β-Galactosidase from K. lactis has been expressed in S. cerevisiae, purified by affinity chromatography and crystallized in its native form.
Keywords: yeast, β-galactosidases, glycoside hydrolase family 2
Abstract
β-Galactosidase from Kluyveromyces lactis catalyses the hydrolysis of the β-galactosidic linkage in lactose. Owing to its many industrial applications, the biotechnological potential of this enzyme is substantial. This protein has been expressed in yeast and purified for crystallization trials. However, optimization of the best crystallization conditions yielded crystals with poor diffraction quality that precluded further structural studies. Finally, the crystal quality was improved using the streak-seeding technique and a complete diffraction data set was collected at 2.8 Å resolution.
1. Introduction
The enzyme β-galactosidase (β-d-galactoside galactohydrolase; EC 3.2.1.23) catalyzes the hydrolysis of the disaccharide lactose to glucose and galactose. Enzymes with this activity are present in microorganisms, plants and animals and have multiple biotechnological applications. β-Galactosidases are useful in the treatment of lactose intolerance (Bhatnagar & Aggarwal, 2007 ▶) and are frequently used in the food industry in order to increase the sweetening power of natural saccharides (Gonzalez Siso, 1996 ▶). They are also employed in the treatment and transformation of cheese whey (Gonzalez Siso, 1996 ▶). The β-galactosidase from the yeast Kluyveromyces lactis (Kl-β-Gal) is one of the most frequently used β-galactosidases in the biotechnological industry owing to its favourable biochemical properties: it has an optimal neutral pH and a higher stability than β-galactosidases from other sources (i.e. fungal galactosidases). Furthermore, K. lactis is an organism that has been designated GRAS (generally recognized as safe) by the American Food and Drug Administration.
Kl-β-Gal (P00723) is encoded by the gene LAC4 (Gene ID 2897170). Although the gene was sequenced in 1992 (Poch et al., 1992 ▶), the structure of the protein has not yet been reported. The molecular weight of the Kl-β-Gal monomer is 119 kDa and it has been shown that only the dimeric and tetrameric forms of the protein are active, with the tetramer being more active than the dimer (Becerra et al., 1998b ▶).
On the basis of sequence, β-galactosidases have been classified into families 1, 2, 35 and 42 of glycosyl hydrolases in CAZy (Cantarel et al., 2009 ▶). Those from eukaryotic organisms are grouped into family 35, with the exceptions of the K. lactis and K. marxianus β-galactosidases (which are 99% identitical), which belong to family 2 together with the prokaryotic β-galactosidases from Escherichia coli and Arthrobacter sp. While the structures of these last two prokaryotic enzymes have been determined (Juers et al., 2000 ▶; Skálová et al., 2005 ▶), no yeast β-galactosidase structure has been reported to date. Although their sequence similarity to the prokaryotic enzymes is significant (48% similarity to that from E. coli and 47% to that from Arthrobacter sp.) there are many differences, particularly some long insertions and deletions, that may play an important role in protein stability and in substrate recognition and specificity. Knowledge of the three-dimensional structure of Kl-β-Gal will provide an important insight into its mechanism of catalysis and should lead to improvement of its biotechnological applications by rational protein engineering. In this study, we describe the expression, purification and preliminary X-ray crystallographic studies of Kl-β-Gal.
