Abstract
Oxidative stress plays a pivotal role in chronic heart failure. SIRT1, an NAD+-dependent histone/protein deacetylase, promotes cell survival under oxidative stress when it is expressed in the nucleus. However, adult cardiomyocytes predominantly express SIRT1 in the cytoplasm, and its function has not been elucidated. The purpose of this study was to investigate the functional role of SIRT1 in the heart and the potential use of SIRT1 in therapy for heart failure. We investigated the subcellular localization of SIRT1 in cardiomyocytes and its impact on cell survival. SIRT1 accumulated in the nucleus of cardiomyocytes in the failing hearts of TO-2 hamsters, postmyocardial infarction rats, and a dilated cardiomyopathy patient but not in control healthy hearts. Nuclear but not cytoplasmic SIRT1-induced manganese superoxide dismutase (Mn-SOD), which was further enhanced by resveratrol, and increased the resistance of C2C12 myoblasts to oxidative stress. Resveratrol's enhancement of Mn-SOD levels depended on the level of nuclear SIRT1, and it suppressed the cell death induced by antimycin A or angiotensin II. The cell-protective effects of nuclear SIRT1 or resveratrol were canceled by the Mn-SOD small interfering RNA or SIRT1 small interfering RNA. The oral administration of resveratrol to TO-2 hamsters increased Mn-SOD levels in cardiomyocytes, suppressed fibrosis, preserved cardiac function, and significantly improved survival. Thus, Mn-SOD induced by resveratrol via nuclear SIRT1 reduced oxidative stress and participated in cardiomyocyte protection. SIRT1 activators such as resveratrol could be novel therapeutic tools for the treatment of chronic heart failure.
Keywords: Cardiac Muscle, Histone Deacetylase, Protein Translocation, Reactive Oxygen Species (ROS), Superoxide Dismutase (SOD), SIRT1, Heart Failure, Resveratrol
Introduction
Heart failure arises as a consequence of various heart diseases, including myocardial infarction, hypertension, and idiopathic dilated cardiomyopathy (DCM).2 The death of cardiomyocytes and the consequent maladaptive changes in the remaining myocytes and extracellular matrix induce the clinical manifestation of heart failure (1). Over the past 20 years, the arsenal of treatments available for heart failure has increased considerably, with the introduction of β blockers, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, aldosterone antagonists, and nonpharmacological therapies including cardiac resynchronization therapy (2). However, even with the very best current therapy, the annual mortality rate among patients with heart failure is still ∼10% (3).
The mitochondrial electron transport chain is the main source of reactive oxygen species (ROS) in most cells (4). Hearts consume large amounts of O2 and yield high levels of ROS (5). Various factors, including angiotensin II and tumor necrosis factor-α, also induce ROS formation, leading to cardiomyocyte death and heart failure (5). Superoxide dismutase (SOD) has a pivotal role in the detoxification of ROS. SOD catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide, which in turn is reduced to water by catalase and glutathione peroxidase (5). Three isoforms of SOD have been identified in mammals: copper-zinc SOD (Cu,Zn-SOD, SOD1), manganese SOD (Mn-SOD, SOD2), and extracellular SOD (SOD3). Although cytoplasmic Cu,Zn-SOD knock-out mice (6) and extracellular SOD knock-out mice (7) lack a deleterious heart phenotype, mice deficient in mitochondrial Mn-SOD have an enlarged heart with endocardial fibrosis, a typical phenotype of DCM, and die within the first 10 days of life (8). Patients with hemochromatosis, in which iron overload induces robust oxidative stress, have a significantly higher prevalence of cardiomyopathy if they have a mutation in the Mn-SOD gene that leads to reduced enzymatic activity (9). Adriamycin increases ROS and induces cardiomyocyte death. However, the cardiotoxicity from adriamycin is less severe in transgenic mice expressing high levels of Mn-SOD than in nontransgenic mice (10). Recently, the FOXO3a (forkhead box O transcription factor 3a)-dependent induction of Mn-SOD by SIRT3, a Sir2 orthologue, was reported to block cardiac hypertrophy (11). Thus, an increase in Mn-SOD levels may provide a prognostic advantage for heart failure patients.
SIRT1, an NAD+-dependent protein/histone deacetylase, is one of the mammalian orthologues of yeast Sir2 (12, 13). SIRT1 deacetylates and activates peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a transcriptional coactivator, resulting in the induction of gluconeogenesis (14) and mitochondrial oxidative phosphorylation (15). PGC-1α has been reported to induce Mn-SOD expression (16). SIRT1 plays an important role in development, and SIRT1-deficient mice exhibit severe developmental abnormalities, such as a small body, heart defects, and exencephaly, and they only infrequently survive postnatally (17, 18). SIRT1 is a nucleocytoplasmic shuttling protein (19, 20). Its nuclear translocation induces neuronal differentiation in neural precursor cells (20). SIRT1 is also involved in cell survival. It binds and inhibits the tumor suppressor p53 and thereby represses apoptosis induced by DNA damage and oxidative stress (12, 13). SIRT1 deacetylates and inhibits poly(ADP-ribose) polymerase-1, an NAD+-dependent enzyme that contributes to caspase-independent myocyte death in failing hearts (21). Consistent with these findings, mild to moderate heart-specific overexpression of SIRT1 in transgenic mice improves the resistance of the heart to stress (22). Moreover, SIRT1 deacetylates and activates the FOXOs under oxidative stress, thereby inducing Mn-SOD expression (23).
