Abstract
GADD45β (growth arrest- and DNA damage-inducible) interacts with upstream regulators of the JNK and p38 stress response kinases. Previously, we reported that the hypertrophic zone of the Gadd45β−/− mouse embryonic growth plate is compressed, and expression of type X collagen (Col10a1) and matrix metalloproteinase 13 (Mmp13) genes is decreased. Herein, we report that GADD45β enhances activity of the proximal Col10a1 promoter, which contains evolutionarily conserved AP-1, cAMP-response element, and C/EBP half-sites, in synergism with C/EBP family members, whereas the MMP13 promoter responds to GADD45β together with AP-1, ATF, or C/EBP family members. C/EBPβ expression also predominantly co-localizes with GADD45β in the embryonic growth plate. Moreover, GADD45β enhances C/EBPβ activation via MTK1, MKK3, and MKK6, and dominant-negative p38αapf, but not JNKapf, disrupts the combined trans-activating effect of GADD45β and C/EBPβ on the Col10a1 promoter. Importantly, GADD45β knockdown prevents p38 phosphorylation while decreasing Col10a1 mRNA levels but does not affect C/EBPβ binding to the Col10a1 promoter in vivo, indicating that GADD45β influences the transactivation function of DNA-bound C/EBPβ. In support of this conclusion, we show that the evolutionarily conserved TAD4 domain of C/EBPβ is the target of the GADD45β-dependent signaling. Collectively, we have uncovered a novel molecular mechanism linking GADD45β via the MTK1/MKK3/6/p38 axis to C/EBPβ-TAD4 activation of Col10a1 transcription in terminally differentiating chondrocytes.
Keywords: Cell/Differentiation, Development Differentiation, Extracellular Matrix/Collagen, Signal Transduction/Protein Kinases/MAP, Transcription/C/EBP, Transcription/Promoter
Introduction
Terminal chondrocyte differentiation involves up-regulation of genes encoding collagen X (Col10a1), matrix metalloproteinase 13 (Mmp13), and alkaline phosphatase by bone morphogenetic proteins and other interacting signaling pathways (1–5). The classical pathway for bone morphogenetic protein signaling involves Smad1 and Smad5 and inhibitory Smad6 and Smad7 (6, 7). Previously, we identified GADD45β (growth arrest- and DNA damage-inducible), also known as MyD118, as one of the most highly up-regulated early gene products induced by BMP-2 (bone morphogenetic protein 2) in chondrocytes via Smad1 and Runx2 synergism (8). We also found that the growth plates of Gadd45β−/− embryos display a compressed hypertrophic zone and reduced bone growth in conjunction with decreased Col10a1 and Mmp13 gene expression (8).
The GADD45 family also includes GADD45α and GADD45γ, which regulate apoptosis and differentiation by modulating cascades of stress-responsive mitogen-activated protein kinases (MAPKs),3 including the p38 and JNK pathways (9). GADD45β protein is known to bind to MTK1/MEKK4, a MAP3K, leading to the phosphorylation of MAP2Ks, such as MKK3 or -6 and MKK4 or -7, followed by activation of the p38 and JNK pathways, respectively (10–12). However, interaction of GADD45β with MTK1 has been reported to inhibit or activate MKK7, followed by inhibition or activation of JNK signaling, depending on the cell type and the availability of upstream signals, such as NF-κB (13–17). In chondrocytes, we found that GADD45β via JNK activation increased MMP13 promoter activity in synergism with Fra1 or Fra2 together with JunB or JunD (8). Thus, our findings and those of others suggest that GADD45β may play an important role in chondrocyte terminal differentiation by modulating both JNK and p38 MAPK signaling cascades.
The MAPK signaling pathways are involved in many cellular processes, such as gene regulation, intracellular metabolism, differentiation, proliferation, mobility, and survival or death (18, 19). MAPKs are activated by MAPK kinases (MAP2K) via phosphorylation of conserved threonine and tyrosine residues in their activation loops, followed by phosphorylation of downstream kinases and targets, including transcription factors that regulate a variety of target genes (20). The roles of MAPKs in chondrogenesis have been investigated in vivo as well as in vitro (reviewed in Refs. 21 and 22). Ablation of the MAPK kinase kinase (MAP3K), MEKK4/MTK1, causes skeletal patterning defects in the mouse embryo (23). Moreover, Col2a1 promoter-driven MKK6 transgene overexpression leads to decreased chondrocyte proliferation and delayed terminal differentiation to hypertrophy (24). Constitutive activation of the MAP2K, MEK1, responsible for ERK activation caused persistence of proliferating chondrocytes and delayed hypertrophic maturation (25), whereas in vitro studies using pharmacological inhibition showed that ERK activation is required for chondrocyte hypertrophy (26). Inhibition of the p38 pathway in vitro also leads to impairment of terminal differentiation of chondrocytes to hypertrophy (27, 28). These results suggest that the MEK1/ERK and MKK3/6/p38 pathways are important for regulating chondrogenesis in the embryonic growth plate. However, the precise mechanisms of action of these kinase cascades have not been defined completely because of the complex regulation at different stages of this process, involving several stimuli, as well as the many downstream transcription factors and target genes (5).
Among the transcription factors implicated in regulating genes related to the terminal hypertrophic chondrocyte phenotype, the Runt domain transcription factor, Runx2, or CBFA1, is a critical factor for the expression of Col10a1 and Mmp13 in vivo and in vitro (29–32). Col10a1 gene activation requires a Runx2 binding site located in its distal promoter (33, 34), as well as a non-consensus Runx2 binding site located in the proximal promoter region (35). In addition, the Fra2 knock-out phenotype is characterized by impaired hypertrophic differentiation of chondrocytes in the embryonic growth plate, along with reduced Col10a1 mRNA expression levels (36). A recent study showed in the CCAAT/enhancer-binding protein β (C/ebpβ) knock-out embryo a delay in hypertrophic differentiation in the growth plate, associated with decreased Col10a1 and Mmp13 mRNA (37), a phenotype similar to that of the Gadd45β−/− embryo (8). Fra2 and C/EBPβ are members of the bZIP family of transcription factors, characterized by a basic region and a leucine zipper domain (38, 39). Transcriptional control by bZIP family members is complex because not only the expression level of each factor but also post-translational modifications determine their effects. Indeed, specific bZIP phosphorylations regulate nuclear translocation, the formation of homo- or heterodimers that determines binding to single or composite sites, and the recruitment of the general transcription machinery to polymerase II transcription initiation complexes (39).
Here, we demonstrate that GADD45β, which co-localizes with C/EBPβ and collagen X in growth plate chondrocytes, is targeted to C/EBPβ and activates the Col10a1 proximal promoter dependent on MKK3/6/p38 signaling. Importantly, we identify the evolutionarily conserved fourth transactivation domain (TAD4) of C/EBPβ as the target of the GADD45β-enhancing effect on Col10a1 promoter activity. Together, our findings indicate that enhancement of C/EBPβ transactivation by GADD45β is one of the mechanisms underlying Col10a1 transcriptional control in terminally differentiating chondrocytes during skeletal development.
EXPERIMENTAL PROCEDURES
Cell Culture
Mouse teratocarcinoma cells, ATDC5, were cultured in Dulbecco's modified Eagle's medium/Ham's F-12 (1:1, v/v; Invitrogen) containing 5% fetal bovine serum, as described previously (8).
Plasmid Constructions
The MMP13-Luc promoter construct was described previously (8). The Col10a1-Luc reporter, pGL4600Int, was a kind gift from Klaus von der Mark, Erlangen, Germany (34, 40). A 2.3-kb fragment from pCol10a1- SAbetageobpA (33) was transferred into the pGL3 basic vector to generate pGL3-Col10a1-(−2128/+201), which was used as template for PCR for deletion mutants employing a common reverse primer (GCCTCGAGGGCAGCTTTCCTGGCTTCCTC) and the following forward primers: Col10a1-(−60/+52), CGGAGCTCGGCTCAGGGGAACTTCATGC; Col10a1-(−34/+52), CGGAGCTCGGAAGAAAACAGTATAAAATAC. Point mutants of the Col10a1 promoter were generated by PCR, and the resultant amplification products were digested with SacI and XhoI and transferred into the Col10a1-(−60/+52) backbone treated with the same enzymes. The PCRs were performed using a common reverse primer (GCCTCGAGGGCAGCTTTCCTGGCTTCCTC) and the following forward primers for Col10a1-(−60/+52) mutants A–G (lowercase letters indicate mutation site): A, CGGAGCTCGGCcCAGGGGAACTTCATGC; B, CGGAGCTCGGCTCAGGGGccCTTCATGC; C, CGGAGCTCGGCTCAGGGGAACTcCATGC; D, CGGAGCTCGGCTCAGGGGAACTTCggGCAATAAGGGAA; E, CGGAGCTCGGCTCAGGGGAACTTCATcCAATAAG; F, CGGAGCTCGGCTCAGGGGAACTTCATGCccTAAG; G, CGGAGCTCGGCTCAGGGGAACTTCATGCAATccGGGAAG. Reporter plasmids of pCRE-Luc and pCEBP-Luc were purchased from Stratagene (catalog numbers 219075 and 240112). AP-1-Luc and expression constructs for AP-1 family members, including Fra2, Jun, JunB, and JunD, were described previously (8). Expression vectors for ATF2, -3, and -4 were obtained from Dr. Ze'ev Ronai (41), Dr. Michael S. Kilberg (42), and Dr. Guozhi Xiao (43), respectively. Mutant human C/EBPβ expression plasmids were generated by two-step PCR mutagenesis using hC/EBPβ/pcDNA3.1(+) as template for the first step and the following primer pairs (lowercase letters indicate mutation site): hC/EBPβ S76A Y78A(SY) for hLAP*SY, forward (CGACTTCgcCCCGTtCCTGGAGCCGCTG) and reverse (CAGCGGCTCCAGGaACGGGgcGAAGTCG); hC/EBPβ F117A L118A(LF) for hLAP*LF, forward (GCACCACGACgcCgcCTCCGACCTCTTCGCCGACGACTAC) and reverse (GTAGTCGTCGGCGAAGAGGTCGGAGgcGgcGTCGTGGTGC); hC/EBPβ T235A, forward (TCCAGCCCGCCCGGCgCGCCGAGCCCCGCT) and reverse (AGCGGGGCTCGGCGCGCCGGGCGGGCTGGA); hC/EBPβ Y274F, forward (CAAGCACAGCGACGAGTtCAAGATCCGGCGCGAGCG) and reverse (CGCTCGCGCCGGATCTTGaACTCGTCGCTGTGCTTG). The second step PCR was performed using TAATACGACTCACTATAGGG (forward) and TAGAAGGCACAGTCGAGGC (reverse). PCR products from the second PCR were digested with EcoRI and ApaI, and fragments were transferred into pcDNA3.1(+). The hC/EBPβ L320R construct was generated by PCR using the hC/EBPβ plasmid as template and the following primer pair: forward, TAATACGACTCACTATAGGG; reverse, GCAGGGTGCTGAGCTCGCGCGACcGCTGCTCCACC. The PCR product was digested with BlpI and EcoRI and transferred into the hC/EBPβ plasmid backbone.
