A specific Lys–Arg cross-link is observed in trimeric barnase crystals.
Keywords: glutaraldehyde, cross-linking, arginine, lysine, barnase
Abstract
In addition to the common use of glutaraldehyde to nonspecifically cross-link protein crystals through lysine residues disposed on the surface of the protein, the use of gentle vapour diffusion of glutaraldehyde offers a convenient way to limit polymerization and to allow slow diffusion throughout the crystal. In the case of trimeric barnase crystals, a specific cross-link was observed between an lysine side chain and an arginine side chain that were spatially disposed at the ideal distance on the protein surface in the three monomers. Here, the direct observation of a specific Lys–Arg cross-link site is reported and a mechanism is proposed for the reaction.
1. Introduction
The stability of protein crystals usually involves a balance between several parameters (pH, temperature, salt concentration, the buffer in the mother liquor etc.). Any slight modification of this balance can bring about an increase in the fragility of the crystals and may cause them to break and/or dissolve completely. A long-known method of tackling this problem is to cross-link crystals with glutaraldehyde, a process that has been well documented since its first applications (Quiocho & Richards, 1964 ▶; Richards & Knowles, 1968 ▶) and that has been optimized in the past decade (Lalonde et al., 1997 ▶; Lusty, 1999 ▶; Heras & Martin, 2005 ▶). Although glutaraldehyde has been widely used, the precise nature of the chemical reaction involved in the reticulation of proteins with glutaraldehyde still remains poorly documented.
Glutaraldehyde, CH2(CH2CHO)2, is a homobifunctional reagent. Its reaction with proteins is commonly assumed to involve the formation of Schiff bases between the two carbonyl ends of glutaraldehyde and positively charged amino groups on the surface of the protein. Research has indicated the best candidate to be the ∊-amino group of lysine side chains (Quiocho & Richards, 1966 ▶; Bishop & Richards, 1968 ▶; Yonath et al., 1977 ▶). In addition, monomeric glutaraldehyde easily polymerizes by condensation, giving rise to mixtures of elongated species that can in turn also cross-link intramolecular and/or intermolecular lysines in a nonspecific manner. The conjugated oligomers that exist in aqueous solution have been reviewed (Peters & Richards, 1977 ▶; Lusty, 1999 ▶). pH has often been reported to play a key role in oligomerization; however, the extent of this role has largely remained undetermined as the literature on the subject provides some contradictory indications (Haas, 1968 ▶; Korn et al., 1972 ▶). A recent modification of the glutaraldehyde protocol by using vapour diffusion instead of soaking (Morozov & Morozova, 1981 ▶; Lusty, 1999 ▶) was found to be a way to limit polymerization around the crystal in the drop; the slow diffusion of the monomer was also found to allow cross-linking throughout the crystal instead of its being limited to the surface.
A recent reinvestigation by soaking crystals of tetragonal lysozyme in glutaraldehyde led to the proposal of two different mechanisms depending on the pH conditions (Wine et al., 2007 ▶). In this case, a characteristic doublet of symmetry-related lysines (Lys13 and Lys13*) are at an ideal distance in the packing for a specific cross-link, as shown by a clear signature in the electron density. These findings complemented previous observations on triclinic lysozyme (Yonath et al., 1977 ▶; Salem et al., 2006 ▶), although they did not implicate the same lysine residues, and gave a reasonable interpretation of the mechanism of the reaction.
In the course of a general study of denaturant agents on the behaviour of protein crystals (Salem et al., 2006 ▶), we investigated the particular case of barnase crystals, which in the presence of glutaraldehyde revealed a new type of specific intermolecular cross-link that involved a unique arginine–lysine doublet.
