The first crystal structure of Leishmania mexicana pyruvate kinase (LmPYK) obtained at a neutral pH. LmPYK was co-crystallized with the small molecule 1,3,6,8-pyrenetetrasulfonic acid, which provides a helpful intermolecular bridge between macromolecules.
Keywords: pyruvate kinase; Leishmania mexicana; 1,3,6,8-pyrenetetrasulfonic acid
Abstract
The inclusion of novel small molecules in crystallization experiments has provided very encouraging results and this method is now emerging as a promising alternative strategy for crystallizing ‘problematic’ biological macromolecules. These small molecules have the ability to promote lattice formation through stabilizing intermolecular interactions in protein crystals. Here, the use of 1,3,6,8-pyrenetetrasulfonic acid (PTS), which provides a helpful intermolecular bridge between Leishmania mexicana PYK (LmPYK) macromolecules in the crystal, is reported, resulting in the rapid formation of a more stable crystal lattice at neutral pH and greatly improved X-ray diffraction results. The refined structure of the LmPYK–PTS complex revealed the negatively charged PTS molecule to be stacked between positively charged (surface-exposed) arginine side chains from neighbouring LmPYK molecules in the crystal lattice.
1. Introduction
The inherent difficulty in crystallizing many biological macromolecules still requires innovative approaches. One such method is the inclusion of small molecules in the crystallization conditions which have the ability to promote lattice formation through stabilizing intermolecular interactions in protein crystals. The success of such a collection of small molecules has been demonstrated for quite a few protein candidates (McPherson & Cudney, 2006 ▶) with various methods of lattice stabilization (Larson et al., 2007 ▶), leading quickly to their availability from Hampton Research (Silver Bullets). Here, we report the use of the small molecule 1,3,6,8-pyrenetetrasulfonic acid (PTS; Fig. 2d), which provides a helpful molecular link between Leishmania mexicana PYK (LmPYK) macromolecules in the crystal (similar to that seen for Silver Bullets; Larson et al., 2007 ▶), promoting the rapid formation of a stable crystal lattice at neutral pH with greatly improved X-ray diffraction results.
2. Materials and methods
2.1. Protein expression and purification
LmPYK was overexpressed and purified using a modified version of the published protocol (Rigden et al., 1999 ▶), all purification steps were performed at 281 K. Briefly, LmPYK samples were concentrated and buffer-exchanged (PD-10 column; Amersham Biosciences) into buffer A [50 mM triethanolamine–HCl (TEA) buffer pH 7.2, 20 mM KCl, 20% glycerol] using standard protocols. LmPYK samples were loaded onto a (35 ml) DEAE Sepharose ion-exchange column pre-equilibrated in buffer A at 0.5 ml min−1. The column was washed (3.0 ml min−1) with ten column volumes of buffer A. LmPYK was eluted over a five column-volume elution gradient (0–60%) with buffer B (50 mM TEA buffer pH 7.2, 200 mM KCl, 20% glycerol). Fractions containing LmPYK were pooled, concentrated and buffer-exchanged into buffer C (20 mM TEA buffer pH 7.2, 20% glycerol). LmPYK samples were concentrated to 30 mg ml−1 and aliquots were stored at 253 K for up to three months.
2.2. Crystallization and data collection
Purified LmPYK aliquots (30 mg ml−1) were diluted to 15 mg ml−1 using a buffer containing 20 mM triethanolamine–HCl pH 7.2 and 1 mM PTS. Single crystals of LmPYK complexed with PTS were obtained at 277 K by vapour diffusion using the hanging-drop technique. The drops were formed by mixing 1.5 µl protein solution with 1.5 µl well solution composed of 12–16% PEG 8000, 20 mM triethanolamine–HCl buffer pH 7.2, 50 mM MgCl2, 100 mM KCl and 10% glycerol. The drops were equilibrated against a reservoir filled with 0.5 ml well solution. Crystals grew to maximum dimensions (1.0 × 0.2 × 0.1 mm) after 24–48 h. Prior to data collection, crystals were equilibrated for 14 h over a well solution composed of 14–18% PEG 8000, 20 mM triethanolamine–HCl buffer pH 7.2, 50 mM MgCl2, 100 mM KCl and 25% glycerol, which eliminated the appearance of ice rings. Intensity data were collected (ϕ scans of 1° over 220°) to a resolution of 2.1 Å from a single crystal flash-frozen in liquid nitrogen at 100 K on beamline ID23-1 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. Data were processed with MOSFLM (Leslie, 2002 ▶) and scaled with SCALA (Evans, 2006 ▶).