SIRT1 is predominantly expressed in the cytoplasm in adult cardiomyocytes (19), but its nuclear localization is necessary for it to regulate gene expression. Cytoplasmic SIRT1 may serve as a reservoir for nuclear SIRT1; it may be translocated into the nucleus on demand in some pathological conditions. In this study, we investigated the subcellular localization of SIRT1 in failing hearts.
EXPERIMENTAL PROCEDURES
Transfection
This study was approved by the Human and Animal Research Ethics Committees, Sapporo Medical University. Transient transfection was performed using a Nucleofector kit (Amaxa). siRNAs (100 nmol/liter) were transfected twice into C2C12 cells with an interval of 24 h, with or without the simultaneous transfection of EGFP-fused plasmids. Twenty-four h after the second introduction of siRNA and/or plasmids, the cells were treated with resveratrol (40 or 100 μmol/liter) or vehicle for 12 h and then exposed to antimycin A for 8 h (20 or 100 μmol/liter) or angiotensin II (100 μmol/liter) for 12 h to induce oxidative stress. For each gene, we purchased three different siRNAs (B-Bridge, see supplemental Methods), which we used as a mixture (100 nmol/liter) to knock down expression efficiently. In experiments examining the effect of resveratrol on acetyl-H3 levels, the cells were treated with 50 nmol/liter tricostatin A for 24 h before harvesting. To measure ROS levels, the cells were incubated with 7 μm dichlorofluorescein diacetate added to the culture medium for 10 min at 37 °C, washed twice with phosphate-buffered saline, and then examined by confocal microscopy (Bio-Rad Radiance 2100MP).
Immunostaining
Human heart specimens were obtained at autopsy from a patient who died of New York Heart Association class IV heart failure due to DCM at Sapporo Medical University Hospital. Immunostaining was carried out as described previously (19). Five sections separated by at least 50 μm from each other were analyzed. For the analysis of hamster hearts, immunostained areas from 10 separate sections per heart from four hamsters in each group were compared. The amount of nuclear SIRT1, fibrosis, and MnSOD protein levels were quantified as described in supplemental Methods.
Hamsters and Diet
Male 4-week-old TO-2 and control golden hamsters were purchased from Bio Breeders, individually housed, and fed a standard control diet (Oriental Yeast, Tokyo) ad libitum. At 6 weeks of age, the TO-2 and golden hamsters were randomly assigned to one of two groups: control groups receiving the control diet and resveratrol-treated groups receiving control diet supplemented with resveratrol (4 g/kg control diet).
Statistical Analysis
Results are presented as means ± S.E. Differences were tested by an unpaired t test and analysis of variance. When the overall analysis of variance indicated a significant difference, multiple comparisons were conducted by the Student-Newman-Keuls post hoc test. Survival rates were monitored and compared using the Kaplan-Meier survival analysis and the log-rank test. A p value of <0.05 was considered significant.
RESULTS
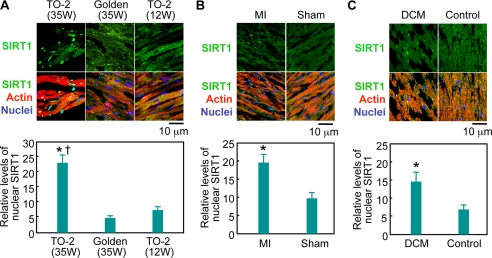
The subcellular localization of SIRT1 was examined in the heart of TO-2 hamsters, which have a genetic defect in the δ-sarcoglycan gene and spontaneously develop DCM (24). The expression levels of nuclear SIRT1 in the cardiomyocytes from TO-2 hamsters with DCM were much higher than in control hearts (Fig. 1A). Quantitative analysis of immunostained sections revealed that the amount of nuclear SIRT1 in failing hearts significantly increased, by 370 and 209%, respectively, compared with its level in the hearts of control hamsters and young TO-2 hamsters that had not yet developed heart failure (Fig. 1A). Furthermore, an exclusively nuclear expression of SIRT1 was observed in 3.2% of cardiomyocytes in the failing hearts of TO-2 hamsters (n = 3,000, supplemental Fig. 1A). In contrast, this nuclear accumulation of SIRT1 was absent in control and in young TO-2 hamsters (n = 3,000 each). In rats, 4 weeks after myocardial infarction, the cardiomyocytes also showed the nuclear accumulation of SIRT1. The expression levels of nuclear SIRT1 in rat hearts after myocardial infarction were twice as high as in sham-operated hearts (n = 4,000 each, Fig. 1B) with 7.0% of cardiomyocytes expressing SIRT1 exclusively in the nucleus (n = 4000, supplemental Fig. 1B). We further examined the heart of a patient who died of New York Heart Association class IV heart failure due to DCM. Western blotting showed that the failing heart expressed SIRT1 at a similar level to a control heart (supplemental Fig. 1C). Quantitative analysis showed a 2.1-fold increase in the intensity of nuclear SIRT1 immunostaining in the DCM heart compared with the control heart (n = 4,000 each, Fig. 1C). In the failing heart, 1.7% of the cardiomyocytes expressed SIRT1 only in the nucleus (n = 4,000), whereas SIRT1 was diffusely distributed in the control heart (Fig. 1C). Thus, significantly higher levels of SIRT1 were expressed in the nucleus of cardiomyocytes in three different models of failing hearts compared with control hearts.