2. Material and methods
2.1. Cross-linked crystals
Barnase (Bacillus amyloliquefaciens ribonuclease) was expressed by culturing Escherichia coli strain XL1-Blue containing the recombinant plasmid pMT1002 with the wild-type barnase gene and was purified as described previously (Hartley, 1988 ▶). Barnase crystallizes as a trimer in the trigonal space group P32 and was refined at a resolution of 1.5 Å (Martin et al., 1999 ▶). Coordinates are available from the PDB (PDB code 1a2p). Crystals were routinely obtained following the standard procedure described in Martin et al. (1999 ▶) using ammonium sulfate (AS) as the precipitating agent. However, glutaraldehyde is not stable in the presence of AS and in our case a yellowish sticky layer appeared around the crystals which made any further manipulation/extraction impossible. To avoid this problem, crystals were transferred into a PEG 400 solution (40% PEG 400, 50 mM HEPES buffer pH 7.5–8.0) for a period of 12 h prior to cross-linking experiments. Solvent exchange proceeded without any change in crystal habit, diffraction power or mosaicity. The glutaraldehyde cross-linking reaction was performed according to Lusty (1999 ▶). Glutaraldehyde was introduced into the reservoir to a final concentration of 0.5% and the crystallization drop was replaced over the reservoir for a period of 12 h (overnight) to achieve complete reticulation by vapour diffusion. The crystals were harvested as usual. The native published barnase structure (1a2p) was obtained at room temperature. For comparative reasons, all data recordings for cross-linked crystals were performed under the same conditions in standard glass capillaries.
2.2. Data recording and refinement
Diffraction data were recorded on the W32 beamline (wavelength 0.964 Å) at the LURE synchrotron facility, Orsay, France using a MAR 345 image-plate detector. One crystal was used to obtain a complete data set. Diffraction data were processed using DENZO from the HKL suite of programs and were scaled using SCALEPACK (Otwinowski & Minor, 1997 ▶). The final data statistics are shown in Table 1 ▶. The starting model was the published 1a2p structure (Martin et al., 1999 ▶) without water molecules. The three independent molecules were refined using the SHELXL program (Sheldrick, 2008 ▶) at the maximum resolution (1.95 Å), with 6% of the data being excluded from refinement for R free calculations. Rebuilding, location of water molecules and density-map analyses were performed with the O or Coot programs (Jones et al., 1991 ▶; Emsley & Cowtan, 2004 ▶). Validation of the final model was performed with PROCHECK (Laskowski et al., 1993 ▶). The coordinates were deposited in the PDB as entry 3kch. A summary of the refinement statistics is reported in Table 1 ▶.
Table 1. Summary of data collection and refinement.
Values in parentheses are for the highest resolution shell.
| Space group | P32 |
| Unit-cell parameters (Å) | a = b = 58.97, c = 82.14 |
| Z | 9 |
| Resolution (Å) | 21–1.94 (2.05–1.94) |
| Mosaicity (°) | 0.19 |
| No. of measured reflections | 85323 (11520) |
| No. of unique reflections | 22894 (3253) |
| Completeness (%) | 97.8 (87.2) |
| Rmerge† (%) | 4.3 (20.2) |
| Mean I/σ(I) | 23.2 (3.7) |
| No. of observed reflections [with I ≥ 3σ(I)] | 21733 |
| Refinement | |
| Resolution limits (Å) | 10–1.94 |
| No. of protein atoms | 8577 |
| No. of water molecules | 256 |
| No. of additional atoms in cross-links | 15 |
| R factor [22107 Fo ≥ 4σ(Fo)] | 0.144 |
| R factor (all 22894 data) | 0.148 |
| Rfree (1080 Fo) | 0.185 |
| Average B factors (Å2) | |
| Protein atoms, main chain | 14.9 |
| Protein atoms, side chains | 20.2 |
| Cross-link atoms | 28.6 |
| Water molecules | 31.6 |
| R.m.s. deviations from ideality | |
| 1–2 bonds (Å) | 0.007 |
| 1–3 bonds (Å) | 0.022 |
| Planes (Å) | 0.35 |
| Nonzero chiral volumes (Å3) | 0.046 |
| Zero chiral volumes (Å3) | 0.034 |
R
merge = 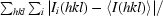
 , where I
i(hkl) is the ith observation of reflection hkl.
, where I
i(hkl) is the ith observation of reflection hkl.
