Abstract
Pulmonary collectins, surfactant proteins A (SP-A) and D (SP-D), play important roles in innate immunity of the lung. Legionella pneumophila is a bacterial respiratory pathogen that can replicate within macrophages and causes opportunistic infections. L. pneumophila possesses cytolytic activity, resulting from insertion of pores in the macrophage membrane upon contact. We examined whether pulmonary collectins play protective roles against L. pneumophila infection. SP-A and SP-D bound to L. pneumophila and its lipopolysaccharide (LPS) and inhibited the bacterial growth in a Ca2+-dependent manner. The addition of LPS in the culture blocked the inhibitory effects on L. pneumophila growth by the collectins, indicating the importance of LPS-collectin interaction. When differentiated THP-1 cells were infected with L. pneumophila in the presence of SP-A and SP-D, the number of permeable cells was significantly decreased, indicating that pulmonary collectins inhibit pore-forming activity of L. pneumophila. The number of live bacteria within the macrophages on days 1–4 after infection was significantly decreased when infection was performed in the presence of pulmonary collectins. The phagocytosis experiments with the pH-sensitive dye-labeled bacteria revealed that pulmonary collectins promoted bacterial localization to an acidic compartment. In addition, SP-A and SP-D significantly increased the number of L. pneumophila co-localized with LAMP-1. These results indicate that pulmonary collectins protect macrophages against contact-dependent cytolytic activity of L. pneumophila and suppress intracellular growth of the phagocytosed bacteria. The promotion of lysosomal fusion with Legionella-containing phagosomes constitutes a likely mechanism of L. pneumophila growth suppression by the collectins.
Keywords: Bacteria, Innate Immunity, Lectin, Lung, Macrophage, Phagocytosis, Pulmonary Surfactant, Legionella, Collectin, Surfactant Proteins A and D
Introduction
Pulmonary collectins, hydrophilic surfactant proteins A and D (SP-A3 and SP-D, respectively), belong to the C-type lectin superfamily (1) and have been implicated in the regulation of pulmonary host defense and inflammation (2, 3). Pulmonary surfactant is a mixture of lipids and proteins that covers alveolar surfaces and keeps alveoli from collapsing. Four specific surfactant proteins have been described. Hydrophobic surfactant proteins B and C play critical roles in biophysical functions of surfactant (4). SP-A and SP-D are now well recognized to be important members that constitute innate immunity in the lung (2, 3).
The structure of pulmonary collectins is characterized by four domains that consist of an N terminus involved in interchain disulfide bonding, a collagen-like domain, a coiled coil neck domain, and a carbohydrate recognition domain (CRD) (5). SP-A forms a bouquet-like structure consisting of six trimeric subunits. SP-D exhibits cruciform structure consisting of four trimeric subunits. Engineered genetic defects in the pulmonary collectins of mice have demonstrated the important functions of these collectins in protecting the lung from microbial infections and inflammation (6, 7). We have shown previously that SP-A and SP-D regulate pulmonary inflammation through interaction with pattern recognition receptors, including Toll-like receptors, CD14 and MD-2 (8–11). Pulmonary collectins also directly interact with microbes, including Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, and Mycoplasma pneumoniae, and inhibit their growth (12, 13).
Legionella pneumophila is a bacterial respiratory pathogen that can replicate within human alveolar macrophages (14). L. pneumophila is found ubiquitously in freshwater environments, and its aerosolization causes opportunistic infections, including severe pneumonia, Legionnaire's disease, and a less severe flu-like disease, Pontiac fever (15, 16). Phagocytosed bacteria are generally killed in phagolysosome, where an acidic pH and lysosomal enzymes digest the bacteria. However, L. pneumophila possesses an ability to manipulate host cell processes (17) in order to survive within the macrophage. L. pneumophila establishes an intracellular replicative niche within an endoplasmic reticulum-derived compartment protected from phagolysosomal destruction (18), resulting in its intracellular growth and its pathogenesis. A type IV secretion system is one of the virulence factors indispensable for intracellular growth of L. pneumophila (17, 19).
The purposes of this study were 1) to determine whether pulmonary collectins directly interact with L. pneumophila and affect its growth, 2) to determine whether the collectins modulate the contact-dependent cytolytic activity of L. pneumophila on macrophage, and 3) to investigate whether the collectins affect the intracellular growth of L. pneumophila in macrophages. In this study, we show macrophage-dependent and -independent activities of pulmonary collectins against L. pneumophila. We demonstrate that pulmonary collectins directly bind L. pneumophila and attenuate its growth in a Ca2+-dependent manner. In addition, SP-A and SP-D protect macrophages against pore-forming activity of L. pneumophila and suppress its intracellular growth. The promotion of lysosomal fusion with Legionella-containing phagosomes constitutes a likely mechanism of L. pneumophila growth suppression by the collectins.
EXPERIMENTAL PROCEDURES
Collectins
The 1.13-kb cDNA for human SP-A1 and the 1.181-kb cDNA for human SP-D were inserted into pEE14 plasmid vectors, and recombinant human SP-A and SP-D were expressed in CHO-K1 cells using the glutamine synthetase gene amplification system (20), as described previously (10, 21). CHO-K1 cells expressing SP-A and SP-D were grown in glutamate-free Glasgow minimum essential medium (Sigma) containing 10% dialyzed fetal calf serum and methionine sulfoximine (50 μm for SP-A and 25 μm for SP-D) for gene amplification. For protein purification, the cells were transferred into serum-free EXCELL 302 medium (SAFC Biosciences, Lenexa, KS) and cultured for 3 days. The medium was collected, and three additional harvests were carried out, allowing 24–48 h of culture between harvests. The medium adjusted to pH 7.4 with 1 m Tris buffer (pH 9.0) was finally filtered with a glass fiber filter and applied to a mannose-Sepharose 6B column. The proteins were eluted with 20 mm Tris buffer (pH 7.4) containing 50 mm EDTA for SP-A or with 20 mm Tris buffer (pH 7.4) containing 0.5 m NaCl and 50 mm EDTA for SP-D and dialyzed against 5 mm Tris buffer (pH 7.4) for SP-A or 5 mm Tris buffer (pH 7.4) containing 0.15 m NaCl for SP-D. The physical forms of the recombinant collectins used in this study were observed by electron microscopy with rotary shadow (11). SP-A appeared to form a typical bouquet-like arrangement. SP-D appeared to form a cruciform dodecamer and multimerized oligomer consisting of SP-D molecules associated at their N termini.
Bacteria
L. pneumophila serotype I was clinically isolated and grown on a buffered charcoal yeast extract (BCYE) agar plate (BD BBL) and recovered. The bacteria were washed and suspended with 0.02% (v/v) glycerol in PBS. Bacterial solution was subdivided into many tubes and stored at −80 °C (14). The thawed bacterial stock was used in each experiment. Before the experiment, one of the stocks was thawed and plated on agar plate to check the viability of bacteria and to determine the CFU of the stock. Once the stock was thawed, remaining bacteria were discarded to avoid the freeze-thaw cycle.