FIGURE 1.
Nuclear expression of SIRT1 in failing hearts. Left ventricular sections were stained with an anti-SIRT1 antibody (green), phalloidin-TRITC (actin, red), and Hoechst 33342 (nucleus, blue). A, representative immunostainings of 35-week-old TO-2 hamsters suffering from severe heart failure, control golden hamsters, and 12-week-old TO-2 hamsters that had not yet developed heart failure. B, non-necrotic, viable areas of rat hearts were analyzed 4 weeks after myocardial infarction (MI) or a sham operation (Sham). C, immunostaining of left ventricular muscles from a DCM patient and a patient without heart disease. In the bottom panels, quantitative analyses of the level of nuclear SIRT1 relative to that of cytoplasmic SIRT1 are shown. *, p < 0.05 versus age-matched (35-week-old) golden hamsters, sham-operated rats, or control patient. †, p < 0.05 versus young (12-week-old) TO-2 hamsters that had not yet developed heart failure; W, weeks.
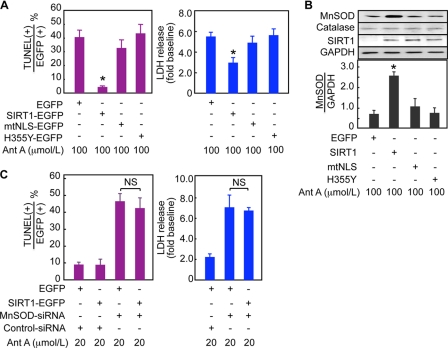
To clarify the functional role of nuclear SIRT1, C2C12 myoblast cells were transfected with expression constructs for nucleus-directed (SIRT1-EGFP), cytoplasm-directed (mtNLS-EGFP), or dominant-negative (H355Y-EGFP) SIRT1 (19). The cells were then exposed to antimycin A, which inhibits complex III of the mitochondrial electron transport chain and increases superoxide production. The overexpression of SIRT1-EGFP markedly reduced the number of apoptotic cells (Fig. 2A and supplemental Fig. 2A) and the LDH activity in the culture medium (Fig. 2A), compared with the overexpression of EGFP alone. The overexpression of nuclear SIRT1 also reduced the number of cells showing necrotic features, i.e. the breakdown of the cytoplasmic and nuclear membranes (supplemental Fig. 2, B and C). However, in cells overexpressing mtNLS-EGFP, which bears mutations in two SIRT1 nuclear-localization signals and is therefore restricted to the cytoplasm (19), the number of apoptotic cells and level of LDH release were similar to those in the control EGFP-expressing cells (Fig. 2A). The overexpressed dominant-negative SIRT1 (H355Y-EGFP) also failed to suppress the antimycin A-induced cell death despite its nuclear localization (Fig. 2A and supplemental Fig. 2, A–C), demonstrating that the cytoprotective function of nuclear SIRT1-EGFP required the enzymatic activity of SIRT1.
FIGURE 2.
An indispensable role of Mn-SOD for the cell-protective function of nuclear SIRT1. C2C12 cells expressing EGFP, SIRT1-EGFP (nuclear SIRT1), mtNLS-EGFP (cytoplasmic SIRT1), or H355Y-EGFP (dominant-negative SIRT1) were exposed to 100 μmol/liter Ant A for 8 h. A, the percentage of TUNEL-positive cells in EGFP-positive cells and LDH activity in the culture medium from four separate series of experiments are shown. B, representative immunoblots of C2C12 cells overexpressing each plasmid and quantitative analyses of the Mn-SOD expression. C, C2C12 cells expressing EGFP or SIRT1-EGFP were transfected with control siRNA or Mn-SOD siRNA and then exposed to 20 μmol/liter Ant A for 8 h. The percentage of EGFP-positive cells that were TUNEL-positive and the LDH activity in the culture medium from four separate series of experiments are shown. *, p < 0.05 versus EGFP-transfected cells. NS, no significant difference; LDH, lactate dehydrogenase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
SIRT1 activates FOXOs and PGC-1α (14, 25, 26), both of which up-regulate Mn-SOD (16, 23). Nuclear SIRT1 may therefore induce Mn-SOD expression. Indeed, a Western blot analysis showed that SIRT1-EGFP significantly increased Mn-SOD (Fig. 2B). On the other hand, catalase and glutathione peroxidase, which also participate in the detoxification of ROS, were not increased by SIRT1-EGFP (Fig. 2B). Immunostaining also showed that the expression levels of Mn-SOD increased in the C2C12 cells overexpressing SIRT1-EGFP, but not in cells overexpressing EGFP, mtNLS-EGFP, or H355Y-EGFP (supplemental Fig. 2D).