3. Discussion
3.1. Barnase-monomer arrangements in the native and cross-linked structures
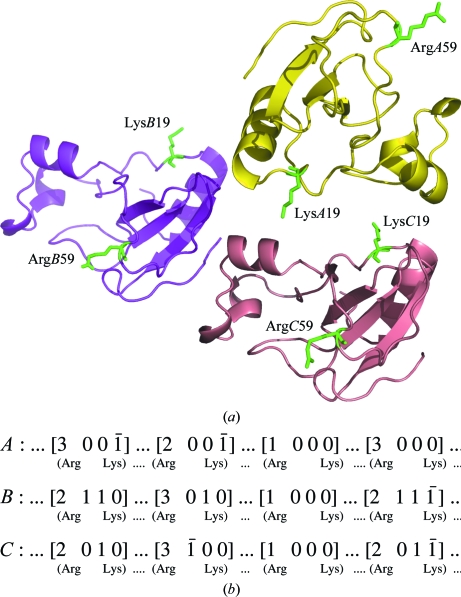
The barnase trimer is shown in Fig. 1 ▶(a). The monomer is the active form and displays the characteristic fold of bacterial RNases, with four helices wrapping a bundle of four β-strands on one side. The glutaraldehyde cross-link is known to be a highly nonspecific process that involves most of the lysine residues distributed on the surface of the protein. In the case of lysozyme this was evident from mass spectroscopy, in which dimers, trimers etc. could be detected (Wine et al., 2007 ▶). In the case of barnase, if a particular lysine is similarly engaged in a cross-link in all three monomers it should be possible to come to some conclusions about the specificity of this particular cross-link.
Figure 1.
(a) The arrangement of barnase molecules in the crystal. The two Lys19 and Arg59 residues involved in cross-links are highlighted in each molecule; they are located on opposite sides of the monomers. They build three infinite covalent networks along the z axis. (b) The three infinite networks of connected symmetry-related molecules; the symmetry codes for each are given in Table 2 ▶.
A comparison of the native and cross-linked structures did not reveal important differences, with an overall r.m.s.d. of about 0.26 Å (Cα atoms) for the three chains. However, the Zn atom of the native structure was lost in the cross-linked structure, presumably during mother-liquor exchange. As a result, one of the zinc ligands, Glu60, is observed to be disordered in two of the three molecules of cross-linked barnase.
3.2. Lys–Arg cross-links are specific in barnase
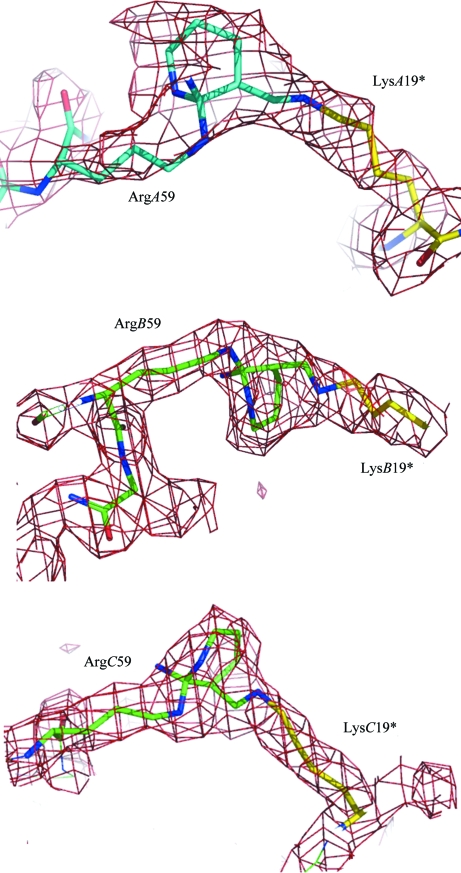
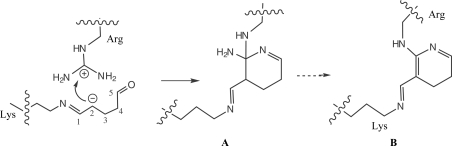
The electron density in the cross-linked barnase is particularly clear: it shows the formation of a six-membered ring in place of the guanidinium moiety of Arg59 and an anti Schiff base with N∊ of a symmetry-related Lys19 (Fig. 2 ▶). This signature is the only difference between the native and cross-linked structures and is identical in the three barnase monomers (Table 2 ▶). As such, this signature demonstrates the specificity of the cross-linking, with Lys19 N∊–Arg59 Cζ distances of about 3–3.6 Å. From comparison with the native structure, the three covalent cross-links are found have no effect on the orientation and distances of the barnase main chains, as shown by the respective Arg59 Cα–Lys 19* Cα distances reported in Table 2 ▶. However, the side chains of the two residues involved in the cross-links are strongly affected. In the native structure the two side chains are randomly oriented towards the pool of solvent, with high thermal factors (in some cases the Arg side chain is poorly defined). In the cross-linked structure these side chains are clearly observed as well as the bridging system. The model used to fit the extra bridges shown in Fig. 2 ▶ corresponds to compound A as shown in Fig. 3 ▶. Only one stereoisomer is shown; however, the formation of the six-membered ring is certainly not stereospecific as it includes a carbocation. One may also take into account the other stereoisomers at C2 and Cζ or a mixture of these.