Cell Culture
The human monocyte cell line THP-1 was obtained from RIKEN CELL BANK (Tsukuba, Japan) and cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum. The cells were seeded in appropriate culture dishes and treated with 100 ng/ml phorbol 12-myristate 13-acetate (Sigma) for 3 days to induce differentiation toward adherent macrophage-like cells (22).
Primary human monocytes were isolated from fresh peripheral whole blood obtained from healthy volunteers (Hokkaido Red Cross Blood Center, Sapporo, Japan) according to the method of Ferguson et al. (23), as described previously (24). Briefly, mononuclear cells were isolated from heparinized blood on Ficoll gradients, and monocyte-derived macrophages (MDMs) were purified by adherence. The MDMs were cultured for 5 days in the presence of 10% pooled AB+ human serum (Sigma).
Biotinylation of Collectins
Biotinylation of recombinant human SP-A and SP-D was performed using sulfo-N-hydroxysuccinimide-biotin (Pierce) according to the manufacturer's instructions.
Binding of Collectins to L. pneumophila
The biotinylated SP-A or SP-D (100 ng) was incubated at 37 °C for 1 h with or without L. pneumophila (106 CFU) in 50 μl of 5 mm Tris buffer (pH 7.4) containing 0.15 m NaCl and 2% (w/v) BSA in the presence of 2 mm CaCl2 or 5 mm EDTA. After the incubation, the mixture of bacteria and protein was washed three times with PBS containing 0.1% (v/v) Triton X-100 by centrifugation at 5,000 rpm for 5 min. The bacterial pellet obtained by a final centrifugation was suspended in 20 μl of PBS and 5 μl of SDS sample buffer. The suspension was boiled for 5 min and was centrifuged, and the supernatant obtained was subjected to SDS-PAGE. Immunoblotting analysis was next performed to detect SP-A and SP-D co-sedimented with the bacteria. Proteins on the gel were transferred onto a polyvinylidene difluoride membrane (Millipore), which was then incubated with HRP-conjugated streptavidin at room temperature for 30 min. The protein bands were visualized using a chemiluminescence reagent (SuperSignal, Pierce) according to the manufacturer's instructions.
The binding study was also performed to examine the effects of anti-SP-A or anti-SP-D monoclonal antibodies on the collectin binding to L. pneumophila. Monoclonal antibodies were prepared and characterized as described previously (25, 26). The bacterial suspension (106 CFU in 40 μl of PBS/well) was put into the microtiter wells (Immulon 1B, Thermo) and dried under UV irradiation. After washing the wells with PBS, nonspecific binding was blocked with 20 mm Tris buffer (pH 7.4) containing 0.15 m NaCl, 2 mm CaCl2, and 2% (w/v) BSA (blocking buffer). The biotinylated SP-A (5 μg/ml) or SP-D and antibodies (20 μg/ml) in the blocking buffer were preincubated for 60 min, and the protein mixture was then added into the wells, followed by the incubation with the bacteria at 37 °C for 1 h. The wells were washed three times with PBS containing 0.1% (v/v) Triton X-100 and 3% (w/v) skim milk. The wells were then incubated with HRP-labeled streptavidin, and the peroxidase reaction was finally performed using o-phenylenediamine as a substrate. The binding of SP-A or SP-D to L. pneumophila was detected by measuring absorbance at 492 nm.
Isolation of LPS from L. pneumophila
LPS was isolated from L. pneumophila by a method based on that described by Uchida and Mizushima (27). LPS isolated from L. pneumophila was subjected to SDS-PAGE under reducing conditions and stained with silver.
Binding of Collectins to LPS
LPS (500 ng/well) in 20 μl of ethanol was put onto microtiter wells, and the solvent evaporated in the ambient air. After nonspecific binding was blocked with the blocking buffer, the wells were incubated at 37 °C for 3 h with the indicated concentrations of biotinylated SP-A or SP-D in the blocking buffer. The wells were then washed with the blocking buffer and further incubated with HRP-conjugated streptavidin at room temperature for 30 min. The binding of SP-A and SP-D was detected by measuring the absorbance at 492 nm using o-phenylenediamine as a substrate for the peroxidase reaction.
Bacterial Growth in AYE Broth
L. pneumophila (10 or 100 CFU/ml) was incubated in N-(2-acetamide)-2-aminoethanesulfonic acid-buffered yeast extract (AYE) broth in the presence of SP-A (10 μg/ml), SP-D, or BSA at 37 °C for 0–5 days. The growth of L. pneumophila in AYE broth was determined by measuring absorbance at 600 nm (turbidity assay) or by counting the number of colonies on the BCYE agar plate after the bacterial suspension was spread onto the BCYE agar plate and incubated at 37 °C for 4 days (colony assay). In some experiments, 2.5 mm EGTA or 10 μg/ml LPS derived from L. pneumophila was added into AYE broth containing L. pneumophila.
Cytotoxicity Assay
The formation of pores in the macrophage membrane induced upon L. pneumophila contact was examined by ethidium bromide staining as described previously (28). THP-1 cells (5 × 105/well) in a 24-well plate were infected with L. pneumophila at an MOI of 0.1–100 for 20 min at 37 °C in the absence or presence of 20 μg/ml pulmonary collectin. After the incubation, the adherent cells were washed with PBS and stained with 25 μg/ml ethidium bromide and 5 μg/ml acridine orange. All cells were stained with acridine orange, whereas an intact macrophage membrane excluded ethidium bromide. Pores formed in the membrane upon L. pneumophila contact allow diffusion of ethidium bromide, where it stains chromosomal DNA. The cells were observed by fluorescence microscopy, and pore-forming activity was determined as the percentage of macrophages stained with ethidium bromide in total macrophages counted.
Intracellular Growth of L. pneumophila
THP-1 cells or MDMs (5 × 104/well) in a 96-well plate were infected for 20 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of SP-A (10 μg/ml), SP-D, or BSA. The cells were washed with PBS five times and incubated in RPMI containing 10% fetal calf serum for 0–4 days at 37 °C in a 5% CO2 atmosphere. After the incubation, the cells were lysed with H2O, plated onto the BCYE agar plate, and incubated at 37 °C for 4 days. Following this, the number of colonies on the agar plate was counted.
Analysis of Phagocytosed L. pneumophila
The intracellular fate of phagocytosed L. pneumophila was analyzed using pHrodoTM (Invitrogen) labeling. The pH-sensitive, rhodamine-based pHrodoTM dye is a specific sensor of phagocytic events; it is nonfluorescent at neutral pH and bright red in acidic environments, such as phagolysosomes. For pHrodoTM labeling, L. pneumophila (4 × 109 CFU) in 1.5 ml of 0.1 m NaHCO3 was incubated for 45 min in the dark with 0.5 mg of amine-reactive pHrodoTM (succinimidyl ester form) in 75 μl of DMSO. After the incubation, 1.5 ml of Hanks' balanced salt solution (Sigma) was added into the mixture to stop the reaction, and the mixture was centrifuged at 14,000 rpm for 60 s. The bacterial pellet obtained was washed three times with Hanks' balanced salt solution.
