Abstract
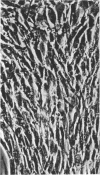
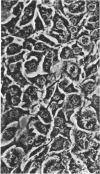
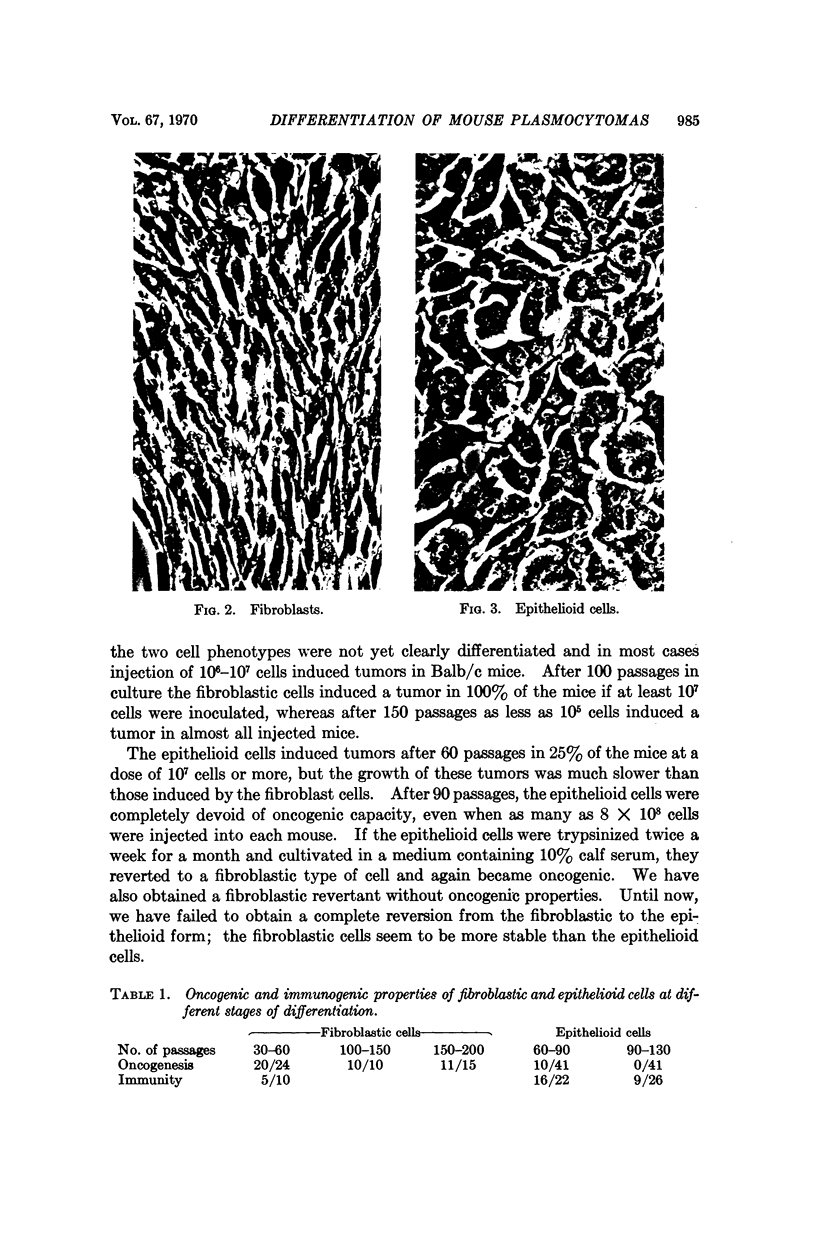
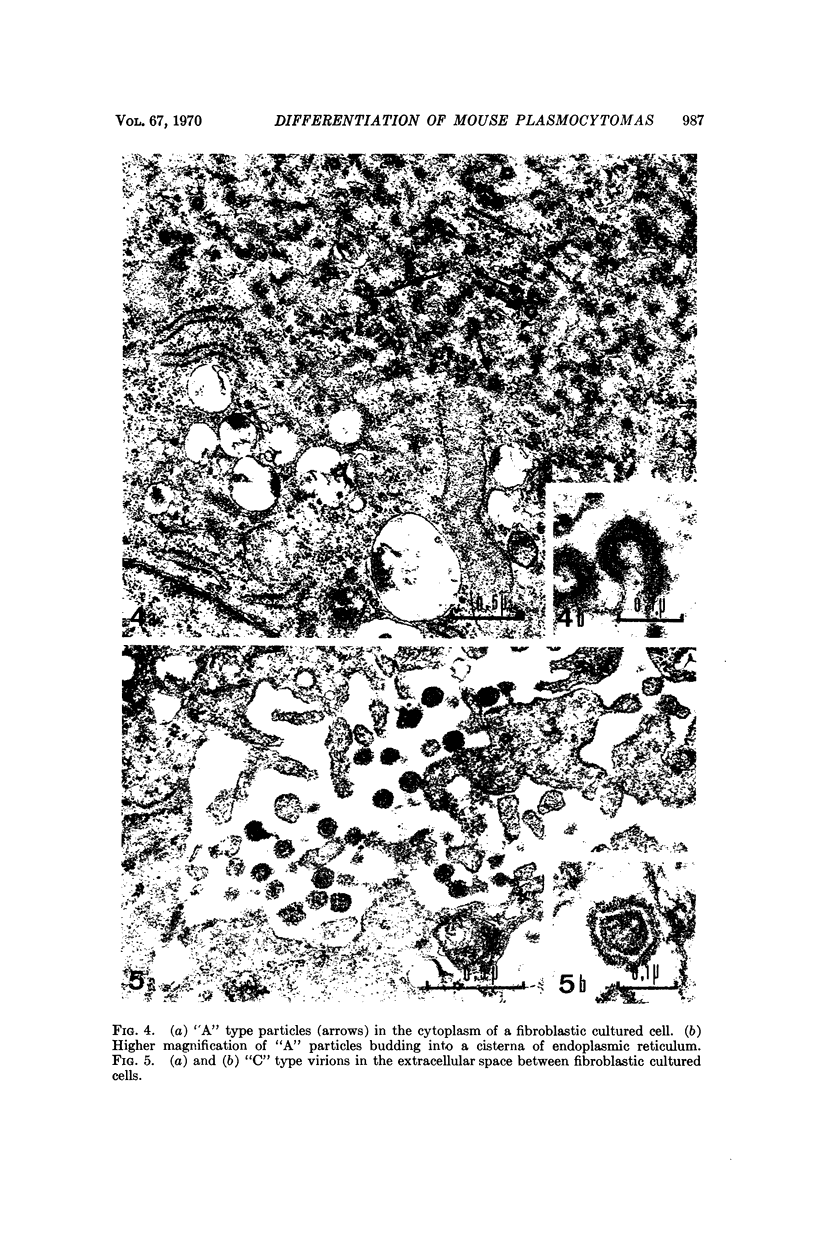
Mouse myelomas from Balb/c and C3H mice were established in tissue culture for more than 200 passages. When allowed to attach to a surface, they differentiated into two stabilized forms, one of which was fibroblast-shaped, grew without contact inhibition, and could be transplanted back to mice. The other had the morphology of epithelial cells, showed contact inhibition, and was not transplantable back to mice. For one strain (MOPC 173) it was demonstrated that both types of cells synthesize molecules with idiotypic determinants of the original myeloma protein. The relationship between the host cells and a leukemia-type virus present in the original tumor cells has been studied during different stages of cellular differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahn R. D., Cahn M. B. Heritability of cellular differentiation: clonal growth and expression of differentiation in retinal pigment cells in vitro. Proc Natl Acad Sci U S A. 1966 Jan;55(1):106–114. doi: 10.1073/pnas.55.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., McCULLOCH E. A. Virus-like bodies in a transplantable mouse plasma cell tumour. Nature. 1958 Apr 26;181(4617):1213–1213. doi: 10.1038/1811213b0. