Abstract
Dlx5 plays an important role in the embryonic development of mineralized tissues. We hypothesized that Dlx5 also functions in regulating post-natal bone formation in mice. To prove this hypothesis, we infected 5-day-old bone sialoprotein (BSP)/avian retroviral receptor gene (TVA) transgenic mice with replication-competent retroviral vectors expressing wild-type Dlx5 (RCAS-Dlx5WT) and mutated Dlx5 at arginine (R) 31 of its homeodomain (RCAS-Dlx5RH). Immunohistochemistry indicated that RCAS-Dlx5WT increased BSP and osteopontin (OPN) expression, whereas it decreased that of osteocalcin (OC). RCAS-Dlx5RH mediated opposite effects. Semi-quantitative RT-PCR confirmed these results. Ex vivo overexpression of RCAS-Dlx5WT in BSP/TVA calvarial cells promoted, whereas that of RCAS-Dlx5RH inhibited, mineralized nodule formation as compared with that in control cells. Our results suggest that Dlx5 promotes expression of early markers of osteogenic differentiation and increases mineralization post-natally.
Keywords: Dlx5 (distal-less-related gene), homeodomain, BSP/TVA transgenic mice, extracellular matrix proteins, mineralization
INTRODUCTION
Homeodomains are 60-amino-acid-long DNA-binding domains that play a critical role in the genetic control of development. Homeodomains are extremely conserved during evolution, and they show a conserved structure consisting of 3 helical regions (Gehring et al., 1994). The arginine substitution in helix 2 (R31, located at position 31 of the homeodomain) disrupts 1 of 2 arginines that directly interact with the phosphate backbone of the α-strand of the core DNA motif (Gehring et al., 1994), and point mutation at this position has been reported to be associated with human congenital diseases, with alterations in human mineralized tissues (D'Elia et al., 2001). For example, R31 mutation in Msx1 leads to selective tooth agenesis (Vastardis et al., 1996), in Msx2 to defective skull ossification (Wilkie et al., 2000), in LMX1B to nail patella syndrome (including abnormalities of bone, joints, fingernails, and kidneys) (McIntosh et al., 1998), and in PITX2 to Rieger syndrome (including dental hypoplasia, anomalies of the anterior chamber of the eye, and a protuberant umbilicus) (Semina et al., 1996) (APPENDIX Fig.).
Distal-less related genes (Dlx) encode homeodomain-containing proteins that play roles in craniofacial patterning, sensory organ morphogenesis and osteogenesis (Merlo et al., 2000). One of the members of the Dlx family, Dlx5, is expressed in developing skeletal elements, discrete neuronal tissues, and teeth (Simeone et al., 1994). The expression of Dlx5 exhibited a stage-specific pattern, with maximal expression occurring in the final stages of osteoblast differentiation in vitro, when the extracellular matrix mineralizes (Ryoo et al., 1997). Dlx5 is also expressed at the onset of chondrocyte maturation and may regulate the process by promoting conversion of premature proliferating chondrocytes into hypertrophying chondrocytes (Ferrari and Kosher, 2002). Lack of a functional Dlx5 in mice results in developmental malformation, including shorter snout, open fontanelle, shorter Meckel's cartilage, defective skull, cleft palate, and deformed mandible (Acampora et al., 1999).
Since mice lacking Dlx5 die soon after birth (Depew et al., 2002), the in vivo involvement of Dlx5 in regulating osteoblast differentiation and bone formation post-natally requires the use of alternative experimental strategies, such as the BSP/TVA model described in our study. In this system, a 4.9-kb murine bone sialoprotein (BSP) promoter is linked to an avian retroviral receptor gene (TVA), and only cells that normally express BSP will express TVA in the BSP/TVA model, and hence will be targeted for viral infection with replication-competent retroviral vectors (RCAS) (Li et al., 2005). BSP is a major non-collagenous protein in mineralized tissues and is expressed in mineralizing tissues (Fisher et al., 1990; Chen et al., 1992; Paz et al., 2005). In the current study, we analyzed the involvement of Dlx5 in regulating matrix protein expression and mineralization post-natally by infecting BSP/TVA mice with RCAS vectors expressing the wild-type sequence of Dlx5 (RCAS-Dlx5WT) or a homeodomain-mutated form of Dlx5 (RCAS-Dlx5RH). The mutated form of Dlx5 (RCAS-Dlx5RH) contained a point mutation of R into H at position 166 (R31 of the homeodomain). We chose this residue for mutagenesis based on the evidence mentioned above that, in several transcription factors whose homeodomain is closely related to that of Dlx5, such as Msx1, Msx2, LMX1B, and PITX2, mutation of this R31 has been associated with alterations in human mineralized tissues. In this study, it was hypothesized that Dlx5 also functions in regulating post-natal bone formation.
MATERIALS & METHODS
BSP/TVA Transgenic Mouse Line
The establishment of the BSP/TVA homozygous transgenic line has been described previously (Li et al., 2005). Mice were maintained and used in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals, prepared by the Institute on Laboratory Animal Resources, National Research Council, and by guidelines established by the Institutional Animal Care and Use Committee of the Tufts-New England Medical Center in Boston, MA.
