Summary
Retinoic acid (RA) generated by Raldh2 in paraxial mesoderm is required for specification of the posterior hindbrain, including restriction of Hoxb1 expression to presumptive rhombomere 4 (r4). Hoxb1 expression requires 3′ and 5′ RA response elements for widespread induction up to r4 and for r3/r5 repression, but RA has previously been detected only from r5–r8, and vHnf1 is required for repression of Hoxb1 posterior to r4 in zebrafish. We demonstrate in mouse embryos that an RA signal initially travels from the paraxial mesoderm to r3, forming a boundary next to the r2 expression domain of Cyp26a1 (which encodes an RA-degrading enzyme). After Hoxb1 induction, the RA boundary quickly shifts to r4/r5, coincident with induction of Cyp26c1 in r4. A functional role for Cyp26c1 in RA degradation was established through examination of RA-treated embryos. Analysis of Raldh2−/− and vHnf1−/− embryos supports a direct role for RA in Hoxb1 induction up to r4 and repression in r3/r5, as well as an indirect role for RA in Hoxb1 repression posterior to r4 via RA induction of vHnf1 up to the r4/r5 boundary. Our findings suggest that Raldh2 and Cyp26 generate shifting boundaries of RA activity, such that r3–r4 receives a short pulse of RA and r5–r8 receives a long pulse of RA. These two pulses of RA activity function to establish expression of Hoxb1 and vHnf1 on opposite sides of the r4/r5 boundary.
Keywords: Retinoic acid, Hindbrain, Segmentation, Raldh2 (Aldh1a2), Cyp26, Hoxb1, Hnf1 (Tcf2), Mouse
Introduction
Retinoic acid (RA) is generated from vitamin A (retinol) in a metabolic process of fundamental importance for development of mammalian embryos as well as other chordate embryos (Duester, 2000; Clagett-Dame and DeLuca, 2002). RA serves as a ligand that controls the action of nuclear RA receptors (Mangelsdorf et al., 1994; Kastner et al., 1995) either in cells where it is synthesized or in neighboring cells via paracrine signaling (Mic et al., 2002). RA plays an important role in development of the posterior central nervous system, including rhombomeric pattern formation in the hindbrain, as previously reviewed (Gavalas and Krumlauf, 2000; Gavalas, 2002; Maden, 2002). Hox homeobox genes exhibit segmental expression in the developing hindbrain and are intimately involved in the formation and identity of the eight rhombomeres that constitute the mouse hindbrain (Krumlauf, 1993; Wilkinson, 1993). Several members of the mammalian Hox gene family are direct targets of RA signaling during hindbrain development, including Hoxb1 (Simeone et al., 1990), and studies in amphioxus indicate that RA regulation of Hox genes is conserved in chordates (Holland and Holland, 1996). Prior to rhombomeric boundary formation, Hoxb1 is initially expressed throughout the posterior hindbrain up to the boundary between presumptive rhombomeres 3 and 4 (r3/r4), but soon becomes restricted to r4 (Wilkinson et al., 1989). Gene targeting in mice has revealed that Hoxb1 expression in r4 is required for facial motor neuron differentiation in this segment (Goddard et al., 1996; Studer et al., 1996).
The transcriptional regulation of Hox genes has been examined extensively as previously reviewed (Lufkin, 1996). It has been discovered that the mouse Hoxb1 gene is regulated by a retinoic acid response element (RARE) located 3′ to the promoter that is required for early widespread induction in the posterior hindbrain up to the presumptive r3/4 boundary (Marshall et al., 1994). This is consistent with the more recent demonstration that Hoxb1 expression anterior to the node requires cell to cell signaling and does not rely on proliferative expansion of Hoxb1-expressing cells at the level of the node (Forlani et al., 2003). Interestingly, another RARE located 5′ to the promoter has been demonstrated to be required for repression of Hoxb1 in r3 and r5 to provide restricted expression in r4 (Studer et al., 1994). In addition, an autoregulatory element has been found in the Hoxb1 promoter that is important for maintenance of r4 expression (Pöpperl et al., 1995). However, RA activity has not previously been detected anterior to r5 (see below) and other factors have been found to regulate Hoxb1 r4 restriction. In zebrafish, repression of Hoxb1 in the posterior hindbrain up to r5 has been demonstrated to depend upon the homeodomain protein encoded by vHnf1 (variant of Hepatocyte nuclear factor 1; Tcf2 – Mouse Genome Informatics), which is expressed in the posterior hindbrain up to the r4/r5 boundary (Wiellette and Sive, 2003). Recent studies also indicate that RA is required for expression of zebrafish vHnf1 in the posterior hindbrain (Hernandez et al., 2004). In addition, zebrafish iro7 encodes an iroquois homeodomain protein (homologous to mouse Irx3) expressed in the anterior hindbrain down to r4, and mutual repression of iro7 and vHnf1 positions the r4/r5 boundary (Lecaudey et al., 2004). Thus, it is unclear if mouse Hoxb1 induction and r4 restriction involves direct effects of RA signaling on the Hoxb1 promoter as RA has not been detected anterior to r5, plus it is unknown if RA may have indirect effects on mouse Hoxb1 r4 restriction through regulation of vHnf1 or Irx3 to set the r4/r5 boundary.
In the mouse, RA is first generated at embryonic day 7.5 (E7.5) just prior to the onset of hindbrain development (Rossant et al., 1991; Ang et al., 1996). RA synthesis for mouse development between E7.5-E8.25 is controlled by retinaldehyde dehydrogenase 2, encoded by Raldh2 (Aldh1a2 – Mouse Genome Informatics), expressed in the trunk paraxial mesoderm destined to become somites (Niederreither et al., 1999; Mic et al., 2002). The timing and location of the initial expression of Raldh2 coincides with the onset of posterior neural development, and RA as well as Raldh2 are indeed required for posterior hindbrain development and Hoxb1 expression in amniote embryos (Maden et al., 1996; Dickman et al., 1997; White et al., 1998; Niederreither et al., 2000; Dupé and Lumsden, 2001) as well as Xenopus (Blumberg et al., 1997; Kolm et al., 1997; Van der Wees et al., 1998; Chen et al., 2001) and zebrafish embryos (Begemann et al., 2001; Grandel et al., 2002; Kudoh et al., 2002). This suggests that RA generated in the trunk paraxial mesoderm by Raldh2 travels anteriorly into the hindbrain. It has been further suggested that a gradient of RA may exist across the hindbrain with the high point located posteriorly where the paraxial mesoderm lies adjacent to the posterior hindbrain. Examination of RA activity in mouse embryos carrying an RA-reporter transgene (RARE-lacZ) has revealed that RA activity is limited to the posterior portion of the embryo at the late primitive streak stage (E7.5) (Rossant et al., 1991), but the exact location of its anterior limit was not determined. At E8.25-E9.25, the RARE-lacZ RA signal has been shown to be present in the posterior hindbrain up to r5 (Sakai et al., 2001; Mic et al., 2002), but there has been no clear indication that RA activity is ever present further anterior in mouse embryos. This issue has not been resolved in other vertebrate embryos because of the lack of an appropriate RA reporter. As RA activity has not been convincingly demonstrated in r3 and r4, it remains unclear whether the Hoxb1 RARE DNA control elements described above require RA to function in those segments. A failure to observe RA activity in r3 and r4 may be due to expression of RA-degrading P450s in the hindbrain. Indeed, three RA-degrading P450s encoded by Cyp26a1 (Fujii et al., 1997; Hollemann et al., 1998; Swindell et al., 1999; Kudoh et al., 2002), Cyp26b1 (MacLean et al., 2001; Reijntjes et al., 2003) and Cyp26c1 (Tahayato et al., 2003; Reijntjes et al., 2004) are expressed in dynamic patterns during hindbrain development in several vertebrate embryos.
We now provide further insight into the mechanism of RA action during establishment of r3/r4/r5 gene expression boundaries through analysis of RARE-lacZ, Raldh2−/− and vHnf1−/− mouse embryos. We demonstrate the existence of dynamic shifting boundaries of hindbrain RA activity during Hoxb1 induction/repression that correspond to the Cyp26a1 and Cyp26c1 expression patterns. We show that RA is transiently present throughout the posterior hindbrain up to the r2/r3 boundary (abutting the anterior Cyp26a1 expression domain) and that this RA is needed to induce Hoxb1 expression throughout the posterior hindbrain up to the presumptive r3/r4 boundary and to induce vHnf1 (a repressor of Hoxb1) up to the presumptive r4/r5 boundary. However, subsequent to induction of Hoxb1 and vHnf1, the boundary of RA activity is quickly shifted to r4/r5, coincident with initiation of Cyp26c1 expression in r4, and this coincides with strict limitation of Irx3 and vHnf1 expression to opposite sides of the r4/r5 boundary, plus restriction of Hoxb1 expression to r4. Studies on Raldh2−/− and vHnf1−/− embryos indicate that RA is required for induction of Hoxb1 and vHnf1, and that vHnf1 is required to repress Hoxb1 posterior to r4. Analysis of RA-treated embryos supports a functional role for Cyp26c1 in RA degradation. Our findings thus provide evidence that RA activity exists in the appropriate location to directly induce Hoxb1 throughout the posterior hindbrain and to directly repress Hoxb1 in r3 and r5 through previously described 3′ and 5′ RAREs (Marshall et al., 1994; Studer et al., 1994). We also demonstrate that RA-mediated repression of Hoxb1 to r4 also functions indirectly through RA induction of its repressor vHnf1.
Materials and methods
Mouse strains
As the Raldh2−/− genotype is embryonic lethal by E11.5, this line was maintained as a heterozygous Raldh2−/+ line as previously described (Mic et al., 2002). A line of Raldh2−/+ mice carrying the RA-reporter transgene RAREhsplacZ (RARE-lacZ) (Rossant et al., 1991) on one chromosome has also been described (Mic et al., 2002). This strain was mated to generate an Raldh2−/+ line carrying RARE-lacZ on both homologous chromosomes (homozygous). For analysis of RARE-lacZ expression in Raldh2−/− embryos, matings were performed between male mice homozygous for RARE-lacZ with female mice lacking RARE-lacZ, resulting in embryos that were all heterozygous for RARE-lacZ. We also generated a wild-type line homozygous for the RARE-lacZ transgene in order to increase the signal strength of the transgene. For enhanced analysis of RARE-lacZ expression in wild-type embryos, matings were performed between males and females homozygous for RARE-lacZ to generate embryos homozygous for RARE-lacZ.