MTK1 and the kinase-dead mutant (MTK1KD), MTK1K/R, were described previously (10). The following plasmids were purchased from Addgene: MKK6KD mutant, pCDNA3 FLAG-MKK6(K82A) (Addgene ID 13519) (44); MKK7ala mutant, pCDNA3 FLAG-MKK7a1(Ala) (Addgene ID 13518) (45); constitutively active mutants of MKK6 and 7, pcDNA3- FLAG-MKK6(glu) (Addgene ID 13518) and pcDNA3 FLAG MKK7a1(glu) (Addgene ID 14539); the JNK activation loop phosphorylation site mutant, pcDNA3 FLAG-Jnk1a1(apf) (Addgene ID 13846) (46); and pWay21-MKP1-FL (Addgene ID 13469) (47). Other plasmids obtained from Addgene were pcDNA3.1(−) mouse C/EBPα (Addgene ID 12550), C/EBPβ (Addgene ID 12557), C/EBP LIP (Addgene ID 12561), and C/EBPδ (Addgene ID 12559). The pFC-MEK3 plasmid was obtained from Stratagene (catalog number 219089).
The plasmids for pcDNA3-FLAG-WT-p38α and p38α mutant, containing a phosphorylation site mutation in the activation loop (p38αapf), were kind gifts from Dr. Jiahuai Han (48). Plasmids for full-length GADD45β (HA-MyD118/ pcDNA3) were kind gifts from Dr. Dan A. Liebermann (49). The pRL-TK (E2241) and pRL-SV40 (E2231) vectors were purchased from Promega. The PathDetect in vivo signal transduction pathway trans-reporting system for c-Jun was purchased from Stratagene (catalog number 219000). pFA-mC/EBPβ (mLAP-(1–275)) was made by transferring a fragment from pcDNA3.1-mC/EBPβ into pFA-CMV trans-reporter cloning vector (catalog number 219036) after digestion with BglII and EcoRI. pFA-mC/EBPβ(1–159) was made by blunt-end self-ligation of a backbone without a BmgBI-BglII fragment (residues 159–275) from the pFA-mC/EBPβ construct and creating a new stop codon after amino acid 159, using the Takara blunting kit (catalog number 6025). For pFA-mC/EBPβ-(1–81), the PCR products using pcDNA3.1-mC/EBPβ as template and forward (TAATACGACTCACTATAGGG) and reverse (CAAGATCTCACGGCTTCTTGCTCGGCTTGGCGC) primers were transferred into the pFA-CMV, as above. mLAPΔSacII-(53–120) and hLAPΔSacII-(47–182) were made by self-ligation of backbone (mLAP and hLAP, respectively) after excision of the SacII fragment. The sequences of all constructs were confirmed by DNA sequencing performed at the Beth Israel Deaconess Medical Center DNA sequencing facility or Cornell University Life Sciences Core Laboratories Center.
Transfection and Reporter Assays
Transient transfection experiments were performed in ATDC5 cells using Lipofectamine PLUSTM reagent (Invitrogen), as described previously (8). Cells were seeded 24 h prior to transfection in 24-well tissue culture plates at 1.25 × 104 cells/cm2 in Dulbecco's modified Eagle's medium/F-12 containing 5% fetal bovine serum. Transfections were carried out in serum-free medium with a total of no more than 475 ng of plasmid DNA, including 300 ng of luciferase reporter construct per well.
Chromatin Immunoprecipitation (ChIP) Assay
The ChIP-IT Express enzymatic kit (catalog number 53009, Active Motif) was used to perform ChIP assays, according to the manufacturer's instructions with minor modifications. Briefly, ATDC5 cells infected with lentiviral vectors containing siRNA-GFP (siGFP) or siRNA-GADD45β (si45β) (8) were plated on 150-cm dishes and differentiated in Dulbecco's modified Eagle's medium/Ham's F-12 medium containing 5% fetal bovine serum and 1% ITS+ Premix (catalog number 354352, BD Biosciences) for 1 and 2 weeks. Cells were cross-linked with 1% formaldehyde for 10 min at room temperature; nuclei were isolated, and chromatin was enzymatically sheared for 8 min at 37 °C, resulting in chromatin fragments of 250–1000 bp. Chromatin was precleared by incubation with 25 μl of protein G magnetic beads and 5 μg of nonspecific (control) rabbit IgG (catalog number 2729; Cell Signaling) for 2 h at 4 °C with rotation. After preclearing and removal of the protein G magnetic beads, the lysates were incubated at 4 °C for 16 h with 5 μg of rabbit anti-C/EBPβ antibody (catalog number sc-150X, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)) or normal rabbit IgG (catalog number 2729, Cell Signaling), and 10 μl of the precleared chromatin was stored to be used as assay input. After reverse cross-linking of the DNA-protein complexes, the DNA was purified using DNA minicolumns (catalog number 28104, Qiagen). The final DNA preparations were subjected to PCR analysis using 5 μl of the eluted DNA and the following set of primers: 5′-CCGTTAGGACTTCCCACCAT-3′ (sense) and 5′-CAGGTAAGCCTCGTCTGAGG-3′ (antisense), spanning from −114 to +91 of the proximal mouse Col10a1 promoter, containing the C/EBP binding motif. The PCR products were resolved on a 2.5% agarose gel and confirmed by DNA sequencing at the Cornell University Life Sciences Core Laboratories Center. Additional real-time PCR analysis was performed using the Opticon 2 real time PCR detector system (Bio-Rad) in order to better detect changes of the C/EBPβ binding to the amplified Col10a1 promoter region.
Real-time Reverse Transcription-PCR Analysis
Total RNA (100 ng) isolated using the RNeasy Plus Mini Kit (catalog number 74134, Qiagen) from 2-week differentiated siGFP- and si45β-ATDC5 cells was reverse transcribed, as previously described (8, 50). Amplifications were carried out using SYBR Green I-based real-time PCR on the Opticon 2 real-time PCR detector system (Bio-Rad), as described previously (8, 50), using the following primers: mouse Col10a1, 5′-TGCCCGTGTCTGCTTTTACTGTCA-3′ (sense) and 5′-TCAAATGGGATGGGGGCACCTACT-3′ (antisense); mouse β-actin, 5′-TGTTACCAACTGGGACGACA-3′ (sense) and 5′-GGGGTGTTGAAGGTCTCAAA-3′ (antisense); mouse glyceraldehyde-3-phosphate dehydrogenase, 5′-CCATGGAGAAGGCCGGGG-3′ (sense) and 5′-CAAAGTTGTCATGGATGACC-3′ (antisense). The data were calculated as the ratio of Col10a1 to β-actin, and glyceraldehyde-3-phosphate dehydrogenase was utilized as an additional housekeeping control.
Western Blotting Analysis
After incubation without or with BMP-2 at 100 ng/ml for 15 and 30 min, the siGFP- and si45β-ATDC5 cells were collected by scraping, and total protein was extracted as described previously (8). The cell lysates were analyzed on Western blots using antibodies against phospho-p38 (catalog number 9211, Cell Signaling) or total p38 (catalog number 9212, Cell Signaling) and β-actin (catalog number A5441, Sigma) was used as an additional loading control.
Immunohistochemistry
Hind limbs were collected from C57BL/6 mouse embryos, fixed for 24 h in 4% paraformaldehyde, embedded in paraffin wax, and cut into 6-μm sections. Tissue sections were deparaffinized in xylene and rehydrated through an ethanol series. IHC was performed, as described previously (8, 37), using the primary antibodies goat polyclonal anti-GADD45β (catalog number sc-8776, Santa Cruz Biotechnology, Inc.; 1:400 dilution, 0.5 μg/ml final concentration) and rabbit polyclonal anti-C/EBPβ (sc-150X, Santa Cruz Biostechnology, Inc.; 1:2000 dilution). Rabbit polyclonal antibody to collagen type X (1:1000 dilution) was kindly supplied by Prof. Ray Boot-Handford (Wellcome Trust Centre for Cell-Matrix Research, Manchester, UK). For negative controls, normal rabbit (sc-2027) or goat (sc-2028) IgG were used in place of the primary antibodies. Vectastain Elite ABC kit (Vector Laboratories, Inc.) was used as described by the manufacturer.