To examine the function of Mn-SOD, we used Mn-SOD siRNA. The expression of MnSOD siRNA reduced the protein level of Mn-SOD to 25% of that in control cells (supplemental Fig. 3A) and did not affect the deacetylation of histone H3 promoted by the overexpression of nuclear SIRT1 (supplemental Fig. 3B). The Mn-SOD siRNA substantially enhanced the cell death induced by 100 μmol/liter antimycin A, in that most cells detached from the culture dish even in the presence of nuclear SIRT1 (supplemental Fig. 3C). Therefore, we switched to a lower dose of antimycin A, 20 μmol/liter, which induced only negligible cell death in the absence of Mn-SOD siRNA (Fig. 2C). When Mn-SOD siRNA was expressed, the overexpression of SIRT1-EGFP inhibited neither the apoptosis nor the increase in LDH release induced by antimycin A (Fig. 2C and supplemental Fig. 3D). Thus, the cell-protective function of nuclear SIRT1 required Mn-SOD.
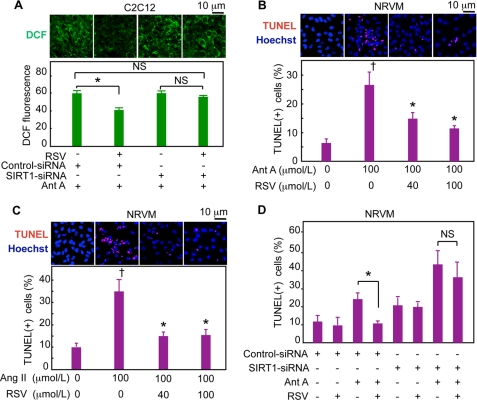
Resveratrol, a well known antioxidant, is a potent activator of SIRT1 (27). We next examined whether SIRT1 is involved in the antioxidative and cell-protective functions of resveratrol. Resveratrol significantly attenuated the increase in ROS levels induced by antimycin A (Fig. 3A and supplemental Fig. 4) and angiotensin II (supplemental Fig. 4), as shown by the significantly lower level of dichlorofluorescein staining. The knockdown of SIRT1 by siRNA (supplemental Fig. 5) completely inhibited the decrease in ROS levels by resveratrol in C2C12 cells (Fig. 3A), indicating that the antioxidative function of resveratrol depends on SIRT1. SIRT1 is known to deacetylate histone H3. Resveratrol promoted the deacetylation of histone H3 in C2C12 cells and neonatal rat ventricular myocytes (NRVMs, supplemental Fig. 6, A and B), and this deacetylation was blocked by SIRT1 siRNA (supplemental Fig. 6C). Resveratrol significantly suppressed the apoptosis of NRVMs induced by antimycin A (Fig. 3B) and by angiotensin II (Fig. 3C). The expression of SIRT1 siRNA abolished the antiapoptotic function of resveratrol in NRVMs (Fig. 3D and supplemental Fig. 7A) and C2C12 cells (supplemental Fig. 7B). Thus, both the antioxidative and antiapoptotic functions of resveratrol completely depended on SIRT1 in these experimental conditions.
FIGURE 3.
Resveratrol inhibits oxidative stress-induced cell death via SIRT1 in vitro. A, SIRT1 siRNA inhibits the antioxidative function of resveratrol (RSV). C2C12 cells expressing SIRT1 siRNA or control siRNA were pretreated with RSV or vehicle for 4 h and then exposed to 100 μmol/liter Ant A for 3 h. Representative images of dichlorofluorescein fluorescence and quantitative analyses of the fluorescence intensity are shown. B and C, NRVMs were pretreated with RSV or vehicle for 4 h and then exposed to 100 μmol/liter antimycin A for 8 h (B) or angiotensin II (Ang II) for 12 h (C). Representative immunostaining images and the percentage of TUNEL-positive cells are shown. D, SIRT1 siRNA inhibits the antiapoptotic function of RSV. NRVMs expressing SIRT1 siRNA or control siRNA were pretreated with RSV or vehicle for 4 h and then exposed to Ant A for 8 h. The percentages of TUNEL-positive cells are shown. †, p < 0.05 versus untreated cells; *, p < 0.05 versus cells exposed to Ant A or angiotensin II in the absence of RSV; NS, no significant difference; DCF, 2′,7′-dichlorofluorescein.
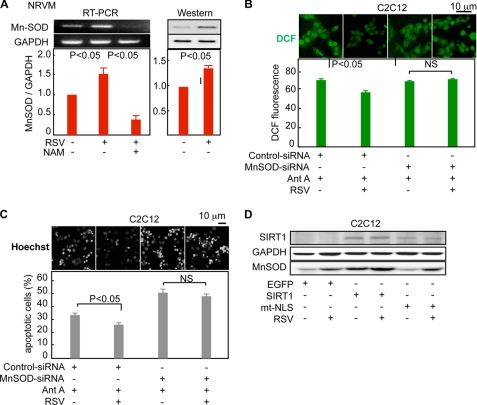
Resveratrol may induce Mn-SOD via the activation of SIRT1. Indeed, resveratrol significantly increased the mRNA and protein levels of Mn-SOD in NRVMs (Fig. 4A and supplemental Fig. 8). Nicotinamide, an SIRT1 inhibitor, suppressed the resveratrol-mediated increase in Mn-SOD mRNA and, in fact, reduced its expression to levels below that of the control cells (Fig. 4A). In addition, the Mn-SOD siRNA blocked resveratrol's reduction of the ROS levels (Fig. 4B) and inhibition of apoptosis (Fig. 4C). Resveratrol had no effect on either the expression level of SIRT1 (Fig. 4D and supplemental Fig. 9) or its subcellular localization (supplemental Fig. 9). Because nuclear but not cytoplasmic SIRT1 induced Mn-SOD expression (Fig. 2B), the expression levels of nuclear SIRT1 are likely to determine the levels of Mn-SOD induced by resveratrol. In fact, the treatment of C2C12 cells overexpressing nuclear SIRT1 with resveratrol further enhanced the already elevated Mn-SOD levels (Fig. 4D). Moreover, the overexpression of cytoplasmic SIRT1 did not affect the Mn-SOD levels, and resveratrol induced similar levels of Mn-SOD regardless of the presence or absence of cytoplasmic SIRT (Fig. 4D). These results indicated that resveratrol decreased ROS and promoted cell survival via Mn-SOD induction, and this protection was strongly influenced by the expression levels of nuclear SIRT1.