In order to examine vHnf1−/− embryos during hindbrain development, we used a conditional knockout of vHnf1 described previously (Coffinier et al., 2002). We also used the Mox2-Cre (MORE) transgene, which expresses Cre throughout the epiblast following implantation but not in extra-embryonic tissues (Tallquist and Soriano, 2000). Conditional vHnf1−/− embryos were generated from matings between mice homozygous for a conditional vHnf1 allele flanked by loxP sites with mice that were heterozygous for the null allele of vHnf1 (Coffinier et al., 1999) and also carried a Mox2-Cre allele.
Embryo genotyping and staging
Embryos from timed matings were genotyped by PCR analysis of yolk sac DNA. Embryos were staged according to morphology as previously described (Downs and Davies, 1993; Forlani et al., 2003) and were assigned the following embryonic day numbers with noon on the day of vaginal lug detection being considered embryonic day 0.5 (E0.5): early headfold (E7.4), headfold (E7.5-E7.75), late headfold (E7.8-7.9), one to three somites (E8.0), four to six somites (E8.25), seven to ten somites (E8.5) and 11–14 somites (E8.75).
Rescue of mutant embryos with a physiological dose of RA
Rescue of Raldh2−/− embryos by maternal dietary RA supplementation was performed as previously described with an RA dose that has previously been demonstrated to be in the normal physiological range (Mic et al., 2003). Briefly, all-trans-RA (Sigma) was dissolved in corn oil and mixed with powdered mouse chow to provide a final concentration of 0.1 mg/g for treatment from E6.75-E8.25 or from E6.75 to the point of analysis for embryos analyzed prior to E8.25. Such food was prepared fresh twice each day (morning and evening) and provided ad libitum. For mice analyzed after E8.25, mice were returned to standard mouse chow at E8.25 until the point of analysis.
Treatment of wild-type embryos with excess RA
Pregnant wild-type mice were treated with exogenous RA similar to a previous description (Conlon and Rossant, 1992). Following timed matings, female mice were orally administered all-trans-RA (Sigma) at a dose of 20 mg/kg body weight dissolved in 0.2 ml corn oil at 12 m on day 7 (E7.5). Embryos were analyzed 18 hours after dosing (E8.25).
Whole-mount in situ hybridization
Embryonic mRNAs were detected by whole-mount in situ hybridization using the alkaline phosphatase substrate NBT-BCI as described (Wilkinson, 1992). Antisense RNA robes were generated from mouse cDNAs encoding Raldh2 (Haselbeck et al., 1999), Hoxb1 (Hunt et al., 1991), vHnf1 (Coffinier et al., 1999), Irx3 (Cohen et al., 2000), Cyp26a1 and Cyp26c1 (Tahayato et al., 2003), Krox20 (Wilkinson, 1993), and Epha2 (Becker et al., 1994).
Detection of retinoic acid activity
Detection of RA activity was performed in embryos carrying the RARE-lacZ RA-reporter transgene, which laces lacZ (encoding β-galactosidase) under the transcriptional control of a retinoic acid response element (RARE) (Rossant et al., 1991). β-Galactosidase staining was performed for 1 hour with the substrate Salmon-gal (6-chloro-3-indolyl-β-D-galactopyranoside) (Labscientific) to produce a red reaction product; in some cases, staining was performed with the substrate X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) to produce a blue-green reaction product. Double staining to examine both RA activity (RARE-lacZ) and mRNA localization was performed by first staining 1 hour for β-galactosidase using Salmon-gal, followed by processing for whole-mount in situ hybridization as described (Tajbakhsh and Houzelstein, 1995).
Results
r3/r4/r5 gene expression boundaries in the early hindbrain
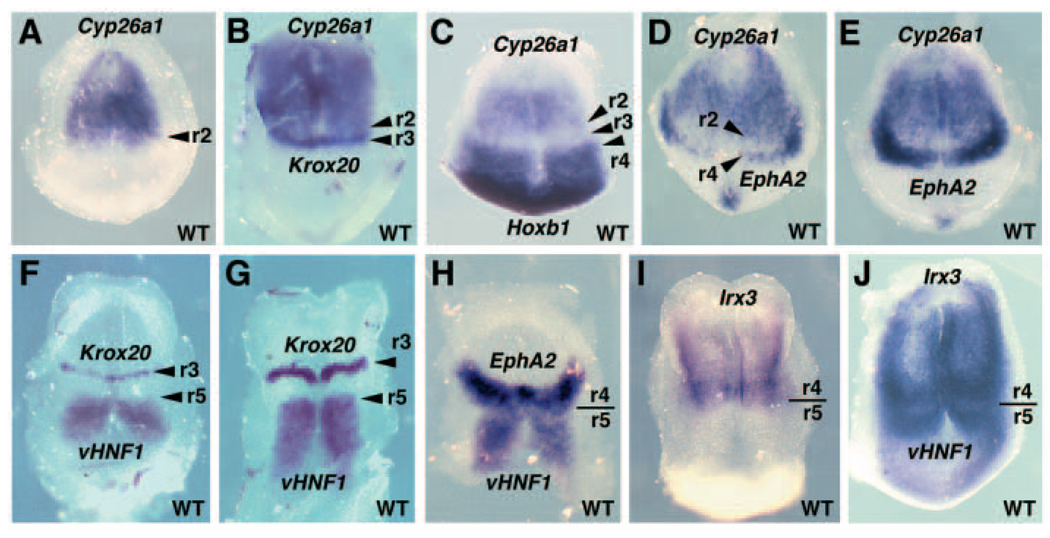
Disruption of Cyp26a1 encoding an RA-degrading P450 enzyme has previously demonstrated that this gene functions in RA degradation for the anterior neural plate (Sakai et al., 2001; Abu-Abed et al., 2001). Transient expression of Cyp26a1 has been previously reported in the anterior neural plate of mouse headfold stage embryos (Fujii et al., 1997; MacLean et al., 2001), but the location of its posterior boundary has not been defined. In order to define this boundary, we compared expression of Cyp26a1 with that of Krox20 (Egr2 – Mouse Genome Informatics), which is a marker for presumptive r3 at the headfold stage (Wilkinson, 1993), with Hoxb1 which is expressed continuously throughout the posterior hindbrain up to the presumptive r3/r4 boundary at the headfold stage (Wilkinson et al., 1989), and with Epha2 which is an early hindbrain marker limited to presumptive r4 and the adjacent mesoderm (Becker et al., 1994). Owing to the early stages examined here, the rhombomeric (r) territories of gene expression described throughout the results will refer to presumptive rhombomeres. Embryos double-stained for Cyp26a1 and Hoxb1 expression exhibited a gap between the two domains, indicating that Cyp26a1 is not expressed in r3, whereas embryos double-stained for Cyp26a1 and Krox20 exhibited no gap between these two domains, suggesting that Cyp26a1 is expressed posteriorly to r2 (Fig. 1A–C). Double-staining for Cyp26a1 and Epha2 revealed a gap between their expression domains where presumptive r3 lies (Fig. 1D–E). These results demonstrate that Cyp26a1 is expressed in the anterior hindbrain down to the r2/r3 boundary.
Fig. 1.
Boundaries of Cyp26a1, vHnf1 and Irx3 expression in the early mouse hindbrain. Anterior is oriented towards the top in all panels, and all embryos are wild type (WT). (A–E) Cyp26a1 mRNA at E7.75-E8.0 is localized anteriorly with its posterior extent to presumptive rhombomere 2 (r2) of the hindbrain, as determined by whole-mount in situ hybridization. Shown is an E7.75 embryo stained for expression of Cyp26a1 (A), and an E8.0 embryo double-stained for expression of Cyp26a1 and Krox20, a known marker for r3 (B). Also shown is an E7.75 embryo double-stained for expression of Cyp26a1 and Hoxb1, a known marker for r4 (C); neither gene is expressed in r3. Double-staining for Cyp26a1 and Epha2 (a marker for presumptive r4 and the adjacent mesoderm) at E7.75 (D) and E7.9 (E) demonstrates a space between the two expression domains where presumptive r3 lies; expression of Epha2 in the mesoderm adjacent to r4 overlaps the lateral regions of presumptive r3 from this view point. (F,G) Expression of vHnf1 is localized posteriorly with its anterior border at the r4/r5 boundary Shown are embryos double-stained for expression of vHnf1 and Krox20 at E8.0 when Krox20 is expressed only in r3 (F) and at E8.25 when Krox20 is expressed strongly in r3 and weakly in r5 (G). There is a gap between Krox20 and vHnf1 expression at both stages, indicating that vHnf1 expression extends to r5 but not into r4. (H) Double staining for Epha2 and vHnf1 shows that vHnf1 is expressed in r5 up to the r4/r5 boundary. (I) Expression of Irx3 at E8.25 is localized anteriorly with a posterior border at the r4/r5 boundary when compared with the expression pattern of vHnf1 (G). (J) Double staining for Irx3 and vHnf1 expression at E8.0 provides evidence that these two expression domains meet at r4/r5.
In zebrafish embryos, vHnf1 has been found to be expressed in the posterior hindbrain up to the r4/r5 boundary (Wiellette and Sive, 2003; Hernandez et al., 2004). In mouse embryos, vHnf1 expression has been reported in the posterior hindbrain at E8.0-E8.5 (Coffinier et al., 1999; Barbacci et al., 1999), but its anterior boundary has not been defined. E8.0 mouse embryos double stained for vHnf1 and Krox20 (which is limited to r3 at E8.0) exhibited a gap between the two domains, indicating that vHnf1 is not expressed in r4 (Fig. 1F). A similar gap was observed at E8.25 when Krox20 is expressed strongly in r3 and weakly in r5, indicating that vHnf1 expression overlaps the r5 domain of Krox20 (Fig. 1G; also see Fig. 2M for Krox20 r5 expression). Double-staining for Epha2 (which is limited to r4) and vHnf1 revealed that their expression domains meet without a gap (Fig. 1H). These results indicate that vHnf1 is expressed in the posterior hindbrain up to the r4/r5 boundary.