Statistical Analysis
Data are reported as mean ± S.D. of at least three independent experiments, each performed in triplicate. Statistical analysis was performed by Student's t test with p values of <0.05 considered significant.
RESULTS
Conserved Half-sites for AP-1, CREB/ATF, and C/EBP Binding in the Col10a1 Proximal Promoter
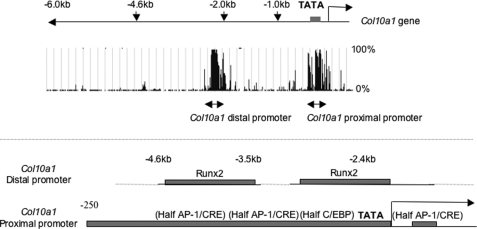
Comparative analyses of DNA sequences from multiple species using the University of California Santa Cruz Genome Browser (Human Assembly, March 2006) identified two evolutionarily conserved regions in the Col10a1 promoter, one in the distal promoter region and the other in the proximal region (Fig. 1). The conserved regions contained a Runx2 binding site in the distal promoter, previously described to be functional in vivo (33, 51), and a proximal Runx2 binding site, reported to be functional by in vitro assay (35). In addition, we found conserved half-sites for binding AP-1 (TGA(C/G)TCA), CREB/ATF (TGACGTCA), and C/EBP (ATTGCGCAAT) in the proximal promoter (Fig. 1). Previously, we demonstrated that GADD45β enhanced AP-1 reporter activity with either JunD or JunB in synergism with Fra1 or Fra2 (8). Therefore, we examined the effects of GADD45β on gene reporters driven by consensus sequences recognized by AP-1, CREB/ATF, and C/EBP family members, respectively. Fra2/JunD transactivation of the AP-1 reporter was further enhanced by GADD45β (Table 1), as expected from our previous report (8). GADD45β also enhanced c-Jun-dependent transactivation but not in the presence of Fra2 (Table 1, top). Moreover, GADD45β increased ATF2-driven CRE reporter activity and C/EBPα, -β, and -δ transactivation of the C/EBP reporter by almost 2-fold (Table 1, middle and bottom). Collectively, these in vitro results using consensus reporters indicate that GADD45β can synergize with not only Fra2/JunD but also with ATF and C/EBP family members that could interact with conserved sites in the Col10a1 proximal promoter region.
FIGURE 1.
Structural homologies between human and mouse Col10a1 promoters. Homologies between the 6-kb fragment of the human and the mouse 5′-flanking region of Col10a1 were analyzed using the University of California Santa Cruz Genome Browser (Human Assembly, March 2006). Schemes of the highly conserved proximal and distal regions of the Col10a1 promoter are represented below.
TABLE 1.
Effects of GADD45β on AP-1, CRE, and C/EBP reporter activities
ATDC5 cells were transfected with 300 ng of AP-1-Luc (top), CRE-Luc (middle), or C/EBP reporter (bottom) and 25 ng of plasmid encoding the indicated family member or GADD45β, alone or together, in a total of 400 ng/well. The values of firefly luciferase activities are normalized to Renilla luciferase activities of pRL-TK and shown as -fold induction compared with control (empty vector). Means ± S.D. from three or more independent experiments are shown.
| Control | c-Jun | JunB | JunD | |
|---|---|---|---|---|
| Control | 1.00 | 5.87 ± 1.83 | 1.60 ± 0.16 | 1.17 ± 0.13 |
| Fra2 | 4.18 ± 0.99 | 6.77 ± 2.00 | 2.92 ± 0.57 | 6.78 ± 1.58 |
| Gadd45β | 2.01 ± 0.38 | 10.71 ± 2.38 | 1.08 ± 0.22 | 1.90 ± 0.32 |
| Fra2/Gadd45β | 8.10 ± 1.54 | 6.50 ± 2.63 | 3.70 ± 1.24 | 10.23 ± 1.38a,b |
| Control | ATF2 | ATF3 | ATF4 | |
|---|---|---|---|---|
| Control | 1.00 | 1.58 ± 0.19 | 0.95 ± 0.13 | 1.53 ± 0.31 |
| GADD45β | 2.78 ± 0.25 (2.78) | 4.58 ± 1.06c (2.91) | 1.83 ± 0.54c (1.92) | 3.04 ± 0.93c (1.98) |
| Control | C/EBPα | C/EBPβ | C/EBPδ | |
|---|---|---|---|---|
| Control | 1.00 | 24.13 ± 5.79 | 7.05 ± 1.59 | 5.11 ± 1.05 |
| GADD45β | 1.47 ± 0.12 (1.47) | 43.38 ± 11.26d (2.00) | 15.13 ± 4.57d (2.40) | 10.61 ± 3.27d (2.34) |
a p < 0.01 versus Fra2/Gadd45b.
b p < 0.01 versus Fra2/JunD.
c p < 0.01 versus GADD45b.
d p < 0.01 for C/EBPα, C/EBPβ, and C/EBPδ, respectively, versus GADD45β.
GADD45β Promotes C/EBP Transactivation of the Col10a1 and MMP13 Promoters
To examine whether GADD45β could participate in transactivating the Col10a1 promoter, we used a Luc reporter construct driven by 4.6 kb of Col10a1 promoter sequence, which produces strong expression in hypertrophic chondrocytes (33, 34, 40, 51, 52), and another Luc reporter with 1.6 kb of the MMP13 promoter as a reference control (8). First, co-transfection of Fra2 and GADD45β, alone or in combination, revealed little or no response of the Col10a1 promoter compared with the positive response of MMP13-Luc (Table 2, top). ATF2 overexpression alone had no effect, whereas GADD45β induced both Col10a1 and MMP13 promoter activation in synergism with ATF2 (Tables 2, third and bottom parts). Importantly, co-transfection of GADD45β with C/EBPα, -β, or -δ also enhanced the activities of both promoters compared with transfections with C/EBP alone (Table 2, third and bottom parts). These results prompted us to investigate the C/EBP family as candidate downstream targets of GADD45β in the regulation of Col10a1 gene expression during chondrocyte terminal differentiation.
TABLE 2.
Effects of GADD45β on transactivation of Col10a1 (4.6 kb) or MMP13 (1.6 kb) promoter activity with ATF2, Fra2, and C/EBP family members
ATDC5 cells were transfected with reporter plasmid (300 ng) of Col10a1 (4.6 kb)-Luc (top three parts) or MMP13 (1.6 kb)-Luc (first, second, and fourth parts) and plasmids encoding Fra2 (first part), ATF2 (second part), or C/EBPα, C/EBPβ, or C/EBPδ (third and fourth part), alone or together with GADD45β in a total of 400 ng/well. Values of firefly luciferase activities are normalized to values of Renilla luciferase activities of pRL-TK and shown as -fold induction compared with control. Means ± S.D. from three or more independent experiments are shown. In the top part, p < 0.01 was considered as significant.
|
Col10a1 (4.6 kb) |
MMP13 (1.6 kb) |
|||
|---|---|---|---|---|
| Control | Fra2 | Control | Fra2 | |
| Control | 1.00 | 0.93 ± 0.08 | 1.00 | 2.20 ± 0.21 |
| GADD45β | 4.76 ± 0.83 | 4.35 ± 0.86 | 5.29 ± 0.62 | 13.33 ± 2.12a |
|
Col10a1 (4.6 kb) |
MMP13 (1.6 kb) |
|||
|---|---|---|---|---|
| Control | ATF2 | Control | ATF2 | |
| Control | 1.00 | 0.97 ± 0.28 | 1.00 | 0.93 ± 0.08 |
| GADD45β | 4.04 ± 1.30 | 9.30 ± 3.44b | 4.23 ± 0.64 | 7.03 ± 1.18a |
|
Col10a1 (4.6 kb) |
||||
|---|---|---|---|---|
| Control | C/EBPα | C/EBPβ | C/EBPδ | |
| Control | 1.00 | 18.77 ± 6.82 | 24.29 ± 10.12 | 17.32 ± 6.66 |
| GADD45β | 2.59 ± 0.41 (2.59) | 57.01 ± 21.91b (3.03) | 81.70 ± 31.18b (3.36) | 72.84 ± 29.25b (4.21) |
|
MMP13 (1.6 kb) |
||||
|---|---|---|---|---|
| Control | C/EBPα | C/EBPβ | C/EBPδ | |
| Control | 1.00 | 9.97 ± 2.06 | 7.26 ± 1.55 | 9.28 ± 2.45 |
| GADD45β | 2.64 ± 0.50 (1.92) | 20.80 ± 6.56a (2.06) | 14.84 ± 6.56a (2.04) | 21.75 ± 6.67a (2.34) |
a p < 0.01 for MMP13.
b p < 0.01 for Col10a1.