THP-1 cells (5 × 105/well) in a 24-well plate were infected with pHrodoTM-labeled L. pneumophila at an MOI of 1.0 for 10–60 min in the presence of SP-A (10 μg/ml), SP-D, or BSA. After the infection, the cells were washed and examined by fluorescence microscopy. The results were expressed as the percentage of macrophages containing red-fluorescent bacteria in total macrophages counted. At least 100 macrophages were counted in each specimen.
Immunocytochemistry
THP-1 cells (104/well) were differentiated with phorbol 12-myristate 13-acetate on collagen-coated glass base dishes (35 mm; Iwaki, Asahi Techno Glass, Chiba, Japan) for 3 days. The cells were infected for 30 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of SP-A (10 μg/ml), SP-D, or BSA. After the incubation, the cells were washed three times with PBS, fixed with 4% (w/v) paraformaldehyde at room temperature for 20 min, and then permeabilized with 0.2% (v/v) Triton X-100 for 2 min. After rinsing with PBS, the cells were incubated at room temperature for 60 min with anti-L. pneumophila monoclonal antibody (Affinity BioReagents) and either anti-SP-A, anti-SP-D, or anti-LAMP-1 (lysosome-associated membrane protein-1) antibody (Sigma), followed by the incubation with Alexa594 (red)-conjugated anti-mouse IgG and Alexa488 (green)-conjugated anti-rabbit IgG (Molecular Probes, Inc., Eugene, OR) at room temperature for 60 min. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma). The specimens were examined and photographed with an Olympus IX 71 inverted microscope (Olympus Co., Tokyo, Japan) and confocal laser-scanning microscope Zeiss LSM 510 (Carl Zeiss, Jena, Japan).
RESULTS
SP-A and SP-D Interact with L. pneumophila through the Carbohydrate Recognition Domain
We first examined whether pulmonary collectins interact with L. pneumophila. The biotinylated SP-A and L. pneumophila were incubated in the presence of 2 mm Ca2+, and the bacteria were sedimented. The protein co-precipitated with the bacteria was examined by immunoblotting. SP-A was detected in the bacterial pellet (Fig. 1A, Legionella +, lane 2), indicating that SP-A directly binds to L. pneumophila. When the protein and the bacteria were incubated in the presence of 5 mm EDTA instead of 2 mm Ca2+, no protein band was detected in the pellet fraction (Fig. 1A, Legionella +, lane 3), demonstrating that the binding of SP-A to L. pneumophila is Ca2+-dependent. SP-D was also co-sedimented with L. pneumophila in the presence of Ca2+, but no protein band of SP-D was detected in the presence of EDTA (Fig. 1B). Taken together, these results indicate that SP-A and SP-D bind L. pneumophila in a Ca2+-dependent manner.
FIGURE 1.
SP-A and SP-D bind to L. pneumophila through the carbohydrate recognition domain. A and B, biotinylated SP-A or SP-D (100 ng) was incubated at 37 °C for 60 min with or without L. pneumophila (106 CFU) in the presence of 2 mm Ca2+ or 5 mm EDTA. After the incubation, the bacterial pellet was obtained by centrifugation, and immunoblotting analysis was performed to detect SP-A or SP-D co-sedimented with the bacteria, as described under “Experimental Procedures.” Lane 1, control SP-A or SP-D alone; lane 2, SP-A or SP-D incubated in the presence of Ca2+; lane 3, SP-A or SP-D incubated in the presence of EDTA. C and D, effect of anti-collectin monoclonal antibodies on the binding to L. pneumophila (106 CFU/well) coated onto microtiter wells. The biotinylated SP-A or SP-D at 5 μg/ml and antibodies at 20 μg/ml were preincubated and then further incubated with the bacteria. The binding of SP-A and SP-D to the bacteria was finally detected by the incubation with HRP-labeled streptavidin, as described under “Experimental Procedures.” The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with control IgG. The insets indicate immunoblotting analysis of the collectins co-sedimented with the bacteria. pAb, polyclonal antibody.
The effects of anti-SP-A monoclonal antibodies on the SP-A binding to L. pneumophila coated onto microtiter wells were examined. Antibodies PE10 and PC6 blocked the binding of SP-A to L. pneumophila (Fig. 1C). Inclusion of antibodies PE10 and PC6 also inhibited co-precipitation of SP-A with the bacteria (Fig. 1C, inset). This indicates that SP-A interacts with L. pneumophila through the CRD of SP-A because the epitopes for antibodies PE10 and PC6 are located at the CRD (29). Anti-SP-D monoclonal antibodies 7A10 and 6B2 but not 7C6 blocked the binding of SP-D to L. pneumophila (Fig. 1D). Because the epitopes for antibodies 7A10 and 6B2 and antibody 7C6 are located at the CRD and the neck domain, respectively (30), the results indicate that SP-D also interacts with L. pneumophila through the CRD.
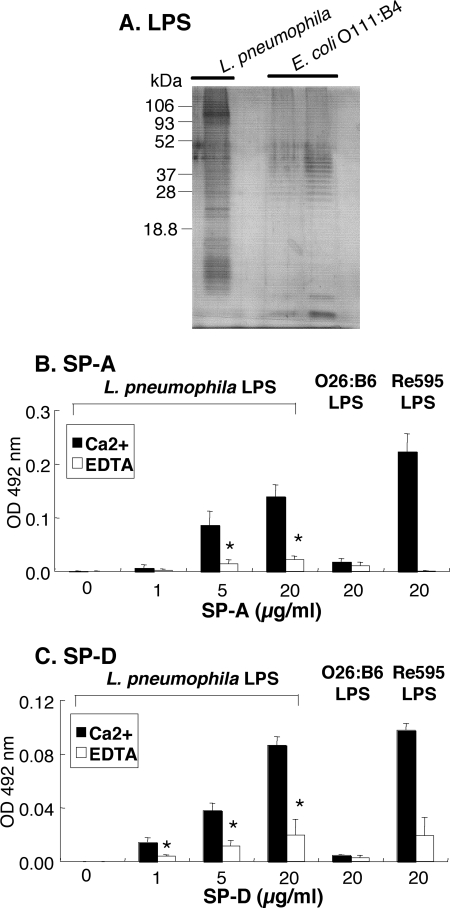
Pulmonary Collectins Bind to LPS Derived from L. pneumophila
We next isolated LPS derived from L. pneumophila to determine which components of L. pneumophila the collectins bind to. The LPS fraction was analyzed by SDS-PAGE and visualized by silver staining (Fig. 2A). The LPS exhibited ladder-like bands containing polysaccharide chains. The binding study with pulmonary collectins was performed using LPS coated onto microtiter wells. SP-A and SP-D bound to the solid phase LPS in a concentration-dependent manner in the presence of 2 mm Ca2+ (Fig. 2, B and C). O26:B6 LPS and Re595 LPS were used as controls. Both collectins bind to Re595 LPS but not to O26:B6 LPS as described previously (9, 11). The addition of 5 mm EDTA instead of 2 mm Ca2+ significantly attenuated their binding to LPS, indicating that the binding of pulmonary collectins to L. pneumophila LPS is Ca2+-dependent.