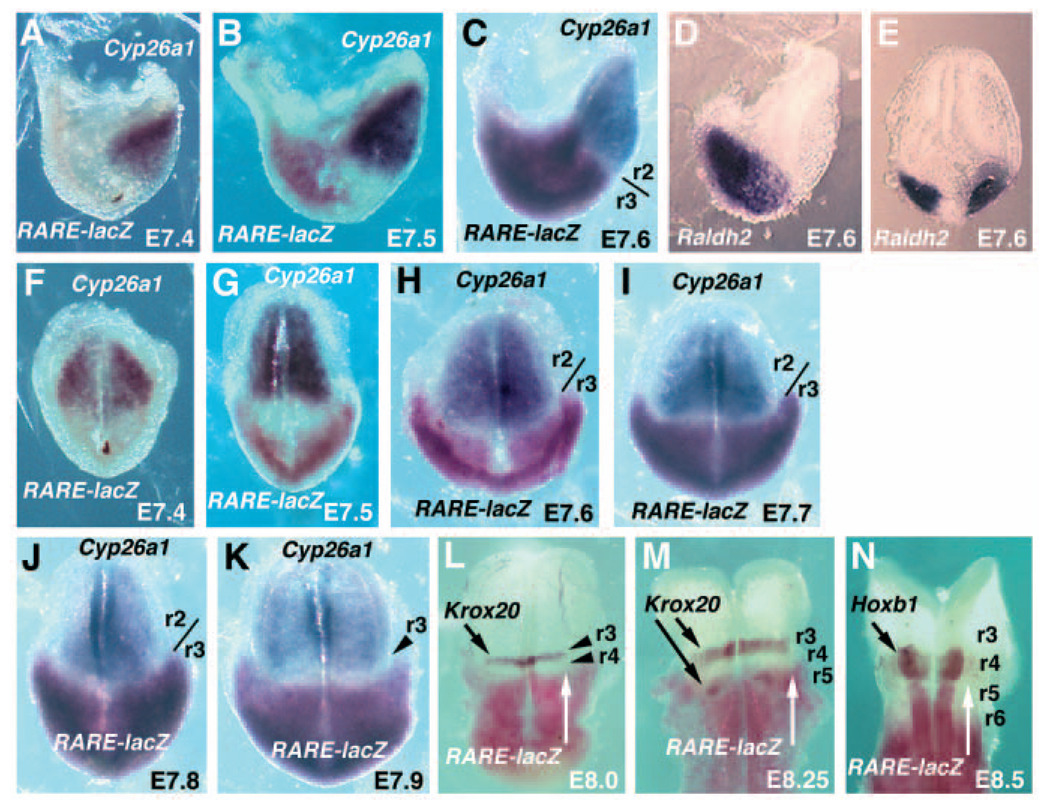
Fig. 2.
Boundaries of hindbrain RA activity along the anteroposterior axis. All embryos are homozygous for the RARE-lacZ RA-indicator transgene; stages are indicated on each panel. Anterior is oriented towards the right in A–D (lateral views) and towards the to in E–O (dorsal views). (A–C) Embryos were double-stained for RARE-lacZ expression (β-galactosidase activity), which is observed posteriorly, followed by Cyp26a1 expression (in situ hybridization), which is observed anteriorly with its posterior border at r2. RARE-lacZ expression is first seen at E7.5 (B) and the r2/r3 division marked in C indicates the anterior extent of RARE-lacZ, which reaches r3 at E7.6. (D,E) Raldh2 expression detected posteriorly in the paraxial mesoderm at E7.6. (F–K) Dorsal view of embryos double-stained for expression of RARE-lacZ and Cyp26a1 from E7.4-E7.9. RARE-lacZ expression has reached r3 by E7.6 (H), but begins to clear from r3 by E7.9 (K). (L–N) Double-stained embryos showing RARE-lacZ expression (white arrows indicate its anterior extent at the r4/r5 boundary) and expression of either Krox20 in r3 at E8.0 (L), Krox20 in both r3 and r5 at E8.25 (M) and Hoxb1 in r4 at E8.5 (N).
The zebrafish iro7 gene is expressed in the anterior hindbrain down to the r4/r5 boundary (Lecaudey et al., 2004). The mouse homolog, Irx3, is also expressed in the anterior neural plate (Bosse et al., 1997), but its anterior boundary is not defined. At E8.25, Irx3 was expressed in the anterior hindbrain down to approximately the r4/r5 boundary (Fig. 1I) when compared with the previously established r5 expression border of vHnf1 at E8.25 (Fig. 1G). Double-staining for Irx3 and vHnf1 expression at E8.0 provided further evidence that the posterior border of Irx3 expression is in r4 as it lies directly adjacent to the vHnf1 r5 domain (Fig. 1J).
Shifting boundaries of RA activity along the hindbrain
RA synthesis during early mouse hindbrain development is controlled by Raldh2 expressed in the paraxial mesoderm (Niederreither et al., 2000), and RA degradation is controlled at least in art by Cyp26a1 expressed in the anterior neural plate (Sakai et al., 2001; Abu-Abed et al., 2001). A similar situation exists in Xenopus, chick and zebrafish embryos (Hollemann et al., 1998; Swindell et al., 1999; Kudoh et al., 2002). This has led to the hypothesis that RA synthesized in the paraxial mesoderm may diffuse anteriorly across the hindbrain until it meets the Cyp26a1 expression domain, perhaps establishing a posterior-high gradient of RA activity across the hindbrain. Such RA is presumably needed for induction of various genes including Hoxb1 whose expression in r4 requires two retinoic acid response elements (RAREs) located 3′ and 5′ of the promoter (Marshall et al., 1994; Studer et al., 1994). Among the various vertebrate embryo models of hindbrain development, the mouse is unique in that an RA-reporter transgene (RARE-lacZ) has been constructed that allows RA activity to be detected during the early stages of hindbrain development (Rossant et al., 1991). RARE-lacZ expression is completely eliminated in the hindbrain of Raldh2−/− embryos and can be induced in all embryonic cells of wild-type embryos following treatment with excess RA, thus demonstrating that this transgene is indeed a faithful reporter for endogenous RA (Niederreither et al., 1999; Mic et al., 2002). Previous studies using RARE-lacZ embryos have not defined the anterior boundary of RA activity during the headfold stages, although it is clear that RA is present in the hindbrain up to the r5 boundary at E8.25-E9.25 (Sakai et al., 2001; Mic et al., 2002). Here, we have examined wild-type headfold stage embryos to determine if RA exists anterior to r5.
As our studies above have now defined r2/r3 as the posterior boundary of Cyp26a1 expression, we examined embryos double-stained for Cyp26a1 and RARE-lacZ to determine the anterior extent of RA activity in the mouse hindbrain. At E7.4, expression of Cyp26a1 expression was observed, but not RARE-lacZ (Fig. 2A). At E7.5, weak RARE-lacZ expression was observed with an ill-defined anterior boundary near the node, but by E7.6 RARE-lacZ expression now extended further anterior up to the Cyp26a1 domain with a well-defined boundary at r2/r3 (Fig. 2B,C). The initiation of RARE-lacZ expression correlates with the initiation of Raldh2 expression as we observed no Raldh2 expression at E7.4, weak expression at E7.5 and strong expression at E7.6 in the posterior paraxial mesoderm (Fig. 2D–E; data not shown). Dorsal views of a series of double-stained embryos from E7.4-E7.8 demonstrates the initiation of RARE-lacZ expression at E7.5, and the formation of an r2/r3 boundary of RA activity from E7.6-E7.8 (Fig. 2F–J). The border formed between Cyp26a1 and RARE-lacZ expression is very dynamic, but embryos at E7.8 provided evidence that the two domains do temporarily abut without an intervening gap (Fig. 2J). At E7.9, we observed a clearing of RARE-lacZ expression in r3, indicating the occurrence of a posterior shift in the RA boundary to ~r3/r4 (Fig. 2K). E8.0 and E8.25 embryos double stained for Krox20 and RARE-lacZ revealed that RA activity had retreated to an r4/r5 boundary by E8.0 as the anterior boundary of RARE-lacZ expression overlapped that of Krox20 in r5 (Fig. 2L–M). Thus, RA activity clears from r4 by E8.0-E8.25. E8.5 embryos double-stained for Hoxb1 (now limited to r4) and RARE-lacZ demonstrated that RA activity still remained in r5 and further posterior, thus indicating that a stable RA activity boundary at r4/r5 was being maintained (Fig. 2N). These findings indicate that RA does not form a stable gradient across the early hindbrain, but instead displays shifting boundaries of RA activity first at r2/r3 then at r4/r5. Our results also demonstrate that RA exists transiently in r3 and r4 during the time when Hoxb1 expression initiates in the hindbrain.
RA induction of Cyp26c1 in r4
Although the expression domain of Cyp26a1 is positioned properly to account for the formation of an r2/r3 boundary of RA activity, it cannot account for the subsequent shift to r4/r5. Previous studies have shown that a related enzyme encoded by Cyp26c1 is expressed in r2 and r4 as well as the adjacent head mesoderm in E8.0-E8.5 mouse embryos (Tahayato et al., 2003). A third related mouse enzyme encoded by Cyp26b1 is expressed in r3 and r5 at approximately E8.25, but this is clearly after Cyp26c1 expression is already apparent in r4 (MacLean et al., 2001) (I.O.S. and G.D., unpublished).