C/EBPβ Co-localizes with GADD45β and Collagen Type X in the Embryonic Growth Plate
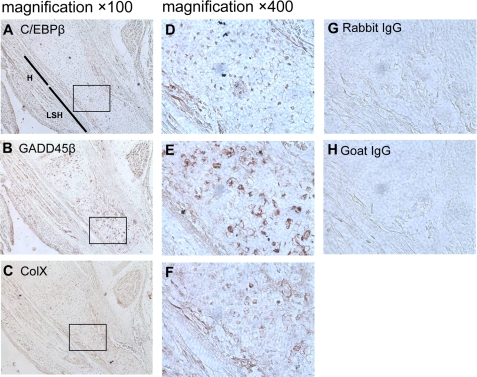
Because C/EBPβ expression has been linked previously to chondrocyte terminal differentiation (37, 53), we next examined the in situ expression of the C/EBP family and GADD45β in the embryonic growth plate by immunohistochemistry. C/EBPβ was highly expressed in late proliferative and prehypertrophic growth plate chondrocytes at embryonic day 15.5, consistent with the findings of Hirata et al. (37) at embryonic day 16.5, but also in the late stage hypertrophic zone (Fig. 2, A and D). Furthermore, C/EBPβ co-localized with both GADD45β (Fig. 2, B and E) and collagen type X (Fig. 2, C and F) in the early and late hypertrophic zones. Strong nuclear staining for C/EBPβ was evident, whereas GADD45β protein was present in both cytoplasm and nuclei. Interestingly, C/EBPα, C/EBPδ, and C/EBPϵ were also expressed with variable expression levels in this region (data not shown), suggesting that other C/EBP members may also participate in regulating Col10a1 expression. Taken together with our in vitro data, the co-localization of GADD45β with C/EBPβ further supports the view that Col10a1 may be a downstream target of GADD45β and C/EBPβ during chondrocyte maturation in the embryonic growth plate.
FIGURE 2.
C/EBPβ distribution in mouse embryonic growth plates. Embryonic growth plates of left limbs (embryonic day 15.5) were stained using antibodies against C/EBPβ (A and D), GADD45β (B and E), collagen X (C and F), and control rabbit (G) and goat (H) IgG, as described under “Experimental Procedures.” The boxes in A–C designate the areas magnified in D–F, showing the late stage hypertrophic (LSH) zone marked with a line in A, compared with the hypertrophic (H) zone. Original magnifications were ×100 (A–C) and ×400 (D–H).
The Synergistic Effect of GADD45β and C/EBPβ Requires a C/EBPβ Binding Motif in the Col10a1 Proximal Promoter
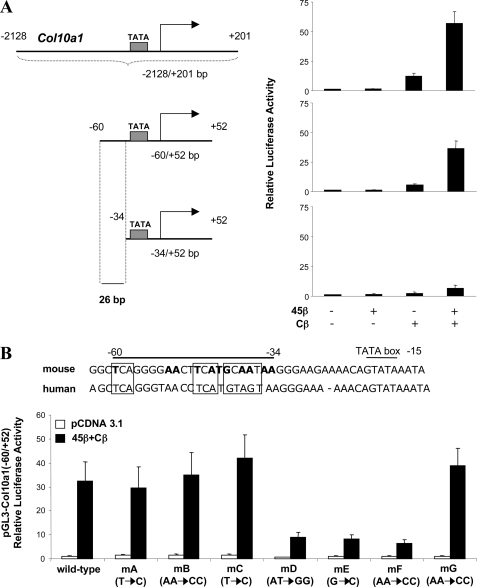
To identify the DNA elements responsible for Col10a1 transcriptional control by GADD45β and C/EBPβ, Col10a1 promoter mutants were constructed and tested in reporter assays. A Col10a1-(−60/+52)-Luc reporter construct lacking the 5′ promoter region spanning bp −2128 to −60 still responded strongly to GADD45β and C/EBPβ synergism compared with the full-length Col10a1 promoter (Fig. 3A). However, the activity of the Col10a1-(−34/+52)-Luc reporter with deletion to −34 bp was strongly reduced, indicating that the sequence spanning −60 to −34 bp contains sites responsive to GADD45β and C/EBPβ (Fig. 3A). We, therefore, made point mutations to delineate the C/EBPβ binding sites (Fig. 3B). Whereas there was no significant difference between wild type and mutants without co-transfection with GADD45β and C/EBPβ, mutations E and F in one C/EBP binding site strongly disrupted the activation of the Col10a1 promoter (80% reduction) in response to C/EBPβ and GADD45β. In addition, mutation D in a non-C/EBP consensus site also caused a significant reduction (Fig. 3B).
FIGURE 3.
Identification of the Col10a1 promoter element responsive to the synergistic action of C/EBPβ and GADD45β. A, ATDC5 cells were co-transfected with deletion mutants of the Col10a1 promoter (−2128/+201, −60/+52, and −34/+52) in the pGL3 reporter vector (300 ng) and 25 ng of each expression vector encoding mouse C/EBPβ (Cβ) or GADD45β (45β), alone or in combination, in a total amount of 375 ng/well. B, the regions with homologies in the mouse and human Col10a1 promoter region (−60/−34 bp) responsive to the synergistic action of C/EBPβ and GADD45β are indicated by a line above. The two half-sites for CREB or AP-1 and the C/EBP half-site are indicated as square boxes. Sites in the mouse sequence that are highlighted in boldface type correspond to substituted sites in the mutant constructs used in the transfections shown in the lower panel. Col10a1 promoter constructs (300 ng) containing mutations in the proximal region were co-transfected with empty vector or 25 ng of expression vector encoding mouse C/EBPβ or GADD45β, alone or in combination, in a total amount of 375 ng/well in ATDC5 cells. Luciferase activities were normalized to pRL-TK reporter activities and shown as -fold induction compared with control (empty vector). Experiments were done in triplicate with data shown as means ± S.D.
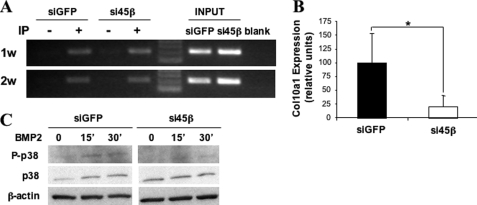
To investigate if GADD45β affected the binding of C/EBPβ to the endogenous Col10a1 proximal promoter, ChIP assays were performed with ATDC5 cells, in which we previously described the ablation of GADD45β expression by stable transduction with lentiviral vectors expressing an siRNA targeted to GADD45β versus the siGFP-matched control cell population (8). As shown in Fig. 4A, no difference was noted in C/EBPβ binding to its proximal site in the endogenous Col10a1 promoter in siGFP control versus GADD45β knockdown ATDC5 cells after culturing them under differentiating conditions for 1 or 2 weeks, and the same result was also verified by real-time PCR analysis (not shown). Similar to the results that we observed previously in micromass cultures of primary mouse rib chondrocytes, the expression of Col10a1 was reduced in the 2-week differentiated ATDC5 cells stably expressing siRNA-GADD45β (Fig. 4B). These results indicate that the C/EBP binding site (GCAAT) is responsible for Col10a1 promoter activation by GADD45β and C/EBPβ and also implies the involvement of the flanking region through homo- or heterodimerization (54–57).
FIGURE 4.
GADD45β knockdown decreases p38 phosphorylation and Col10a1 mRNA levels, without affecting C/EBPβ binding to the Col10a1 promoter in vivo. A, siGFP and si45β-ATDC5 cells were differentiated for 1 week (1w) and 2 weeks (2w). Chromatin was cross-linked and enzymatically sheared, and after reverse cross-linking of the DNA-protein complexes, the precleared lysates were incubated with antibodies against C/EBPβ (+) or normal rabbit IgG (−) overnight at 4 °C. The mouse Col10a1 promoter region was PCR-amplified using primers spanning from −114 to +91, and the PCR products were resolved on a 2.5% agarose gel. Data are representative of two independent experiments performed in duplicate. B, total RNA isolated from 2-week differentiated siGFP- and si45β-ATDC5 was analyzed by real-time PCR. Each value was normalized to β-actin in the same sample and shown as mean ± S.D. *, p < 0.05. C, the siGFP- and si45β-ATDC5 cells were incubated with 100 ng/ml BMP-2 for the indicated times, and total lysates were analyzed on Western blots using antibodies against phospho-p38 (P-p38), total p38 (p38), and β-actin.
Because the binding of C/EBPβ to the endogenous Col10a1 proximal promoter was not affected by GADD45β knockdown, we then investigated whether GADD45β-dependent phosphorylation events could modulate C/EBPβ activity independent of DNA binding. Because C/EBPβ is known to be a direct substrate of p38 kinase (58–60), we analyzed whether p38 activation was differentially modulated in siGFP control versus si45β-ATDC5 cells after BMP-2 treatment. Indeed, although BMP-2 treatment enhanced p38 phosphorylation in siGFP control cells, it failed to do so in the GADD45β knockdown cells (Fig. 4C). Taken together, the above results are consistent with GADD45β-mediated Col10a1 modulation working through p38 to enhance the transcriptional activity of DNA-bound C/EBPβ.