2. Materials and methods
2.1. Expression and purification
The LAC4 gene (Gene ID 2897170) was amplified by PCR from the pLX8 plasmid and cloned in the YEpFLAG vector (Eastman Kodak Company) as reported previously (Becerra et al., 2001 ▶). The construct was used to transform Saccharomyces cerevisiae BJ3505 cells (Eastman Kodak Company) using the procedure of Ito et al. (1983 ▶). Cells were incubated at 303 K and 250 rev min−1 for 96 h in a 2 l Erlenmeyer flask containing 1 l YPHSM modified medium [1%(w/v) glucose, 3%(v/v) glycerol, 1%(w/v) yeast extract and 8%(w/v) peptone]; these conditions increased the protein expression. Cells were collected by centrifugation (5000g for 10 min at 274 K), resuspended in 0.1 M KH2PO4, 1.2 M sorbitol and incubated at 303 K for 3 h with lyticase (2 mg per gram wet weight) in order to obtain the protoplasts (Jigami et al., 1986 ▶), from which protein extracts were prepared as described previously (Becerra et al., 1998a ▶). Kl-β-Gal was purified with anti-FLAG M2 affinity gel (Sigma Chemical, USA) and concentrated with Ultra-4 (Millipore, UFC 803024) as reported previously (Rodríguez et al., 2008 ▶). The purified protein, with the FLAG peptide (SDYKDDDDK) attached to its N-terminus, was concentrated to 7 mg ml−1 in 0.05 M Tris–HCl, 0.150 M NaCl and 0.002 M DTT. The homogeneity of the purified protein sample was analyzed (Fig. 1 ▶) by SDS–PAGE (Laemmli, 1970 ▶).
Figure 1.
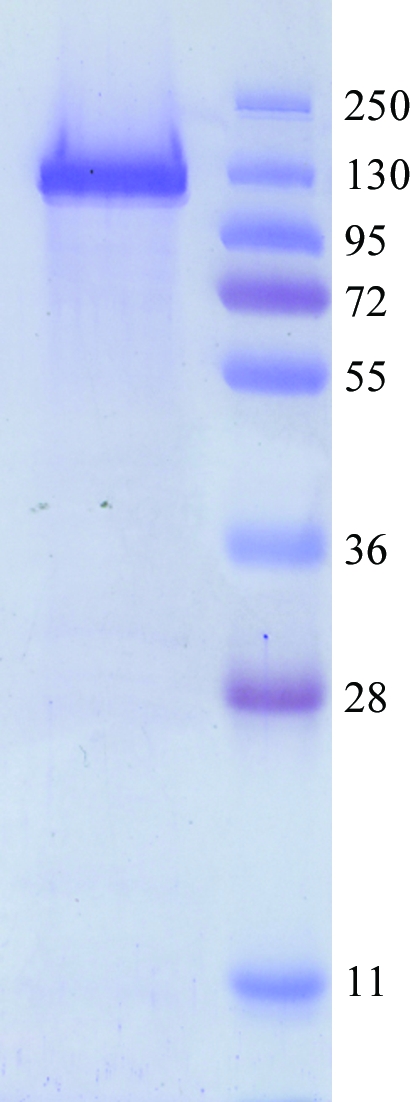
SDS–PAGE analysis of the purified sample of β-galactosidase (119 kDa).
2.2. Crystallization
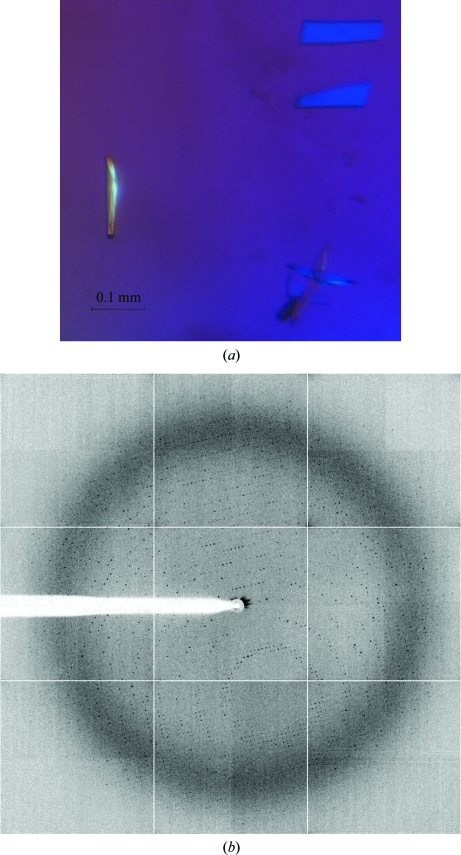
Crystallization conditions were initially explored using high-throughput techniques with a NanoDrop robot (Innovadyne Nanodrop I) and commercially available screens. Crystal Screen, Crystal Screen II, Crystal Screen Lite, SaltRx and Index Screen from Hampton Research, PACT Suite and JCSG+ Suite from Qiagen and JB Screen Classic from Jena Biosciences were assayed using the sitting-drop vapour-diffusion method at 291 K. Drops consisting of 0.25 µl precipitant and 0.25 µl pure Kl-β-Gal (3.5 mg ml−1 in 0.05 M Tris–HCl, 0.150 M NaCl and 0.002 M DTT) were equilibrated against 80 µl reservoir solution on sitting-drop microplates (Innovaplate SD-2). Small crystals grew in several conditions from the PACT Suite screen when 7%(v/v) glycerol was added to the protein stock solution prior to setup of the experiment. Initial hits were then tested on Cryschem (Hampton Research) sitting-drop plates by mixing 1 µl protein solution with 1 µl precipitant solution and equilibrating against 500 µl reservoir solution. Crystallization trials in the presence of agarose were performed by adding 0.2% agarose to the drop using a preheated stock of 0.4% agarose in precipitant solution. Crystallization conditions were optimized further and small plate-shaped crystals grew in 23–27%(w/v) polyethylene glycol (PEG) 3350, 0.1 M bis-tris pH 7.5, 0.2 M sodium tartrate. Streak-seeding (Stura & Wilson, 1991 ▶) performed under these conditions gave better-quality crystals that were suitable for X-ray diffraction experiments (Fig. 2 ▶).