Figure 2.
The three intermolecular cross-linking sites at barnase interfaces. In the trimer, the three Arg–Lys links each correspond to a pair of equivalent symmetry-related molecules (A–A*, B–B* and C–C*; see Table 2 ▶). Weighted mF o − DF c maps contoured at 1.5σ above the mean are shown.
Table 2. Perturbations induced by glutaraldehyde.
| Molecules | First residue | Second residue† | Cα—Cα distances‡ | N∊—Cζ distances‡ |
|---|---|---|---|---|
| A–A* | LysA19 | ArgA59* [3 0 0 0] | 13.13/12.49 | 7.01/3.65 |
| B–B* | LysB19 | ArgB59* [2 1 1 −1] | 12.67/12.01 | 8.86/2.84 |
| C–C* | LysC19 | ArgC59* [2 0 1 −1] | 12.38/12.00 | 9.49/3.34 |
The operators of the symmetry-related monomers are given in square brackets and follow the usual notation of the P32 space group: the symmetry number (1, x, y, z; 2, −y, x − y, 2/3 + z; 3, −x + y, −x, 1/3 + z) is followed by the three translations.
The first value corresponds to native barnase and the second to the cross-linked structure.
Figure 3.
Putative mechanism of formation of the Lys–Arg cross-link as deduced from the observed electron density, using the monomer of glutaraldehyde as the primary reagent. An equivalent ring formation would also result when using the ene-aldehyde group of an oligomer formed by chain condensation of glutaraldehyde. The C2 atom (activated by either the α-ene group and/or an external water molecule) is represented activated as a carbanion. Nucleophilic attack at the positively charged Cζ atom of arginine is followed by ring closure onto one of the NH2 groups to yield A, which may eventually give rise to B by β-elimination.
In addition to Lys19 and Arg59, barnase contains seven further lysines as well as four further arginines. In the three independent molecules none of these were found to be connected to extra density that is relevant to the glutaraldehyde reaction, with the exception of LysA27, which was dubious and was refined as a residue that was disordered over two positions. Moreover, two of these residues, Lys66 and Arg69, are suitably oriented and close enough to be cross-linked by glutaraldehyde. The electron densities did not reveal any connection between these two residues, indicating that the Lys19–Arg59 doublet is a specific and unique site in barnase.