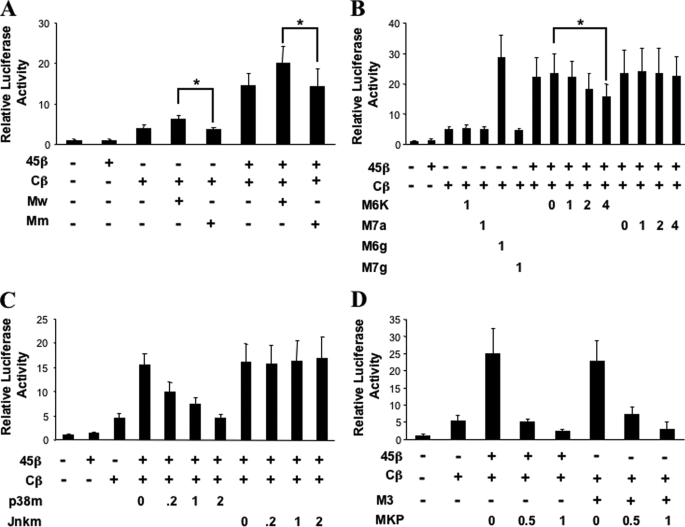
GADD45β Enhances C/EBPβ Activity via MTK1/MKK3/6/p38 Signaling
To better delineate the mechanisms underlying the enhancement of C/EBPβ transactivation by GADD45β, we compared the activities of wild type, dominant negative, or dominant active kinase mutants in co-transfections with the Col10a1-(−60/+52)-Luc reporter. First, wild type MTK1 (Mw) overexpression further enhanced the activation of the Col10a1 reporter co-transfected with GADD45β and C/EBPβ expression plasmids, whereas the kinase-dead MTK1K/R mutant (Mm) did not (Fig. 5A). To identify the required MAP2K that delivers the signal from GADD45β to C/EBPβ, the MKK6KD mutant or the MKK3ala, MKK4ala, and MKK7ala mutants with phosphoacceptor amino acid threonine or tyrosine changed to alanine were used in reporter assays. Interestingly, overexpression of MKK6KD (M6K; Fig. 5B) or MKK3ala (data not shown) reduced the ability of GADD45β to enhance C/EBPβ-mediated Col10a1 transactivation in a dose-dependent manner, whereas overexpression of MKK4ala (data not shown) or MKK7ala (M7a; Fig. 5B) did not. Furthermore, constitutively active mutants, MKK6glu (M6g; Fig. 5B) and MKK3 (M3; Fig. 5D), but not MKK7glu (M7g; Fig. 5B), mimicked the GADD45β-dependent enhancement of C/EBPβ transactivation of the Col10a1 promoter. These results suggest that GADD45β interaction with MTK1 promotes downstream phosphorylation events via MKK6 or MKK3 that activate C/EBPβ. In confirmation of this interpretation, the dominant negative phosphorylation site mutant, p38αapf (p38m), but not the JNKapf mutant (Jnkm), reduced the GADD45β-dependent enhancement of C/EBPβ activity on the Col10a1 promoter (Fig. 5C). Finally, overexpression of MKP1, a specific MAPK phosphatase (47), diminished C/EBPβ transactivation of the Col10a1 promoter induced by GADD45β or MKK3 (M3) (Fig. 5D). Together, these results provide strong evidence that phosphorylation events in the MTK1/MKK3/6/p38α cascade are critical for GADD45β signaling to C/EBPβ- and GADD45β-dependent transactivation of Col10a1 by C/EBPβ.
FIGURE 5.
The MTK1/MKK3/6/p38α cascade mediates the GADD45β-driven C/EBPβ activation of the Col10a1 promoter. ATDC5 cells were co-transfected with 300 ng of the Col10a1-(−60/+52) promoter and 25 ng of expression vector encoding mouse C/EBPβ (Cβ) or GADD45β (45β), alone or together, and 50 ng of expression plasmid encoding either wild type MTK1 (Mw) or mutant MTK1K/R (Mm) (A); with 25 ng of expression vector encoding mouse C/EBPβ and 50 ng of MKK6KD (M6K), MKK7ala (M7a), MKK6glu (M6g), or MKK7glu (M7g) (lanes 4–7) or 25 ng of each expression vector encoding mouse C/EBPβ and GADD45β with increasing amounts (0, 25, 50, and 100 ng, indicated as factors of 0, 1, 2, and 4) of expression vector encoding MKK6KD or MKK7ala (lanes 8–16) (B); with 25 ng of each expression vector encoding mouse C/EBPβ and GADD45β with increasing amounts (0, 5, 25, and 50 ng, indicated as 0, 0.2, 1, and 2) of p38αapf (p38m) or Jnka1apf (Jnkm) expression vectors (lanes 4–11) (C); and 25 ng of each expression vector encoding mouse C/EBPβ and GADD45β or MKK3 (M3) and increasing amounts (0, 12.5, or 25 ng, indicated as 0, 0.5, and 1) of plasmid encoding MKP-1 (MKP) (D). Luciferase activities were normalized to pRL-TK reporter activities and shown as fold induction compared with control (empty vector). Means ± S.D. of triplicates from three independent experiments are shown. * indicates p < 0.01.
C/EBPβ Threonine 235 Mutation Disrupts Its Activation by GADD45β, as Assessed with a C/EBP Consensus-Luc Reporter but Not in the Context of the Col10a1 Promoter
p38 is known to phosphorylate C/EBPβ at threonine 235 (60). Unexpectedly, cotransfection of the C/EBPβ T235A mutant did not significantly affect Col10a1-(−60/+52) reporter activity (Fig. 6A), whereas it did disrupt the GADD45β-enhanced transactivation of the pCEBP-Luc reporter (Fig. 6B). In contrast, the C/EBPβ mutants, Y274F and L320R, which affect DNA binding and protein dimerization, respectively (55, 61), decreased the GADD45β-dependent activities of both Col10a1 and C/EBP reporters (Fig. 6, A and B). These results indicate that the promoter context is important for the permissive effects of GADD45β on transactivation of the Col10a1 promoter by C/EBPβ and that the protein dimerization domains of C/EBPβ are important for driving its activation by GADD45β.
FIGURE 6.
Differential effects of threonine 235 on Col10a1 and C/EBP reporter activities. 25 ng of human C/EBPβ mutants (hCβ), threonine 235 to alanine (T235A), tyrosine 274 to phenylalanine (Y274F), and leucine 320 to arginine (L320R) were co-transfected in the absence or presence of 25 ng of expression vector encoding GADD45β (45β) using 300 ng of Col10a1-(−60/+52) (A) or pCEBP reporter vector (B) in a total of 375 ng/well in ATDC5 cells. Luciferase activities were normalized to pRL-TK reporter activities and shown as -fold induction compared with control (empty vector). Means ± S.D. of triplicates from three independent experiments are shown. *, p < 0.01. NS, not significant.
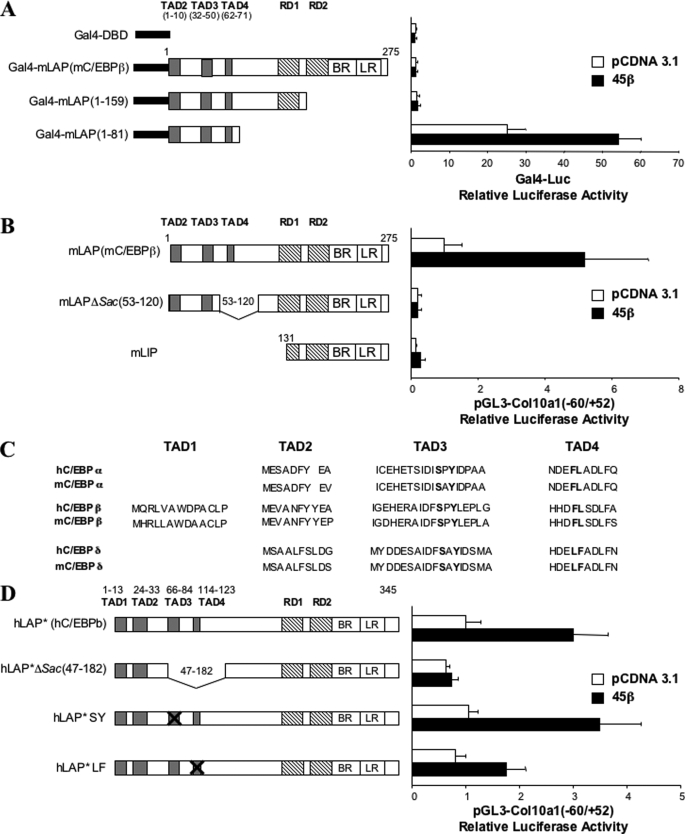
GADD45β Enhances C/EBPβ Function via Its N-terminal Transactivation Domain (TAD)
To identify the C/EBPβ domain responsive to GADD45β activation, we fused murine wild type C/EBPβ (mLAP) and truncated C/EBPβ sequences (1–159 and 1–81) to the yeast Gal4 DNA binding domain and examined their activities on a Gal4-Luc reporter (Fig. 7A). The Gal4-mLAP-(1–81) chimera was highly responsive to GADD45β activation, whereas Gal4-mLAP and Gal4-mLAP-(1–159) constructs both showed very low transactivation and responsiveness to GADD45β (Fig. 7A). The Gal4-mLAP result was in accord with a previous report that identified two repression domains, RD1 and RD2, between the C/EBPβ activation and DNA binding domains (62). Further assays using TAD-(1–81) and MAPK mutants, alone or together, showed that the GADD45β-enhanced C/EBPβ-TAD activity was diminished by MKK6KD overexpression (data not shown). Moreover, Col10a1 promoter transactivation experiments with the mLAP-TAD4 deletion mutant, mLAPΔSacII-(53–120), and mLIP, a C/EBPβ-inhibitory isoform lacking all TAD domains (Fig. 7B), revealed that the TAD4 domain was necessary for transactivating the Col10a1 promoter as well as being essential for responsiveness of C/EBPβ to GADD45β (Fig. 7B). In addition, deletion of the TAD3 and -4 domains in the full-length human C/EBPβ protein (Fig. 7C), hLAP*ΔSacII(47–182), ablated its response to GADD45β (Fig. 7D). Because the TAD3 and -4 domains are conserved among all C/EBP family members (Fig. 7C) and as additional confirmation of the TAD4 requirement, we also examined the activities of the TAD3-SY and TAD4-LF site-directed mutants in the context of the full-length human C/EBPβ protein (Fig. 7D). The TAD4-LF mutant, but not TAD3-SY, blunted the GADD45β-dependent enhancement of C/EBPβ-driven Col10a1 promoter activity (Fig. 7D). Co-transfection of mLAP (Fig. 7B) or hLAP (Fig. 7D) together with GADD45β significantly enhanced the activity of the Col10a1 reporter above that observed with either species of C/EBPβ alone. Wild type murine C/EBPβ had a stronger transactivating effect than the human homologue in these experiments due to the latter's reduced expression in ATDC5 murine cells (data not shown). Collectively, these experiments indicate that GADD45β enhances C/EBPβ transactivation of the Col10a1 promoter via the evolutionarily conserved C/EBPβ-TAD4 domain.