2.3. Structure determination
The structure of the LmPYK–PTS complex was solved by molecular replacement using the program Phaser (McCoy et al., 2007 ▶). A monomer from the previously determined tetrameric structure of LmPYK (PDB code 1pkl; Rigden et al., 1999 ▶) was divided into two search domains. Domain 1 (residues Pro87–Pro187, a complete B-domain) and domain 2 (residues 1–86, 188–481 and 489–498) served as search models. There was a clear molecular-replacement solution with two monomers in the crystallographic asymmetric unit, with the crystallographic twofold generating a tetramer. Only one of the crystallographically independent B-domains had interpretable electron density. The resulting model was then subjected to ten cycles of rigid-body refinement using the program REFMAC (Murshudov et al., 1997 ▶). The PTS molecule was added where clear unbiased F o − F c electron density was observed and the model was then subjected to several rounds of restrained refinement using tight geometrical restraints; manual adjustments were made using Coot (Emsley & Cowtan, 2004 ▶), resulting in R and R free values of 26% and 29%, respectively. Water molecules were added to the model using Coot and after several rounds of restrained refinement (using loose geometrical restraints) the final model yielded R and R free values of 18.91% and 23.91%, respectively. The B-domain for which no clear electron density was observed was modelled in an identical position to that of the well defined B-domain and a final round of refinement yielded R and R free values of 18.55% and 23.88%, respectively. All atoms were modelled with full occupancy and with individual atomic B factors. The geometry of the model was assessed using MolProbity (Davis et al., 2007 ▶). Although electron density was well defined for Thr296 (a key active-site residue), it exhibited geometry outside the Ramachandran plot, which is common in many PYK structures. This is primarily owing to a restricted geometry which facilitates interactions with active-site ligands. The coordinates of the LmPYK–PTS complex have been deposited in the Protein Data Bank as entry 3ktx.
3. Results and discussion
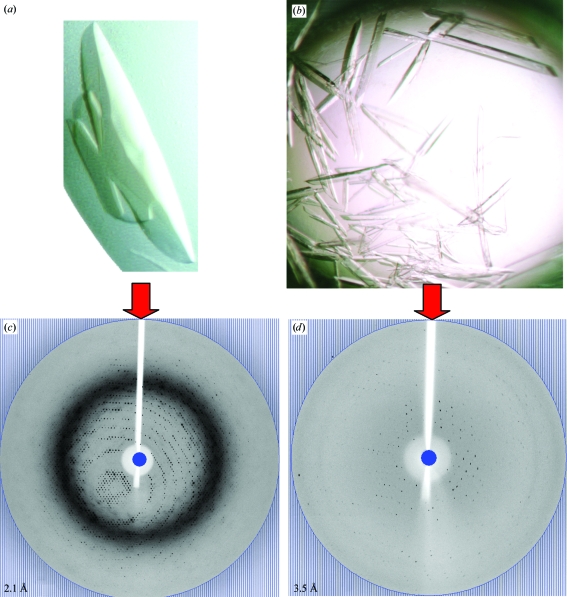
LmPYK crystals grown in the presence of 0.25 mM PTS (Fig. 1 ▶ a) diffracted to a maximum resolution of 2.1 Å (Fig. 1 ▶ c; see Table 1 ▶ for data-collection and refinement statistics). LmPYK–PTS data were indexed in the orthorhombic space group I222. There are two monomers per asymmetric unit (ASU) and the biologically relevant tetramer is generated by a crystallographic twofold rotation axis. Ordered electron density was only observed for one of the two B-domains, which is not uncommon in PYK structures (Valentini et al., 2002 ▶; Wernimont et al., unpublished work) as this domain is highly flexible (average B factor of 52 Å2, with a range from 43 to 64 Å2) and as a result is often found in various orientations in PYK structures (Larsen et al., 1994 ▶; Valentini et al., 2002 ▶; Christofk et al., 2008 ▶). For completion, the missing B-domain in chain B was modelled in an identical position to that of chain A. Ordered electron density was not observed for the effector loops (residues Ala481–Tyr488), which are normally disordered when the effector molecule is unbound (Rigden et al., 1999 ▶). Both chains were similar, with a root-mean-square deviation of 0.15 Å for all Cα atoms of residues 1–498 and an r.m.s.d. of 0.15 Å for all Cα atoms of residues 1–87 and 188–480 (excluding the B-domains). The LmPYK–PTS tetramer was superimposed onto the inactive LmPYK 1pkl (chains EFGH), resulting in an r.m.s. fit for all Cα atoms of 0.72 Å, indicating that the LmPYK–PTS structure was in the inactive (T) conformation.