FIGURE 2.
SP-A and SP-D bind to Legionella-derived LPS. A, LPS (500 ng) isolated from L. pneumophila was subjected to SDS-PAGE and stained with silver. E. coli O111:B4 LPS was used as control (left lane, 100 ng; right lane, 500 ng). B and C, the indicated concentrations of SP-A or SP-D were incubated with LPS isolated from L. pneumophila (500 ng/well), O26:B6 LPS, or Re595 LPS in the presence of 2 mm CaCl2 (filled bar) or 5 mm EDTA (open bar), followed by the incubation with HRP-labeled streptavidin, as described under “Experimental Procedures.” The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with the value in the presence of Ca2+.
SP-A and SP-D Inhibit the Growth of L. pneumophila in a Ca2+-dependent Manner
We examined whether pulmonary collectins affect the growth of L. pneumophila in AYE broth, using two different assays. First, the bacterial growth was determined by measuring turbidity (absorbance at 600 nm) of the medium. When 100 CFU/ml L. pneumophila was cultured for 1–5 days with 10 μg/ml SP-A or BSA in AYE broth, which contains 1.44 mm Ca2+, the turbidity on days 3 and 5 in the SP-A-containing bacterial culture was significantly reduced compared with that in the BSA-containing culture (Fig. 3A). When the culture was started at 10 CFU/ml L. pneumophila, the turbidity was not increased throughout the observation in the presence of SP-A, indicating that SP-A inhibits the growth of L. pneumophila. SP-D also significantly inhibited the increase of the turbidity in the bacterial culture (Fig. 3B). When the bacterial growth was also determined by the colony assay, pulmonary collectins significantly inhibited the growth of L. pneumophila on days 1, 2, and 5 (Fig. 3, C and D).
FIGURE 3.
SP-A and SP-D attenuate the growth of L. pneumophila. A–D, L. pneumophila (10 and 100 CFU/ml) was cultured at 37 °C in AYE broth in the presence of 20 μg/ml SP-A (A and C, open squares), SP-D (B and D, open squares), or BSA (filled diamonds) for 0–5 days. The growth of the bacteria was determined by measuring turbidity at 600 nm (A and B) or by counting the colony number on the BCYE agar plate after the bacterial suspension was spread (C and D), as described under “Experimental Procedures.” E, L. pneumophila (100 CFU/ml) was cultured for 5 days at 37 °C in AYE broth containing 1.44 mm CaCl2 (filled bar) or 2.5 mm EGTA (open bar) in the presence of 20 μg/ml SP-A, SP-D, or BSA. F, L. pneumophila (100 CFU/ml) was cultured for 5 days at 37 °C in AYE broth containing 5 μg/ml SP-A, SP-D, or BSA in the presence (open bar) or the absence (filled bar) of 10 μg/ml Legionella-derived LPS. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA (A–D), with Ca2+ (E), or with LPS (−) (F).
The effects of EGTA on the inhibition of the bacterial growth by the collectins were examined. Inclusion of 2.5 mm EGTA blocked the inhibitor activities of the collectins on L. pneumophila growth (Fig. 3E), whereas EGTA did not exhibit a significant effect on the bacterial growth in the presence of BSA. The results indicate that the growth inhibition by the collectins is Ca2+-dependent.
The role of LPS in collectin-mediated growth inhibition was next examined. The addition of L. pneumophila-derived LPS in the culture blocked the inhibitory effects on L. pneumophila growth by the collectins (Fig. 3F). Taken together with the results of the LPS binding, these results indicate the importance of LPS-collectin interaction in the inhibition of bacterial growth.
Pulmonary Collectins Protect Macrophages against Contact-dependent Cytolytic Activity of L. pneumophila
L. pneumophila possesses cytolytic activity, resulting from the insertion of pores in the macrophage membrane upon contact (31). THP-1 cells were infected with L. pneumophila at an MOI of 100 for 20 min in the presence of BSA, SP-A, or SP-D, and the cells were stained with ethidium bromide and acridine orange. The permeabilized macrophages were stained with ethidium bromide. The number of the macrophages stained with ethidium bromide (permeable cells) was clearly decreased in the presence of SP-A or SP-D when compared with that in the presence of BSA (Fig. 4A). The percentages of permeable cells infected at an MOI of 10 and 100 were significantly lower in the presence of pulmonary collectins than that in the presence of BSA (Fig. 4B). These results indicate that pulmonary collectins inhibit pore-forming activity of L. pneumophila.
FIGURE 4.
Pulmonary collectins inhibit pore-forming activity of L. pneumophila in macrophages. THP-1 cells (5 × 105/well) were infected with L. pneumophila at an MOI of 100 (A) or 0.1–100 (B) for 20 min at 37 °C in the presence of 20 μg/ml SP-A (open diamonds), SP-D (open circles), or BSA (filled triangles). After infection, the adherent cells were washed, stained with ethidium bromide and acridine orange, and observed by fluorescence microscopy, as described under “Experimental Procedures.” The results in B are expressed as the percentage of permeable cells (ethidium bromide-stained) in total cells counted. At least 100 macrophages were counted in each specimen. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA. The filled square indicates the percentage of permeable cells without L. pneumophila.
Pulmonary Collectins Suppress Intracellular Growth of L. pneumophila
After THP-1 cells were infected with L. pneumophila, phagocytosed L. pneumophila was stained with anti-L. pneumophila antibody. SP-A and SP-D significantly increased the phagocytosis of L. pneumophila when the percentages of Legionella-containing cells were calculated (Fig. 5A). We next examined the number of live bacteria after they were phagocytosed by THP-1 cells and MDMs. When the number of live bacteria on day 0, immediately after the infection, was determined by colony assay, the colony number was significantly greater in the presence of SP-A or SP-D than that in the presence of BSA (Fig. 5B), indicating that more L. pneumophila are alive inside the cell immediately after the bacteria are phagocytosed in the presence of pulmonary collectins than in the presence of BSA. These results are consistent with those obtained by counting the number of macrophages containing L. pneumophila (Fig. 5A). However, further culture for 1–4 days after the infection in the presence of the collectins resulted in a significant reduction of live L. pneumophila compared with that in the presence of BSA (Fig. 5C). When the colony numbers were expressed as percentages of control CFU (BSA) on each day in comparison with that in the BSA group (Fig. 5D), the collectin groups on days 1–4 exhibited only 12–35% as many colonies as found with the BSA group, although the collectin groups on day 0 showed higher percentages than the BSA group. These results demonstrate that SP-A and SP-D attenuate intracellular growth of L. pneumophila in macrophages.