FIGURE 7.
GADD45β enhances C/EBPβ activity via TAD4. A, mouse C/EBPβ deletion constructs fused with the GAL4-DBD (black bar) are shown. Transactivation domains (TAD1, -2, -3, -4), also known as activation domain modules (ADM1, -2, and -3 for TAD2, -3, and -4, respectively), and repression domains (RD1 and -2) are represented as gray boxes and dashed boxes, respectively. Basic region (BR) and leucine zipper region (LR) are indicated (57). Reporter assays were carried out in ATDC5 cells co-transfected with 300 ng of pRL-TK containing the Gal4 binding site and 50 ng of the GAL4-fused mC/EBPβ constructs, alone or in combination with 25 ng of GADD45β expression vector. Luciferase activities were normalized to pRL-SV40 reporter activities and shown as -fold induction compared with control (empty vector). B, wild-type mouse C/EBPβ (mLAP); mLAPΔSacII-(53–120), and mLIP constructs are represented. Col10a1-(−60/+52) transactivation was analyzed in co-transfection experiments with 25 ng of mLAP, mLIP, or mLAPDSacII, alone or in combination with GADD45β expression plasmid. Luciferase activities were normalized to pRL-TK reporter activities and shown as -fold induction compared with control (mLAP). C, human and mouse TAD1, -2, -3, and -4 homologies among the C/EBP family. SY and FL amino acids are highlighted in boldface type. D, full-length human C/EBPβ (hLAP*), hLAP*ΔSacII(47–182), and point SY and LF mutants are depicted. The Col10a1-(−60/+52) transactivation was analyzed in co-transfection experiments with 25 ng of hLAP*, hLAP*ΔSacII, or SY or LF mutant, alone or in combination with GADD45β expression plasmid. Luciferase activities were normalized to pRL-TK reporter activities and shown as -fold induction compared with control (hLAP*). Means ± S.D. of triplicates from three independent experiments are shown for A, B, and D.
DISCUSSION
Molecular Basis of GADD45β Enhancement of C/EBPβ-dependent Col10a1 Promoter Transactivation
In this study, we showed that GADD45β regulates the expression of genes involved in terminal chondrocyte differentiation, Col10a1 and MMP13, by modulating C/EBPβ activity in a manner dependent on the functional binding sites of each promoter and the molecular interplay among signaling molecules and transcription factors in ATDC5 cells (Tables 1 and 2). Although GADD45β modulates transactivation of consensus promoters by a number of bZIP class transcription factors, including AP-1, CREB/ATF, and C/EBP family members (Table 1), the Col10a1 promoter responded specifically to C/EBP family members in synergism with GADD45β (Table 2). Site-directed mutagenesis of the conserved sites in the Col10a1 promoter showed that the proximal C/EBPβ site was functional for the GADD45β/C/EBPβ response, and ChIP assays revealed C/EBPβ binding to this site in the endogenous Col10a1 promoter. As we expected and consistent with our data indicating that GADD45β-dependent signaling modulates C/EBPβ activity by phosphorylation, the binding of C/EBPβ was not affected by siRNA-Gadd45β knockdown, whereas Col10a1 mRNA levels were decreased significantly in those cells (Fig. 4, B and C).
GADD45β Regulation of Genes Involved in Terminal Chondrocyte Differentiation and the Role of C/EBPβ
GADD45β modulates stress-responsive MAPKs, such as p38 and JNK, by binding to MTK1, which activates MAP2Ks (10, 11, 63, 64). Our results indicate that GADD45β acts via the MTK1/MKK3/6/p38 cascade to enhance C/EBPβ activation of the Col10a1 promoter (Fig. 5A), further demonstrating the important role of this pathway in chondrocyte terminal differentiation. Interestingly, mutant mice harboring the MTK1 kinase-inactive mutant allele, which is incompetent for p38 signaling, display abnormalities in axial skeletogenesis, although the role of MTK1 in terminal chondrocyte differentiation was not addressed (23). In addition, transgenic mice expressing a Col2a1 promoter/enhancer-driven, constitutively active MKK6 mutant have shorter limbs associated with delayed chondrocyte terminal differentiation and reduced Col10a1 expression (24), suggesting that excessive MKK6 signaling inhibits chondrocyte proliferation and subsequent differentiation. In contrast, enforced MKK6 activation of the p38 pathway in synovial fibroblasts induces terminal chondrocyte differentiation markers, including type X collagen, and mineralization (65). Moreover, in vitro studies with specific p38 inhibitors also substantiate the importance of p38 signaling for chondrocyte differentiation to hypertrophy (27, 28). Due to these contradictory observations, the role of p38 signaling in chondrocyte terminal differentiation has not been definitively established. Several variables may influence p38-dependent phenotypic changes because excessively high or low p38 activity could differentially modulate hypertrophic processes in the growth plate depending on the availability of transcription factors that could activate or repress target genes (21). Indeed, after treatment with BMP-2, a positive activator of both GADD45β and p38, si45β-ATDC5 cells exhibited defective p38 phosphorylation, indicating that p38 participates in the signaling pathway that goes from GADD45β to Col10a1.
In this study, we showed that bZIP family members ATF2, Fra2, and C/EBPβ, known to be involved in endochondral ossification processes (53, 66), are downstream GADD45β targets (Tables 1 and 2). ATF2 mutant mice present with skeletal abnormalities in proliferating growth plate chondrocytes (67). In contrast, Fra2 knock-out mice display delayed hypertrophic chondrocyte differentiation characterized by decreased Col10a1 gene expression (36). However, in data not shown in that study, Fra2 failed to bind to the distal AP-1 sites (36), which may serve as silencers in vivo (51). Here, we demonstrate that, unlike the MMP13 promoter, the Col10a1 promoter does not respond to Fra2, either with or without GADD45β, further confirming that the 4.6-kb Col10a1 promoter is not a Fra2 target (Table 2, top). Interestingly, the C/EBPβ promoter is regulated directly by Fra2 in other cell types (68), and our finding that C/EBPβ activates the Col10a1 promoter via the proximal GADD45β-C/EBP responsive element (GCRE)1 (Fig. 3B) suggests that Fra2-induced C/EBPβ protein expression, together with GADD45β, may account for Fra2-regulated Col10a1 expression in the embryonic growth plate (36).
The Gadd45β and C/ebpβ knock-out mice display similar phenotypic changes in the embryonic growth plate, characterized by a delay in terminal chondrocyte differentiation along with decreased expression of genes linked to this process, including Col10a1 and Mmp13 (8, 37, 69). In addition, the embryonic limbs of C/ebpβ knock-out mice display impaired osteogenesis (53). GADD45β acts early in the MAPK pathway as a positive switch to facilitate the up-regulation of downstream target genes, such as Col10a1 and MMP13, by activation of Fra2/JunD, ATF2, or C/EBP (Table 2). In addition, GADD45α and GADD45γ also activate the p38 pathway to induce Col10a1 and MMP13 promoter activities in cooperation with C/EBP family members in ATDC5 cells (data not shown). Further, GADD45γ is highly expressed in hypertrophic growth plate chondrocytes (data not shown), suggesting the possibility of partially compensatory or redundant systems for GADD45β signaling to C/EBP proteins and their regulation of genes related to chondrocyte terminal differentiation.
We identified the highly conserved TAD4 of all C/EBP family members (70–72) as the C/EBPβ domain required for the GADD45β-dependent enhancement (Fig. 7). TAD4 is known to be essential for direct binding of C/EBP-TAD to the TAF2B and TBP components of the general RNA polymerase II transcriptional apparatus (70) and also for interaction of C/EBPβ with CBP and p300 transcriptional coactivators and SWI/SNF chromatin remodeling factors (73–77). Thus, GADD45β activation of C/EBPβ-TAD4 function via MTK1/MKK3/6/p38 signaling suggests a general mechanism for facilitating processes driven by transcription factors containing TAD4-like domains, such as c-Jun (72). C/EBPβ-TAD4 activation by GADD45β via p38 kinase or downstream kinases, such as CK2 (78, 79), could conceivably involve more than one mechanism, including (i) phosphorylation of C/EBPβ to recruit TAF2B and TBP, (ii) phosphorylation of TAF2B or TBP to induce interactions between C/EBPβ-TAD and the polymerase II transcription complexes, (iii) C/EBPβ-TAD4 phosphorylation to enhance its recruitment of coactivator or chromatin remodeling complexes, or (iv) interactions of C/EBPβ-TAD and TAF2B or TBP to produce a conformational change making this complex a better kinase substrate. Each of these possibilities could explain our observations showing that the conserved C/EBP-TAD4 domain is required for the GADD45β-enhanced transactivation of Col10a1 by C/EBPβ in ATDC5 cells.