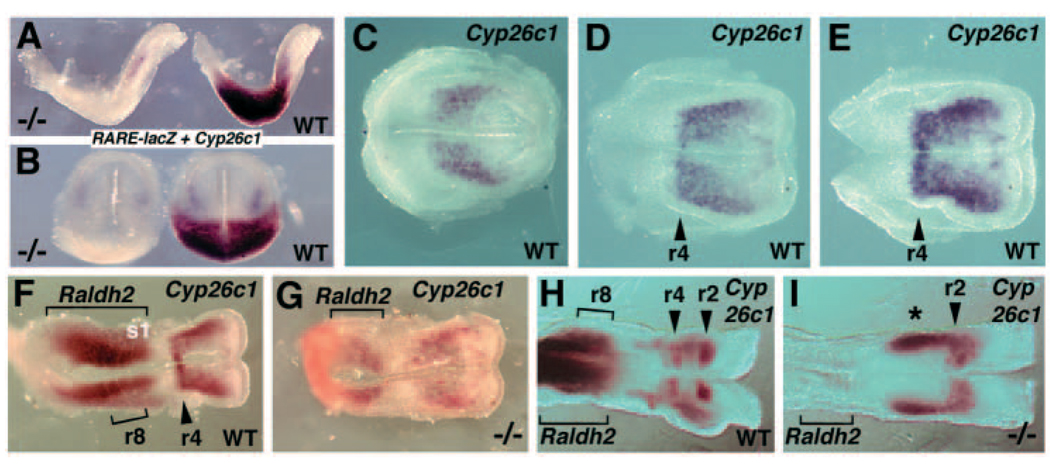
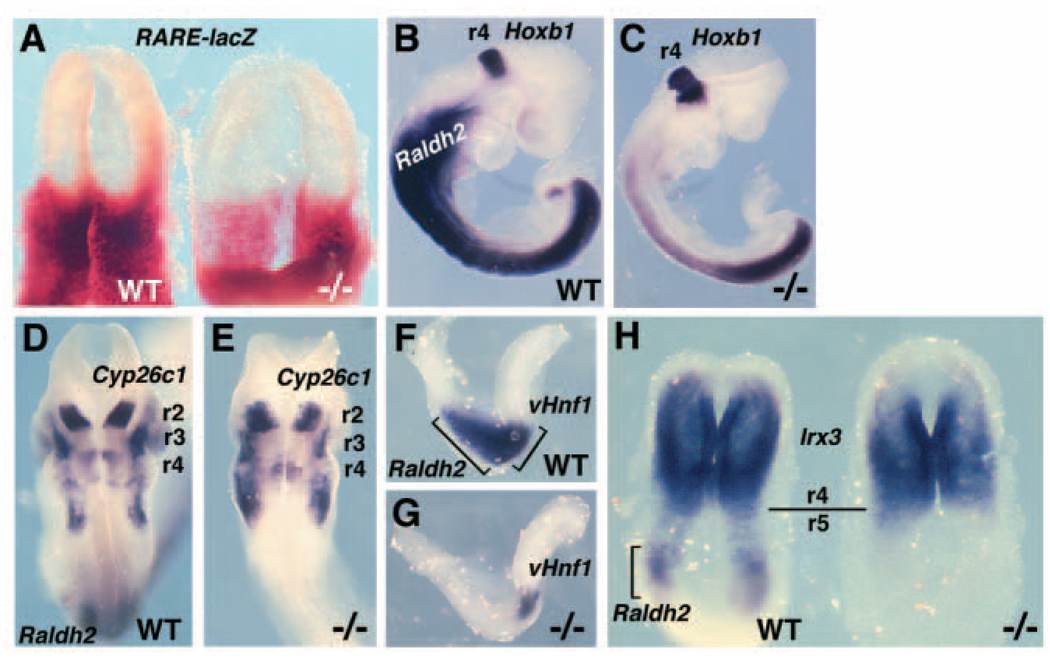
Wild-type E7.75 headfold embryos double-stained for Cyp26c1 and RARE-lacZ revealed a domain of Cyp26c1 expression limited to the head mesoderm anterior to the r2/r3 boundary of RA activity, and thus not in a position to regulate RA levels in the hindbrain at this stage (Fig. 3A,B). E7.75 Raldh2−/− embryos double stained for Cyp26c1 and RARE-lacZ demonstrated that RARE-lacZ expression was completely lost when Raldh2 function was lost, whereas Cyp26c1 expression was maintained in the head mesoderm (Fig. 3A,B). Wild-type embryos stained for Cyp26c1 expression from E7.75-E8.0 revealed that the early domain of head mesoderm expression intensifies up to E8.0 and that expression in r4 begins at about E7.9 then intensifies at E8.0 (Fig. 3C–E). Comparison of E8.25-E8.5 wild-type and Raldh2−/− embryos double-stained for Cyp26c1 and Raldh2 demonstrated that a loss of RA synthesis results in a loss of Cyp26c1 expression in r4 but expression remains in r2 and head mesoderm (n=5; Fig. 3F–I). Cyp26c1 expression in the head mesoderm of Raldh2−/− embryos is intensified and expanded posteriorly, and the r2 expression domain is wider along the anteroposterior axis (Fig. 3H–I). Thus, Cyp26c1 expression initiates in r4 at approximately the same time that we observe a shift in RA activity from r2/r3 to r4/r5, and RA is required for r4 induction of Cyp26c1.
Fig. 3.
RA generated by Raldh2 is required for Cyp26c1 expression in r4. Anterior is oriented towards the right in all panels except B, where anterior is towards the top. (A,B) Lateral (A) and dorsal (B) views of E7.75 Raldh2−/− (−/−) and wild-type (WT) embryos double stained for expression of RARE-lacZ (β-galactosidase activity) observed posteriorly followed by staining for Cyp26c1 mRNA (whole-mount in situ hybridization) observed anteriorly. The Raldh2−/− embryo lacks RARE-lacZ expression, but Cyp26c1 expression at this stage (head mesoderm) is not affected. (C–E) Cyp26c1 expression in dorsal view of wild-type embryos at E7.75 (C), E7.9 (D) and E8.0 (E) showing its initial expression in r4, with the remaining expression occurring in head mesoderm. (F–I) Dorsal view of double staining for expression of Raldh2 (posterior) and Cyp26c1 (anterior) in Raldh2−/− and wild-type embryos at E8.25 (F–G) and E8.5 (H–I). Raldh2−/− embryos (marked by a large reduction in Raldh2 mRNA) lack the r4 domain of Cyp26c1 expression while retaining the r2 expression domain normally observed by E8.5. Raldh2−/− embryos also exhibit an abnormal posterior extension of Cyp26c1 expression in head mesoderm at E8.5 marked by an asterisk (I). The anterior extent of Raldh2 mRNA in wild-type embryonic mesoderm is somite 1 (s1), which is adjacent to rhombomere 8 (r8) at E8.25 (F).
RA is required for early expansion of Hoxb1 expression to r3/r4 border
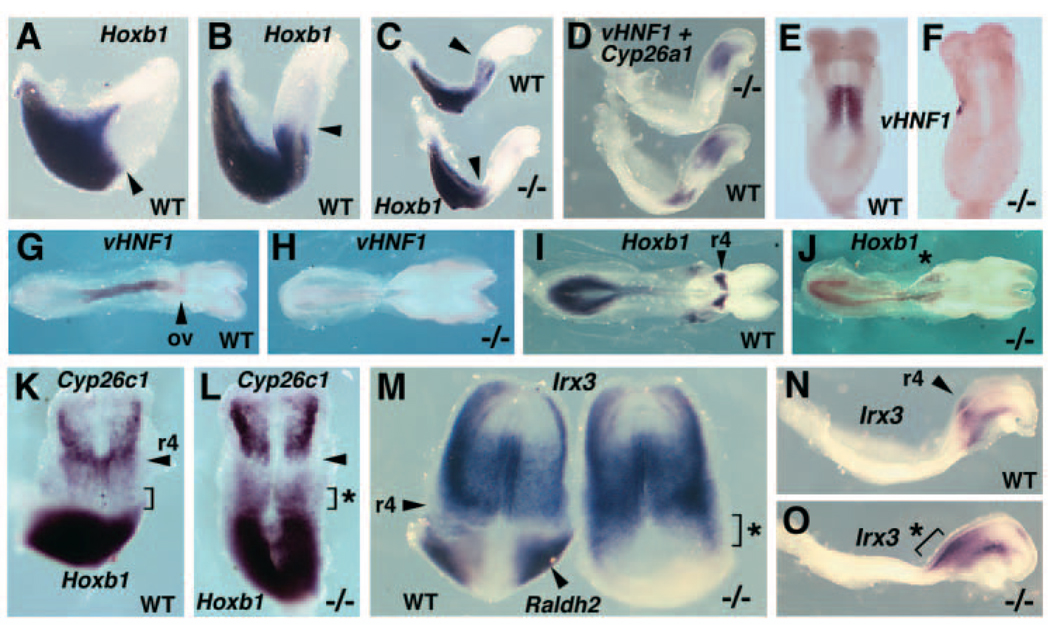
RA generated by Raldh2 has previously been shown to be required for r4 expression of Hoxb1 in E8.25-E8.5 mouse embryos (Niederreither et al., 2000). We examined the effect of a loss of RA synthesis on the early anterior expansion of Hoxb1 expression. The normal expansion of Hoxb1 expression from the node to the r3/r4 boundary is shown in wild-type embryos examined from E7.5-E8.0 (Fig. 4A–C). By E8.0, Hoxb1 is continuously expressed from the node to r3/r4 and has not yet been restricted to r4 (Fig. 4C). Raldh2−/− embryos at E8.0 completely lacked the anterior expansion of Hoxb1 to r3/r4, thus resembling wild-type E7.5 embryos in which Hoxb1 expression was limited anteriorly to a region near the node (n=3; Fig. 4A,C). These findings demonstrate that RA generated by Raldh2 is required for the initial expansion of Hoxb1 expression throughout the hindbrain up to r3/r4.
Fig. 4.