FIGURE 4.
Resveratrol up-regulates Mn-SOD through nuclear SIRT1 in vitro. A, RSV (100 μmol/liter) up-regulated both Mn-SOD mRNA (left panel) and protein (right panel), and nicotinamide (NAM) (5 mmol/liter), an SIRT1 inhibitor, canceled the effect of RSV and further reduced the expression levels of Mn-SOD in NRVMs (left panel). Representative ethidium bromide-stained agarose gel (left panel) and immunoblots (right panel) and the quantitative data from four independent experiments are shown. B and C, Mn-SOD siRNA inhibited the antioxidative and antiapoptotic functions of RSV. B, representative images and quantification of dichlorofluorescein fluorescence from four independent series of experiments. Experiments were performed as described in Fig. 3A, except that the MnSOD siRNA was transfected instead of the SIRT1 siRNA. C, C2C12 cells expressing the MnSOD siRNA or control siRNA were pretreated with 100 μmol/liter of RSV or vehicle for 4 h and then exposed to 100 μmol/liter Ant A for 8 h. Representative images and the percentage of apoptotic cells showing nuclear condensation from four independent experiments are shown. Nuclei were stained with Hoechst 33342. D, resveratrol further augmented the Mn-SOD induction by nuclear SIRT1. C2C12 cells expressing EGFP, SIRT1-EGFP (nuclear SIRT1), or mtNLS-EGFP (cytoplasmic SIRT1) were treated with 100 μmol/liter resveratrol for 12 h, and then the cells were exposed to 100 μmol/liter antimycin A for 3 h before being used in the immunoblot analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT, reverse transcription; DCF, 2′,7′-dichlorofluorescein.
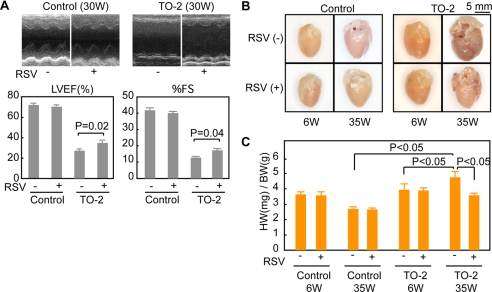
Because the cardiomyocytes of failing hearts express nuclear SIRT1, resveratrol may have a more potent effect on failing hearts than on healthy ones. Resveratrol was orally administered to control golden hamsters and TO-2 hamsters from the age of 6 weeks. The average daily caloric intake of the animals was not affected by the administration of resveratrol (supplemental Fig. 10A). At the age of 30 weeks, we examined the cardiac function of control and TO-2 hamsters. Ventricular wall motion was severely impaired in TO-2 hamsters fed the control diet, but the impairment was significantly less pronounced in the animals treated with resveratrol (Fig. 5A). The administration of resveratrol attenuated the deterioration of cardiac function in TO-2 hamsters, as revealed by their significantly higher left ventricular ejection fraction (LVEF) (34.4 ± 2.2% versus 27.9 ± 1.6%, n = 15 each) and fractional shortening (17.3 ± 1.1% versus 12.7 ± 0.8%, n = 15 each) compared with those of the TO-2 hamsters fed the control diet (Fig. 5A). Resveratrol did not affect the cardiac wall motion or function in control golden hamsters (Fig. 5A), and it did not affect the heart rate in either the golden hamsters (371 ± 5 versus 371 ± 8) or the TO-2 hamsters (381 ± 5 versus 387 ± 6).
FIGURE 5.
Resveratrol attenuated the deterioration of cardiac function and hypertrophy in TO-2 hamsters. A, echocardiography of control golden hamsters and TO-2 hamsters fed the control or RSV-containing diet (4 g/kg control diet). The left ventricular ejection fraction (LVEF) and fractional shortening (%FS) of fifteen animals in each group were examined. Representative M-mode left ventricular short axis images (top panels) and statistical analyses (bottom panels) are shown. B, representative whole heart images of hamsters excised at 6 or 35 weeks of age. Note that the morphological features of heart failure, including spherical ventricles and enlarged atria, were less evident in the TO-2 hamsters treated with resveratrol, at the age of 35 weeks. C, quantitative analyses of the heart weight (HW)/body weight (BW) ratio. Note that the increase in HW/BW was significantly suppressed by resveratrol in the TO-2 hamsters. W, week.