FIGURE 5.
Pulmonary collectins attenuate intracellular growth of L. pneumophila. A, uptake of L. pneumophila. THP-1 cells (5 × 104/well) were infected for 30 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of 10 μg/ml SP-A or SP-D or BSA. After the infection, the cells were washed and fixed. The cells were incubated with anti-L. pneumophila antibody, as described under “Experimental Procedures.” The specimens were examined by an Olympus IX 71 inverted microscope. The results are expressed as the percentage of macrophages that contain L. pneumophila in total THP-1 cells counted. At least 100 macrophages were counted in each specimen. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA. B, live bacteria immediately after infection. THP-1 cells or MDMs (5 × 104/well) were infected for 20 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of 10 μg/ml SP-A, SP-D, or BSA. The cells were washed, lysed with H2O, and plated onto a BCYE agar plate, and the number of colonies was counted, as described under “Experimental Procedures.” The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA. C and D, intracellular growth. THP-1 cells and MDMs were infected for 20 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of 10 μg/ml SP-A (open triangles, gray bars), SP-D (open squares, black bars), or BSA (filled diamonds, white bars). The cells were washed and further cultured for 0–4 days at 37 °C in a 5% CO2 atmosphere. After the incubation, the cells were lysed, and the number of live bacteria was determined, as described above. The CFU determined on days 0–4 are presented in C. In D, the colony number on each day is also expressed as the percentage of control CFU (BSA) in comparison with that in the BSA group. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA.
We next examined whether pulmonary collectins are associated with L. pneumophila after they are taken up in macrophages. THP-1 cells were infected for 30 min with L. pneumophila in the presence of pulmonary collectins, and the bacteria and the collectins were stained with each antibody. Observation with fluorescence microscopy showed that pulmonary collectins were co-localized with L. pneumophila in THP-1 cells (Fig. 6A, SP-A and SP-D). Antibody to SP-A or SP-D did not recognize any intracellular compartments in macrophages that were infected with L. pneumophila in the presence of BSA (Fig. 6A, BSA). When the cells were observed by confocal microscopy, intracellular L. pneumophila was clearly co-localized with SP-A or SP-D inside the cell (Fig. 6B).
FIGURE 6.
Pulmonary collectins are co-localized with L. pneumophila in THP-1 cells. THP-1 cells (5 × 104 for A, 104 for B) were infected for 30 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of 10 μg/ml SP-A, SP-D, or BSA. After the infection, the cells were washed, fixed, and incubated with anti-L. pneumophila antibody and either anti-SP-A antibody or anti-SP-D antibody, followed by incubation with Alexa594- and Alexa488-conjugated secondary antibody, as described under “Experimental Procedures.” The cell nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. The specimens were examined by an Olympus IX 71 inverted microscope (A) and a confocal laser-scanning microscope (B). Surfaces of the macrophages are labeled with white dotted lines.
The pH-sensitive, rhodamine-based pHrodoTM dye is a specific sensor of phagocytic events. It is nonfluorescent at neutral pH but bright red in acidic environments. We examined the phagocytosis of L. pneumophila labeled with pHrodoTM in THP-1 cells. After a 30-min infection, the macrophages were observed by fluorescence microscopy. Infection with SP-A or SP-D clearly increased the number of bright red macrophages (Fig. 7A). The percentages of positive cells were significantly increased in the infection with pulmonary collectins compared with that with BSA (Fig. 7B). The increase of the positive cell number was time-dependent and reached a plateau at 50–60 min. Infection with SP-A or SP-D gave an approximately 3-fold increase of macrophages containing red-fluorescent bacteria compared with that with BSA. These results indicate that SP-A and SP-D stimulate the localization of L. pneumophila to the acidic compartment in the cell.
FIGURE 7.
Pulmonary collectins promote localization of L. pneumophila to an acidic compartment. THP-1 cells (5 × 105/well) in a 24-well plate were infected with pHrodoTM-labeled L. pneumophila at an MOI of 1.0 for 30 min (A) or for 10–60 min (B) in the presence of 10 μg/ml SP-A (open triangles), SP-D (open squares), or BSA (filled diamonds). After the infection, the cells were washed and examined by fluorescence microscopy, as described under “Experimental Procedures.” The results are expressed as the percentage of macrophages containing red-fluorescent bacteria in total macrophages counted. At least 100 macrophages were counted in each specimen. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA.
We next examined whether pulmonary collectins increase the number of L. pneumophila co-localized with lysosome using anti-L. pneumophila antibody and anti-LAMP-1 antibody. Co-localization of L. pneumophila with LAMP-1 was evident in the collectin-treated groups (Fig. 8A). Although the collectin-treated cells contained a number of L. pneumophila, most of them were merged with LAMP-1, indicating that SP-A and SP-D significantly increase LAMP-1-positive L. pneumophila. In contrast, the BSA-treated cell contained many bacteria that were not merged with LAMP-1. When L. pneumophila merged with LAMP-1 inside the cells was counted, its percentage (the number of L. pneumophila merged with LAMP-1/total number of L. pneumophila × 100) was significantly higher in the collectin-treated groups than that in the BSA-treated group (Fig. 8B). Approximately 80% of L. pneumophila inside the cells were merged with LAMP-1 in the collectin-treated cells, whereas only 8.5% of L. pneumophila were merged with LAMP-1 in the control cells. This suggests that L. pneumophila replicates intracellularly in the control cells, with the consequence that LAMP-1-positive bacteria were decreased, and is consistent with the results showing that the number of live bacteria in the BSA-treated cells increased on day 1 (Fig. 5, C and D). Taken together, these results indicate that SP-A and SP-D increase the number of L. pneumophila co-localized with lysosome.
FIGURE 8.
Pulmonary collectins increase the number of L. pneumophila co-localized with LAMP-1 in THP-1 cells. THP-1 cells (5 × 104) were infected for 30 min at 37 °C with L. pneumophila at an MOI of 1.0 in the presence of 10 μg/ml SP-A, SP-D, or BSA. Twenty-four hours after the infection, the cells were washed, fixed, and incubated with anti-L. pneumophila antibody and anti-LAMP-1 antibody, followed by the incubation with Alexa594- and Alexa488-conjugated secondary antibody, as described under “Experimental Procedures.” The cell nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride. The specimens were examined by an Olympus IX 71 inverted microscope (A) (magnification, ×800). B, the percentage of L. pneumophila merged with LAMP-1 in total bacteria counted. At least 100 bacteria were counted in each specimen. The data shown are the means ± S.D. from three separate experiments. *, p < 0.01 when compared with BSA.