3.3. Mechanism of formation of the Lys–Arg bridge
The mechanism of glutaraldehyde-bridge formation has recently been reinvestigated (Wine et al., 2007 ▶) in order to address an apparent discrepancy: the length of the glutaraldehyde monomer is about 7.5 Å, while the bridge between two lysines, when observed, is about 3 Å in length, which contradicts the formation of a Schiff base at both ends of the reagent. Instead, the α-ene-aldehyde group resulting from oligomerization/polymerization of glutaraldehyde was invoked by the authors. In the case of the Lys–Arg doublet cross-linked by vapour diffusion a similar situation would be the case, but in contrast to soaking conditions the vapour-diffusion protocol used here favours the slow diffusion of the more volatile monomer at low concentration through the crystal in the drop. Under these conditions, the oligomerization of glutaraldehyde, which is a concentration-dependent process, is expected to be less rapid than reaction of the monomer (or at least oligomers of low molecular weight) with positively charged residues on the surface of the protein. Looking at the electron density, the standard Schiff base appears to be the first step in the reaction on the lysine side. On the arginine side, the formation of the six-membered ring probably arises from a nucleophilic attack on the guanidinium Cζ atom of arginine by the C2 ene-activated carbon of glutaraldehyde (formally represented as a carbanion in Fig. 3 ▶). The additional and unexpected ring formation on the arginine side could arise from the formation of a further Schiff base between one of the nearest ∊-aldehyde groups of the glutaraldehyde monomer/oligomer and the second amino group of arginine (Fig. 3 ▶). Whatever the primary step, the end-product of cyclization would be either compound A, the result of a further β-elimination of the exo NH2 group (compound B) or a mixture of both (Fig. 3 ▶). In Fig. 2 ▶, the Lys–Arg bridges were tentatively modelled as the result of this reaction and found to reasonably fit the three extra electron densities bridging the Lys–Arg doublets. It is interesting to note that each of the three cross-links connects two equivalent molecules (i.e. A to A*, B to B* and C to C*), with the formation of three infinite and independent linear networks throughout the crystal packing along the z axis according to the scheme shown in Fig. 1 ▶(b).
Supplementary Material
PDB reference: barnase cross-linked by glutaraldehyde, 3kch
Acknowledgments
We would like to thank all the scientists and the staff of the late LURE Laboratory in Orsay.
References
- Bishop, W. H. & Richards, F. M. (1968). J. Mol. Biol.33, 415–421. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Haas, D. J. (1968). Biophys. J.8, 549–555. [DOI] [PMC free article] [PubMed]
- Hartley, R. W. (1988). J. Mol. Biol.202, 913–915. [DOI] [PubMed]
- Heras, B. & Martin, J. L. (2005). Acta Cryst. D61, 1173–1180. [DOI] [PubMed]
- Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. (1991). Acta Cryst. A47, 110–119. [DOI] [PubMed]
- Korn, A. H., Feairheller, S. H. & Filachione, E. M. (1972). J. Mol. Biol.65, 525–529. [DOI] [PubMed]
- Lalonde, J. J., Navia, M. A. & Margolin, A. L. (1997). Methods Enzymol.286, 443–464.
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst.26, 283–291.
- Lusty, C. J. (1999). J. Appl. Cryst.32, 106–112.
- Martin, C., Richard, V., Salem, M., Hartley, R. & Mauguen, Y. (1999). Acta Cryst. D55, 386–398. [DOI] [PubMed]
- Morozov, V. N. & Morozova, T. Y. (1981). Biopolymers, 20, 451–467. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–325. [DOI] [PubMed]
- Peters, K. & Richards, F. M. (1977). Annu. Rev. Biochem.46, 523–551. [DOI] [PubMed]
- Quiocho, F. A. & Richards, F. M. (1964). Proc. Natl Acad. Sci. USA, 52, 833–839. [DOI] [PMC free article] [PubMed]
- Quiocho, F. A. & Richards, F. M. (1966). Biochemistry, 5, 4062–4076.
- Richards, F. M. & Knowles, J. R. (1968). J. Mol. Biol.37, 231–233. [DOI] [PubMed]
- Salem, M., Mauguen, Y. & Prangé, T. (2006). Biochem. Biophys. Acta, 1764, 903–912. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wine, Y., Cohen-Hadar, N., Freeman, A. & Frolow, F. (2007). Biotechnol. Bioeng.98, 711–718. [DOI] [PubMed]
- Yonath, A., Sielecki, A., Moult, J., Podjarny, A. & Traub, W. (1977). Biochemistry, 16, 1413–1417. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: barnase cross-linked by glutaraldehyde, 3kch
PDB reference: barnase cross-linked by glutaraldehyde, 3kch