Figure 2.
(a) Crystal of β-galactosidase grown in 23–27%(w/v) PEG 3350, 0.1 M bis-tris pH 7.5 and 0.2 M sodium tartrate by streak-seeding from previous crystals. (b) X-ray diffraction pattern obtained using a synchrotron source. The outer resolution shell is 2.8 Å (2.0 Å at the edge of the detector).
2.3. X-ray data collection and processing
All crystals were cryoprotected before being flash-cooled to 100 K in liquid nitrogen. The mother liquor was substituted by cryoprotectant solution consisting of the crystallization solution containing 20%(v/v) glycerol. Diffraction data were collected using synchrotron radiation on the ID23-1 beamline at the European Synchrotron Radiation Facility (ESRF, Grenoble) using an ADSC Quantum Q315r detector fixed at 415.9 mm and a wavelength of 0.979 Å. The exposure time was set to 0.3 s and the oscillation range was 0.5° per image. The diffraction data collected were processed with MOSFLM (Leslie, 1992 ▶) and scaled using the CCP4 package (Collaborative Computational Project, Number 4, 1994 ▶).
3. Results and discussion
Previous crystallization experiments carried out in our laboratory with a full-factorial experimental design (Rodríguez et al., 2008 ▶) led to the growth of Kl-β-Gal crystals but of rather poor diffraction quality. Trials of different modifications in protein preparation led to an improvement in the purity of the protein sample. For example, although various methods of cell disruption were tried, protein crystals only grew from material obtained from cells pre-treated with lyticase. The initial protein concentration in the crystallizations was 7 mg ml−1 but, after persistent protein precipitation, 7%(v/v) glycerol was added to the protein samples and the protein concentration was reduced to 3.5 mg ml−1 in order to increase its solubility. Repetition of some of the screening tests yielded small plate-like crystals which grew within two weeks in several conditions from the PACT Suite screen with PEG 3350 as the main precipitant agent and a pH of between 6.5 and 8. Subsequently, more than 1000 optimization trials were performed, varying the pH and the PEG type/concentration and using different salts. Clusters of medium-size crystals grew in 23–27%(w/v) PEG 3350, 0.1 M bis-tris pH 7.5 and 0.2 M sodium tartrate. The diffraction of these crystals was weak, hence further improvement of the crystallization conditions was necessary. Neither the use of additives (Additive Screen from Hampton Research) nor the use of different experimental setups (hanging drop, microbatch and agarose-containing drops) improved crystal growth; however, crystal streak-seeding proved to be crucial in obtaining diffraction-quality crystals (Fig. 2 ▶).