DISCUSSION
Pulmonary collectins have been shown to interact directly with a broad spectrum of microbes, including bacteria, fungi, and viruses, and to inhibit their growth (12, 13, 32). In addition, the collectins modulate macrophage functions. SP-A and SP-D regulate inflammation by interaction with cell surface receptors, including Toll-like receptors, CD14, signal inhibitory regulatory protein α, and calreticulin/CD91 (8–11, 33). Pulmonary collectins enhance phagocytosis of bacteria by macrophages through opsonic and non-opsonic effects, which may contribute to bacterial clearance in the lung. SP-A binds to bacteria, including Staphylococcus aureus, K. pneumoniae, Streptococcus pneumoniae, and Haemophilus influenzae and enhances their phagocytosis by macrophages (34–37). The non-opsonic effect includes the direct interaction of the collectins with macrophages that results in the increased cell surface localization of phagocytic receptors, mannose receptor, and scavenger receptor A (24, 38, 39). In this study, we have shown macrophage-dependent and -independent activities of pulmonary collectins against L. pneumophila.
Pulmonary collectins inhibit the growth of Gram-negative bacteria, including E. coli, K. pneumoniae, and E. aerogenes by increasing membrane permeability (13). L. pneumophila is also a type of Gram-negative bacteria, and this study shows that SP-A and SP-D inhibit the growth of this bacterium in AYE broth (see Fig. 3). The collectins do not aggregate L. pneumophila (data not shown). Consistently, the inhibition of E. coli growth is independent of the bacterial aggregation caused by the collectins (13). The L. pneumophila-derived LPS blocks the inhibitory effect of the collectins on L. pneumophila growth (see Fig. 3F) as a previous study has shown that growth inhibition of E. coli by the collectins is blocked by J5 LPS (13). Because LPS derived from L. pneumophila (see Fig. 2) and from E. coli (13) is a ligand for pulmonary collectins, these results indicate that interaction of the collectins with LPS on the bacteria is important for the bacterial growth inhibition. This is consistent with the results showing that the activities of SP-A and SP-D to bind L. pneumophila and LPS derived from L. pneumophila and to inhibit its growth are Ca2+-dependent (see Figs. 1 (A and B), 2 (B and C), and 3E).
L. pneumophila possesses cytolytic activity that results from the insertion of pores in the macrophage membrane upon contact. The Legionella-containing phagosome bypasses the normal endocytic pathway. A type IV secretion system encoded by dot (defective in organelle trafficking)/icm (intracellular multiplication) genes is essential to this process (17). This study demonstrates that pulmonary collectins inhibit the pore-forming activity of L. pneumophila in macrophages (see Fig. 4). A previous study (40) has revealed that L. pneumophila with the dotA mutation, which does not possess pore-forming activity (31), is co-localized well with LAMP-1 and does not grow inside macrophage, whereas the majority of wild-type L. pneumophila phagosomes do not acquire LAMP-1. Thus, the protection of macrophages against membrane injury induced by the dot/icm system may be a possible mechanism by which pulmonary collectins inhibit intracellular growth of L. pneumophila. However, the pore-forming activity is not sufficient for L. pneumophila phagosome trafficking and intracellular growth (31). L. pneumophila lacking the icmW gene retains pore-forming activity but is co-localized with LAMP-1, and its intracellular growth is attenuated. It remains to be elucidated whether pulmonary collectins affect the products of dot/icm genes.
When THP-1 cells are infected with L. pneumophila in the presence of pulmonary collectins, the bacteria are co-localized with the collectins (see Fig. 6), indicating that SP-A and SP-D bind L. pneumophila and exist as a collectin-L. pneumophila complex inside the cell. Preincubation of the collectins with macrophages and washing of the cells before the addition of L. pneumophila result in the failure to inhibit intracellular growth of the bacteria (data not shown), suggesting that opsonization of the bacteria with the proteins and their interactions are critical for the collectin-mediated suppression of intracellular L. pneumophila growth. Consistent with this idea, precoating L. pneumophila with SP-A and SP-D, followed by removal of unbound collectins, is sufficient to protect macrophages from pore-forming activity of L. pneumophila and to modulate the routing to an acidic lysosomal compartment. When differentiated THP-1 cells were infected with the bacteria precoated with SP-A, SP-D, and BSA, the percentages of permeabilized macrophages were 13.3 ± 1.9% (mean ± S.D., n = 3), 15.0 ± 3.5%, and 23.5 ± 2.6%, respectively. The phagocytosis experiments with L. pneumophila labeled with pHrodoTM showed that infection with the bacteria precoated with SP-A and SP-D significantly increased the proportion of bright red macrophages (23.2 ± 4.9% (mean ± S.D., n = 3) and 21.7 ± 2.9%, respectively) when compared with BSA (8.4 ± 3.9%).
L. pneumophila escapes from lysosome-mediated destruction and survives within macrophages (17). The phagocytosis experiments with the pH-sensitive, rhodamine-based pHrodoTM dye-labeled L. pneumophila have revealed that pulmonary collectins promote the localization of L. pneumophila to an acidic compartment after they are taken up into macrophages (see Fig. 7). Because the collectins are co-localized with L. pneumophila inside the cell (see Fig. 6), it is possible to assume that pulmonary collectins bring L. pneumophila to the acidic compartment (i.e. lysosomes) in the cell. However, this raises a possibility that the increased number of macrophages containing red-fluorescent bacteria is due to the increased phagocytosis of bacteria by the collectins because immunostaining of macrophages phagocyting L. pneumophila with anti-L. pneumophila antibody shows that SP-A and SP-D increase the percentage of Legionella-containing macrophages (see Fig. 5A). Thus, we investigated whether L. pneumophila is co-localized with LAMP-1 after it is taken up by macrophages in the presence of pulmonary collectins. The results clearly demonstrate that SP-A and SP-D increase the number of L. pneumophila co-localized with LAMP-1 (see Fig. 8). This excludes the possibility that the increase of macrophages containing red-fluorescent bacteria is not solely due to the increased phagocytosis. Thus, it is likely that pulmonary collectins promote the lysosomal fusion with Legionella-containing phagosomes, and intracellular growth of L. pneumophila is consequently inhibited. Consistent with this idea, merely 1 day of culture exhibits significant reduction of live bacteria in macrophages infected with pulmonary collectins, although more bacteria are alive in the cells immediately after the infection with the collectins (see Fig. 5).
Approximately 20% of bacteria are not merged with LAMP-1 when infected with pulmonary collectins (see Fig. 8B), suggesting that these bacteria may not be killed in phagolysosomes but may be directed to the compartment protected from phagolysosomal destruction. Actually, a small number of bacteria are still alive even on day 4, although the infection with the collectins significantly reduced the number of live bacteria inside the cells (see Fig. 5, C and D). This would allow for delayed growth of the bacteria. On the other hand, the collectins also inhibit the bacterial growth outside the macrophages before they are taken up by the cells (see Fig. 3). Pulmonary collectins may play important roles at the initial step of host defense against L. pneumophila infection. Thus, it is possible to assume that pulmonary collectins antagonize Legionella infection both before and after the bacteria are phagocytosed by macrophages in order to decrease the number of bacteria infected into the lung and to prevent the expansion of the infection.