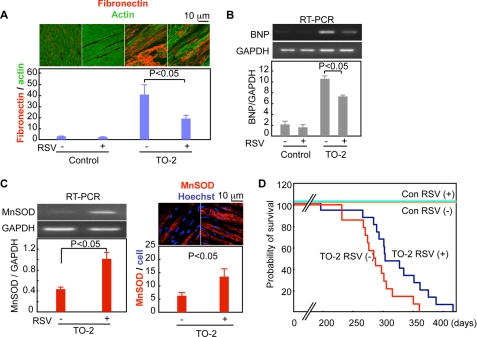
The hearts of control TO-2 hamsters at the age of 35 weeks showed spherical ventricles and enlarged atria with an increased heart weight to body weight ratio. These morphological features of heart failure were less evident in TO-2 hamsters treated with resveratrol (Fig. 5B), whose heart weights were significantly lower than those of the control TO-2 hamsters (Fig. 5C). The administration of resveratrol significantly attenuated the loss of cardiomyocytes and the development of interstitial fibrosis (Fig. 6A). It also alleviated the up-regulation of B-type natriuretic peptide (an index of the severity of heart failure) mRNA in the cardiomyocytes of 35-week-old TO-2 hamsters (Fig. 6B). Resveratrol affected neither the expression levels of SIRT1 nor its subcellular localization in the heart (data not shown). The levels of acetyl-histone H3 in the hearts of resveratrol-fed TO-2 hamsters were significantly lower than in control hamsters (supplemental Fig. 10B), suggesting that the cardiac SIRT1 of the TO-2 hamsters was activated by the oral administration of resveratrol. Resveratrol increased the Mn-SOD mRNA levels by >2-fold in the left ventricles of TO-2 hamsters compared with the levels in TO-2 hamsters fed the control diet (Fig. 6C). Immunohistochemical examinations also showed an increase in the Mn-SOD levels in the hearts of TO-2 hamsters fed resveratrol (Fig. 6C).
FIGURE 6.
Resveratrol suppresses the deterioration of the failing heart and reduces the mortality of TO-2 hamsters. A, cardiac fibrosis. Left ventricular sections of TO-2 hamsters were stained with an anti-fibronectin antibody (red) and phalloidin-fluorescein isothiocyanate (actin, green). Representative images and the quantitative analyses of data from four animals in each group are shown. B, B-type natriuretic peptide (BNP) mRNA expression in the heart. Representative reverse transcription-PCR images and quantitative analysis from four animals in each group are shown. C, representative reverse transcription (RT)-PCR of cardiac Mn-SOD mRNA and Mn-SOD immunostaining of the left ventricles. Quantitative analyses of the data from four animals in each group are shown. D, Kaplan-Meier survival curves. The Hazard ratio for RSV treatment was 0.41 (x2 = 4.54, p = 0.03) versus control diet in the TO-2 hamsters. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Con, control.
The lifespan of TO-2 hamsters fed resveratrol was significantly extended compared with that of the control TO-2 hamsters (average lifespan = 318 ± 13 versus 287 ± 10 days, p < 0.05) (supplemental Fig. 10C). A Cox proportional hazards regression showed that resveratrol reduced the risk of death in TO-2 hamsters by 59% (Hazard ratio = 0.41, p = 0.03) (Fig. 6D). Overall, the oral administration of resveratrol suppressed the progression of heart failure and improved the survival of TO-2 hamsters.
DISCUSSION
Normal adult mouse cardiomyocytes express eight times more SIRT1 in the cytoplasm than in the nucleus (19). Similarly, normal human cardiomyocytes predominantly expressed SIRT1 in their cytoplasm (Fig. 1C and data not shown). We found that chronic heart failure induced a nuclear translocation of SIRT1 in cardiomyocytes. The nuclear accumulation of SIRT1 is likely to be an adaptive mechanism of cardiomyocytes against heart failure, as indicated by the potent cell protective effect of nuclear SIRT1 against ROS. Previously, we used the pharmacological inhibition of phosphoinositide 3-kinase (PI3K) to exclude SIRT1 from the nucleus (19) and observed that insulin-like growth factor 1, an upstream activator of PI3K, dose-dependently promoted the nuclear localization of SIRT1 in NRVMs (data not shown). These findings indicate that PI3K is a candidate for signaling to induce the nuclear localization of SIRT1 in failing hearts. PI3K is activated in the cardiomyocytes of hypertrophic and failing hearts (28), and enhanced PI3K activity prolongs the lifespan of mice with DCM, whereas the diminished activity of this kinase shortens it by ∼50% (29). Therefore, a PI3K-enhanced increase in nuclear SIRT1 levels may have contributed to the prognostic advantage of the mice. The expression level of nuclear SIRT1 in the failing hearts was different from cell to cell (Fig. 1). Because many cardiomyocytes in failing hearts still express abundant cytoplasmic SIRT1, agents that promote the nuclear translocation of SIRT1 may elicit additional cardioprotective function when combined with resveratrol.