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Leduc E. H., Avrameas S., Bouteille M. Ultrastructural localization of antibody in differentiating plasma cells. J Exp Med. 1968 Jan 1;127(1):109–118. doi: 10.1084/jem.127.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Hanaoka M. Immunoglobulin synthesis by cultured mouse myeloma cells. J Immunol. 1969 Jun;102(6):1486–1497. [PubMed] [Google Scholar]
- Pettengill O. S., Sorenson G. D. Murine myeloma cells in suspension culture. Exp Cell Res. 1967 Sep;47(3):608–613. doi: 10.1016/0014-4827(67)90017-1. [DOI] [PubMed] [Google Scholar]
- Przybylski R. J., Blumberg J. M. Ultrastructural aspects of myogenesis in the chick. Lab Invest. 1966 May;15(5):836–863. [PubMed] [Google Scholar]
- Rabinowitz Z., Sachs L. The formation of variants with a reversion of properties of transformed cells. I. Variants from polyoma-transformed cells grown in vivo. Virology. 1969 Jun;38(2):336–342. doi: 10.1016/0042-6822(69)90375-4. [DOI] [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. 3. Blocks in defective synthesis. J Mol Biol. 1968 Dec;38(3):273–288. doi: 10.1016/0022-2836(68)90386-0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin assembly in a mouse myeloma. Proc Natl Acad Sci U S A. 1968 Jun;60(2):683–690. doi: 10.1073/pnas.60.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Munro A., Ohno S. Immunoglobulin biosynthesis. I. A myeloma variant secreting light chain only. J Mol Biol. 1968 Dec;38(3):253–262. doi: 10.1016/0022-2836(68)90384-7. [DOI] [PubMed] [Google Scholar]