SP-A and SP-D exist as surfactant components in the alveolar hypophase, which is the epithelial lining fluid of the alveolus. Because the alveolar hypophase cannot be directly measured, it is difficult to determine the concentrations of pulmonary collectins in vivo. Nevertheless, their concentrations can be estimated based on the recovery of the proteins in the bronchoalveolar lavage fluids and the extrapolated volume (100–1000 μl/lung) of the epithelial lining fluid of the alveolus (41, 42). The calculated concentrations of SP-A in the alveolar hypophase range from 180 to 1800 μg/ml, and the SP-D concentration was calculated as ∼63 μg/ml (43–47). The levels of pulmonary collectins appear to vary in diseased states, indicating complicated responses under conditions of physiological stress. Intratracheal administration of LPS increases the levels of SP-A and SP-D in rats (48). The SP-A concentration in the lavage fluid increases in AIDS-related pneumonia (49), whereas it decreases in some bacterial pneumonia (43). Although one cannot yet determine the exact concentrations of pulmonary collectins in the hypophase of healthy and diseased states, the collectin concentrations used in this study are within the best estimates of the physiological ranges.
In this study, we used purified SP-A and SP-D. Because these collectins are constituents of pulmonary surfactant, it was determined if purified surfactant executes the same functions as purified collectins. Pulmonary surfactant was purified from bronchoalveolar lavage fluids of rats by sodium bromide gradient centrifugation. When differentiated THP-1 cells were infected with L. pneumophila in the presence of purified surfactant (50 μg of protein/ml) and BSA, the percentages of permeabilized macrophages were 10.9 ± 1.7% (mean ± S.D., n = 3) and 27.7 ± 7.6%, respectively. In addition, the phagocytosis experiments with L. pneumophila labeled with pHrodoTM showed that infection with purified surfactant significantly increased the proportion of bright red macrophages (28.3 ± 4.3%; mean ± S.D., n = 3) when compared with that with BSA (12.2 ± 1.8%). These results indicate that purified surfactant executes the same functions as purified SP-A and SP-D, with respect to L. pneumophila-induced pore-forming activity and modulation of the routing to an acidic lysosomal compartment.
In conclusion, pulmonary collectins directly interact with L. pneumophila through the CRD and inhibit the bacterial growth in a Ca2+-dependent manner. In addition, SP-A and SP-D protect macrophages against cytolytic activity of L. pneumophila upon membrane contact. Pulmonary collectins attenuate intracellular growth of L. pneumophila in macrophages. Infection with the collectins promotes co-localization of L. pneumophila with LAMP-1 and localization to an acidic compartment. The promotion of lysosomal fusion with Legionella-containing phagosomes constitutes a likely mechanism of L. pneumophila growth suppression by the collectins.
Acknowledgment
Fresh peripheral whole blood was a generous gift from the Hokkaido Red Cross Blood Center (Sapporo, Japan).
This work was supported by Grants-in-aid for Scientific Research 20790574 (to S. A.) and 20390232 and 19041062 (to Y. K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
- SP-A
- surfactant protein A
- SP-D
- surfactant protein D
- LPS
- lipopolysaccharide
- CRD
- carbohydrate recognition domain
- AYE
- N-(2-acetamide)-2-aminoethanesulfonic acid-buffered yeast extract
- BCYE
- buffered charcoal yeast extract
- MDM
- monocyte-derived macrophage
- PBS
- phosphate-buffered saline
- BSA
- bovine serum albumin
- CFU
- colony-forming units
- HRP
- horseradish peroxidase
- MOI
- multiplicity of infection
- LAMP-1
- lysosome-associated membrane protein-1.
REFERENCES
- 1.Day A. J. (1994) Biochem. Soc. Trans. 22, 83–88 [DOI] [PubMed] [Google Scholar]
- 2.Kuroki Y., Takahashi M., Nishitani C. (2007) Cell. Microbiol. 9, 1871–1879 [DOI] [PubMed] [Google Scholar]
- 3.Wright J. R. (2005) Nat. Rev. Immunol. 5, 58–68 [DOI] [PubMed] [Google Scholar]
- 4.Whitsett J. A., Weaver T. E. (2002) N. Engl. J. Med. 347, 2141–2148 [DOI] [PubMed] [Google Scholar]
- 5.Kuroki Y., Voelker D. R. (1994) J. Biol. Chem. 269, 25943–25946 [PubMed] [Google Scholar]
- 6.LeVine A. M., Bruno M. D., Huelsman K. M., Ross G. F., Whitsett J. A., Korfhagen T. R. (1997) J. Immunol. 158, 4336–4340 [PubMed] [Google Scholar]
- 7.LeVine A. M., Whitsett J. A., Gwozdz J. A., Richardson T. R., Fisher J. H., Burhans M. S., Korfhagen T. R. (2000) J. Immunol. 165, 3934–3940 [DOI] [PubMed] [Google Scholar]
- 8.Murakami S., Iwaki D., Mitsuzawa H., Sano H., Takahashi H., Voelker D. R., Akino T., Kuroki Y. (2002) J. Biol. Chem. 277, 6830–6837 [DOI] [PubMed] [Google Scholar]
- 9.Sano H., Sohma H., Muta T., Nomura S., Voelker D. R., Kuroki Y. (1999) J. Immunol. 163, 387–395 [PubMed] [Google Scholar]
- 10.Yamada C., Sano H., Shimizu T., Mitsuzawa H., Nishitani C., Himi T., Kuroki Y. (2006) J. Biol. Chem. 281, 21771–21780 [DOI] [PubMed] [Google Scholar]
- 11.Yamazoe M., Nishitani C., Takahashi M., Katoh T., Ariki S., Shimizu T., Mitsuzawa H., Sawada K., Voelker D. R., Takahashi H., Kuroki Y. (2008) J. Biol. Chem. 283, 35878–35888 [DOI] [PubMed] [Google Scholar]
- 12.Piboonpocanun S., Chiba H., Mitsuzawa H., Martin W., Murphy R. C., Harbeck R. J., Voelker D. R. (2005) J. Biol. Chem. 280, 9–17 [DOI] [PubMed] [Google Scholar]