Viral Vector Construction
We used an avian retroviral receptor (TVA)-based RCAS retroviral system (Federspiel and Hughes, 1997; Holland and Varmus, 1998). The RCAS empty vector and RCAS-GFP construct were kindly provided by Dr. Stephen Hughes (NCI-Frederick, MD, USA). Intact Dlx5 cDNA was kindly provided by Dr. Stephen E. Harris (University of Texas, Health Sciences Center at San Antonio). The PCR product of Dlx5 was ligated into Flag-pcDNA3 expression vector at BamHI and XbaI sites. The mutated Dlx5 (Dlx5RH) was generated by in vitro mutagenesis with a GeneTailor Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA, USA). To generate the RCAS viral constructs, we subcloned the 900-bp Hind III-Xba I fragments of pcDNA3-FLAG-Dlx5 or mutated Dlx5 into a Cla I site in RCAS, using a CLA 12NCO shuttle vector. The viral constructs containing wild-type Dlx5 and its mutated form were named RCAS-Dlx5WT and RCAS-Dlx5RH, respectively. The RCAS empty vector was used as a negative control. To titer infection efficiency in the in vitro experiments, we used RCASGFP as a positive control and monitored green fluorescence in the infected cells by fluorescence microscopy.
Production of High-titer Viral Stocks and Infection of BSP/TVA Transgenic Mice
RCAS constructs were transfected into the established chicken fibroblast cell line DF1 (CL-12203), to produce viral stocks as previously described (Tu et al., 2006). Viral supernatant was concentrated, and the viral pellet was re-suspended to a titer of 108 cfu/mL and stored at −80°C before use. Thirty 5-day-old BSP/TVA mice (10 mice in each group) were injected intraperitoneally with 500 µL of RCAS-Dlx5WT, RCAS-Dlx5RH, or empty vector viral stocks, respectively, as described (Li et al., 2005).
In vitro Transduction of RCAS-Dlx5WT and RCAS-Dlx5RH into BSP/TVA Calvarial Cells
An osteoblast cell line from BSP/TVA mice was established from calvarial bone as previously described (Ecarot-Charrier et al., 1989). Cells were routinely cultured in α-modified essential medium (α-MEM) containing 10% fetal bovine serum and 1% penicillin/streptomycin. Incubation of RCAS-Dlx5WT and RCAS-Dlx5RH with calvarial cells was performed as previously described (Tu et al., 2006).
RNA Isolation and Reverse-transcriptase Polymerase Chain-reaction (RT-PCR) Analysis
Total RNA was isolated with TRIzol Reagent (GibcoBRL/Life Technologies, Gaithersburg, MD, USA) and was used for RTPCR with SuperScript™ one-step RT-PCR with platinum Taq (Invitrogen). Specific primers for mouse BSP, OPN, ALP, OC, and GAPDH have been described previously (Li et al., 2005). PCR products were photographed and quantified with UVP Image software (UVP, Inc., Upland, CA, USA). GAPDH amplification was performed for normalization purposes.
Western Blot Analysis
Preparation of protein lysates, SDS-PAGE, and Western blot analyses were performed as previously described (Chen et al., 1992; Valverde et al., 2005). Detection of overexpressed Dlx5 or mutated Dlx5 was carried out with rabbit anti-FLAG polyclonal antibody (anti-FLAG®, 2.5 µg/mL; Sigma, St. Louis, MO, USA) and a horseradish-peroxidase-conjugated goat anti-rabbit IgG antibody (US Biological, Swampscott, MA, USA). Detection of β-actin was used as a loading control. The immunoreactions were detected by chemiluminescence with a BioImaging System (UVP).
Immunohistochemistry
Tissues were processed for immunohistochemistry as described previously (Meinel et al., 2005). Immunohistochemical studies were performed with rabbit polyclonal antibodies against BSP (a gift from Dr. L. Fisher, NIH/NIDCR), OPN (a gift from Dr. L. Fisher, NIH/NIDCR), and OC (from US Biological, Swampscott, MA, USA) at dilutions of 1:200 – 1:300. A non-specific IgG was used as negative control. Slides were assessed and photographed by light microscopy (Carl Zeiss, Inc., Oberkochen, Germany). The localization and intensity of immunohistochemical staining in bone tissues were studied by modified semi-quantitative methods, as previously described (Chen et al., 1991; Paz et al., 2005). In brief, on each slide there were tissue sections from all 3 groups. The relative intensities of immunohistochemical staining were classified as intense (+++), moderate (++), weak (+), or negative (−). For each slide, the intensity of staining in the control group was classified as moderate, and staining intensity and tissue sections from the other 2 groups were compared with this standard. Stronger staining than control was classified as intense (+++), lower as weak (+), and no staining as negative. All slides were coded to prevent the introduction of examiner bias.
