) Hepatology 49, 627–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodama Y., Taura K., Miura K., Schnabl B., Osawa Y., Brenner D. A. (2009) Gastroenterology 136, 1423–1434 [DOI] [PubMed] [Google Scholar]
- 49.Bajt M. L., Farhood A., Lemasters J. J., Jaeschke H. (2008) J. Pharmacol. Exp. Ther. 324, 8–14 [DOI] [PubMed] [Google Scholar]