Figure 1.
Improvement of LmPYK crystal quality on crystallization in the presence of PTS. (a, b) Light-microscope photographs of LmPYK crystals in the presence (a) and absence (b) of PTS. (c, d) A typical 1° oscillation image obtained during data collection from LmPYK crystals grown in the presence (c) and absence (d) of PTS. The edge of the oscillation image corresponds to the blue circle and the maximum resolution of the image is shown in the bottom left corner.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the outer shell.
| Data collection | |
| Space group | I222 |
| Unit-cell parameters (Å) | a = 122, b = 130, c = 165 |
| Wavelength (Å) | 1.0 |
| Resolution (Å) | 2.1 |
| No. of reflections | 696295 |
| No. of unique reflections | 76957 |
| Rmerge (%) | 7.60 (52.70) |
| Rmeas (%) | 8.00 (56.10) |
| Rp.i.m. (%) | 2.70 (18.90) |
| I/σ(I) | 20.10 (4.20) |
| Completeness (%) | 99.90 (100.00) |
| Refinement | |
| Resolution range | 20.69–2.10 |
| No. of reflections in working set | 73091 |
| No. of reflections in test set | 3865 |
| Rcryst (%) | 18.55 |
| Rfree (%) | 23.88 |
| Average B factor (protein) (Å2) | 44.68 |
| No. of protein atoms | 7506 |
| No. of water molecules | 639 |
| R.m.s.d. from ideal geometry | |
| Bond lengths (Å) | 0.02 |
| Bond angles (°) | 1.82 |
| Ramachandran plot | |
| Allowed (%) | 98.20 [958/976] |
| Generously allowed (%) | 99.80 [974/976] |
| Disallowed (%) | 0.20 [2/976] |
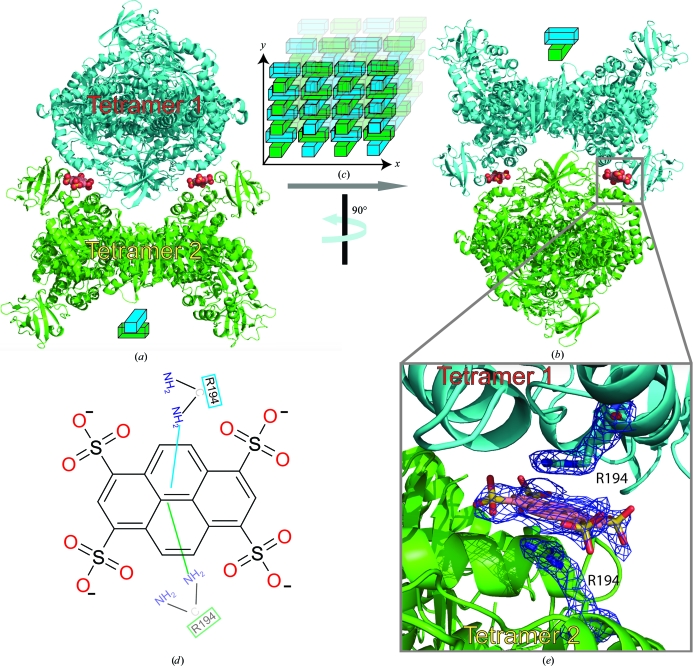
The incorporation of two PTS molecules within the crystal lattice, at the junction between two crystallographically related LmPYK tetramer molecules (Figs. 2 ▶ a and 2 ▶ b), allows tetramers to pack along the crystallographic twofold screw axis, forming a PTS-stabilized lattice (Fig. 2 ▶ c). The two tetramers are drawn together by two PTS molecules, which form a negatively charged bridge between two positively charged arginine side chains (Fig. 2 ▶ e). An important feature of cocrystallizing LmPYK with PTS is the very significant decrease in the time taken (from two weeks to 2 d) to grow high-quality diffracting LmPYK crystals. The addition of PTS resulted in the growth of large crystals within 24–48 h (Fig. 1 ▶ a) which diffracted to a greatly improved resolution of 2.1 Å (compare Figs. 1 ▶ c and 1 ▶ d, with and without PTS). Sufficient data could not be obtained from LmPYK crystals grown under similar conditions but in the absence of PTS; therefore, a structural comparison of PYK in the presence and absence of PTS could not be completed.
Figure 2.