Mineralization Assays in vitro
In vitro alizarin red staining was performed essentially as described (Tu et al., 2006), after calvarial cells were maintained in culture medium supplemented with 5 mM β-glycerophosphate and 50 µg/mL of ascorbic acid for 14 days. Digital images of the stained cultures were taken at 10× magnification. Bone nodule number was counted in 6 different fields of each well and in 3 independent experiments.
Statistical Analysis
Results are expressed as mean ± SE of 3 or more independent experiments. We used one-way ANOVA to test significance, using the software package Statgraphic statistical graphics system (STSC, Inc., Rockville, MD, USA). Values of p lower than 0.05 were considered statistically significant.
RESULTS
BSP/TVA-driven Overexpression of Dlx5 and Mutated Dlx5
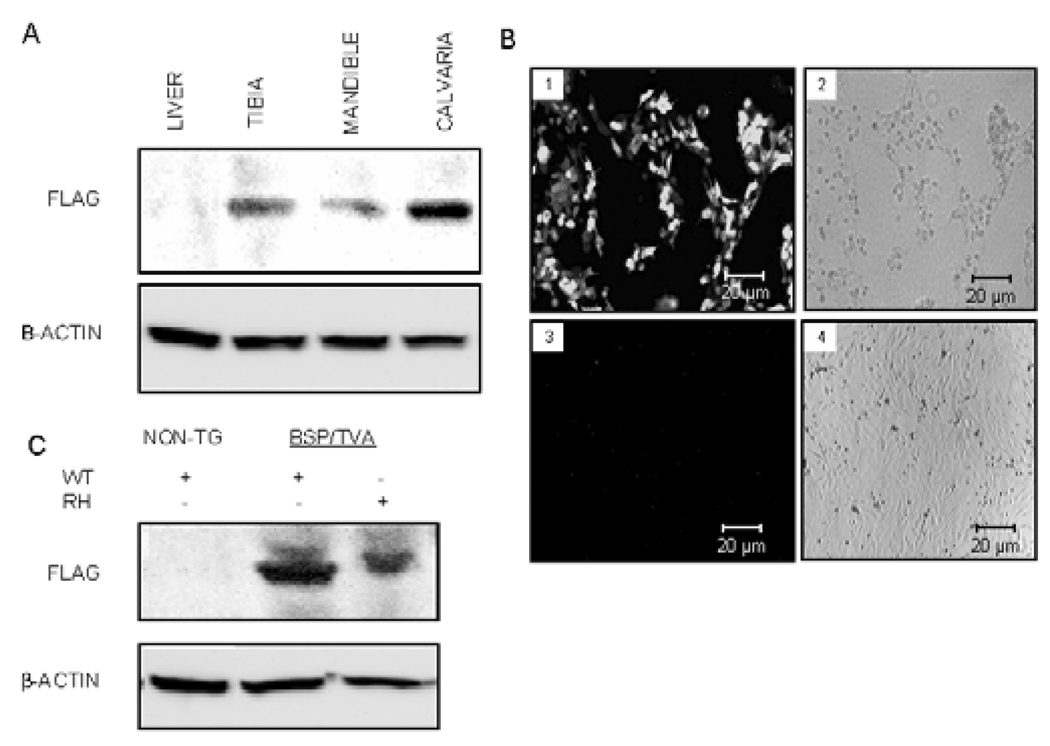
To confirm the tissue specificity of viral targeting, we performed Western blot analysis with an anti-FLAG antibody and/or detection of GFP fluorescence. Infection of BSP/TVA mice with RCAS-Dlx5 led to positive anti-FLAG immunoreaction in bone tissues, but not in the liver. To confirm further that the viral targeting would be restricted to cells expressing TVA, we performed ex vivo infection studies with RCAS-GFP in calvarial cells. Whereas more than 80% of the calvarial cells from BSP/TVA mice exhibited green fluorescence, calvarial cells from wild-type mice were GFP negative (Fig. 1B). In addition, calvarial cells from BSP/TVA mice that were infected ex vivo with RCAS-Dlx5WT and RCAS-Dlx5RH showed a positive immunoreaction for the FLAG antibody, whereas those from wild-type mice demonstrated a lack of immunoreaction in Western blot analysis (Fig. 1C).
Figure 1.