Requirement of RA for induction of Hoxb1 and vHnf1, and for repression of Irx3. Anterior is towards the right (A–D, G–J,N–O) or towards the to (E–F,K–M). (A–C) Hoxb1 expression normally exhibits an anterior extension into the hindbrain, but this extension is eliminated in Raldh2−/− embryos. Arrowheads indicate the anterior extent of Hoxb1 expression, which is at the level of the node in a wild-type embryo at E7.5 (A), and anterior to the node in the hindbrain at E7.75 (B) and E8.0 (C). The anterior expansion of Hoxb1 expression into the hindbrain is eliminated in an Raldh2−/− embryo (C). (D) Double in situ hybridization at E8.0, showing a lateral view of vHnf1 expression posteriorly in the hindbrain and Cyp26a1 expression anteriorly. The Raldh2−/− embryo lacks vHnf1 expression, but Cyp26a1 is not affected by a loss of RA. (E,F) A dorsal view of vHnf1 expression at E8.25, showing a complete loss of vHnf1 mRNA in an Raldh2−/− embryo. (G,H) vHnf1 expression at E8.5 is normally present in the anterior spinal cord, as well as the posterior hindbrain up to the otic vesicle (ov), but an Raldh2−/− embryo lacks vHnf1 mRNA in these neural tissues. (I,J) Neural expression of Hoxb1 at E8.5 is normally limited to r4 in the hindbrain and to the posterior spinal cord, but an Raldh2−/− embryo lacks the r4 expression domain and instead exhibits a weak expression domain shifted to the most posterior region of the hindbrain (asterisk). (K,L) Double-staining for expression of Cyp26c1 (anterior) and Hoxb1 (posterior) at E8.25 demonstrates that both overlap in r4 of wild-type embryo, but that the Raldh2−/− embryo lacks expression of both in the region where r4 should have developed (arrowhead). The brackets indicate a region where the Raldh2−/− embryo retains expression of Hoxb1 in the most posterior region of the hindbrain (asterisk), whereas this domain of Hoxb1 expression is normally lost by E8.25. (M) Double staining for expression of Irx3 and Raldh2 in E7.9 wild-type and Raldh2−/− embryos demonstrates the normal posterior border of Irx3 expression at r4 separated from the Raldh2 domain, whereas the mutant exhibits a loss of Raldh2 expression and an expansion of Irx3 expression into the posterior hindbrain (bracket and asterisk). (N,O) Irx3 expression at E8.25 is normally present in the anterior hindbrain with a posterior limit at r4 (arrowhead), but an Raldh2−/− embryo exhibits a posterior expansion of Irx3 expression to the hindbrain/spinal cord junction (bracket and asterisk).
RA regulates vHnf1 and Irx3 during r4/r5 boundary formation
Recent studies in zebrafish suggest that two homeodomain genes, vHnf1 and iro7 (Irx3), lay crucial roles in establishment of the r4/r5 boundary and r4 restriction of Hoxb1 expression (Wiellette and Sive, 2003; Lecaudey et al., 2004; Hernandez et al., 2004). Those studies demonstrated that vHnf1 functions as a repressor for Hoxb1, that vHnf1 and iro7 function as mutual repressors on opposite sides of the r4/r5 boundary, and that RA is required for vHnf1 expression. We examined these genes in mouse embryos that lack RA synthesis. E8.0 wild-type and Raldh2−/− embryos double stained for Cyp26a1 and vHnf1 demonstrated that a loss of RA results in a complete loss of vHnf1 expression in the hindbrain, but no effect on Cyp26a1 expression in the anterior neural plate (n=3; Fig. 4D). Raldh2−/− embryos examined at E8.25-E8.5 lacked vHnf1 expression in the hindbrain as well as the spinal cord (n=3; Fig. 4E–H).
Raldh2−/− embryos at E8.25-E8.5 lacked the r4 domain of Hoxb1 expression, but exhibited residual Hoxb1 expression at the posterior hindbrain/spinal cord junction that would normally be eliminated by this stage (n=3; Fig. 4I–L). Raldh2−/− embryos double stained for Cyp26c1 and Hoxb1 demonstrated a complete loss of expression of both genes in r4, but retention of Hoxb1 expression at the posterior hindbrain/spinal cord junction lying just posterior to the remaining head mesodermal domain of Cyp26c1 (Fig. 4K–L). This residual posterior domain of Hoxb1 expression may be the remnants of that observed near the node in E8.0 Raldh2−/− embryos (Fig. 4C). These findings suggest that mouse vHnf1 may function in repression of Hoxb1, as the loss of vHnf1 observed in Raldh2−/− embryos may result in a failure to repress this RA-independent posterior domain of Hoxb1 expression.
Raldh2−/− embryos examined at E7.9-E8.25 exhibited a posterior expansion of Irx3 expression in the hindbrain (n=6; Fig. 4M–O). Whereas Irx3 expression in the anterior hindbrain normally displayed an r4 posterior boundary, a loss of RA synthesis resulted in an expansion posteriorly throughout the hindbrain to the spinal cord junction. This abnormal posterior domain of Irx3 expression lies at approximately the same location where Hoxb1 expression is abnormally retained in the mutant posterior hindbrain (compare Fig. 4J with Fig. 4O). These results suggest that the effect of RA on mouse Irx3 expression may be due to the requirement of RA for expression of vHnf1, which may function (as it does in zebrafish) as a repressor of both Irx3 and Hoxb1 in the posterior hindbrain up to the r4/r5 boundary.
Requirement of vHnf1 for r4/r5 gene expression boundary
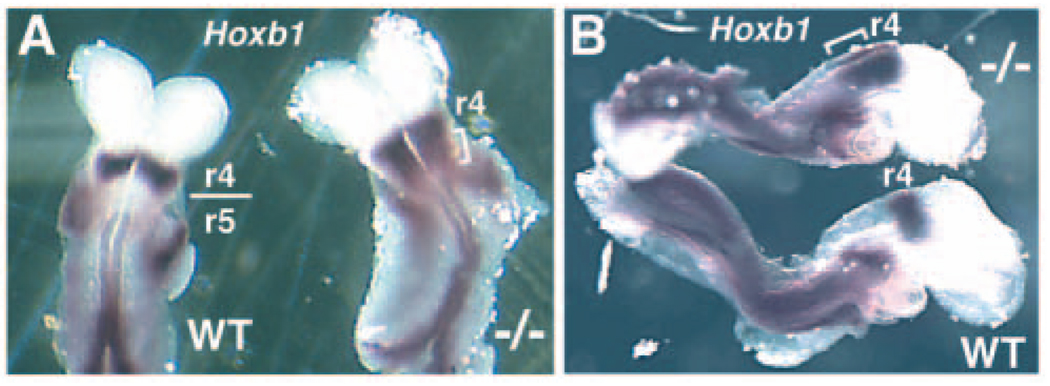
We examined the effect of a loss of vHnf1 function on expression of Hoxb1 in the mouse hindbrain. Disruption of mouse vHnf1 has previously been shown to result in an extra-embryonic defect of visceral endoderm formation, leading to embryonic lethality prior to hindbrain formation (Coffinier et al., 1999; Barbacci et al., 1999). A conditional vHnf1 mutant mouse has allowed the function of this gene to be examined at later stages (Coffinier et al., 2002). Here, conditional vHnf1−/− embryos were generated from matings with mice containing the Mox2-Cre (MORE) transgene, which stimulates Cre/lox-mediated deletion throughout the epiblast following implantation, but does not affect extra-embryonic tissues (Tallquist and Soriano, 2000). Hoxb1 expression in E8.5 conditional vHnf1−/− embryos was observed posterior to the normal r4/r5 boundary, indicating a failure to restrict Hoxb1 expression to r4 (n=3; Fig. 5A–B). These findings indicate that the function of vHnf1 as a Hoxb1 repressor is conserved in mouse and zebrafish.
Fig. 5.
vHnf1 is required to define r4/r5 gene expression boundary in mouse embryos. (A,B) Dorsal and lateral views of Hoxb1 expression wild-type and conditional vHnf1−/− embryos. The normal r4/r5 boundary of Hoxb1 expression is indicated in wild-type embryos. vHnf1−/− embryos exhibit expansion of Hoxb1 expression posterior to the r4/r5 boundary in the hindbrain (brackets).
Physiological dose of RA rescues r3/r4 and r4/r5 gene expression boundaries
Previous studies have demonstrated that Raldh2−/− embryos can be rescued by low-dose maternal dietary RA supplementation which was quantitated to provide embryonic RA in the normal physiological range (Mic et al., 2003). Following such RA supplementation and analysis at E8.0, we found that RARE-lacZ expression was recovered in Raldh2−/− embryos, and that the pattern of RARE-lacZ expression observed in RA-treated wild-type embryos was indistinguishable from that of untreated wild-type embryos (n=3; Fig. 6A; compare to Fig. 2L). The anterior boundary of RARE-lacZ expression in such RA-rescued Raldh2−/− embryos was similar to that of wild type, suggesting that RA degradation anteriorly by Cyp26 enzymes may be preventing this low dose of RA from acting anteriorly. Following RA supplementation and analysis at E8.75, Raldh2−/− embryos exhibited a recovery of Hoxb1 expression restricted to r4 (n=3; Fig. 6B,C) and a recovery of Cyp26c1 expression in r4 (n=3; Fig. 6D,E). RA supplementation of Raldh2−/− embryos also resulted in a recovery of vHnf1 expression (n=3; Fig. 6F–G) and the Irx3 expression domain was now restricted posteriorly to the r4/r5 boundary (n=4; Fig. 6H).
Fig. 6.
Maternal dietary RA supplementation rescues r3/r4 and r4/r5 gene expression boundaries in Raldh2−/− embryos. Embryos were subjected to low-dose maternal dietary RA supplementation from E6.75-E8.0 (A,F–H) or from E6.75-E8.25 (B–E). (A) RARE-lacZ expression in RA-treated wild-type and Raldh2−/− embryos at E8.0. The Raldh2−/− embryo exhibits an anterior border of RARE-lacZ expression similar to that observed in a wild-type littermate, although the intensity of staining was less than that observed in the wild-type embryo. (B–E) RA treatment of wild-type and Raldh2−/− embryos followed by analysis at E8.75 demonstrates that RA administration rescues r4 expression of Hoxb1 (B,C) and Cyp26c1 (D,E) in Raldh2−/− embryos. RA-treated wild-type embryos exhibited relatively normal expression of Hoxb1 and Cyp26c1. All embryos were also simultaneously stained for Raldh2 expression, which appeared only in wild-type embryos as indicated. (F–H) RA treatment of Raldh2−/− embryos followed by analysis at E8.0 results in posterior hindbrain expression of vHnf1 (F–G) and relatively normal r4/r5 restriction of Irx3 (H). Embryos were double stained for Raldh2 expression, which was observed only in wild-type embryos (brackets).