Resveratrol has been proposed to be responsible for the cardioprotective effects of red wine, invoked by the so-called French paradox (30). Resveratrol enhances SIRT1 activity by increasing the affinity of SIRT1 for NAD+ and for acetylated peptides by an allosteric interaction (27) and suppresses the cell death induced by various oxidative stressors (31). In the present study, we found that the antioxidative and cell-protective functions of resveratrol depended on SIRT1 because SIRT1 siRNA cancelled the resveratrol-mediated decrease in ROS levels (Fig. 3A) and cell survival (Fig. 3D and supplemental Fig. 7B). SIRT1 has been shown to protect cardiomyocytes by inhibiting p53 and poly(ADP-ribose) polymerase-1 (21, 31, 32). We showed here that the Mn-SOD induced by nuclear SIRT1 and resveratrol plays an indispensable role in this protection. Mn-SOD was important for the decrease in ROS levels (Fig. 4B) and cell survival (Fig. 4C) promoted by resveratrol and has been shown to protect cardiomyocytes from oxidative stress and to prevent cardiomyopathy (8–10). Together, these findings strongly indicate that the elevated levels of cardiac Mn-SOD in TO-2 hamsters treated with resveratrol (Fig. 6C) plays a significant role in the cardioprotection and survival of the animals with failing hearts. The effectiveness of resveratrol's induction of Mn-SOD was determined by the expression level of nuclear SIRT1 (Fig. 4D). Recently, the administration of resveratrol to healthy mice was shown to increase Mn-SOD expression in the brain but not the heart (33). We observed that the oral administration of resveratrol to normal animals with healthy hearts had little effect on left ventricular function, cardiac hypertrophy, fibrosis, or B-type natriuretic peptide expression (Figs. 5 and 6). These findings indicate that resveratrol has a minimal effect on healthy cardiomyocytes, in which SIRT1 is predominantly located in the cytoplasm.
The plasma concentration of resveratrol in mice fed 200–400 mg resveratrol/kg/day was reported to be 44–526 nmol/liter (15). In the present study, the plasma concentration of resveratrol was estimated at 30–200 nmol/liter, which was lower than that used in our in vitro experiments (Figs. 3 and 4). However, resveratrol significantly decreased the acetyl-histone H3 levels in the heart of TO-2 hamsters, indicating that the dose of resveratrol used in the present study was sufficient to activate SIRT1 in cardiomyocytes (supplemental Fig. 10B). Previous experiments showed that resveratrol is much more potent when given orally to animals than when incubated with cultured cells in vitro (34, 35). This might be explained, at least in part, by the tissue accumulation of resveratrol resulting from its hydrophobicity. The average daily intake of resveratrol by the hamsters was ∼145 mg/kg of body weight, which corresponds to the daily ingestion of as much as 9 g in humans. Recently, small molecule activators of SIRT1 that are 1,000-fold more potent than resveratrol have been developed (36). Similar to resveratrol, these compounds improve insulin resistance and diabetes in mice and rats (36). Other SIRT1 activators, as well as resveratrol, should be considered for clinical applications.
Current therapies using β blockers, angiotensin II type 1 receptor blockers, angiotensin-converting enzyme inhibitors, and aldosterone blockers mainly aim at neurohumoral factors that are maladaptively activated in failing hearts. Because these medications neither directly detoxify ROS nor induce Mn-SOD, the protective function resveratrol for cardiomyocytes is distinct. Thus, a combination regimen that uses SIRT1 activators along with the above-mentioned medications may turn out to be an improvement on current therapeutic approaches.
Supplementary Material
This work was supported in part by a Grant-in-aid for scientific research (20590869), a grant from a National Project, “Knowledge Cluster Initiative,” (2nd stage, “Sapporo Biocluster Bio-S”) and Program for developing the supporting system for upgrading the education and research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants from The Special Fund for Medical Research from Sapporo Medical University and the Sapporo Medical University Foundation for Promotion of Medical Science, and the Northern Advancement Center for Science and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Figs. 1–10, and additional references.
- DCM
- dilated cardiomyopathy
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- siRNA
- small interfering RNA
- EGFP
- enhanced green fluorescent protein
- NRVM
- neonatal rat ventricular myocyte
- PI3K
- phosphoinositide 3-kinase
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- RSV
- resveratrol
- Ant A
- antimycin A
- mtNLS
- mutant nuclear localization signal.