BSP/TVA-driven Dlx5 or mutated Dlx5 overexpression in mineralized tissues and calvarial cultures. Western blot analyses were performed with tissues from BSP/TVA mice 4 days after infection with RCAS-Dlx5 (i.e., on day 9) and calvarial cells 3 days after in vitro infection with RCAS-Dlx5 or RCAS-Dlx5RH. (A) Expression analysis upon in vivo delivery of Dlx5 viral constructs. BSP/TVA mice were infected with RCAS-Dlx5, and expression of overexpressed Dlx5 was detected with an anti-FLAG antibody on day 9. An antibody for β-actin was used as a loading control. Protein lysates were obtained from tibial, mandibular, and calvarial tissues isolated from BSP/TVA mice infected with the RCAS-Dlx5 construct and from a representative soft tissue (i.e., liver). (B) GFP expression analyses upon ex vivo infection of RCAS-GFP into calvarial cells from BSP/TVA transgenic and non-transgenic mice. Calvarial cells from BSP/TVA mice (1,2) or wild-type mice that did not express TVA (2,4) were transfected with the RCASGFP construct. We used GFP expression to titer infection efficiency and to confirm the specificity of the overexpression to cells that express TVA 3 days after the infection. The in vitro-infected cells were photographed by fluorescence (1,3) and phase contrast (2,4) microscopy. Green fluorescence was detected in infected cells from BSP/TVA mice (1), but not in those of normal mice, due to the lack of TVA expression (3). (C) Western blot analysis upon ex vivo delivery of viral constructs. The analysis was performed with protein lysates from calvarial cells derived from non-transgenic mice (without TVA expression) before and after being infected ex vivo with RCAS-Dlx5. Calvarial cells from BSP/TVA mice were infected ex vivo with RCAS-Dlx5WT or RCAS-Dlx5RH viral constructs. Expression of exogenous Dlx5 constructs and the loading control β-actin were detected as described above.
Dlx5 Overexpression Alters the Expression Pattern of Extracellular Matrix Proteins
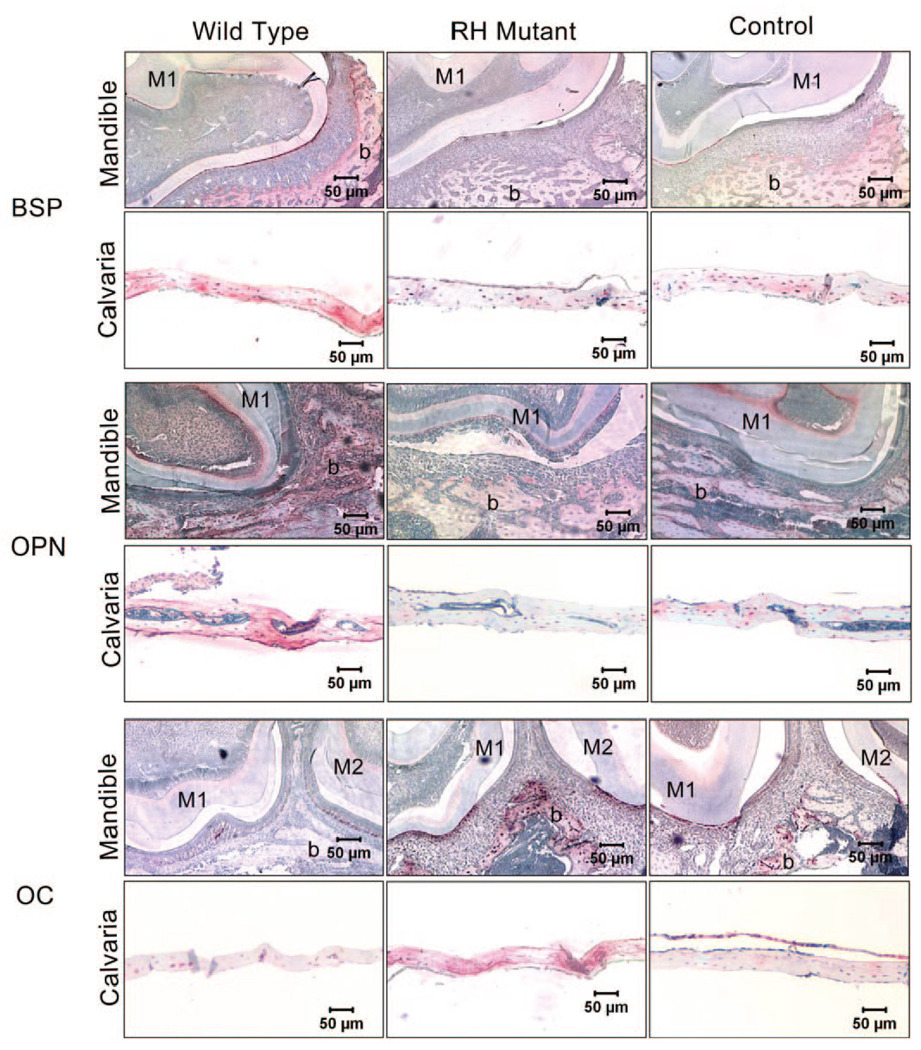
Nine days after infection of BSP/TVA transgenic mice, we performed immunohistochemical analyses to evaluate the bone phenotype and expression of the extracellular matrix proteins BSP, OPN, and OC. Infection with RCAS-Dlx5WT resulted in an increase in BSP and OPN expression levels, and a decrease in those of OC in calvarial and mandibular tissues, as compared with levels in control animals. In contrast, infection with RCAS-Dlx5RH led to a decrease in BSP and OPN and an increase in OC expression levels, as compared with levels in control mice (Fig. 2).
Figure 2.