These observations indicate that a low dose of exogenous RA can mimic the local endogenous RA synthesis function of Raldh2 in paraxial mesoderm. The uneven distribution of RA in treated Raldh2−/− embryos follows the normal pattern probably because of Cyp26a1 and Cyp26c1, which are expressed relatively normally (Fig. 4D; Fig. 6E). It is clear that anterior cells of the embryo are responsive to RA, as previous studies have shown that treatment of embryos with a high dose of RA induces RARE-lacZ in all cells of the embryo (Rossant et al., 1991; Mic et al., 2002). Thus, it is clear that RA is normally functioning locally in a paracrine fashion and that a low dose of RA can mimic this paracrine function, whereas a high dose of RA results in abnormal systemic distribution.
Excess RA induces anterior shifts in Cyp26c1 domain and Hoxb1/vHnf1 boundary
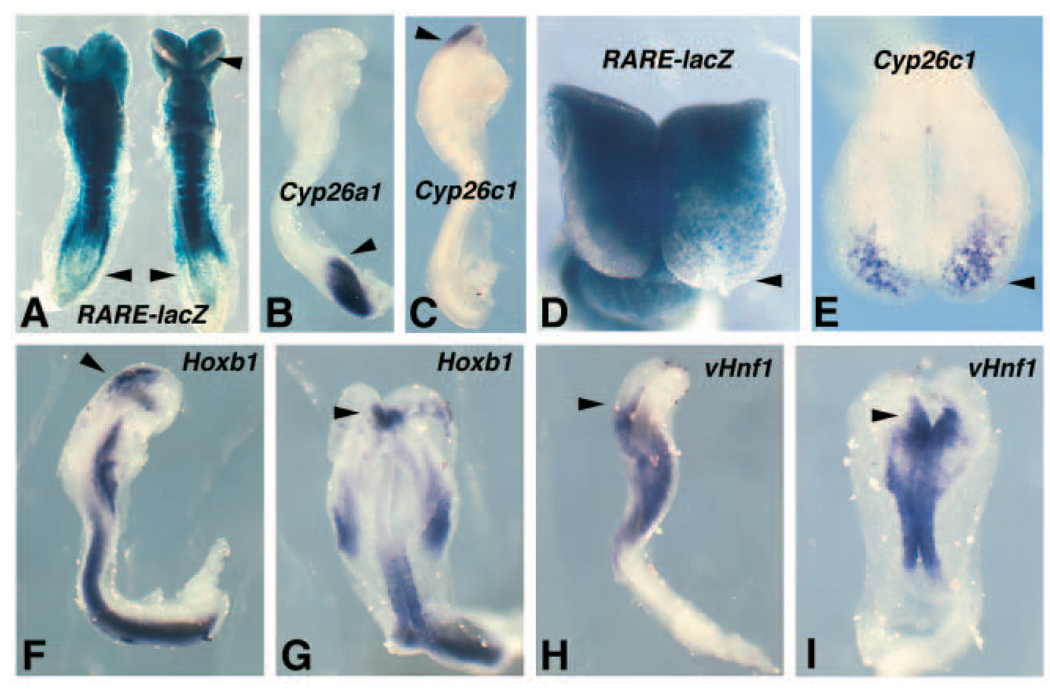
Our results suggest that Cyp26c1 may provide an RA degradation function in r4 to limit the anterior border of RA activity, and that this border may be crucial for establishing a border between Hoxb1 and vHnf1 at the r4/r5 boundary. Genetic studies of the related genes Cyp26a1 and Cyp26b1 have revealed that both have a function in RA degradation (Sakai et al., 2001; Abu-Abed et al., 2001; Yashiro et al., 2004), but genetic studies for Cyp26c1 have not been reported. In order to test this potential function for Cyp26c1, we treated RARE-lacZ embryos with a teratogenic dose of RA to disrupt the normal boundary of RA activity. Previous studies have shown that a 20 mg/kg dose of RA administered at E7.5 leads to induction of RARE-lacZ expression throughout the anterior region of the embryo when examined 6 hours after treatment (Rossant et al., 1991), thus eliminating the normal RA boundary in the hindbrain. When we administered a 20 mg/kg dose of RA at E7.5, we found that some embryos examined at E8.25 (18 hours after treatment) still exhibited a shift in RARE-lacZ expression completely to the anterior tip (n=9/15), but that some embryos exhibited clearing of RARE-lacZ expression at the anterior-most region of the embryo (n=6/15) and that all embryos exhibited reduced RARE-lacZ expression posteriorly in the tailbud (Fig. 7A). Cyp26a1 expression following this treatment was observed in the tailbud where RARE-lacZ expression was reduced, consistent with its known function in RA degradation (n=3; Fig. 7B); expression was similar to that in untreated embryos, which normally downregulate Cyp26a1 anteriorly and upregulate expression in the tailbud by E8.25 (MacLean et al., 2001). However, this RA treatment resulted in a shift in Cyp26c1 expression to the anterior-most region of the embryo (n=7; Fig. 7C; compare with untreated control in Fig. 3F). A closer Comparison demonstrates that this new Cyp26c1 expression domain lies in approximately where RARE-lacZ expression first begins to clear anteriorly (Fig. 7D–E). This provides evidence that Cyp26c1 is functioning to degrade RA in this anteriorly shifted domain.
Fig. 7.
Teratogenic dose of RA induces anterior shifts in the Cyp26c1 expression domain and the Hoxb1/vHnf1 expression boundary. All embryos were subjected to a 20 mg/kg dose of RA at E7.5, then analyzed 18 hours later at E8.25 (see Fig 2–Fig 4 for untreated controls). (B,C,F,H) lateral view; (G,I) dorsal view; (A) ventral view; (D,E) anterior view. (A) RARE-lacZ expression is observed to the anterior tip of some embryos (left embryo), but in other embryos RARE-lacZ expression is reduced at the anterior tip (upper arrowhead in right embryo). RARE-lacZ expression in all embryos is greatly reduced posteriorly in the tailbud (lower arrowheads) when compared with the trunk. (B–C) Cyp26a1 expression is observed in the tailbud (arrowhead) similar to untreated embryos (MacLean et al., 2001), whereas Cyp26c1 expression has been shifted to the anterior-most region of the embryo (arrowhead). A lateral view shows that most Cyp26c1 expression is neural, with mesodermal expression being greatly reduced. (D,E) Comparison of anterior expression patterns of RARE-lacZ and Cyp26c1 demonstrates that the abnormal domain of Cyp26c1 expression lies in the region where RARE-lacZ expression first clears anteriorly. (F,G) The anterior domain of Hoxb1 expression (arrowhead) has been shifted further anteriorly from where it should normally reside at r4. (H–I) The anterior border of vHnf1 expression (arrowhead) has been shifted further anteriorly. This abnormal anterior border of vHnf1 expression lies posterior to the abnormal expression domains of Cyp26c1 and Hoxb1.
Previous studies have shown that a 20 mg/kg RA dose administered at E7.5 results in an anterior shift in hindbrain Hoxb1 expression observed 18 hours later at E8.25 (Conlon and Rossant, 1992). We also found that Hoxb1 expression in the brain was shifted anteriorly with this RA treatment (n=5; Fig. 7F,G), plus we observed that vHnf1 expression was also shifted anteriorly under these conditions (n=6; Fig. 7H,I). RA treatment resulted in a coordinate shift in the Hoxb1 and vHnf1 expression domains such that the new posterior border of Hoxb1 expression in the brain lies at approximately the same location as the new anterior border of vHnf1 expression (Fig. 7G,I). These findings provide evidence that Cyp26c1 functions to restrict the anterior boundary of RA, and that this RA boundary helps determine the location of the Hoxb1/vHnf1 boundary.
Discussion
Mechanism of RA action during establishment of hindbrain Hoxb1 expression
The studies reported here provide new information on how RA-synthesizing and RA-degrading enzymes cooperate in regulation of hindbrain gene expression, resulting in restricted expression domains for Hoxb1 and its repressor vHnf1, as well as formation of an Irx3/vHnf1 boundary at r4/r5. Previous genetic studies have demonstrated that Raldh2 functions in RA synthesis (Niederreither et al., 1999; Mic et al., 2002) and that P450 enzymes encoded by the Cyp26 family function as RA-degrading enzymes (Sakai et al., 2001; Abu-Abed et al., 2001; Yashiro et al., 2004), but the effect of these enzymes on the spatiotem oral localization of RA activity in the hindbrain has been unclear. By carefully examining mouse head-fold stage embryos carrying the RARE-lacZ RA-reporter transgene, we have shown that RA activity coincides with the onset of Raldh2 expression in the trunk paraxial mesoderm and that RA is secreted from these cells and travels anteriorly. Furthermore, double-labeling experiments have revealed that distinct boundaries of RA activity are created by expression of Cyp26a1, which provides a short-lived r2/r3 boundary, and later of Cyp26c1, which provides a long-lived r4/r5 boundary. In addition, through analysis of Raldh2−/− mouse embryos, we discovered that hindbrain expression of vHnf1 is completely eliminated and that Irx3 expression expands posteriorly, thus indicating that repression of Hoxb1 and Irx3 posterior to r5 is indirectly controlled by RA via induction of vHnf1. This conclusion was supported by our observation that vHnf1−/− embryos exhibit expansion of Hoxb1 expression posterior to r4.