REFERENCES
- 1.McMurray J. J., Pfeffer M. A. (2005) Lancet 365, 1877–1889 [DOI] [PubMed] [Google Scholar]
- 2.Neubauer S. (2007) N. Engl. J. Med. 356, 1140–1151 [DOI] [PubMed] [Google Scholar]
- 3.Cleland J. G., Daubert J. C., Erdmann E., Freemantle N., Gras D., Kappenberger L., Tavazzi L. (2005) N. Engl. J. Med. 352, 1539–1549 [DOI] [PubMed] [Google Scholar]
- 4.Balaban R. S., Nemoto S., Finkel T. (2005) Cell 120, 483–495 [DOI] [PubMed] [Google Scholar]
- 5.Giordano F. J. (2005) J. Clin. Invest. 115, 500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elchuri S., Oberley T. D., Qi W., Eisenstein R. S., Jackson Roberts L., Van Remmen H., Epstein C. J., Huang T. T. (2005) Oncogene 24, 367–380 [DOI] [PubMed] [Google Scholar]
- 7.Sentman M. L., Granström M., Jakobson H., Reaume A., Basu S., Marklund S. L. (2006) J. Biol. Chem. 281, 6904–6909 [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H., Wallace D. C., Epstein C. J. (1995) Nat. Genet. 11, 376–381 [DOI] [PubMed] [Google Scholar]
- 9.Valenti L., Conte D., Piperno A., Dongiovanni P., Fracanzani A. L., Fraquelli M., Vergani A., Gianni C., Carmagnola L., Fargion S. (2004) J. Med. Genet. 41, 946–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yen H. C., Oberley T. D., Vichitbandha S., Ho Y. S., St. Clair D. K. (1996) J. Clin. Invest. 98, 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundaresan N. R., Gupta M., Kim G., Rajamohan S. B., Isbatan A., Gupta M. P. (2009) J. Clin. Invest. 119, 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigis M. C., Guarente L. P. (2006) Genes Dev. 20, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 13.Longo V. D., Kennedy B. K. (2006) Cell 126, 257–268 [DOI] [PubMed] [Google Scholar]
- 14.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 15.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 16.St.-Pierre J., Drori S., Uldry M., Silvaggi J. M., Rhee J., Jäger S., Handschin C., Zheng K., Lin J., Yang W., Simon D. K., Bachoo R., Spiegelman B. M. (2006) Cell 127, 397–408 [DOI] [PubMed] [Google Scholar]
- 17.Cheng H. L., Mostoslavsky R., Saito S., Manis J. P., Gu Y., Patel P., Bronson R., Appella E., Alt F. W., Chua K. F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10794–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBurney M. W., Yang X., Jardine K., Hixon M., Boekelheide K., Webb J. R., Lansdorp P. M., Lemieux M. (2003) Mol. Cell. Biol. 23, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanno M., Sakamoto J., Miura T., Shimamoto K., Horio Y. (2007) J. Biol. Chem. 282, 6823–6832 [DOI] [PubMed] [Google Scholar]
- 20.Hisahara S., Chiba S., Matsumoto H., Tanno M., Yagi H., Shimohama S., Sato M., Horio Y. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15599–15604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajamohan S. B., Pillai V. B., Gupta M., Sundaresan N. R., Birukov K. G., Samant S., Hottiger M. O., Gupta M. P. (2009) Mol. Cell. Biol. 29, 4116–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcendor R. R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., Tian B., Wagner T., Vatner S. F., Sadoshima J. (2007) Circ. Res. 100, 1512–1521 [DOI] [PubMed] [Google Scholar]
- 23.Kops G. J., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W., Coffer P. J., Huang T. T., Bos J. L., Medema R. H., Burgering B. M. (2002) Nature 419, 316–321 [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto A., Ono K., Abe M., Jasmin G., Eki T., Murakami Y., Masaki T., Toyo-oka T., Hanaoka F. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 13873–13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Horst A., Tertoolen L. G., de Vries-Smits L. M., Frye R. A., Medema R. H., Burgering B. M. (2004) J. Biol. Chem. 279, 28873–28879 [DOI] [PubMed] [Google Scholar]
- 26.Daitoku H., Hatta M., Matsuzaki H., Aratani S., Ohshima T., Miyagishi M., Nakajima T., Fukamizu A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 28.Dorn G. W., 2nd, Force T. (2005) J. Clin. Invest. 115, 527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMullen J. R., Amirahmadi F., Woodcock E. A., Schinke-Braun M., Bouwman R. D., Hewitt K. A., Mollica J. P., Zhang L., Zhang Y., Shioi T., Buerger A., Izumo S., Jay P. Y., Jennings G. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renaud S., de Lorgeril M. (1992) Lancet 339, 1523–1526 [DOI] [PubMed] [Google Scholar]
- 31.Pillai J. B., Isbatan A., Imai S., Gupta M. P. (2005) J. Biol. Chem. 280, 43121–43130 [DOI] [PubMed] [Google Scholar]
- 32.Alcendor R. R., Kirshenbaum L. A., Imai S. I., Vatner S. F., Sadoshima J. (2004) Circ. Res. 95, 971–980 [DOI] [PubMed] [Google Scholar]
- 33.Robb E. L., Winkelmolen L., Visanji N., Brotchie J., Stuart J. A. (2008) Biochem. Biophys. Res. Commun. 372, 254–259 [DOI] [PubMed] [Google Scholar]
- 34.Casper R. F., Quesne M., Rogers I. M., Shirota T., Jolivet A., Milgrom E., Savouret J. F. (1999) Mol. Pharmacol. 56, 784–790 [PubMed] [Google Scholar]
- 35.Sun C., Zhang F., Ge X., Yan T., Chen X., Shi X., Zhai Q. (2007) Cell Metab. 6, 307–319 [DOI] [PubMed] [Google Scholar]
- 36.Milne J. C., Lambert P. D., Schenk S., Carney D. P., Smith J. J., Gagne D. J., Jin L., Boss O., Perni R. B., Vu C. B., Bemis J. E., Xie R., Disch J. S., Ng P. Y., Nunes J. J., Lynch A. V., Yang H., Galonek H., Israelian K., Choy W., Iffland A., Lavu S., Medvedik O., Sinclair D. A., Olefsky J. M., Jirousek M. R., Elliott P. J., Westphal C. H. (2007) Nature 450, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.