Immunohistochemical analysis of extracellular matrix proteins OPN, BSP, and OC in bone tissues of BSP/TVA overexpressing mutated Dlx5 (RH mutant), wild-type Dlx5 (Wild-type), and empty vector (Control). Nine days after infection of BSP/TVA with viral constructs, bones were isolated, and immunohistochemistry was performed to determine the expression of OPN, BSP, and OC in calvarial and mandibular tissues. Expression levels of OPN, BSP, and OC in mandibles were shown in transverse sections of the developing first and/or second molars and the surrounding tissues at the cementumenamel junction. In control mandibular tissues, BSP and OCN were both moderately expressed in the cells (osteoblasts and osteocytes) and in bone matrix associated with the alveolar bone surface. BSP expression in mandibular tissues isolated from the wild-type group was up-regulated in the alveolar bone surface area, and moderate expression can be detected in the odontoblast layer. However, BSP expression in mandibular tissues isolated from RH mutant group was down-regulated, and there is no evident BSP expression above background. In contrast, OCN expression in the wild-type group was weaker than that in the RH mutant group. OPN expression in control mandibular tissues can also be detected in the alveolar bone surface (osteoblasts, osteocytes, and bone matrix), as well as the odontoblast layer. OPN expression in the corresponding areas in the wild-type group and the RH mutant group was up-regulated and down-regulated, respectively. Expressions of BSP, OPN, and OCN are all evident in the osteocytes and bone matrix of control calvarial tissues. While BSP and OPN expression levels are increased in the wild-type group and decreased in the RH mutant group, OCN expression level is down-regulated in the wild-type group and up-regulated in the RH mutant group. M1, the first mandibular molar; M2, the second mandibular molar; b, bone.
Overexpression of Dlx5 and Mutated Dlx5 in BSP/TVA Calvarial Cells Determined Different Expression Patterns of Extracellular Matrix Proteins
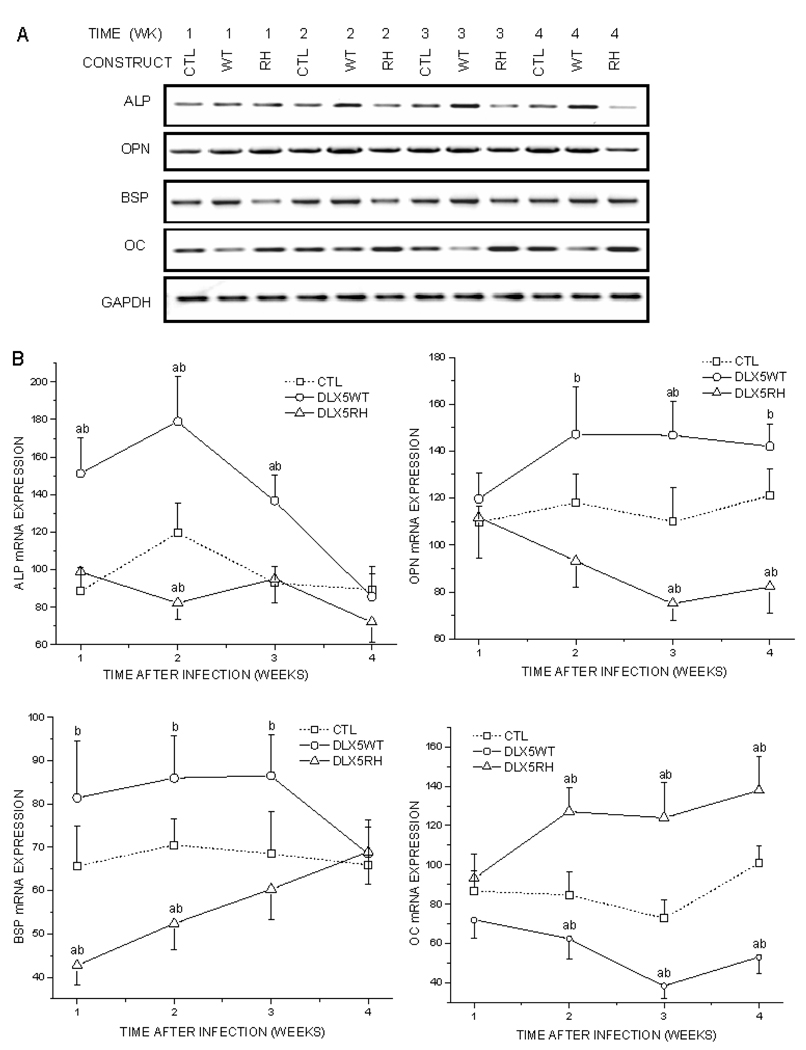
Calvarial tissues were also subjected to semi-quantitative RTPCR analyses (Fig. 3). Infection with RCASDlx5WT increased BSP and ALP within the first 3 wks after infection, as compared with control cells. In addition, it increased OPN and decreased OC expression levels from the 2nd to the 4th wks after infection. Infection with RCAS-Dlx5RH resulted in the significant up-regulation of OC expression levels from the 2nd to the 4th wks after infection. RCAS-Dlx5RH also decreased the expressions of BSP, OPN, and alkaline phosphatase (ALP) at some of the time-points at which the RCAS-Dlx5WT effects were significant.
Figure 3.