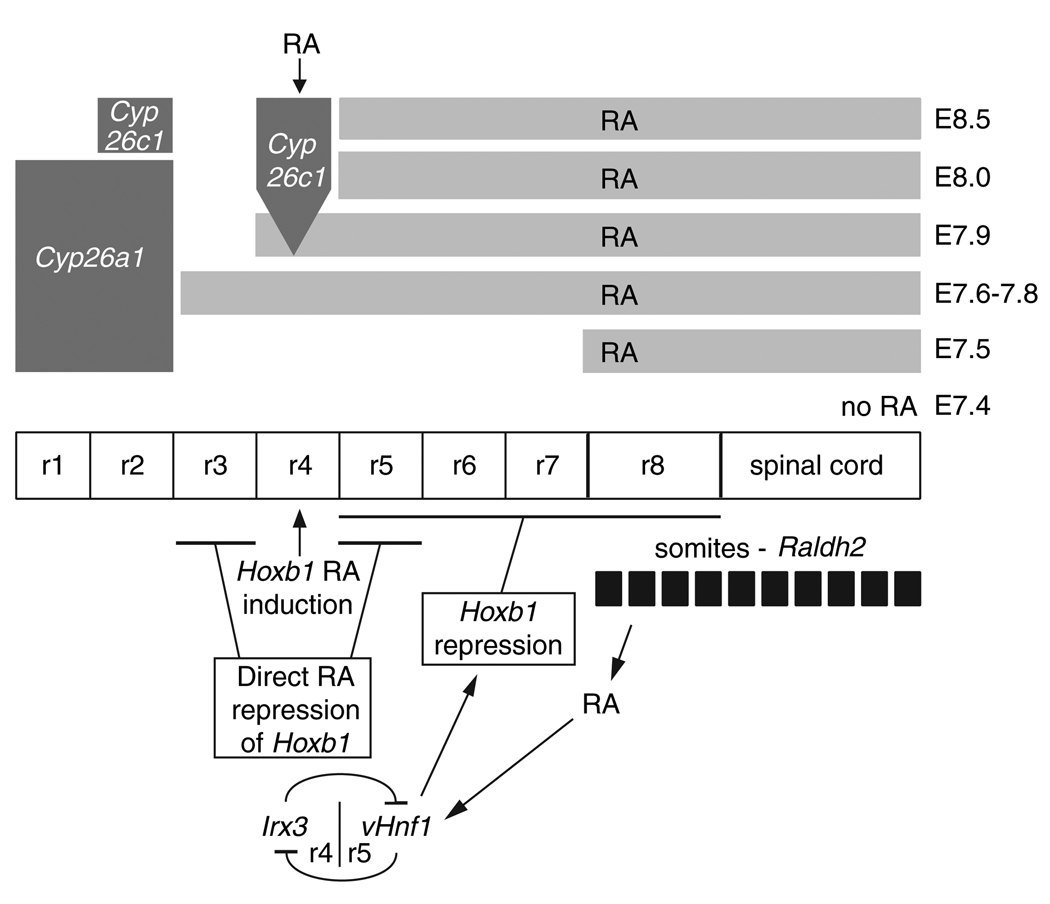
Previous studies on chick embryos treated with RA receptor antagonists provided evidence that hindbrain patterning requires graded responses to RA, with a higher RA concentration being required posteriorly (Dupé and Lumsden, 2001). Our findings with mouse embryos demonstrate that the length of RA exposure is also important for regulating hindbrain gene expression as RA activity was observed for only 6–8 hours in r3–r4 (E7.6-E7.9), but for at least 20 hours in r5–r8 (E7.6-E8.5). The studies we present also make it clear that a stable RA gradient is not established across the hindbrain, but that the initial gradient of RA entering the posterior hindbrain is converted by RA-degrading enzymes into RA boundaries that shift over time such that anterior tissues receive a short pulse of RA and posterior tissues receive a long pulse of RA.
Our studies also reveal that the initial RA boundary at r2/r3 is independent of RA activity, as Cyp26a1 expression does not require RA, but that the shift to an r4/r5 boundary is dependent upon RA to activate Cyp26c1 expression in r4. Our findings provide evidence that RA is present in presumptive r3, r4 and r5 to directly regulate the activity of RA receptors binding the 3′ and 5′ RAREs of Hoxb1, which have previously been shown to be required for induction up to the r3/r4 border and repression in r3/r5, respectively (Marshall et al., 1994; Studer et al., 1994). Interestingly, the RA present in r5 can repress Hoxb1 not only directly through the previously established 5′ RARE, but also indirectly by inducing vHnf1 in the posterior hindbrain up to the r4/r5 border. A model of hindbrain gene expression incorporating the role of shifting boundaries of RA activity is presented in Fig. 8.
Fig. 8.
Role of shifting RA boundaries during posterior hindbrain segmentation. A model for RA action during mouse hindbrain development is shown. Our findings demonstrate that RA generated by Raldh2 in the paraxial mesoderm travels anteriorly to presumptive r3 and r4 during establishment of Hoxb1 expression, but that this is very transient. Initially, RA forms an early anterior boundary at r2/r3 (next to the r2 border of Cyp26a1 expression) followed soon after by a late anterior boundary at r4/r5 next to the r4 border of Cyp26c1 expression). RA is therefore present in r3/r4/r5 to directly regulate Hoxb1 induction and repression through previously described 3′ and 5′ RAREs (Marshall et al., 1994; Studer et al., 1994). We also demonstrate that vHnf1 requires RA for posterior hindbrain expression, and that vHnf1 is needed to limit the posterior extent of Hoxb1 expression to help establish the r4/r5 expression boundary for Hoxb1. Thus, RA acts directly to induce Hoxb1 expression and then RA acts both directly and indirectly (through induction of vHnf1) to restrict Hoxb1 expression to r4. Also shown is a mutual repression between Irx3 and vHnf1 at the r4/r5 boundary, which has been demonstrated in zebrafish (Lecaudey et al., 2004) and is supported by our findings in mouse.
It is unclear how RA-liganded RA receptors bound to the 5′ RARE of Hoxb1 result in r3/r5 repression whereas RA-liganded RA receptors bound to the 3′ RARE of Hoxb1 stimulate widespread induction in the posterior hindbrain up to r4. It is possible that additional transcriptional regulatory proteins expressed in r3 and/or r5 bind near the 5′ RARE and interact with an RA-liganded RA receptor in such a fashion that results in Hoxb1 repression in r3 and r5 rather than induction. In addition, as a loss of Cyp26a1 function in mouse embryos results in ectopic expression of Hoxb1 in r3 (but not r5) and presumably higher RA activity in r3 (Sakai et al., 2001; Abu-Abed et al., 2001), this suggests that the 5′ RARE is insufficient to repress Hoxb1 in r3. Perhaps the relative activities of the 3′ and 5′ RAREs are determined by levels of RA such that a higher than normal level of RA in r3 disrupts 5′ RARE repression and/or allows 3′ RARE induction of Hoxb1.
RA signaling is required for neural expression of vHnf1
Our findings shed more light on the mechanism whereby vHnf1 is induced in the posterior hindbrain. We find that RA activity is required for induction of mouse vHnf1 as previously reported for zebrafish vHnf1 (Hernandez et al., 2004). However, other previous studies have suggested that Hoxb1 may be sufficient to induce vHnf1 in zebrafish (Choe and Sagerström, 2004). Our studies suggest that Hoxb1 is not sufficient for vHnf1 induction as we find persistent expression of Hoxb1 in the posterior hindbrain and anterior spinal cord of Raldh2−/− embryos, but a complete lack of vHnf1 expression in this domain of Hoxb1 expression. Thus, we suggest that RA signaling is needed to activate vHnf1. A direct effect of RA is plausible as a DR1 retinoid response element has been identified in the promoter region of mouse vHnf1 (Power and Cereghini, 1996). As the vHnf1 DR1 response element was found to bind retinoid receptors less efficiently than a DR5 retinoid response element (such as those present near the RARβ and Hoxb1 genes), it was suggested that vHnf1 may be less responsive to RA than genes with DR5 retinoid response elements (Power and Cereghini, 1996). We suggest that this lower responsiveness of vHnf1 to RA may be crucial to the mechanism whereby its expression domain is limited to a more posterior boundary than that of Hoxb1. The r5 anterior limit of vHnf1 expression is in fact closer to the paraxial mesodermal source of RA than the r4 anterior limit of Hoxb1, and we have demonstrated that RA activity is transient in r4 but more long lived in r5.
Hoxb1 expression in the absence of RA activity
Studies on quail embryos reported that vitamin A deficiency (VAD) results in a complete absence of r4–r8, with the hindbrain consisting of an enlarged r1–r3 (devoid of Hoxb1 expression) joined to the anterior spinal cord, which retained expression of Hoxb1 (Maden et al., 1996). A similar phenotype was also observed in mouse Raldh2−/− embryos at E8.25-E8.5, which lacked the characteristic r4 stripe of Hoxb1 expression, but retained a diffuse domain of Hoxb1 expression in the posterior-most region of the hindbrain next to the spinal cord junction (Niederreither et al., 2000). However, results from chick embryos treated with retinoid receptor antagonists suggested that RA deficiency leads to elimination of r5–r8 but with an enlarged r4 remaining because of residual expression of Hoxb1 and other r4 markers in the posterior-most region of the hindbrain adjacent to the spinal cord (Dupé and Lumsden, 2001). Results from VAD rat embryos also suggested that r4 is not totally eliminated (Baybutt et al., 2000). The residual Hoxb1 expression observed in the posterior hindbrain of mouse Raldh2−/− embryos was originally not interpreted as an indication of r4 character, possibly owing to the observation of an extension of the Krox20 r3 expression domain all the way to the spinal cord junction (Niederreither et al., 2000). We note that the r4 expression domain of Cyp26c1 is completely missing in the posterior hindbrain of Raldh2−/− embryos (but an expanded r2 expression domain was observed), providing further evidence that at least some aspects of r4 character have been lost in the absence of RA activity. In addition, residual expression of Hoxb1 in the posterior hindbrain of Raldh2−/− embryos may be due to the loss of vHnf1 expression, which normally functions to repress Hoxb1 in that location. Rather than being an indicator of r4 character, this residual Hoxb1 expression may be the remnants of that which normally occurs independent of RA in posterior neuroectoderm up to the level of the node prior to anterior expansion of Hoxb1 expression into the hindbrain (Forlani et al., 2003).
Conserved function for RA boundaries
The model of mouse hindbrain RA activity proposed here is likely to be conserved in other vertebrate embryos. Raldh2 expression occurs in the trunk paraxial mesoderm of all vertebrate embryos analyzed and Cyp26 homologs expressed in the hindbrain exist as well (see Introduction). Studies on zebrafish embryos have demonstrated that vHnf1 functions as a repressor of Hoxb1 in r5 (Wiellette and Sive, 2003) and it was recently reported that RA is required for vHnf1 expression in the zebrafish hindbrain (Hernandez et al., 2004). In addition, zebrafish Irx3 (iro7) and vHnf1 function as mutual repressors needed to establish an r4/r5 expression boundary (Lecaudey et al., 2004). Thus, shifting boundaries of RA activity that regulate the spatiotem oral expression patterns of Hoxb1, vHnf1 and Irx3 may be a general feature of vertebrate hindbrain development. However, there have been no reports of methods to localize RA activity in the hindbrain of other vertebrates to directly test this. Thus, our studies highlight the importance of using mouse embryos carrying the RARE-lacZ transgene as a model system to decipher RA function, as this is the only system in which the location of RA activity can be determined during development.