We also found that the repressor domain, RD1, blocked both the GADD45β-enhancing effects and the activity of the full-length C/EBPβ in the Gal4 system (Fig. 7), in accord with a prior report showing that RD1 inhibits the TAD activity of C/EBPβ (62). The latter authors also hypothesized that DNA binding through the bZIP domain could promote a structural change that interrupts the putative RD1-TAD interaction to unmask the TAD domain, whereas the TAD domain in a non-DNA bound protein would remain inaccessible (62). Accordingly, DNA binding by C/EBPβ and/or its homo- or heterodimerization may be required to expose its TAD, allowing for its activation by GADD45β and leading to Col10a1 promoter induction. On the other hand, RD2 is also important for C/EBP-mediated promoter responses to a number of signaling pathways, including RAS, ERK, GSK3β, and p38 (38, 39, 80), which disrupt RD2 interactions with either the C/EBP DNA-binding or TAD domain, leading to derepression of C/EBPβ function (62, 81). However, although the RD2 threonine 235 is a p38 substrate, mutating that residue did not affect Col10a1 promoter activity, whereas it did disrupt C/EBP-Luc induction (Fig. 6). These results highlight the importance of dynamic intra- and intermolecular interplay in the context of the Col10a1 promoter. This interpretation is supported by our finding that GADD45β knockdown in differentiating ATDC5 cells did not affect the binding of C/EBPβ to the endogenous Col10a1 promoter. Armed with these new findings, future work will involve elucidating the mechanism of action of TAD4 in regulating the transcription of hypertrophic target genes in response to GADD45β signaling.
Taken together, we have shown co-localization of GADD45β and C/EBPβ in hypertrophic chondrocytes and GADD45β-dependent activation of C/EBPβ-TAD via the MTK1/MKK3/6/p38 signaling pathway, leading to Col10a1 promoter activation at the level of its proximal GCRE1 site (Fig. 8). We speculate that GADD45β promotes or maintains chondrocyte hypertrophy by enhancing C/EBPβ-TAD function to coordinately induce the expression of terminal differentiation-related genes, including Col10a1 and MMP13. Thus, our results provide new insights into the molecular mechanisms underlying the regulation of the Col10a1 promoter that are applicable to other genes associated with chondrocyte terminal differentiation in the embryonic growth plate.
FIGURE 8.
Schematic representation of GADD45β-driven C/EBPβ modulation of Col10a1 during chondrocyte terminal differentiation. GADD45β through the MTK1/MKK3/6/p38 axis may promote chondrocyte terminal differentiation via p38-dependent phosphorylation of the C/EBPβ-TAD4 to promote protein-protein interactions with CBP-p300 or TAF2B/TBP to recruit the polymerase II complex and enhance transcriptional activation of Col10a1. GADD45β-C/EBP-responsive element 1 (GCRE) is the binding site for C/EBPβ that we identified in the Col10a1 promoter (TCATGCAATA). TF, transcription factor.
Acknowledgments
We thank the following individuals for kindly providing plasmids: Dr. Dan A. Liebermann (Temple University, Fels Institute for Cancer Research, Philadelphia, PA), Dr. Klaus von der Mark (University of Erlangen-Nuremberg, Erlangen, Germany), Dr. Peter F. Johnson (NCI-Frederick, National Institutes of Health), Dr. Anton Bennett (Yale University School of Medicine, New Haven, CT), Dr. Roger Davis (University of Massachusetts Medical School, Worcester, MA), Dr. Jiahuai Han (Scripps Research Institute, La Jolla, CA), Dr. Qiping Zheng (Baylor College of Medicine, Houston, TX), Dr. Ze'ev Ronai (Burnham Institute, La Jolla, CA), Dr. Michael S. Kilberg (University Florida College of Medicine, Gainesville, FL), and Dr. Guozhi Xiao (University of Pittsburgh).
This work was supported, in whole or in part, by National Institutes of Health Grant AG022021 (to M. B. G.), CA85467 (to T. A. L.), and GM066882 (to K. B. M.). This work was also supported by Department of Defense Grant PC051217 (to L. F. Z.) and an Arthritis Foundation postdoctoral fellowship (to M. O.).
- MAPK
- mitogen-activated protein kinase
- JNK
- c-Jun N-terminal kinase
- TAD
- transactivation domain
- ERK
- extracellular signal-regulated protein
- siRNA
- small interfering RNA
- siGFP
- siRNA-GFP
- si45β
- siRNA-GADD45β.
REFERENCES
- 1.D'Angelo M., Yan Z., Nooreyazdan M., Pacifici M., Sarment D. S., Billings P. C., Leboy P. S. (2000) J. Cell Biochem. 77, 678–693 [PubMed] [Google Scholar]
- 2.Minina E., Wenzel H. M., Kreschel C., Karp S., Gaffield W., McMahon A. P., Vortkamp A. (2001) Development 128, 4523–4534 [DOI] [PubMed] [Google Scholar]
- 3.Yoon B. S., Pogue R., Ovchinnikov D. A., Yoshii I., Mishina Y., Behringer R. R., Lyons K. M. (2006) Development 133, 4667–4678 [DOI] [PubMed] [Google Scholar]
- 4.Wu X., Shi W., Cao X. (2007) Ann. N.Y. Acad. Sci. 1116, 29–49 [DOI] [PubMed] [Google Scholar]
- 5.Adams S. L., Cohen A. J., Lassová L. (2007) J. Cell Physiol. 213, 635–641 [DOI] [PubMed] [Google Scholar]
- 6.Chen D., Zhao M., Mundy G. R. (2004) Growth Factors 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 7.Retting K. N., Song B., Yoon B. S., Lyons K. M. (2009) Development 136, 1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijiri K., Zerbini L. F., Peng H., Correa R. G., Lu B., Walsh N., Zhao Y., Taniguchi N., Huang X. L., Otu H., Wang H., Wang J. F., Komiya S., Ducy P., Rahman M. U., Flavell R. A., Gravallese E. M., Oettgen P., Libermann T. A., Goldring M. B. (2005) J. Biol. Chem. 280, 38544–38555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman B., Liebermann D. A. (2009) J. Cell Physiol. 218, 26–31 [DOI] [PubMed] [Google Scholar]
- 10.Takekawa M., Saito H. (1998) Cell 95, 521–530 [DOI] [PubMed] [Google Scholar]
- 11.Mita H., Tsutsui J., Takekawa M., Witten E. A., Saito H. (2002) Mol. Cell Biol. 22, 4544–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyake Z., Takekawa M., Ge Q., Saito H. (2007) Mol. Cell Biol. 27, 2765–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papa S., Zazzeroni F., Bubici C., Jayawardena S., Alvarez K., Matsuda S., Nguyen D. U., Pham C. G., Nelsbach A. H., Melis T., De Smaele E., Tang W. J., D'Adamio L., Franzoso G. (2004) Nat. Cell Biol. 6, 146–153 [DOI] [PubMed] [Google Scholar]
- 14.Papa S., Monti S. M., Vitale R. M., Bubici C., Jayawardena S., Alvarez K., De Smaele E., Dathan N., Pedone C., Ruvo M., Franzoso G. (2007) J. Biol. Chem. 282, 19029–19041 [DOI] [PubMed] [Google Scholar]
- 15.Gupta M., Gupta S. K., Hoffman B., Liebermann D. A. (2006) J. Biol. Chem. 281, 17552–17558 [DOI] [PubMed] [Google Scholar]
- 16.Svensson C. I., Inoue T., Hammaker D., Fukushima A., Papa S., Franzoso G., Schett G., Corr M., Boyle D. L., Firestein G. S. (2009) Arthritis Rheum. 60, 3229–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massa P. E., Li X., Hanidu A., Siamas J., Pariali M., Pareja J., Savitt A. G., Catron K. M., Li J., Marcu K. B. (2005) J. Biol. Chem. 280, 14057–14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuevas B. D., Abell A. N., Johnson G. L. (2007) Oncogene 26, 3159–3171 [DOI] [PubMed] [Google Scholar]
- 19.Gerits N., Kostenko S., Moens U. (2007) Transgenic Res. 16, 281–314 [DOI] [PubMed] [Google Scholar]
- 20.Brancho D., Tanaka N., Jaeschke A., Ventura J. J., Kelkar N., Tanaka Y., Kyuuma M., Takeshita T., Flavell R. A., Davis R. J. (2003) Genes Dev. 17, 1969–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobick B. E., Kulyk W. M. (2008) Birth Defects Res. C Embryo Today 84, 131–154 [DOI] [PubMed] [Google Scholar]
- 22.Goldring M. B., Tsuchimochi K., Ijiri K. (2006) J. Cell Biochem. 97, 33–44 [DOI] [PubMed] [Google Scholar]
- 23.Abell A. N., Rivera-Perez J. A., Cuevas B. D., Uhlik M. T., Sather S., Johnson N. L., Minton S. K., Lauder J. M., Winter-Vann A. M., Nakamura K., Magnuson T., Vaillancourt R. R., Heasley L. E., Johnson G. L. (2005) Mol. Cell Biol. 25, 8948–8959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R., Murakami S., Coustry F., Wang Y., de Crombrugghe B. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami S., Balmes G., McKinney S., Zhang Z., Givol D., de Crombrugghe B. (2004) Genes Dev. 18, 290–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provot S., Nachtrab G., Paruch J., Chen A. P., Silva A., Kronenberg H. M. (2008) Mol. Cell Biol. 28, 344–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanton L. A., Sabari S., Sampaio A. V., Underhill T. M., Beier F. (2004) Biochem. J. 378, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanton L. A., Beier F. (2007) Exp. Cell Res. 313, 146–155 [DOI] [PubMed] [Google Scholar]