Semi-quantitative RT-PCR analysis for markers of osteoblast differentiation, including extracellular matrix proteins and ALP. BSP/TVA mice were infected with RCAS-Dlx5WT (WT), RCASDlx5RH (RH), or RCAS empty vector as a control (CTL). Calvarial tissues were isolated for 1 to 4 wks after infection, and their expression levels of ALP, OPN, BSP, and OC were analyzed by semi-quantitative RT-PCR. Expression of GAPDH was used as a loading control. (A) Representative RT-PCR experiment. (B) Normalized mRNA expression of ALP, OPN, BSP, and OC. Results (in arbitrary units) are expressed as the mean ± SE from 3 different experiments. Values of p < 0.05 were considered significantly different (ap < 0.05 vs. CTL at every specific time point; bp < 0.05 WT vs. RH).
Calvarial Cells Overexpressing Mutated Dlx5 Exhibited a Slower Mineralization Rate Than Did Those Overxepressing Wild-type Dlx5
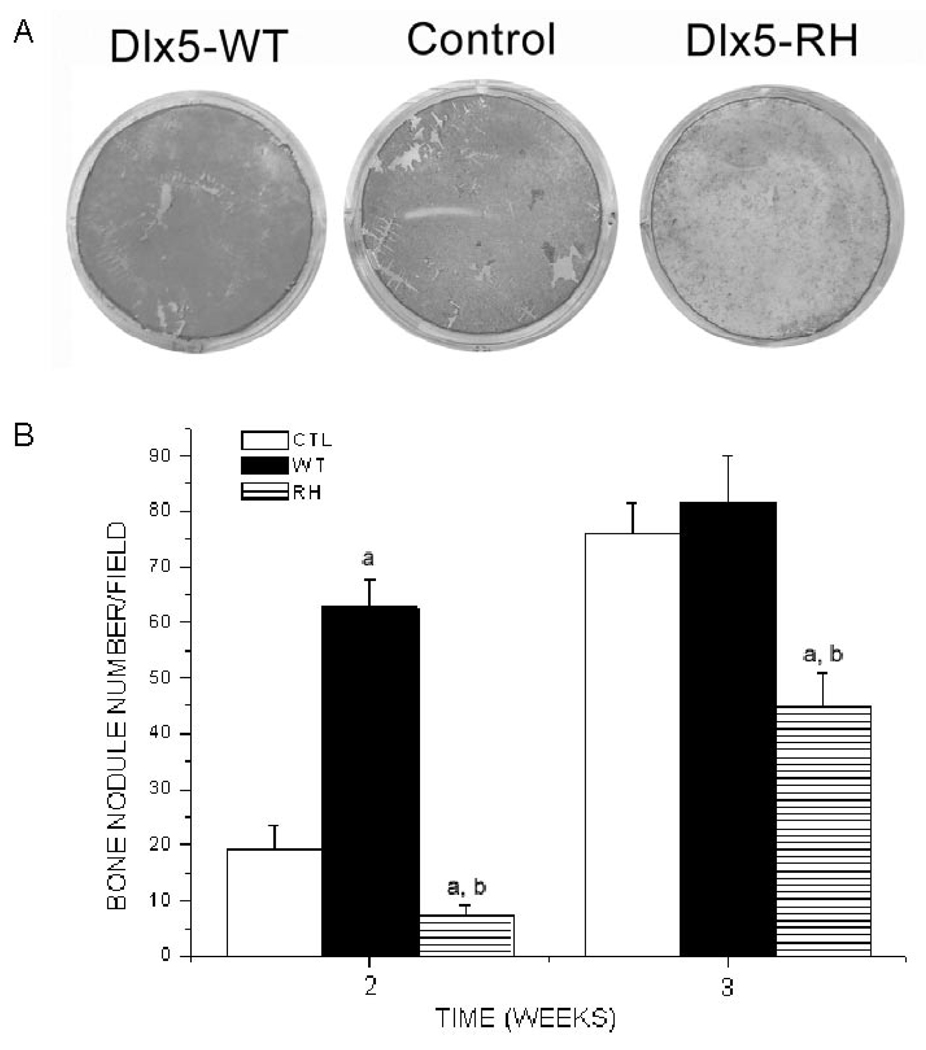
BSP/TVA calvarial cells infected with RCAS-Dlx5WT became consistently mineralized 2 and 3 wks after infection, and even 1 wk after infection in some experiments (Figs. 4A, 4B). Cells infected with RCAS-Dlx5RH exhibited a lower number of bone nodules at all times examined than did cells infected with RCAS-Dlx5WT or control vectors (Figs. 4A, 4B).
Figure 4.