More than 30 crystals were tested until a full data set from a single Kl-β-Gal crystal was collected to 2.8 Å resolution (Fig. 2 ▶). Data processing showed that the crystal belonged to the orthorhombic crystal system, with unit-cell parameters a = 139.97, b = 153.40, c = 216.30 Å. Analysis of the systematic absences in h00, 0k0 and 00l indicated the space group to be P212121. Data-collection statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics.
Values in parentheses are for the outer resolution shell.
| Crystal data | |
| Space group | P212121 |
| Unit-cell parameters | |
| a (Å) | 139.97 |
| b (Å) | 153.40 |
| c (Å) | 216.30 |
| Data collection | |
| Beamline | ID23.1, ESRF |
| Temperature (K) | 100 |
| Wavelength (Å) | 0.979 |
| Resolution (Å) | 125.00–2.80 (2.95–2.80) |
| Data processing | |
| Total reflections | 643048 (91525) |
| Unique reflections | 115027 (16572) |
| Redundancy | 5.6 (5.5) |
| Completeness (%) | 100.0 (99.9) |
| I/σ(I) | 7.3 (2.3) |
| Mean I/σ(I) | 15.9 (5.2) |
| Rmerge† (%) | 10.7 (35.7) |
| Rp.i.m.‡ (%) | 4.9 (16.4) |
| Molecules per ASU | 4 |
| Matthews coefficient (Å3 Da−1) | 2.45 |
| Solvent content (%) | 49.83 |
R
merge = 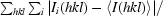
 , where I
i(hkl) is the ith measurement of reflection hkl and 〈I(hkl)〉 is the weighted mean of all measurements.
, where I
i(hkl) is the ith measurement of reflection hkl and 〈I(hkl)〉 is the weighted mean of all measurements.
R
p.i.m. = 
 , where N is the redundancy for reflection hkl.
, where N is the redundancy for reflection hkl.
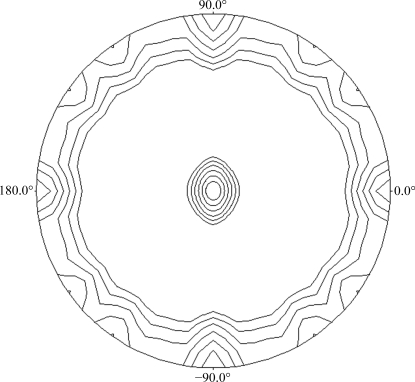
Analysis of the Matthews coefficient (Matthews, 1968 ▶), assuming the presence of either a dimer or a tetramer in the asymmetric unit, indicated a solvent content of 74.91 or 49.83% and a V M coefficient of 4.90 or 2.45 Å3 Da−1, respectively. The self-rotation functions calculated using POLARRFN (Kabsch et al., 1976 ▶) from the CCP4 package, with a radius of integration of 62 Å and resolution limits of 50–5 Å, showed two peaks in the κ = 180° section of the stereographic projection (Fig. 3 ▶), revealing noncrystallographic twofold symmetry that could be compatible with a tetrameric state of the protein. Structure determination was carried out using the structure of Arthrobacter sp. β-galactosidase (PDB code 1yq2; Skálová et al., 2005 ▶) as the template for creating the model for molecular replacement using the program CHAINSAW (Stein, 2008 ▶) within the CCP4 suite and the LAC4 sequence (nonconserved residues were pruned to the γ atom). The sequence homology between these two proteins is 47% (32% identity). Molecular replacement was performed with the program MOLREP (Vagin & Teplyakov, 1997 ▶) using reflections within the resolution range 30–2.8 Å and a radius of integration of 62 Å. A single solution containing four monomers in the asymmetric unit with a final correlation coefficient of 0.27 and an R factor of 0.55 was obtained, confirming a tetrameric quaternary structure of this enzyme. Refinement and building of the Kl-β-Gal model, which contains as many as 4000 amino-acid residues, is in progress.