Stabilization of the LmPYK crystal lattice as a result of cocrystallization with PTS. (a, b) Two orthogonal views of two LmPYK tetramers observed in the crystal lattice stabilized by PTS (shown as spheres). The orientations of the LmPYK tetramers are shown as blocks and repetition of LmPYK in this PTS-stabilized manner forms the crystal lattice (c). (d) A schematic drawing of a PTS molecule and the stacking interactions of Arg194 with pyrene. (e) A molecule of PTS lies at the interface between two protein molecules (blue and green) in the LmPYK crystal. The PTS molecule is shown with electron density from a 2F o − F c map. The map was calculated at 2.1 Å and is contoured at 1σ.
LmPYK (Rigden et al., 1999 ▶) and LmPYK Glu451Trp (Tulloch et al., 2008 ▶) crystallized in the presence of ammonium sulfate under acidic conditions (pH 4.0–4.6) resulted in structures with resolution limits of 2.35 and 3.3 Å, respectively. An additional complication caused by crystallizing the allosteric enzyme using ammonium sulfate was the tendency for sulfate ions to compete with the binding of the natural phosphometabolites (adenosine triphosphate, fructose-2,6-bisphosphate and phosphoenolpyruvate) and active-site and effector-site ligands (Tulloch et al., 2008 ▶). The low pH required for the crystallization of LmPYK also presented problems concerning the charged states of amino acids and the subsequent effect upon the conformational state observed in the crystal structures. LmPYK (apo) was crystallized at a more neutral pH (7.3–8.5) using PEG 4000 as the major precipitant (Rigden et al., 1999 ▶). However, these crystals (Fig. 1 ▶ b) were difficult to obtain and only diffracted to a maximum resolution of between 3.4 and 3.5 Å (Fig. 1 ▶ d). PTS provides a useful crosslink between LmPYK macromolecules in the crystal, forming a stable crystal lattice at a neutral pH with greatly improved X-ray diffraction results. The novel properties of PTS as a crystallization additive have been further exploited in the successful determination of structures of LmPYK in complex with various lead inhibitors. Refinement of these structures is currently in progress and will be published at a later date.
Supplementary Material
PDB reference: pyruvate kinase, 3ktx
Acknowledgments
We would like to thank the staff at the ESRF, Grenoble and the staff of the Edinburgh Protein Production Facility (EPPF). This project was funded by the MRC and by the European Commission through its INCO-DEV programme. Use of the Edinburgh Protein Production Facility was supported by The Wellcome Trust and the Scottish University Life Sciences Alliance.
References
- Christofk, H. R., Vander Heiden, M. G., Wu, N., Asara, J. M. & Cantley, L. C. (2008). Nature (London), 452, 181–186. [DOI] [PubMed]
- Davis, I. W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L. W., Arendall, W. B. III, Snoeyink, J., Richardson, J. S. & Richardson, D. C. (2007). Nucleic Acids Res.35, W375–W383. [DOI] [PMC free article] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Larsen, T. M., Laughlin, L. T., Holden, H. M., Rayment, I. & Reed, G. H. (1994). Biochemistry, 33, 6301–6309. [DOI] [PubMed]
- Larson, S. B., Day, J. S., Cudney, R. & McPherson, A. (2007). Acta Cryst. D63, 310–318. [DOI] [PubMed]
- Leslie, A. G. W. (2002). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst.40, 658–674. [DOI] [PMC free article] [PubMed]
- McPherson, A. & Cudney, B. (2006). J. Struct. Biol.156, 387–406. [DOI] [PubMed]
- Murshudov, G. N., Vagin, A. A. & Dodson, E. J. (1997). Acta Cryst. D53, 240–255. [DOI] [PubMed]
- Rigden, D. J., Phillips, S. E. V., Michels, P. A. M. & Fothergill-Gilmore, L. A. (1999). J. Mol. Biol.291, 615–635. [DOI] [PubMed]
- Tulloch, L. B., Morgan, H. P., Hannaert, V., Michels, P. A. M., Fothergill-Gilmore, L. A. & Walkinshaw, M. D. (2008). J. Mol. Biol.383, 615–626. [DOI] [PubMed]
- Valentini, G., Chiarelli, L. R., Fortin, R., Dolzan, M., Galizzi, A., Abraham, D. J., Wang, C., Bianchi, P., Zanella, A. & Mattevi, A. (2002). J. Biol. Chem.277, 23807–23814. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: pyruvate kinase, 3ktx
PDB reference: pyruvate kinase, 3ktx