- 29.Inada M., Yasui T., Nomura S., Miyake S., Deguchi K., Himeno M., Sato M., Yamagiwa H., Kimura T., Yasui N., Ochi T., Endo N., Kitamura Y., Kishimoto T., Komori T. (1999) Dev. Dyn. 214, 279–290 [DOI] [PubMed] [Google Scholar]
- 30.Enomoto H., Enomoto-Iwamoto M., Iwamoto M., Nomura S., Himeno M., Kitamura Y., Kishimoto T., Komori T. (2000) J. Biol. Chem. 275, 8695–8702 [DOI] [PubMed] [Google Scholar]
- 31.Selvamurugan N., Jefcoat S. C., Kwok S., Kowalewski R., Tamasi J. A., Partridge N. C. (2006) J. Cell Biochem. 99, 545–557 [DOI] [PubMed] [Google Scholar]
- 32.Takeda S., Bonnamy J. P., Owen M. J., Ducy P., Karsenty G. (2001) Genes Dev. 15, 467–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Q., Zhou G., Morello R., Chen Y., Garcia-Rojas X., Lee B. (2003) J. Cell Biol. 162, 833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gebhard S., Pöschl E., Riemer S., Bauer E., Hattori T., Eberspaecher H., Zhang Z., Lefebvre V., de Crombrugghe B., von der Mark K. (2004) Matrix Biol. 23, 309–322 [DOI] [PubMed] [Google Scholar]
- 35.Higashikawa A., Saito T., Ikeda T., Kamekura S., Kawamura N., Kan A., Oshima Y., Ohba S., Ogata N., Takeshita K., Nakamura K., Chung U. I., Kawaguchi H. (2009) Arthritis Rheum. 60, 166–178 [DOI] [PubMed] [Google Scholar]
- 36.Karreth F., Hoebertz A., Scheuch H., Eferl R., Wagner E. F. (2004) Development 131, 5717–5725 [DOI] [PubMed] [Google Scholar]
- 37.Hirata M., Kugimiya F., Fukai A., Ohba S., Kawamura N., Ogasawara T., Kawasaki Y., Saito T., Yano F., Ikeda T., Nakamura K., Chung U. I., Kawaguchi H. (2009) PLoS One 4, e4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson P. F. (2005) J. Cell Sci. 118, 2545–2555 [DOI] [PubMed] [Google Scholar]
- 39.Ramji D. P., Foka P. (2002) Biochem. J. 365, 561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebhard S., Hattori T., Bauer E., Bösl M. R., Schlund B., Pöschl E., Adam N., de Crombrugghe B., von der Mark K. (2007) Histochem. Cell Biol. 127, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhoumik A., Takahashi S., Breitweiser W., Shiloh Y., Jones N., Ronai Z. (2005) Mol. Cell 18, 577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Y., Chen H., Siu F., Kilberg M. S. (2003) J. Biol. Chem. 278, 38402–38412 [DOI] [PubMed] [Google Scholar]
- 43.Xiao G., Jiang D., Ge C., Zhao Z., Lai Y., Boules H., Phimphilai M., Yang X., Karsenty G., Franceschi R. T. (2005) J. Biol. Chem. 280, 30689–30696 [DOI] [PubMed] [Google Scholar]
- 44.Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. (1996) Mol. Cell Biol. 16, 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tournier C., Dong C., Turner T. K., Jones S. N., Flavell R. A., Davis R. J. (2001) Genes Dev. 15, 1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. (1994) Cell 76, 1025–1037 [DOI] [PubMed] [Google Scholar]
- 47.Wu J. J., Zhang L., Bennett A. M. (2005) Mol. Cell Biol. 25, 4792–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.New L., Jiang Y., Han J. (2003) Mol. Biol. Cell 14, 2603–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vairapandi M., Azam N., Balliet A. G., Hoffman B., Liebermann D. A. (2000) J. Biol. Chem. 275, 16810–16819 [DOI] [PubMed] [Google Scholar]
- 50.Ijiri K., Zerbini L. F., Peng H., Otu H. H., Tsuchimochi K., Otero M., Dragomir C., Walsh N., Bierbaum B. E., Mattingly D., van Flandern G., Komiya S., Aigner T., Libermann T. A., Goldring M. B. (2008) Arthritis Rheum. 58, 2075–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Q., Keller B., Zhou G., Napierala D., Chen Y., Zabel B., Parker A. E., Lee B. (2009) J. Bone Miner. Res. 24, 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang G., Cui F., Hou N., Cheng X., Zhang J., Wang Y., Jiang N., Gao X., Yang X. (2005) Genesis 42, 33–36 [DOI] [PubMed] [Google Scholar]
- 53.Tominaga H., Maeda S., Hayashi M., Takeda S., Akira S., Komiya S., Nakamura T., Akiyama H., Imamura T. (2008) Mol. Biol. Cell 19, 5373–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chumakov A. M., Silla A., Williamson E. A., Koeffler H. P. (2007) Blood 109, 4209–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller M., Shuman J. D., Sebastian T., Dauter Z., Johnson P. F. (2003) J. Biol. Chem. 278, 15178–15184 [DOI] [PubMed] [Google Scholar]
- 56.Newman J. R., Keating A. E. (2003) Science 300, 2097–2101 [DOI] [PubMed] [Google Scholar]
- 57.Podust L. M., Krezel A. M., Kim Y. (2001) J. Biol. Chem. 276, 505–513 [DOI] [PubMed] [Google Scholar]
- 58.Engelman J. A., Lisanti M. P., Scherer P. E. (1998) J. Biol. Chem. 273, 32111–32120 [DOI] [PubMed] [Google Scholar]
- 59.Ambrosino C., Iwata T., Scafoglio C., Mallardo M., Klein R., Nebreda A. R. (2006) Biochem. J. 396, 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aouadi M., Jager J., Laurent K., Gonzalez T., Cormont M., Binétruy B., Le Marchand-Brustel Y., Tanti J. F., Bost F. (2007) FEBS Lett. 581, 5591–5596 [DOI] [PubMed] [Google Scholar]
- 61.Landschulz W. H., Johnson P. F., McKnight S. L. (1989) Science 243, 1681–1688 [DOI] [PubMed] [Google Scholar]
- 62.Williams S. C., Baer M., Dillner A. J., Johnson P. F. (1995) EMBO J. 14, 3170–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takekawa M., Posas F., Saito H. (1997) EMBO J. 16, 4973–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takekawa M., Tatebayashi K., Itoh F., Adachi M., Imai K., Saito H. (2002) EMBO J. 21, 6473–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seto H., Kamekura S., Miura T., Yamamoto A., Chikuda H., Ogata T., Hiraoka H., Oda H., Nakamura K., Kurosawa H., Chug U. I., Kawaguchi H., Tanaka S. (2004) J. Clin. Invest. 113, 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vale-Cruz D. S., Ma Q., Syme J., LuValle P. A. (2008) Mech. Dev. 125, 843–856 [DOI] [PubMed] [Google Scholar]
- 67.Reimold A. M., Grusby M. J., Kosaras B., Fries J. W., Mori R., Maniwa S., Clauss I. M., Collins T., Sidman R. L., Glimcher M. J., Glimcher L. H. (1996) Nature 379, 262–265 [DOI] [PubMed] [Google Scholar]
- 68.Chang W., Rewari A., Centrella M., McCarthy T. L. (2004) J. Biol. Chem. 279, 42438–42444 [DOI] [PubMed] [Google Scholar]
- 69.Hayashida M., Okazaki K., Fukushi J., Sakamoto A., Iwamoto Y. (2009) Arthritis Rheum. 60, 708–716 [DOI] [PubMed] [Google Scholar]
- 70.Nerlov C., Ziff E. B. (1995) EMBO J. 14, 4318–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pedersen T. A., Kowenz-Leutz E., Leutz A., Nerlov C. (2001) Genes Dev. 15, 3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutherland J. A., Cook A., Bannister A. J., Kouzarides T. (1992) Genes Dev. 6, 1810–1819 [DOI] [PubMed] [Google Scholar]
- 73.Kovács K. A., Steinmann M., Magistretti P. J., Halfon O., Cardinaux J. R. (2003) J. Biol. Chem. 278, 36959–36965 [DOI] [PubMed] [Google Scholar]
- 74.Kowenz-Leutz E., Leutz A. (1999) Mol. Cell 4, 735–743 [DOI] [PubMed] [Google Scholar]
- 75.Mink S., Haenig B., Klempnauer K. H. (1997) Mol. Cell Biol. 17, 6609–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller C., Calkhoven C. F., Sha X., Leutz A. (2004) J. Biol. Chem. 279, 7353–7358 [DOI] [PubMed] [Google Scholar]
- 77.Schwartz C., Beck K., Mink S., Schmolke M., Budde B., Wenning D., Klempnauer K. H. (2003) EMBO J. 22, 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sayed M., Kim S. O., Salh B. S., Issinger O. G., Pelech S. L. (2000) J. Biol. Chem. 275, 16569–16573 [DOI] [PubMed] [Google Scholar]
- 79.Yamaguchi Y., Wada T., Suzuki F., Takagi T., Hasegawa J., Handa H. (1998) Nucleic Acids Res. 26, 3854–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakajima T., Kinoshita S., Sasagawa T., Sasaki K., Naruto M., Kishimoto T., Akira S. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 2207–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowenz-Leutz E., Twamley G., Ansieau S., Leutz A. (1994) Genes Dev. 8, 2781–2791 [DOI] [PubMed] [Google Scholar]