Effects of RCAS-Dlx5WT, RCAS-Dlx5RH, or empty RCAS vector in bone nodule formation of calvarial cells expressing TVA. Calvarial cells from BSP/TVA mice were infected with RCAS empty vector (Control), wild-type Dlx5 (Dlx5-WT), and mutated Dlx5 (Dlx5-RH). The formation of in vitro mineralization nodules was determined by alizarin red-S histochemical staining, and the number of nodules was counted under a microscope at different time-points (1, 2, and 3 wks). (A) Representative example of alizarin red-S staining 2 wks after infection and osteogenic differentiation. (B) Numbers of nodules obtained 2 and 3 wks after infection and osteogenic differentiation. Results are the mean ± SE from 3 different experiments. Values of p < 0.05 were considered significantly different (ap < 0.05 vs. CTL at every time-point; bp < 0.05 RH vs. WT at every time-point).
DISCUSSION
In this study, we analyzed the involvement of Dlx5 in the post-natal expression of osteoblast differentiation markers and in mineralization. Immunohistochemical and RT-PCR analyses indicated that Dlx5 increased BSP, OPN, and ALP expression levels, whereas it decreased those of OC. Increases in the expression of ALP and BSP occurred during wks 1–3, while changes in the expression of OPN and OC were seen in wks 2–4. ALP and BSP are both early markers of osteogenic differentiation, while OPN and OCN are mid- and late markers of osteoblasts, respectively, which might be the reason why changes in expression levels of ALP and BSP appeared earlier than those of OCN and OPN (Chen et al., 1992; Lekic et al., 1996; Volk et al., 2005; Abe et al., 2006). The effects of mutated Dlx5 were opposite to those obtained with wild-type Dlx5. Overexpression of Dlx5 in ROS 17/2.8 osteoblast-like cells was previously found to decrease OC promoter activity and endogenous OC mRNA levels, whereas antisense inhibition of Dlx5 increased OC gene transcription (Ryoo et al., 1997). A different report demonstrated that overexpression of Dlx5 in chick calvarial osteoblast cultures (Tadic et al., 2002) also increased ALP, OPN, and BSP mRNA levels, as described in our study. Loss of OC expression in mice leads to a phenotype marked by higher bone mass and bones of improved functional quality (Ducy et al., 1996), while ALP activity is required for the mineralization process to occur (Murshed et al., 2005). OPN and BSP play roles in both mineralization and bone resorption (Ganss et al., 1999; Sodek et al., 2000; Valverde et al., 2005; Wang et al., 2006). OPN-deficient mice were reported to exhibit increased mineral content and increased crystal size/perfection consistent with in vitro data, indicating that OPN can promote osteoclast recruitment and function and inhibit mineral formation and mineral crystal growth (Sodek et al., 2000). Recombinant BSP can also promote both bone formation and bone resorption (Ganss et al., 1999; Valverde et al., 2005; Wang et al., 2006). When one considers that BSP/TVA bones exhibited a different pattern of extracellular matrix protein expression before or after infection with Dlx5 or mutated Dlx5, but did not exhibit other significant macroscopic differences, it is tempting to speculate that Dlx5 may play a role in controlling the functional quality of post-natal bone by decreasing OC expression. The apparent lack of effects of Dlx5 in affecting bone mass post-natally might be the result of the opposite effects of OC, BSP, and OPN in regulating bone formation and bone resorption.
Overexpression of Dlx5 increased the ability of BSP/TVA calvarial cells to mineralize in vitro, whereas overexpression of mutated Dlx5 decreased their mineralization potential. Homozygous mice lacking Dlx5 gene (Acampora et al., 1999) exhibited defective osteogenesis and an increase in OC expression, pointing to a role for Dlx5 in bone formation during embryonic development. Since a point mutation at R31 of the homeodomain of Dlx5 was found to inhibit mineralization and increase OC expression in our study, it is likely that the effect of this mutation is due to haploinsufficiency, as has been described for other related homeodomain-containing proteins (D'Elia et al., 2001). This notion is supported by the observation that deletion of an entire homeodomain-containing gene elicits a phenotype that is very similar to that induced by point mutations at the homeodomain. However, since mutated Dlx5 was overexpressed post-natally and only in bone-like cells in our study, the phenotype was found to be milder than in the Dlx5 knockout study, but consistent with the role of Dlx5 in promoting osteogenesis.
To summarize, we have demonstrated that Dlx5 promotes the expression of early markers of osteoblast differentiation during post-natal bone development and induces formation of bone nodules. Future studies combining the use of the BSP/TVA transgenic model and of targeted point mutations in homeodomain - containing proteins are expected to lead to a wealth of information about the involvement of these transcription factors in the post-natal regulation of osteogenesis.
ACKNOWLEDGMENTS
This study was supported by NIH grants DE14537 and DE16710 to JC. We appreciate the secretarial assistance of Amanda Fix.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.iadrjournals.org/cgi/content/full/87/1/45/DC1.