Acknowledgments
We thank the following for mouse cDNAs used to re are in situ hybridization robes: R. Krumlauf (Hoxb1), C. Hui (Irx3), M. Petkovich and Cytochroma (Cyp26a1 and Cyp26c1), and D. Wilkinson (Krox20 and Epha2). We also thank J. Rossant for providing RARE-lacZ mice and P. Soriano for the MORE mouse strain. L.G. was supported by a fellowshi from the French Ministry of Research and the pierre et Marie Curie University. This work was funded by National Institutes of Health grant GM62848 (G.D.), and by the Institut Pasteur and the Centre National de la Recherche Scientifique (J.B.).
References
- Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang HL, Deltour L, Hayamizu TF, Zgombic-Knight M, Duester G. Retinoic acid synthesis in mouse embryos during gastrulation and craniofacial development linked to class IV alcohol dehydrogenase gene expression. J. Biol. Chem. 1996;271:9526–9534. doi: 10.1074/jbc.271.16.9526. [DOI] [PubMed] [Google Scholar]
- Barbacci E, Reber M, Ott M-O, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. doi: 10.1242/dev.126.21.4795. [DOI] [PubMed] [Google Scholar]
- Baybutt RC, Hu L, Molteni A. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J. Nutr. 2000;130:1159–1165. doi: 10.1093/jn/130.5.1159. [DOI] [PubMed] [Google Scholar]
- Becker N, Seitanidou T, Murphy P, Mattei MG, Topilko P, Nieto MA, Wilkinson DG, Charnay P, Gilardi-Hebenstreit P. Several receptor tyrosine kinase genes of the Eph family are segmentally expressed in the developing hindbrain. Mech. Dev. 1994;47:3–17. doi: 10.1016/0925-4773(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Blumberg B, Bolado J, Jr, Moreno TA, Kintner C, Evans RM, Papalopulu N. An essential role for retinoid signaling in anteroposterior neural patterning. Development. 1997;124:373–379. doi: 10.1242/dev.124.2.373. [DOI] [PubMed] [Google Scholar]
- Bosse A, Zulch A, Becker MB, Torres M, Gomez-Skarmeta JL, Modolell J, Gruss P. Identification of the vertebrate Iroquois homeobox gene family with overlapping expression during early development of the nervous system. Mech. Dev. 1997;69:169–181. doi: 10.1016/s0925-4773(97)00165-2. [DOI] [PubMed] [Google Scholar]
- Chen YL, Pollet N, Niehrs C, Pieler T. Increased XRALDH2 activity has a posteriorizing effect on the central nervous system of Xenopus embryos. Mech. Dev. 2001;101:91–103. doi: 10.1016/s0925-4773(00)00558-x. [DOI] [PubMed] [Google Scholar]
- Choe S-K, Sagerström CG. Paralog group 1 hox genes regulate rhombomere 5/6 expression of vhnf1, a repressor of rostral hindbrain fates, in a meis-dependent manner. Dev. Biol. 2004;271:350–361. doi: 10.1016/j.ydbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Thépot B, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1β in visceral endoderm differentiation. Development. 1999;126:4785–4794. doi: 10.1242/dev.126.21.4785. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Gresh L, Fiette L, Tronche F, Schutz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- Cohen DR, Cheng CW, Cheng SH, Hui CC. Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech. Dev. 2000;91:317–321. doi: 10.1016/s0925-4773(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Rossant J. Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development. 1992;116:357–368. doi: 10.1242/dev.116.2.357. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur. J. Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Dupé V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Fujii H, Sato T, Kaneko S, Gotoh O, Fujii-Kuriyama Y, Osawa K, Kato S, Hamada H. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. EMBO J. 1997;16:4163–4173. doi: 10.1093/emboj/16.14.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalas A. ArRAnging the hindbrain. Trends Neurosci. 2002;25:61–64. doi: 10.1016/s0166-2236(02)02067-2. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Krumlauf R. Retinoid signalling and hindbrain patterning. Curr. Opin. Genet. Dev. 2000;10:380–386. doi: 10.1016/s0959-437x(00)00100-3. [DOI] [PubMed] [Google Scholar]
- Goddard JM, Rossel M, Manley NR, Capecchi MR. Mice with targeted disruption of Hoxb-1 fail to form the motor nucleus of the VIIth nerve. Development. 1996;122:3217–3228. doi: 10.1242/dev.122.10.3217. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Küchler AM, Schulte-Merker S, Geisler R, et al. Retinoic acid signalling in the zebrafish embryo is necessary during presegmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Haselbeck RJ, Hoffmann I, Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev. Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RE, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Holland ND. Expression of AmphiHox-1 and AmphiPax-1 in amphioxus embryos treated with retinoic acid: Insights into evolution and patterning of the chordate nerve cord and pharynx. Development. 1996;122:1829–1838. doi: 10.1242/dev.122.6.1829. [DOI] [PubMed] [Google Scholar]
- Hollemann T, Chen YL, Grunz H, Pieler T. Regionalized metabolic activity establishes boundaries of retinoic acid signalling. EMBO J. 1998;17:7361–7372. doi: 10.1093/emboj/17.24.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P, Wilkinson D, Krumlauf R. Patterning the vertebrate head: Murine Hox 2 genes mark distinct subpopulations of premigratory and migrating cranial neural crest. Development. 1991;112:43–50. doi: 10.1242/dev.112.1.43. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: What are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kolm PJ, Apekin V, Sive H. Xenopus hindbrain patterning requires retinoid signaling. Dev. Biol. 1997;192:1–16. doi: 10.1006/dbio.1997.8754. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993;9:106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–4346. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Lecaudey V, Anselme I, Rosa F, Schneider-Maunoury S. The zebrafish Iroquois gene iro7 positions the r4/r5 boundary and controls neurogenesis in the rostral hindbrain. Development. 2004;131:3121–3131. doi: 10.1242/dev.01190. [DOI] [PubMed] [Google Scholar]
- Lufkin T. Transcriptional control of Hox genes in the vertebrate nervous system. Curr. Opin. Genet. Dev. 1996;6:575–580. doi: 10.1016/s0959-437x(96)80086-4. [DOI] [PubMed] [Google Scholar]
- MacLean G, Abu-Abed S, Dollé P, Tahayato A, Chambon P, Petkovich M. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech. Dev. 2001;107:195–201. doi: 10.1016/s0925-4773(01)00463-4. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile MH. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Umesono K, Evans RM. The retinoid receptors. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids Biology, Chemistry, and Medicine. 2nd edn. New York: Raven Press; 1994. pp. 319–349. [Google Scholar]
- Marshall H, Studer M, Pöpperl H, Aparicio S, Kuroiwa A, Brenner S, Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc. Natl. Acad. Sci. USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Pöpperl H, Bienz M, Studer M, Chan S-K, Aparicio S, Brenner S, Mann RS, Krumlauf R. Segmental expression of Hoxb-1 is controlled by a highly conserved autoregulatory loop dependent upon exd/pbx. Cell. 1995;81:1031–1042. doi: 10.1016/s0092-8674(05)80008-x. [DOI] [PubMed] [Google Scholar]
- Power SC, Cereghini S. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol. Cell. Biol. 1996;16:778–791. doi: 10.1128/mcb.16.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, Maden M. Expression of the retinoic acid catabolising enzyme CYP26B1 in the chick embryo and its regulation by retinoic acid. Gene Exp. Patt. 2003;3:621–627. doi: 10.1016/s1567-133x(03)00112-1. [DOI] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, Maden M. Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev. Dyn. 2004;230:509–517. doi: 10.1002/dvdy.20025. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Arcioni L, Andrews PW, Boncinelli E, Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Studer M, Pöpperl H, Marshall H, Kuroiwa A, Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265:1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–634. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Thaller C, Sockanathan S, Petkovich M, Jessell TM, Eichele G. Complementary domains of retinoic acid production and degradation in the early chick embryo. Dev. Biol. 1999;216:282–296. doi: 10.1006/dbio.1999.9487. [DOI] [PubMed] [Google Scholar]
- Tahayato A, Dollé P, Petkovich M. Cyp26c1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Exp. Patt. 2003;3:449–454. doi: 10.1016/s1567-133x(03)00066-8. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Houzelstein D. In situ hybridization and β-galactosidase: a powerful combination for analysing transgenic mice. Trends Genet. 1995;11:42. doi: 10.1016/s0168-9525(00)88994-5. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extraembryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Van der Wees J, Schilthuis JG, Koster GH, Diesveld-Schipper H, Folkers GE, van der Saag PT, Dawson MI, Shudo K, van der Burg B, Durston AJ. Inhibition of retinoic acid receptor-mediated signalling alters positional identity in the developing hindbrain. Development. 1998;125:545–556. doi: 10.1242/dev.125.3.545. [DOI] [PubMed] [Google Scholar]
- White JC, Shankar VN, Highland M, Epstein ML, DeLuca PF, Clagett-Dame M. Defects in embryonic hindbrain development and fetal resorption resulting from vitamin A deficiency in the rat are prevented by feeding pharmacological levels of all-trans-retinoic acid. Proc. Natl. Acad. Sci. USA. 1998;95:13459–13464. doi: 10.1073/pnas.95.23.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiellette EL, Sive H. vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain. Development. 2003;130:3821–3829. doi: 10.1242/dev.00572. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]
- Wilkinson DG. Molecular mechanisms of segmental patterning in the vertebrate hindbrain and neural crest. BioEssays. 1993;15:499–505. doi: 10.1002/bies.950150802. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Cook M, Boncinelli E, Krumlauf R. Segmental expression of Hox-2 homeobox-containing genes in the developing mouse hindbrain. Nature. 1989;341:405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev. Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]