- 13.Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J. H., Kim K. S., McCormack F. X. (2003) J. Clin. Invest. 111, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz M. A., Silverstein S. C. (1980) J. Clin. Invest. 66, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann A. F., McDade J. E., Patton C. M., Bennett J. V., Skaliy P., Feeley J. C., Anderson D. C., Potter M. E., Newhouse V. F., Gregg M. B., Brachman P. S. (1981) Am. J. Epidemiol. 114, 337–347 [DOI] [PubMed] [Google Scholar]
- 16.McDade J. E., Shepard C. C., Fraser D. W., Tsai T. R., Redus M. A., Dowdle W. R. (1977) N. Engl. J. Med. 297, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 17.Shin S., Roy C. R. (2008) Cell. Microbiol. 10, 1209–1220 [DOI] [PubMed] [Google Scholar]
- 18.Horwitz M. A. (1983) J. Exp. Med. 158, 2108–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marra A., Blander S. J., Horwitz M. A., Shuman H. A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ausbel F. M., Brent R., Kingston R. E., Moore D. D., Seidman L. G., Smith J. A., Struhl K. (1992) Current Protocols in Molecular Biology, Unit 16.14.1–16.14.13, John Wiley & Sons, Inc., New York [Google Scholar]
- 21.Ohya M., Nishitani C., Sano H., Yamada C., Mitsuzawa H., Shimizu T., Saito T., Smith K., Crouch E., Kuroki Y. (2006) Biochemistry 45, 8657–8664 [DOI] [PubMed] [Google Scholar]
- 22.Schwende H., Fitzke E., Ambs P., Dieter P. (1996) J. Leukoc. Biol. 59, 555–561 [PubMed] [Google Scholar]
- 23.Ferguson J. S., Voelker D. R., McCormack F. X., Schlesinger L. S. (1999) J. Immunol. 163, 312–321 [PubMed] [Google Scholar]
- 24.Kudo K., Sano H., Takahashi H., Kuronuma K., Yokota S., Fujii N., Shimada K., Yano I., Kumazawa Y., Voelker D. R., Abe S., Kuroki Y. (2004) J. Immunol. 172, 7592–7602 [DOI] [PubMed] [Google Scholar]
- 25.Kuroki Y., Fukada Y., Takahashi H., Akino T. (1985) Biochim. Biophys. Acta 836, 201–209 [PubMed] [Google Scholar]
- 26.Nagae H., Takahashi H., Kuroki Y., Honda Y., Nagata A., Ogasawara Y., Abe S., Akino T. (1997) Clin. Chim. Acta 266, 157–171 [DOI] [PubMed] [Google Scholar]
- 27.Uchida K., Mizushima S. (1987) Agric. Biol. Chem. 51, 3107–3114 [Google Scholar]
- 28.Kirby J. E., Vogel J. P., Andrews H. L., Isberg R. R. (1998) Mol. Microbiol. 27, 323–336 [DOI] [PubMed] [Google Scholar]
- 29.Chiba H., Sano H., Saitoh M., Sohma H., Voelker D. R., Akino T., Kuroki Y. (1999) Biochemistry 38, 7321–7331 [DOI] [PubMed] [Google Scholar]
- 30.Nie X., Nishitani C., Yamazoe M., Ariki S., Takahashi M., Shimizu T., Mitsuzawa H., Sawada K., Smith K., Crouch E., Nagae H., Takahashi H., Kuroki Y. (2008) Biochemistry 47, 12873–12885 [DOI] [PubMed] [Google Scholar]
- 31.Zuckman D. M., Hung J. B., Roy C. R. (1999) Mol. Microbiol. 32, 990–1001 [DOI] [PubMed] [Google Scholar]
- 32.McCormack F. X., Gibbons R., Ward S. R., Kuzmenko A., Wu H., Deepe G. S., Jr. (2003) J. Biol. Chem. 278, 36250–36256 [DOI] [PubMed] [Google Scholar]
- 33.Gardai S. J., Xiao Y. Q., Dickinson M., Nick J. A., Voelker D. R., Greene K. E., Henson P. M. (2003) Cell 115, 13–23 [DOI] [PubMed] [Google Scholar]
- 34.Geertsma M. F., Nibbering P. H., Haagsman H. P., Daha M. R., van Furth R. (1994) Am. J. Physiol. 267, L578–L584 [DOI] [PubMed] [Google Scholar]
- 35.Hartshorn K. L., Crouch E., White M. R., Colamussi M. L., Kakkanatt A., Tauber B., Shepherd V., Sastry K. N. (1998) Am. J. Physiol. 274, L958–L969 [DOI] [PubMed] [Google Scholar]
- 36.Kabha K., Schmegner J., Keisari Y., Parolis H., Schlepper-Schaeffer J., Ofek I. (1997) Am. J. Physiol. 272, L344–L352 [DOI] [PubMed] [Google Scholar]
- 37.Tino M. J., Wright J. R. (1996) Am. J. Physiol. 270, L677–L688 [DOI] [PubMed] [Google Scholar]
- 38.Beharka A. A., Gaynor C. D., Kang B. K., Voelker D. R., McCormack F. X., Schlesinger L. S. (2002) J. Immunol. 169, 3565–3673 [DOI] [PubMed] [Google Scholar]
- 39.Kuronuma K., Sano H., Kato K., Kudo K., Hyakushima N., Yokota S., Takahashi H., Fujii N., Suzuki H., Kodama T., Abe S., Kuroki Y. (2004) J. Biol. Chem. 279, 21421–21430 [DOI] [PubMed] [Google Scholar]
- 40.Roy C. R., Berger K. H., Isberg R. R. (1998) Mol. Microbiol. 28, 663–674 [DOI] [PubMed] [Google Scholar]
- 41.Bastacky J., Lee C. Y., Goerke J., Koushafar H., Yager D., Kenaga L., Speed T. P., Chen Y., Clements J. A. (1995) J. Appl. Physiol. 79, 1615–1628 [DOI] [PubMed] [Google Scholar]
- 42.Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. (1986) J. Appl. Physiol. 60, 532–538 [DOI] [PubMed] [Google Scholar]
- 43.Baughman R. P., Sternberg R. I., Hull W., Buchsbaum J. A., Whitsett J. (1993) Am. Rev. Respir. Dis. 147, 653–657 [DOI] [PubMed] [Google Scholar]
- 44.Honda Y., Kuroki Y., Matsuura E., Nagae H., Takahashi H., Akino T., Abe S. (1995) Am. J. Respir. Crit. Care Med. 152, 1860–1866 [DOI] [PubMed] [Google Scholar]
- 45.Tino M. J., Wright J. R. (1999) Am. J. Physiol. 276, L164–L174 [DOI] [PubMed] [Google Scholar]
- 46.van de Graaf E. A., Jansen H. M., Lutter R., Alberts C., Kobesen J., de Vries I. J., Out T. A. (1992) J. Lab. Clin. Med. 120, 252–263 [PubMed] [Google Scholar]
- 47.Wright J. R. (1997) Physiol. Rev. 77, 931–962 [DOI] [PubMed] [Google Scholar]
- 48.McIntosh J. C., Swyers A. H., Fisher J. H., Wright J. R. (1996) Am. J. Respir. Cell Mol. Biol. 15, 509–519 [DOI] [PubMed] [Google Scholar]
- 49.Phelps D. S., Rose R. M. (1991) Am. Rev. Respir. Dis. 143, 1072–1075 [DOI] [PubMed] [Google Scholar]