REFERENCES
- Abe T, Nomura S, Nakagawa R, Fujimoto M, Kawase I, Naka T. Osteoblast differentiation is impaired in SOCS-1-deficient mice. J Bone Miner Metab. 2006;24:283–290. doi: 10.1007/s00774-006-0685-0. [DOI] [PubMed] [Google Scholar]
- Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang Q, McCulloch CA, Sodek J. Immunohistochemical localization of bone sialoprotein in foetal porcine bone tissues: comparisons with secreted phosphoprotein 1 (SPP-1, osteopontin) and SPARC (osteonectin) Histochem J. 1991;23:281–289. doi: 10.1007/BF01045047. [DOI] [PubMed] [Google Scholar]
- Chen J, Shapiro HS, Sodek J. Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res. 1992;7:987–997. doi: 10.1002/jbmr.5650070816. [DOI] [PubMed] [Google Scholar]
- D'Elia AV, Tell G, Paron I, Pellizzari L, Lonigro R, Damante G. Missense mutations of human homeoboxes: a review. Hum Mutat. 2001;18:361–374. doi: 10.1002/humu.1207. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B, Bouchard F, Delloye C. Bone sialoprotein II synthesized by cultured osteoblasts contains tyrosine sulfate. J Biol Chem. 1989;264:20049–20053. [PubMed] [Google Scholar]
- Federspiel MJ, Hughes SH. Retroviral gene delivery. Methods Cell Biol. 1997;52:179–214. [PubMed] [Google Scholar]
- Ferrari D, Kosher RA. Dlx5 is a positive regulator of chondrocyte differentiation during endochondral ossification. Dev Biol. 2002;252:257–270. doi: 10.1006/dbio.2002.0862. [DOI] [PubMed] [Google Scholar]
- Fisher LW, McBride OW, Termine JD, Young MF. Human bone sialoprotein. Deduced protein sequence and chromosomal localization. J Biol Chem. 1990;265:2347–2351. [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Affolter M, Burglin T. Homeodomain proteins. Annu Rev Biochem. 1994;63:487–526. doi: 10.1146/annurev.bi.63.070194.002415. [DOI] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic P, Sodek J, McCulloch CA. Osteopontin and bone sialoprotein expression in regenerating rat periodontal ligament and alveolar bone. Anat Rec. 1996;244:50–58. doi: 10.1002/(SICI)1097-0185(199601)244:1<50::AID-AR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Li L, Zhu J, Tu Q, Yamauchi M, Sodek J, Karsenty G, et al. An in vivo model to study osteogenic gene regulation: targeting an avian retroviral receptor (TVA) to bone with the bone sialoprotein (BSP) promoter. J Bone Miner Res. 2005;20:1403–1413. doi: 10.1359/JBMR.050316. [DOI] [PubMed] [Google Scholar]
- McIntosh I, Dreyer SD, Clough MV, Dunston JA, Eyaid W, Roig CM, et al. Mutation analysis of LMX1B gene in nail-patella syndrome patients. Am J Hum Genet. 1998;63:1651–1658. doi: 10.1086/302165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinel L, Fajardo R, Hofmann S, Langer R, Chen J, Snyder B, et al. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Merlo GR, Zerega B, Paleari L, Trombino S, Mantero S, Levi G. Multiple functions of Dlx genes. Int J Dev Biol. 2000;44:619–626. [PubMed] [Google Scholar]
- Murshed M, Harmey D, Millan JL, McKee MD, Karsenty G. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz J, Wade K, Kiyoshima T, Sodek J, Tang J, Yamauchi M, et al. Tissue- and bone cell-specific expression of bone sialoprotein is directed by a 9.0 kb promoter in transgenic mice. Matrix Biol. 2005;24:341–352. doi: 10.1016/j.matbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ryoo HM, Hoffmann HM, Beumer T, Frenkel B, Towler DA, Stein GS, et al. Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol Endocrinol. 1997;11:1681–1694. doi: 10.1210/mend.11.11.0011. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Pannese M, D'Esposito M, Stornaiuolo A, Gulisano M, et al. Cloning and characterization of two members of the vertebrate Dlx gene family. Proc Natl Acad Sci USA. 1994;91:2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Tadic T, Dodig M, Erceg I, Marijanovic I, Mina M, Kalajzic Z, et al. Overexpression of Dlx5 in chicken calvarial cells accelerates osteoblastic differentiation. J Bone Miner Res. 2002;17:1008–1014. doi: 10.1359/jbmr.2002.17.6.1008. [DOI] [PubMed] [Google Scholar]
- Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006;341:1257–1265. doi: 10.1016/j.bbrc.2006.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20:1669–1679. doi: 10.1359/JBMR.050511. [DOI] [PubMed] [Google Scholar]
- Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE. A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet. 1996;13:417–421. doi: 10.1038/ng0896-417. [DOI] [PubMed] [Google Scholar]
- Volk SW, Diefenderfer DL, Christopher SA, Haskins ME, Leboy PS. Effects of osteogenic inducers on cultures of canine mesenchymal stem cells. Am J Vet Res. 2005;66:1729–1737. doi: 10.2460/ajvr.2005.66.1729. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou HY, Salih E, Xu L, Wunderlich L, Gu X, et al. Site-specific in vivo calcification and osteogenesis stimulated by bone sialoprotein. Calcif Tissue Int. 2006;79:179–189. doi: 10.1007/s00223-006-0018-2. [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, et al. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet. 2000;24:387–390. doi: 10.1038/74224. [DOI] [PubMed] [Google Scholar]