Figure 3.
Plot of the self-rotation function of the β-galactosidase Patterson function using data between 50 and 5 Å and a 62 Å radius of integration in the κ = 180° section. The view is down the c axis. ϕ = 0° and ϕ = 90° correspond to the a and b axes, respectively.
Acknowledgments
RFL received an FPU fellowship from Ministerio de Educación y Ciencia. APR received a María Barbeito fellowship from Xunta de Galicia. Research at Universidade da Coruña was supported by grant 07TAL010103PR from Xunta de Galicia co-financed by FEDER (CEE). General support of the laboratory during 2008–2009 was funded by ‘Programa de axudas para a consolidación e a estruturación de unidades de investigación competitivas da Consellería de Educación e Ordenación Universitaria’ (Xunta de Galicia). Research at Instituto de Química-Física Rocasolano was supported by grant BIO2007-67708-C04-04 from Dirección General de Investigación. This is a product of the project ‘Factoría de Cristalización’ from the program Consolider-Ingenio 2010.
References
- Becerra, M., Cerdan, M. E. & Gonzalez Siso, M. (1998a). Biol. Proced. Online, 1, 48–58. [DOI] [PMC free article] [PubMed]
- Becerra, M., Cerdan, M. E. & Gonzalez Siso, M. (1998b). Biotechnol. Tech.12, 253–256.
- Becerra, M., Díaz Prado, S., Gonzalez Siso, M. & Cerdan, M. E. (2001). Prot. Eng.14, 379–386. [DOI] [PubMed]
- Bhatnagar, S. & Aggarwal, R. (2007). Br. Med. J.334, 1331–1332. [DOI] [PMC free article] [PubMed]
- Cantarel, B., Coutinho, P., Rancurel, C., Bernard, T., Lombard, V. & Henrissat, B. (2009). Nucleic Acids Res.37, D233–D238. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Gonzalez Siso, M. (1996). Bioresour. Technol.57, 1–11.
- Ito, H., Fukuda, Y., Murata, K. & Kimura, A. (1983). J .Bacteriol.153, 163–168. [DOI] [PMC free article] [PubMed]
- Jigami, Y., Muraki, M., Harada, N. & Tanaka, H. (1986). Gene, 43, 273–279. [DOI] [PubMed]
- Juers, D. H., Jacobson, R., Wigley, D., Zhang, X., Huber, R. E., Tronrud, D. & Matthews, B. W. (2000). Protein. Sci.9, 1685–1699. [DOI] [PMC free article] [PubMed]
- Kabsch, W., Kabsch, H. & Eisenberg, D. (1976). J. Mol. Biol.100, 283–291. [DOI] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr 26
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Poch, O., L’Hote, H., Dallery, V., Debeaux, F., Fleer, R. & Sodoyer, R. (1992). Gene, 118, 55–63. [DOI] [PubMed]
- Rodríguez, Á., Leiro, R., Cerdán, M., González Siso, M. & Fernández, M. (2008). J. Mol. Catal. B Enzym.52, 178–182.
- Skálová, T., Dohnálek, J., Spiwok, V., Lipovová, P., Vondráčková, E., Petroková, H., Dušková, J., Strnad, H., Králová, B. & Hašek, J. (2005). J. Mol. Biol.353, 282–294. [DOI] [PubMed]
- Stein, N. (2008). J. Appl. Cryst.41, 641–643.
- Stura, E. & Wilson, I. (1991). J. Cryst. Growth, 110, 271–282.
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst.30, 1022–1025.